Abstract
This review summarizes the recent knowledge obtained on the molecular mechanisms involved in the intrinsic and acquired resistance of cancer cells to current cancer therapies. We describe the cascades that are often altered in cancer cells during cancer progression that may contribute in a crucial manner to drug resistance and disease relapse. The emphasis is on the implication of ATP-binding cassette (ABC) multidrug efflux transporters in drug disposition and antiapoptotic factors, including epidermal growth factor receptor cascades and deregulated enzymes in ceramide metabolic pathways. The altered expression and activity of these signaling elements may have a critical role in the resistance of cancer cells to cytotoxic effects induced by diverse chemotherapeutic drugs and cancer recurrence. Of therapeutic interest, new strategies for reversing the multidrug resistance and developing more effective clinical treatments against the highly aggressive, metastatic, and recurrent cancers, based on the molecular targeting of the cancer progenitor cells and their further differentiated progeny, are also described.
Important advances in the development of novel early diagnostic and prognostic methods and therapeutic treatments of cancers using surgical tumor resection, hormonal therapies, radiotherapy, or adjuvant chemotherapy, alone or in combination, have been achieved in past years.1–13 This has led to a substantial increase in the cure rate for patients diagnosed in the early stages of localized cancers. For patients diagnosed in the late stages of locally invasive and metastatic cancers, the systemic chemotherapeutic regimens represent one of the principal clinical options. In general, the current chemotherapeutic treatments may contribute to enhancing the time to disease progression, overall survival, and quality of life for patients with advanced and aggressive disease states. Unfortunately, current chemotherapeutic treatments for advanced cancers often result in disease relapse and ultimately lead to the death of the patients.2–6,9,11,12,14–19 The development of resistance by cancer cells to hormonal therapies, radiotherapy, and chemotherapeutic drugs, which usually occurs during cancer progression and after long-term treatment, still represents a major challenge in the clinical cure of advanced and metastatic cancer forms. Therefore, this underlines the critical importance of establishing molecular mechanisms involved in the drug disposition and resistance or multidrug resistance (MDR) of cancer cells for improving current therapies against aggressive cancers in the clinics. Numerous works have indicated that the alterations in diverse signaling elements may contribute to high levels of resistance to one or some chemotherapeutic drugs and radiation.20–26 Among them are the elevated expression and activity of ATP-binding cassette (ABC) multidrug efflux pumps, DNA repair enzymes, and growth factor signaling elements, including epidermal growth factor receptor (EGFR), hedgehog, and Wnt/β-catenin, as well as alterations in ceramide metabolism enzymes and changes in expression levels of drug targets or the inactivation of drugs.
Recent lines of evidence have also revealed that the accumulation of genetic or epigenetic alterations, including mutations in adult stem cells or early progeny during their lifespan, may lead to their malignant transformation into leukemic or tumorigenic cancer progenitor cells, also designated as cancer stem cells or cancer-initiating cells.3,12,13,21,27–39 The leukemic or tumorigenic cancer progenitor cells may acquire a more malignant phenotype during cancer progression and give rise to further differentiated cancer cell sub-populations constituting the primary and secondary neoplasms. In support of this, a population of cancer progenitor cells representing a very small fraction of total cancer cells and expressing the specific stem cell-like markers, including CD133, CD44, Sca-1, Oct-3/4, c-KIT, and/or ABC multidrug efflux transporters, has been identified in numerous cancers. Among them are leukemias and diverse human solid tumor types, including skin, lung, brain, breast, ovarian, prostate, pancreatic, gastrointestinal, and colorectal cancers.12,13,15,27,29,32,39–48 It has been observed that a small number of these cancer progenitor cells isolated from the patients’ malignant tissue specimens can initiate and drive leukemia or tumor progression in animal models in vivo, whereas the bulk mass of further differentiated cancer cells was non-leukemic, non-tumorigenic, or only formed, tumors when implanted in very high numbers. 27,29,32,41,42,44,45,47,48 Hence, the differences between the functional properties of leukemic or tumorigenic cancer progenitor cells versus their further differentiated progeny, emphasize the importance of considering the intrinsic properties of these cancer-initiating cells to overcome resistance to current cancer therapies. Indeed, the leukemic or tumorigenic cancer progenitor cells, similar to their normal counterpart, the adult stem cells, generally display an active DNA repair and high-expression levels of some ABC multidrug efflux pumps, antiapoptotic factors, and inhibitors of apoptosis (Figure 1).13,15,21,24,49,50 The intrinsic properties of cancer progenitor cells may provide them a greater resistance to current clinical therapies than that observed for their further differentiated progeny. Furthermore, the reactivation of diverse developmental signaling pathways involved in stem cell self-renewal, including EGFR, hedgehog, and Wnt/β-catenin, which frequently occurs in cancer progenitor cells during cancer initiation and progression, may contribute to their sustained growth, survival, drug resistance, and disease relapse (Figure 1).12,13,25,26,30,31,39,51–53 The host cells, including the tumor fibroblasts, infiltrating macrophage, and endothelial cells, may also influence the behavior of cancer progenitor cells during tumor progression.12,39,54 Thus, the persistence of leukemic or tumorigenic cancer progenitor cells at the primary or metastatic sites after the current clinical treatments may be responsible, at least in part, for the recurrence of the most aggressive cancers. In this matter, we report the molecular mechanisms often associated with the resistance of cancer cells to current cancer therapies and disease relapse. We also describe new therapeutic strategies based on molecular targeting for deregulated signaling elements involved in the survival and resistance of cancer cells, particularly of cancer stem/progenitor cells.
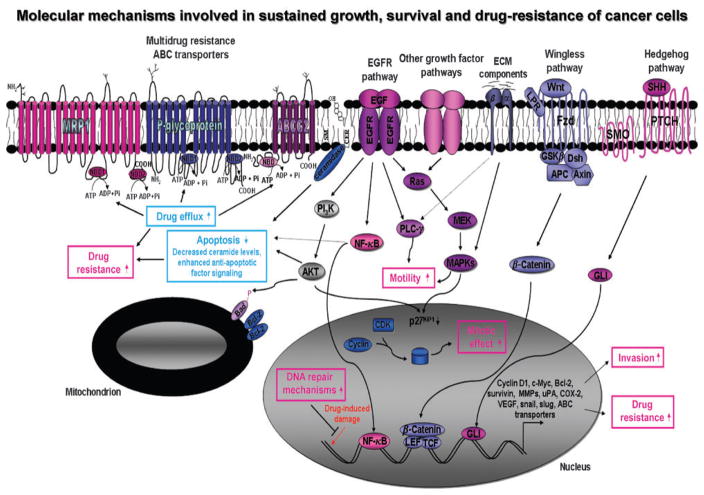
Figure 1.
Schematic showing the possible molecular mechanisms involved in the sustained growth, survival, invasion, and drug resistance of cancer cells. The oncogenic intracellular cascades induced through the activation of distinct growth factor signaling pathways, including EGF–EGFR system, sonic hedgehog SHH/PTCH/GLI, Wnt/β-catenin, and extracellular matrix (ECM) component/integrins, which may provide a critical role for the sustained growth, survival, and invasion of cancer cells, are shown. The upregulated expression levels of certain target gene products, including upregulated antiapoptotic factors Bcl-2 and survivin, matrix metalloproteinases (MMPs), urokinase plasminogen activator (uPA), cyclooxygenase (COX-2), vascular epidermal growth factor (VEGF), transcriptional repressor of E-cadherin (snail and slug), and MDR ABC transporters that can contribute to the malignant transformation of cancer cells and drug resistance are indicated. In addition, the possible drug efflux via the MDR ABC transporters, including MRP1, P-glycoprotein, and brain cancer resistance protein (BCRP/ABCG2), is also shown. APC, adenomatous polyposis coli; CDK, cyclin-dependent kinase; CER, ceramide; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FZD, Frizzled receptor; IGF, insulin-like growth factor; IGF-1R, insulin-like growth factor-1 receptor; LEF, lymphoid enhancer factor; LPR, low-density lipoprotein receptor-related protein; MAPKs, mitogen-activated protein kinase; MEK, extracellular signal-related kinase kinase; NBD, nucleotide binding domain; NF-κB, nuclear factor-κB; PI3K, phosphatidyl inositol 3′-kinase; PLC-γ, phospholipase C-γ; SHH, sonic hedgehog; SM, sphingomyelin; SMO, smoothened; TCF, T-cell factor; uPA, urokinase plasminogen activator; Wnt, wingless.
CANCER THERAPIES AGAINST LOCALLY ADVANCED AND METASTATIC CANCERS
Current clinical cancer therapies
Significant therapeutic advances have led to the cure of patients diagnosed with diverse types of localized cancers in the clinics.1–13 Nevertheless, most patients who undergo potentially curative tumor resection, hormonal therapy, and radiotherapy for advanced and metastatic cancers subsequently relapse because of the persistence of cancer cells in primary tumors and micrometastases.3,11–13,15,19,34,39,43,55–58 Therefore, systemic chemotherapy may represent another option to eradicate the cancer cells at the primary and distant metastatic sites. Several preclinical and clinical trials have been performed to increase the efficacy of current chemotherapeutic regimens used for the treatment of patients with locally advanced and metastatic cancers in the clinics. Although there have been important advances, the current chemotherapeutic regimens remain ineffective against the most advanced and metastatic cancers. This is partly due to dose-limiting toxicity that restricts their use at high doses, and to the development of diverse drug resistance and MDR mechanisms by cancer cells. It has been observed that the combinations of chemotherapeutic drugs, which target distinct oncogenic signaling elements, are generally more effective than individual drugs.2,3,12,13,59 The combination of low doses of drugs may decrease certain side effects associated with the use of high concentrations of single drugs. Of particular interest, the combined use of high-dose chemotherapy, high-dose sequential chemotherapy, or highintensity ionizing radiation with subsequent autologous or allogenic stem cell transplantation support may also constitute alternative effective strategies. These therapeutic approaches are particularly considered among the principal options in the clinics for patients with high-risk or relapsed/refractory cancers. These treatment types can be used to treat leukemias, multiple myeloma, and Hodgkin’s and non-Hodgkin’s lymphomas as well as advanced and metastatic solid tumors such as sarcoma, neuroblastoma, medulloblastoma, retinoblastoma, and lung, breast, kidney, ovarian, and colorectal cancers.13,60–72 For instance, the results from two prospective phase II trials have revealed that high-dose sequential chemotherapy followed by autologous peripheral blood stem cell transplantation resulted in an estimated 12-year overall, disease- and event-free survival of 34, 55, and 30%, respectively, in 46 patients diagnosed with advanced stage peripheral T-cell lymphomas.65 It has also been noted that this treatment type, given a higher rate of long-term complete remission in patients with anaplastic lymphoma-kinase-positive anaplastic large-cell lymphoma and complete remission before autografting, may represent an important factor indicative of a better prognosis of patients.65 Thus, the choice of the therapeutic treatments, including the regimen option, drug, doses, and administration sequence of combined drugs should be optimized and adapted to each patient in a particular clinical setting. The clinical genetic analyses, including the oncogenic gene expression profiling of patients, may notably improve the diagnostic and prognostic tests in certain cancer cases. It may also help to establish the most appropriate types of therapeutic intervention to counteract the recurrence of the disease.
Pharmacogenetic analyses
Numerous pharmacogenetic investigations have revealed that the interindividual differences in genetic polymorphisms and mutations may influence the risk for developing certain cancer types and the patient’s response to a particular therapeutic approach. 73–77 Particularly, the genetic polymorphisms or mutations in the drug targets, the transporters or metabolizing enzymes involved in drug disposition and DNA repair enzymes, may contribute to the variability in drug pharmacokinetic and pharmacodynamic processes and also drug response and observed toxicity.73–75,77,78 For instance, certain polymorphisms in human multidrug transporter gene ABCB1 encoding P-glycoprotein (P-gp) or DNA repair enzymes such as excision-repair cross-complementing group 1 protein might be associated with the chemotherapy response observed in lung cancer patients.79,80 Similarly, the occurrence of EGFR gene polymorphisms or mutations may also influence the response to therapeutic cancer treatment and outcome of patients.74,76
Some studies have also revealed that the risk of disease relapse, which is generally associated with the presence of micrometastases at distant sites, might be predicted by the specific gene expression profile in the primary neoplasm, whose signature may be indicative of the rate of recurrence and the overall survival of patients.14,81–85 Thus, pharmacogenetic analyses in patients’ cancer tissue samples by methods of genotyping, such as traditional DNA sequencing and microarray technology, could aid in determining the potent resistance or response of patients to a particular type of cancer therapy. The gene expression profiling of diverse human cancer tissue samples, made before or after adjuvant chemotherapy, has provided important information on the molecular signatures that may aid in predicting the potent chemoresistant phenotype and the prognosis of patients.86–89 For instance, the microarray analyses of gene expression patterns in leukemic blasts from 360 pediatric patients with acute lymphoblastic leukemia revealed that the gene expression profiles may help to identify the patients at high risk for failing therapeutic treatments.88 The DNA microarray screening of the expression of approximately 21,000 genes in paired tumor samples, taken before and after chemotherapeutic treatment from six patients with predominantly advanced stage, high-grade epithelial ovarian cancers, has revealed that the intrinsic and acquired chemoresistant phenotypes of post-chemotherapeutic tumors, relative to primary tumors, may be attributed to the combined action of different factors.87 These factors may be implicated in regulatory mechanisms of cell proliferation, tumor progression, and chemoresistance.87 In addition, the cytogenetic analyses of 23 cancer cell lines, made resistant to either camptothecin, cisplatin, etoposide (VP-16), doxorubicin, or 1-β-D-arabinofuranosylcytosine, have also revealed that the genomic alterations in the copy numbers of certain genes may be a characteristic related to the acquisition of drug resistance mechanisms in cancer cell lines.90 More specifically, the amplification of several ABC multidrug efflux transporter genes, such as multidrug resistance 1 (MDR1), multidrug resistance-associated protein 1 (MRP1), and antiapoptotic factor Bcl-2, or a decrease in the copy numbers of genes encoding deoxycytidine kinase, DNA topoisomerase I, and DNA topoisomerase II-α were observed in certain drug resistance cancer cells examined, as compared to drug-sensitive parental cancer cells.90 A recent study has also indicated that an increase of MRP1 expression in a subset of patients with breast cancer may be associated with the resistance to standard adjuvant cyclophosphamide, methotrexate, fluorouracil chemotherapy, but it does not alter the response to tamoxifen and goserelin.91 This suggests that the analyses of the MRP1 expression could aid in determining whether the adjuvant endocrine treatment could be beneficial to estrogen receptor alpha (ERα)-positive patients. Thus, on the basis of these observations, it appears that the clinical pharmacogenetic analyses of biomarkers of sensitivity of cancer cells to specific drug treatments could be beneficial in certain cancer cases. For instance, the analyses of EGFR expression levels and the mutational status in biopsy material could be used to select patients who would benefit from a clinical treatment with the selective EGFR inhibitors such as gefitinib. Hence, the identification and molecular targeting of signaling elements involved in self-renewal, invasion, and the drug resistance of cancer cells may constitute a novel promising approach for improving the current chemotherapeutic treatments of patients with aggressive and metastatic cancers in the clinics.15,92 We describe here the molecular mechanisms that may contribute to the intrinsic and acquired resistance phenotypes of cancer cells, and that may influence their sensitivity to conventional cancer therapies and disease relapse.
Drug resistance mechanisms of cancer cells and targeting therapies
A major problem in the clinical treatment of advanced and metastatic cancer forms is the intrinsic or acquisition of a resistant phenotype by cancer cells to current therapeutic treatments.21 Particularly, the acquisition of the MDR phenotype by cancer cells, which may occur after exposition to one specific drug, and may result in the cross-resistance to other structurally and functionally unrelated anticancer drugs.93,94 Drug resistance mechanisms, including the MDR phenomenon, may be associated with alterations in the expression and activity of a complex framework of signaling elements that actively protect the cancer cells from the cytotoxic effects induced by xenobiotics, including chemotherapeutic drugs (Figure 1). Therefore, the assessment of the multiple factors related to the chemotherapeutic drug resistance is necessary for the development of new drugs and therapeutic regimens for a more effective treatment of aggressive and recurrent cancers.
Many metastatic cancer cell lines established from skin, brain, non-small-cell lung (NSCLC), prostate, breast, ovarian, pancreatic, and colorectal cancers show altered expression and activation of diverse signaling cascades. Among them, there are enhanced activation of diverse developmental signaling pathways, including EGFR, hedgehog, and Wnt/β-catenin as well as certain signaling elements, such as PI3K (phosphatidyl inositol 3′-kinase)/Akt, nuclear factor-κB, Bcl-2, cyclooxygenase-2 (COX-2), survivin, snail, slug, and twist. These pathways may contribute to their uncontrolled growth, survival, invasion, and resistance to conventional therapies (Figure 1).12,13,22,23,25,26,30,31,39,51,52,95 The establishment of cancer cell lines showing high levels of drug resistance has allowed researchers to identify certain deregulated gene products that may contribute to the acquired resistance of cancer cells to antineoplastic drugs. Among them are the elevated expression and activity of ABC multidrug efflux pumps, DNA repair enzymes, and growth factor signaling elements, including EGFR, hedgehog, and Wnt/β-catenin. Moreover, the alterations in apoptotic signaling elements such as ceramide metabolism enzymes and changes in expression levels of drug targets and the inactivation of drugs may also provide cancer cells with a high resistance to one or some chemotherapeutic drugs and radiation.20–26 Therefore, specific inhibitory agents that target signaling pathway elements involved in cancer progression, invasion, and resistance to conventional cancer treatments are of therapeutic interest. These agents could be used in novel clinical therapeutic regimens and in improving current treatments against aggressive, metastatic, and recurrent cancers (Table 2).11,25,96 Regardless, we report, in a more detailed manner the implication of ABC multidrug efflux transporters in the drug disposition, EGFR-dependent signaling pathways, and the enzymes involved in the ceramide metabolism as well as the novel therapeutic agents developed for their molecular targeting. Of particular therapeutic interest, we present an overview of the recent knowledge obtained on specific alterations that may contribute to the intrinsic and acquired resistance and decreased response of leukemic or tumorigenic cancer stem/progenitor cells to antineoplastic drugs. We also discuss the critical importance of considering their molecular targeting for improving the current clinical cancer therapies.
Table 2.
Inhibitory agents targeting deregulated signaling elements involved in sustained growth, survival, and drug resistance of cancer cells
| Targeted signaling element | Name of inhibitory agent |
|---|---|
| Growth and survival signaling | |
| EGFR family member inhibitors | |
| Anti-EGFR (erbB1) antibody | mAb-C225, IMC-C225 |
| Anti-EGF antibody | ABX-EGF |
| Antisense oligonucleotide | As-EGFR, As-EGF, As-TGF-α |
| EGFR-TKI | AG1478, gefitinib, erlotinib, EKB-569 |
| Anti-erbB2 antibody | Trastuzumab |
| EGFR-ErbB2-TKI | PKI-166, TAK165, GW572017 (lapatinib) |
| erbB1, erbB2, erbB3, erbB4-TKI | CI1033 |
| EGFR/VEGFR-TKI | AEE788, ZD6474 |
| Other growth factor signaling inhibitors | |
| Hedgehog signaling inhibitor | SMO inhibitor cyclopamine, anti-SHH antibody |
| Wnt signaling inhibitor | Anti-Wnt antibody, WIF-1 |
| Notch signaling inhibitor | γ-secretase inhibitor |
| IGF-1R signaling inhibitor | Adenovirus-IGF-1r/dn, anti-IGF-1R antibody (A12), IGF-1R-TKI (NVP-AEW541) |
| BCR-ABL-TKI | Imatinib mesylate (ST1571), dasatinib, nilotinib |
| Src-family and ABL-TKI | PD180970 |
| Src-family-TKI | CGP-76030 |
| Hormonal signaling inhibitors | |
| AR | Bicalutamide, flutamide |
| ER | Tamoxifen, raloxifen |
| Apoptotic signaling activators | |
| Ceramide generation activator | Docetaxel, paclitaxel, etoposide, doxorubicin, gefitinib, |
| Ceramide synthase activator | PSC833 |
| Ceramidase inhibitor | N-oleoylethanolamine (OE), LCL204, B13, D-e-MAPP |
| GCS inhibitor | D,L-threo-PPMP (PDMP), PPMP, tamoxifen |
| Sphingosine kinase-1 inhibitor | N,N-dimethylsphingosine (DMS), F-12509a |
| Bcl-2 inhibitor | As-Bcl-2, ABT-737 |
| PI3K inhibitor | LY294002, rapamycin, CCI-779 |
| NF-κB inhibitor | IκBα inhibitor, sulfasalazine, bortezomib (PS-341) |
| COX-2 inhibitor | NS-398, etodolax, celecoxib, rofecoxib |
| VEGFR inhibitor | Anti-VEGFR-antibody, SU5416 |
| Drug resistance signaling inhibitors | |
| ABC transporter inhibitors | |
| P-gp/MDR1/ABCB1 | UIC2 monoclonal antibody, antisense oligodeoxynucleotide verapamil, dexverapamil, cyclosporin A, valspodar (PSC-833), quinidine, cinchonine, tamoxifen, toremifene, VX-710 and GF-120918, retinoid X receptor-selective agonist bexarotene (LGD1069, Targretin), dofequidar fumarate, MS-209, VX-710, flavonoids, gefitinib, CI1033 |
| MRP1/ABCC1 | Anti-MRP1 antibody, NSAIDs (indomethacin, sulindac, tolmetin, acemetacin, zomepirac, mefenamic acid), quinidine, MS-209, VX-710, flavonoids |
| BCRP/ABCG2 | Flavonoids, tamoxifen derivatives, gefitinib, C11033, Prazosin |
| Organic cation transporter OCT-1 inhibitor | |
| Drug resistance signaling inhibitor | |
| EGFR-TKI-DR | IGF-1R inhibitor |
| AR/anti-androgen-DR | EGFR inhibitor (gefitinib) |
| ERα/anti-estrogen-DR | EGFR inhibitor (gefitinib), erbB2 inhibitor |
ABC, ATP-binding cassette; AR, androgen receptor, BCRP, breast cancer resistance protein; COX, cyclooxygenase; D-e-MAPP, D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ER, estrogen receptor; GCS, glucosylceramide synthase; IGF-1R, insulin-like growth factor-1 receptor; IκBα, inhibitor κBα; mAb, monoclonal antibody; MDR1, multidrug resistance 1; MRP1, multidrug resistance-associated protein 1; NF-κB, nuclear factor-κB; NSAIDs, non-steroidal anti-inflammatory drugs; OCT-1, organic cation transporter- 1; P-gp, P-glycoprotein; PI3K, phosphoinositide-3 kinase; SHH, sonic hedgehog; SMO, smoothened; TKI, tyrosine kinase inhibitor; VEGFR, vascular epidermal growth factor receptor; WIF-1, Wnt inhibitory factor-1; Wnt, wingless.
Targeting of ABC multidrug efflux pumps
The different members of the ABC multidrug efflux transporter superfamily, which are expressed in a variety of tissues/organs, are constituted of either one or two transmembrane spanning domains involved in drug binding and one or two cytoplasmic nucleotide (ATP)-binding domains (Figure 1).17,24,97–103 One of the physiological roles of these cellular transporters is to pump out diverse endogenous substrates at the expense of ATP hydrolysis. Hence, they may protect the normal tissues from the cytotoxic effects of xenobiotics, including the drugs. Each ABC transporter family, however, possesses the specific structural and functional properties and is able to transport only a specific range of several classes of anticancer drugs (Table 1).17,24,97–103 The best characterized ABC transported superfamily members include MDR1 gene product P-gp, MRPs, such as MRP1 and MRP2, and breast cancer resistance protein (BCRP)/ABCG2, also designated as mitoxantrone resistance (MXR) and placenta-specific ATP-binding cassette protein (ABCP) (Figure 1).20,24,97–104 A substantial contribution of the multidrug transporters, P-gp and MRP1, to basal drug resistance has been shown by using engineered mouse fibroblast cell lines, in which the genes encoding for these proteins were inactivated.20 The cell lines lacking functional P-gp were markedly more sensitive to paclitaxel (PTX, 16-fold), anthracyclines (fourfold), and Vinca alkaloids (threefold) than the wild-type cell lines (Table 1). The fibroblast cell lines lacking both P-gp and MRP1 proteins were also highly sensitive to a large range of drugs, including epipodophyllotoxins (four- to sevenfold), anthracyclines (six- to sevenfold), camptothecin (threefold), and Vinca alkaloids, especially vincristine (28-fold), relative to parental cell lines.20 This suggests that a small change in the expression levels of these drug transporters may have a major repercussion on the sensitivity of cancer cells to chemotherapeutic drugs. In addition, the characterization of diverse cancer cell lines showing an acquired resistance to one or several anticancer drugs has allowed researchers to identify certain ABC transporters that may be activated in malignant cells and contribute to drug resistance. For instance, the enhanced expression of P-gp has been associated with the acquired decreased sensitivity of doxorubicin-resistant DU145 and PC3 prostatic cancer cell sublines to various anticarcinogenic drugs. Among them are etoposide, camptothecin, vinblastine, vincristine, fluorodeoxyuridine, doxorubicin, amsacrine, and melphalan (Table 1).105 The implication of the ABC transporter P-gp in drug resistance was further supported by the observation that the treatment of doxorubicin-resistant DU145 and PC3 cells with a P-gp inhibitor, verapamil, restored their sensitivity to doxorubicin. 105 An immunohistochemical analysis of BCRP/ABCG2 expression levels in 150 untreated tumors has also revealed that this transporter is often expressed in several cancer types, specifically, in the carcinomas of the gastrointestinal tract.106 Moreover, the enhanced expression of the gene and protein levels of BCRP/ABCG2 multidrug efflux transporter has been associated with the enhanced resistance to diverse chemotherapeutic drugs, including mitoxantrone, irinotecan, SN-38, topotecan, and anthracycline (Table 1).24,102 For instance, the enhanced expression of BCRP/ABCG2 protein in SN-38-resistant NSCLC cell line NCI-H23 resulted in a decreased sensitivity to SN-38. This was also accompanied by cross-resistance to other anticancer drugs such as topotecan, etoposide, doxorubicin, and mitoxantrone, relative to the parental cell line.107 More specifically, the cellular accumulation of topotecan was decreased in SN-38-resistant NCI-H23 cells compared with that in the parental SN-38-sensitive NCI-H23 cells, and the treatment of SN-38-resistant NCI-H23 with an inhibitor of BCRP/ABCG2 transporter reversed this effect.107 A high level of ABCA2 transporter, which is localized in the endolysosomal compartment in ovarian and small-cell lung cancer cell lines, has also been related to resistance to estramustine and mitoxantrone, respectively. 100,108,109 This suggests the possible implication of ABCA2 transporter in lysosomal detoxification and MDR phenomenon in these cancer cell lines. Moreover, the results of microarray and real-time polymerase chain reaction analyses have also indicated that the ABCA2 and ABCA3 transporters are overexpressed in pediatric T-cell acute lymphoblastic leukemia and acute myeloid leukemia compared with healthy bone marrow. The enhanced expression of these transporters may contribute to resistance to methotrexate, vinblastine, or doxorubicin.110,111
Table 1.
MDR ABC transporter superfamily members and their cytotoxic drug resistance profile
| Transporter/subfamily name | Cytotoxic drug resistance profile |
|---|---|
| ABCA2 | Estramustine, mitoxantrone (MXT), doxorubicin (Doxo) or adriamycin (Adr), methotrexate, vinblastine |
| ABCA3 | Doxorubicin, methotrexate, vinblastine |
| MDR1 (P-gp)/ABCB1 | Anthracyclines (doxorubicin, daunorubicin, epirubicin), actinomycin D, colchicine, mitomycin C, epipodophyllotoxin derivatives (etoposide (VP-16), teniposide), methotrexate, mitoxantrone, taxanes (paclitaxel (PXT or taxol), docetaxel (taxotere)), Vinca alkaloids (vincristine, vinblastine) |
| MRP1/ABCC1 | Anthracyclines, colchicine, etoposide, methotrexate, paclitaxel, camptothecine, vincristine, vinblastine |
| MRP2/ABCC2 | Irinotecan (camptothecin-11, CPT-11, or camptosar), active metabolite of irinotecan (7-ethyl-10- hydroxycamptothecin, SN-38), cisplatin, doxorubicin, etoposide, methotrexate, vincristine, vinblastine |
| BCRP/ABCG2 | Anthracyclines, bisantrene, 9-amino-camptothecin, irinotecan, SN-38, topotecan (TPT), mitoxantrone, methotrexate, etoposide, epirubicin, flavopiridol |
ABC, ATP-binding cassette; BCRP, breast cancer resistance protein; MDR1, multidrug resistance 1; MRP1, multidrug resistance-associated protein 1; MRP2, multidrug resistance-associated protein 2; P-gp, P-glycoprotein.
ABC transporter protein inhibitors
As ABC transporter superfamily members provide a critical function in the acquisition of an MDR phenotype of cancer cells, much effort has been made to overcome MDR mediated through these drug efflux pumps. Strategies developed to reverse MDR include the use of pharmacological agents that can interact with one or several ABC transporters, thereby inhibiting their functions, monoclonal antibody directed against a specific ABC transporter, and antisense oligonucleotide or oligodeoxynucleotide (Figure 2 and Table 2).17,24,103,112–118 An alternative approach also considered using agents that can inhibit the signaling cascade elements responsible for the enhanced expression of ABC transporters in resistant cancer cells.114 Certain pharmacological agents have been identified and observed to inhibit the transport activity of P-gp, MRPs, and BCRP/ABCG2 efflux pumps, increase the intracellular drug accumulation, and reverse MDR in vitro and in vivo, and in certain cases have reached clinical trials. Unfortunately, few successful treatments have been developed based on their use in combination with other conventional chemotherapeutic drugs. The lack of clinical efficacy of most of the ABC transporter modulatory agents tested appears to be due, in part, to their low oral bioavailability, high cellular extrusion and metabolism, additional cellular targets, and cellular systemic toxicity at effective doses.101,119,120 For instance, P-gp transporter protein inhibitors such as verapamil and cyclosporin A, which are used in other clinical therapeutic applications, as well as their analogues, dexverapamil and valspodar (PSC-833), have been observed to reverse the MDR mediated via the P-gp transporter in a variety of cancer cells in vivo and in vitro.21,121 However, the results of phase III trials have indicated that these agent types show a less convincing response rate in clinical settings.21,121 A recent study has revealed that the combined application of different classes of P-gp modulators at low concentrations could represent a more promising approach for improving their efficacy and decreasing the toxicity associated with the use of high concentrations of these individual agents in vivo. For instance, it has been reported that the conformation-sensitive UIC2 monoclonal antibody directed against membrane P-gp protein partially inhibited the P-gp-mediated substrate transport when used alone (Figure 2). Interestingly, the simultaneous application of UIC2 with other modulators, such as cyclosporin A or valspodar, followed by their removal, resulted in nearly 100% inhibition of P-gp drug efflux pump activity.122 This inhibitory effect was also accompanied by a complete restoration of intracellular accumulation of various P-gp substrates.122 Further ongoing investigations also include the novel specific ABC transporter inhibitory agents acting on both P-pg and MRP1 drug efflux pumps, such as quinoline derivative dofequidar fumarate (MS-209) and biricodar (VX-710).120,121,123 Among them, clinical trials with MS-209, a quinolone-derived sphingomyelin synthase inhibitor, have been performed for the treatment of drug-resistant cancers, including breast cancer and NSCLCs.120,123,124 The results from a phase I trial revealed that the combined use of MS-209 with docetaxel does not significantly affect the non-hematological and hematological toxicities and has little effect on pharmacokinetics of docetaxel in a group of patients with advanced and metastatic cancers.125 Moreover, data from another recent clinical trial have also revealed that orally active MS-209 plus cyclophosphamide, doxorubicin, and fluorouracil (CAF) therapy was well tolerated and improved the progression-free survival in patients with advanced or recurrent breast cancer who had not received previous therapy in comparison with CAF treatment alone.123 Additional results from long-term clinical trials should confirm the potent benefit of including this agent type in conventional chemotherapeutic regimens to reverse MDR and improve the overall survival of patients.
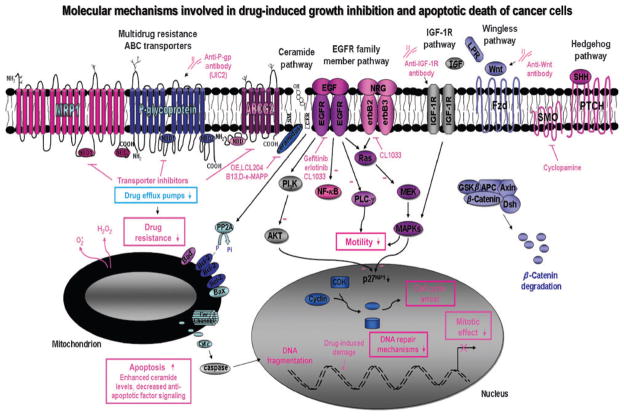
Figure 2.
Proposed molecular targets in cancer cells for the development of novel therapeutic strategies against aggressive and recurrent cancers. The schematic shows the possible antiproliferative and apoptotic effects induced by the tyrosine kinase activity inhibitors, including EGFR (gefitinib and erlotinib) and EGFR/erbB2/erbB3 (CI-1033), as well as by a selective inhibitor of SMO hedgehog signaling element (cyclopamine) and monoclonal antibody directed against Wnt and IGF-1R. Moreover, the reversal of the MDR mediated through ABC transporters by using specific transporter inhibitors and antibody directed against P-glycoprotein drug efflux pump is also shown. Cyt c, cytochrome c; PP2A, protein phosphatase 2A.
Several dietary flavonoids, such as genistein, silymarin, quercetin, biochanin A, daidzein, naringenin, and kaempferol, may also inhibit several ABC transporters, including P-gp-, MRP1-, and BCRP/ABCG2-mediated cellular drug efflux, thereby reversing the drug resistance of cancer cells.126–128 It has also been reported that use of flavonoid cocktails constituted by an equimolar concentration of several flavonoid compounds resulted in additive inhibitory effects on the activity of ABC multidrug efflux pumps.126 This suggests that these natural compounds may constitute the potential adjuvant therapeutic agents to restore the sensitivity of cancer cells to chemotherapeutic drugs. In this matter, it is noteworthy that the flavonoids presented in diets may interfere with treatments that use other ABC transporter inhibitory agents. Additionally, recent lines of evidence have also revealed that certain clinical anticarcinogenic agents, such as anti-estrogens and tyrosine kinase inhibitors (TKIs), may inhibit or completely reverse intrinsic and acquired resistance of cancer cells to distinct chemotherapeutic agents and thereby restore their sensitivity to the cytotoxic effects of drugs. For instance, anti-estrogens, toremifene, tamoxifen, and its derivatives have been observed to increase the sensitivity of breast, lung, gastrointestinal, bladder, and colorectal cancer cell lines to cytotoxic effects induced by anthracyclines, Vinca alkaloids, and taxanes, at least in part, by overcoming P-gp transporter-mediated resistance in these cancer cells.129,130 Particularly, the tamoxifen derivatives may interact with the BCRP/ABCG2 drug efflux pump and thereby enhance the cellular accumulation of topotecan in BCRP-transfected K562 cells (K562/BCRP) and reverse SN-38 and mitoxantrone resistance.131 breakpoint cluster region protein (BCR)-Abelson kinase (ABL) TKI, imatinib mesylate (ST1571), and certain EGFR-TKIs, such as gefitinib and CI1033, as discussed below, have also been reported to act as substrates or inhibitors of functions of P-gp and BCRP/ABCG2 transporters.24,132–141 Hence, these TKIs can reverse MDR mediated by drug efflux pumps in cancer cells in vitro and in vivo.24,132–141 The interaction of TKIs with these ABC transporters expressed in normal tissues may also influence their pharmacokinetics and thereby contribute to resistance and toxicity associated with the use of these agents in vivo.142,143
Targeting of EGFR signaling pathway
The overexpression of EGFR (ErbB1) and its ligands EGF, transforming growth factor-β, heparin-binding EGF-like growth factor, and amphiregulin has been observed to occur during the progression of numerous human aggressive and metastatic cancers, including head and neck squamous cell carcinoma, NSCLC, brain, skin, prostate, breast, ovarian, and gastrointestinal cancers. This event has been associated with the disease relapse and resistance to current hormonal therapies, radiotherapy, and chemotherapies.2,3,12,13,22,23,39,52,95,144–148 The sustained activation of EGFR may lead to the activation of diverse tumorigenic intracellular cascades (mitogen-activated protein kinases, PI3K/Akt, nuclear factor-κB, and phospholipase C-γ) and the enhanced expression of multiple targeted gene products (Bcl-2, survivin, COX-2, vascular epidermal growth factor, urokinase plasminogen activator, and snail) (Figure 1). These cascades may contribute to uncontrolled growth, survival, and invasion of cancer cells. As the EGFR signaling pathway assumes a pivotal role in cancer progression and disease recurrence, many preclinical and clinical investigations have been carried out with the anti-EGFR or EGF antibody, antisense oligonucleotide, and TKIs (Figure 2 and Table 2).2,3,12,13,39,96,146,149–153 Many studies have revealed the beneficial effect of combining these agent types for improving the cytotoxic effects induced by anti-hormonal agents, radiation, and other chemotherapeutic drugs. For instance, it has also been observed that gefitinib, erlotinib, or cetuximab (IMC-C225) may induce additive or synergistic growth inhibitory and apoptotic effects in combination with the anti-androgenic agents, such as bicalutamide, smoothened hedgehog signaling inhibitor (cyclopamine), and diverse chemotherapeutic drugs on diverse cancer cell lines in vitro and in vivo.2,3,12,13,39,144,151–154 Among them are human lung, prostate, ovarian, breast, colorectal, pancreatic, head and neck, bladder, and epidermoid cancer cell lines. Furthermore, the results from a recent investigation have also revealed that gefitinib may inhibit the invasion and migration of tamoxifen-resistant MCF-7 breast cancer cells and restore the sensitivity of MCF-7a cells obtained after long-term estrogen deprivation to tamoxifen and anastrozole.95,155 The gefitinib treatment also restored the sensitivity of hormone-independent and Bcl-2-overexpressing MDR MCF-7/ADR cells to PTX, docetaxel, and IDN S109 in vitro.156 As the activation of insulin-like growth factor-1 receptor (IGF-1R) signaling may lead to the resistance of human breast, prostate, and NSCLC cells to EGFR-TKI such as gefitinib or erlotinib, the inclusion of specific IGF-1R inhibitor, such as anti-IGF-1R antibody or NVP-AEW541, may constitute a strategy to prevent or reverse the resistance of these cancer cell types to these agents (Figure 2 and Table 2).157,158 Gefitinib can delay the emergence of resistance to both estrogen deprivation or anti-estrogen fulvestrant on the ErbB2/Her2/Neu-overexpressing ERα-positive MCF7/HER-2/neu-18 xenograft model in vivo.159 However, the treatment with gefitinib and estrogen deprivation or fulvestrant subsequently led to tumor re-growth, which was accompanied by a loss of ERα, IGF-1R, and an increase of p-HER-2 (phosphorylated human epidermal growth factor receptor 2) and phosphorylated serine|threonine protein kinase (pAKT|PKB) levels (Figure 2 and Table 2).159 This suggests the potential benefit of also including the inhibitors of HER-2 and Akt for patients with ERα/HER-2-positive breast cancer subtypes (Figure 2 and Table 2).159 Moreover, the EGFR inhibitor, PD153035, has also been observed to restore the sensitivity of human melanoma cells and ovarian cancer cells to betulinic acid and PTX, respectively, by inhibiting the drug-induced survivin expression.160,161 Similarly, the inhibition of ErbB1/ErbB2 by using lapatinib (GW572016) also markedly reduced survivin expression and induced apoptosis in ErbB2-overexpressing breast cancer cells compared with trastuzumab and gefitinib alone.162
Several recent studies have indicated that gefitinib may inhibit certain MDR ABC transporter family members, including MDRI/ABCB1 gene product P-gp and BCRP/ABCG2 protein in diverse cancer cell types, overexpressing these drug efflux pumps.135–141 Hence, gefitinib may reverse ABC transporter-mediated MDR and enhance the apparent bioavailability and efficacy of coadministrated drugs that are the substrates of these transporters in vitro and in vivo. For instance, gefitinib may reverse SN-38 resistance in BCRP-transduced human myelogenous leukemia K562 (K562/BCRP) or BCRP-transduced murine lymphocytic leukemia P388 (P388/BCRP) cells in vitro and in vivo.136 Gefitinib may also enhance the sensitivity of MDR BCRP-overexpressing cancer cells, including human PC-6 small-cell lung cancer cells selected with SN-38 (PC-6/SN2-5H), MCF-7 breast cancer cells selected with mitoxantrone (MCF-7/MXT) or BCRP cDNA-transfected MCF-7 cells to topotecan, SN-38, and mitoxantrone.141 The use of gefitinib at clinically relevant concentrations also moderately reversed P-gp-mediated resistance to PTX and docetaxel in P-gp-overexpressing MDR PC-6/PTX small-cell lung cancer and MCF-7/Adr breast cancer cells in vitro.138 Similarly, gefitinib also reversed the resistance to PTX in CLI-Pac cells and doxorubicin in MCF-7/Adr cells, which overexpress P-gp and topotecan resistance in BCRP-overexpressing CL1/TPT and MCF-7/TPT cells. However, these effects did not restore the sensitivity of MRP1-overexpressing MCF-7/VP-16 cells to etoposide.137 This suggests that gefitinib may induce, at least in part, its chemosensitizing effect in MDR cancer cells by inhibiting P-gp and BCRP drug efflux pumps. The coadministration of gefitinib plus a camptothecin derivative, irinotecan (CPT-11), also resulted in an increase of oral bioavailability and tumor growth inhibitory effect induced by irinotecan on a panel of pediatric subcutaneous tumor xenograft models in vivo.140 It has been proposed that this chemosensitizing effect of gefitinib could be mediated, in part, by inhibiting BCRP/ABCG2 transporter activity.
Altogether, these results suggest that the use of EGFR inhibitors, such as gefitinib or CI1033, in combination therapies might have multiple beneficial effects in vitro and in vivo, including the improvement of both bioavailability and antitumoral activities of combined drugs as well as the reversal of MDR of cancer cells by downregulating ABC multidrug efflux pump expression and activity.
Targeting of ceramide signaling pathways
The ionizing radiation, anti-androgens, and many cytotoxic agents, including the chemotherapeutic drugs, alone or in combination therapy, have been reported to mediate, at least in part, their apoptotic/necrotic effects in diverse human cancer cells by enhancing the cellular ceramide levels.3,152,163–170 Among them, there are endogenous lipid anandamide, tumor necrosis factor, tumor necrosis factor-related apoptosis-inducing ligand, docetaxel, PTX, etoposide, doxorubicin, irinotecan, gefitinib, and semisynthetic retinoid, N-(4-hydroxyphenyl) retinamide (or fenretinide). The molecular mechanisms of cytotoxic effects induced by these agents include the activation of de novo ceramide synthesis pathway, acid sphingomyelinase and inhibition of ceramide metabolism (Figure 3). More specifically, the cellular ceramide accumulation in plasma membrane may result in the activation of protein phosphatase 2A and its subsequent translocation to mitochondria where it can contribute to the inactivation of the Bcl-2 protein.163 The ceramide may also participate in the formation of large transmembrane channels (Cer channels) in mitochondria. These effects may subsequently result in permeabilizing mitochondrial outer membrane, releasing cytochrome c and other proapoptotic factors in cytosol, and activation of caspase-dependent and -independent pathways (Figure 2).171–173 It has also been observed that the molecular alterations in cellular ceramide pathways may be responsible, in part, for the resistance of certain cancer cells to chemotherapeutic drugs that mediate their cytotoxic effects via the activation of the cellular ceramide accumulation-induced apoptotic death.163–168,174 For instance, the lack of ceramide generation in response to antimitotic agent, PTX, in the stable human PTX-resistant ovarian carcinoma cell line, CABA-1/PTX, has been associated with PTX resistance.175 More specifically, the enhanced expression and activity of enzymes involved in the metabolism of proapoptotic sphingolipid ceramide, including the acid ceramidase and sphingosine kinase, which participate in subsequent steps to produce antiapoptotic agent, sphingosine- 1-phosphate, may contribute to intrinsic or acquired drug resistance of cancer cells (Figure 3).172,175–179 Similarly, the enhanced activity of glucosylceramide synthase (GCS), the enzyme converting ceramide into glucosylceramide, may also decrease the cellular ceramide levels and rate of apoptotic death of cancer cells. Moreover, the enhanced expression and activity of sphingomyelin synthase 1, which catalyzes the conversion of ceramide and phosphatidylcholine into sphingomyelin and diacylglycerolsphingomyelin, or the decreased function of galactocerebrosidase, which is an enzyme that degrades the galactosylcerebroside into ceramide, may also alter the sensitivity of certain cancer cells to cytotoxic drugs (Figure 3).176,180 On the basis of the major implication of cellular ceramide in triggering the apoptotic death of cancer cells, numerous investigations have been performed for improving the cytotoxic effects of anticarcinogenic drugs by using ceramide metabolism inhibitors to enhance cellular ceramide levels.
Figure 3.

Schematic representation of metabolic pathways involved in the biosynthesis and degradation of the endogenous sphingolipid ceramide. The cellular ceramide (Cer) accumulation induced through the activation of de novo synthesis and sphingomyelin pathways as well as the catabolic pathway involved in ceramide degradation are shown. Moreover, the blockade of the ceramide degradation by using the specific inhibitors of the enzymes that catalyze the transformation of proapoptotic ceramide into its diverse metabolites, including ceramidase, sphingosine kinase, and GCS inhibitors, and whose inhibitory agents can induce the ceramide accumulation-induced apoptotic death in cancer cells are also indicated. D-e-MAPP, D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol; DMS, N,N-dimethylsphingosine; GCS, glucosylceramide synthase; OE, N-oleoylethanolamine; PDMP, D,L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol; PPMP, 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Ceramide metabolism inhibitors
Several studies have revealed that the inhibition of the acid ceramidase activity, the enzyme that hydrolyzes ceramide into its metabolite sphingosine and fatty acid by using N-oleoylethanolamine, LCL204, or B13, potentiated the cytotoxic effects induced by diverse drugs in metastatic and androgen-responsive and-independent prostate cancer cell lines in vitro and in vivo (Figures 2 and 3: Table 2).3,152,181 The MDR associated with doxorubicin and etoposide in HL-60/Doxo and HL-60/VP-16 acute myeloid leukemia cells overexpressing MRP1 and MDR1, respectively, was also reversed by using a specific inhibitor of sphingosine kinase-1, F-12509a.182 Additionally, it has been reported that the upregulated expression of GCS occurring in diverse human cancer cell lines after exposure to drugs, such as doxorubicin (adriamycin), vinblastine, and etoposide, resulted in high-expression levels of glucosylceramide and P-gp protein concomitant with the acquisition of an MDR phenotype.179 Interestingly, the inhibition of GCS by small interfering RNA in doxorubicin-resistant MCF7/AdrR breast cancer cells restored their sensitivity to the DNA-interacting drug, doxorubicin, and other antineoplastic drugs, vinblastine and PTX.183,184 Importantly, the down-regulation of GCS by interfering RNA or using D,L-threo-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol, a potent chemical inhibitor of GCS, also decreased MDR1 expression and enhanced the uptake and cytotoxic effects of chemotherapeutic drugs (Figure 3).183 It is noteworthy that the P-gp transporter modulators such as verapamil, cyclosporin A, and tamoxifen have also been reported to enhance ceramide levels in cancer cells by downregulating the glucosylceramide formation, whereas other agents such as PSC833 may act by stimulating de novo ceramide synthesis pathway (Figure 3 and Table 2).121,124,152,172,176,185–187 Therefore, it is likely that these MDR ABC transporter inhibitory agents may also induce their chemosensitizing effect by promoting the cellular ceramide accumulation-induced apoptotic death.
Hence, in light of these observations, it appears that the alterations in ceramide metabolism in cancer cells may be a determinant factor involved in the MDR phenomenon associated with the use of chemotherapeutic drugs. Moreover, the increase of cellular ceramide levels induced by different cytotoxic agents may also be accompanied simultaneously by downregulating expression or modulation of certain ABC multidrug efflux pumps. The molecular events that are associated with the crosstalk between the ceramide and ABC transporter signaling are not precisely known. However, it is likely that the downregulation of ABC transporter expression induced by certain modulators of ceramide metabolism may contribute, at least in part, to their chemosensitizing effect in particular cancer cell types and vice versa.
Functions of cancer progenitor cells in cancer progression and novel targeting therapies
Numerous lines of evidence indicated that the leukemic or tumorigenic cancer progenitor cells with the stem cell-like properties can assume a critical role by driving leukemia or tumor formation and generating a heterogeneous population of further differentiated cancer cells in primary neoplasms. 3,12,13,15,21,27,29,32,9–45,188–190 The activation of different oncogenic signaling pathways in cancer progenitor cells and their early progeny, as well as the changes in their local microenvironment may also result in their acquisition of a more aggressive phenotype during the progression from localized cancer into invasive and metastatic disease states. More specifically, the tumorigenic cancer progenitor cells may give rise to poorly to moderately differentiated progeny and acquire a migratory behavior during the epithelial–mesenchymal transition program in primary neoplasm. 12,15,39,51,52,92,191 The cancer progenitor cells also possess some intrinsic properties in common with adult stem cells that may allow them to remain in a quiescent and viable state of dormancy under the specific microenvironmental conditions prevalent at the primary and distant metastatic sites after therapeutic treatments.12,13,15,39,43,49,57,58 Consistent with this, several studies revealed that the enhanced expression of stem cell-like gene products in cancer progenitor cells is generally associated with a poor prognosis of patients with different cancer types. Particularly, the sustained activation of EGFR, hedgehog, Wnt/β-catenin, and integrins in cancer progenitor cells during cancer progression may lead to an enhanced expression of several oncogenic gene products, such as matrix metalloproteinases, urokinase plasminogen activator, COX-2, vascular epidermal growth factor, and transcription repressors of E-cadherin (snail and slug). These signaling elements may contribute to their unlimited growth and survival and also their resistance to current therapeutic treatments (Figure 1).12,13,30,31,39,51,52,57,58,192 These oncogenic events are consistent with the cancer stem/progenitor cell model of carcinogenesis, in which accumulating genetic alterations leading to a deregulated activation of diverse oncogenic cascades by multiple growth factors in cancer progenitor cells and their early progeny may serve critical functions for leukemic graft or tumor growth, metastasis, and disease relapse.12,13,15,21,33,34,39,193,194
In addition, certain established cancer cell lines derived from brain, lung, liver, breast, ovarian, prostate, and gastrointestinal tumors and maintained in culture for a long period of time may also represent a heterogeneous population of cancer cells. It has been observed that only a small sub-population may correspond to highly leukemic or tumorigenic cancer progenitor cells with the stem cell-like properties. This small sub-population of cancer progenitor cells may be isolated from well-established cell lines by fluorescence-activated cell shorting technique based on their expression of specific stem cell-like surface markers, such as CD34, Sca-1, CD133, and CD44 as well as their high ability to efflux fluorescent Hoechst dye compared with other cancer cell populations.21,50,97,100,104 In this regard, this very small sub-population of cancer progenitor cells generally expresses the higher levels of stemness genes, such as ABC multidrug efflux transporters, EGFR, hedgehog signaling elements, and β-catenin, than the bulk mass of differentiated cancer cells. Additionally, this very small sub-population of cells is generally more leukemic or tumorigenic and is able to generate leukemic grafts or tumors in vivo at a low number of cells compared with their further differentiated progeny. More specifically, a small cell sub-population designated as side population (SP) cells, which generally possesses stem cell-like properties, including a high Hoechst dye efflux capacity, because of an elevated expression levels of ABC multidrug efflux pumps relative to non-SP fraction, has been detected in diverse mammalian cancer cell lines and cancer cells from tumor tissues (Table 2).46,47,50,189,195–201 It has been reported that SP cells, but not non-SP cells, could generate both SP and non-SP cells in culture by asymmetric division and were largely responsible for the in vivo leukemic graft or tumor formation established from these cancer cell lines in animal models in vivo. Similarly, the in vitro and in vivo characterization of CD44+/high-positive prostatic cancer cells LAPC-4, -9, DU145, and PC3 cells isolated from the total parental cell population revealed that this cancer cell sub-population was more proliferative, invasive, and tumorigenic than the CD44-negative or CD44low-positive cancer cell sub-population. 188,190 Taken together, these observations emphasize the importance of considering the presence of this small sub-population of more leukemic or tumorigenic cancer progenitor cells when these cancer cell lines are used to test the anticarcinogenic effects of novel combinations of drugs. This should allow us to obtain a better estimation of efficacy of antitumoral drugs to eliminate the cancer-initiating cells found in available cancer cell lines.
Drug resistance of cancer progenitor cells
Several recent lines of evidence have indicated that a small population of leukemic or tumorigenic cancer progenitor cells possessing stem cell-like properties may be more resistant to chemotherapy than their further differentiated progeny. More specifically, high-expression levels of ABC multidrug efflux transporters and antiapoptotic signaling factors (Bcl-2, EGF–EGFR, and sonic hedgehog (SHH)-PTCH) and alterations in tumor suppressor gene products such as p53, combined with the active DNA repair mechanisms in cancer progenitor cells, may confer on them their intrinsic or acquired resistance to hormonal therapies, radiotherapy, and chemotherapeutic drugs (Figure 1 and Table 1 ).21,24,86,97,98,100,101,202,203 Regardless, the occurrence of different genetic or epigenic alterations, including mutations and the activation of different oncogenic cascades in cancer progenitor cell sub-populations during cancer initiation and progression, may be partly responsible for the intratumoral heterogeneity or diversity of cancer subtypes. 12,13,33,39,45,52,81–83,193,204 In particular, the acquisition of mesenchymal and migratory properties by poorly differentiated or moderately differentiated cancer progenitor cells expressing the basal or intermediate epithelial cell markers, respectively, during the epithelial–mesenchymal transition program may result in highly aggressive cancer forms.12,39,44,45,51,52,165,199,203–206 Of critical therapeutic importance, these different cancer-initiating progenitor cell subsets, with different mutations and oncogenic properties, may also respond with different sensitivity to cytotoxic drug treatment. Thereby, they can contribute to the partial response or resistance to current therapies observed in different subsets of patients.44,45,52,165,199,203–209 This new model on the heterogeneity of cancer progenitor cells has important repercussions for the design of new effective diagnostic methods and preventive and curative treatments. Indeed, the model implicates that specific therapeutic regimens may be effective to eradicate only certain leukemic or tumorigenic cancer progenitor cells within a cancer subtype, whereas other sub-populations of cancer progenitor cells may be more resistant to treatments. For instance, the malignant transformation of the most primitive ERα-negative cancer progenitor cells may result in the formation of poorly differentiated and aggressive tumor types with a basal phenotype.204 Hence, the persistence of ERα-negative cancer progenitor cells may contribute to resistance to anti-estrogen therapy and disease relapse.204 In contrast, the enhanced expression of ERα in cancer progenitor cells in other breast cancer subtypes may represent a determinant factor that may influence their greater sensitivity and response to selective anti-estrogen therapies, including tamoxifen and aromatase inhibitors, than their clinical inefficacy against ERα-negative cancer cells. Furthermore, it has been observed that the expression levels of CD133 biomarker, which may be associated with the presence of CD133-positive cancer progenitor cells, were significantly higher in recurrent glioblastoma multiforme (GBM) tissues obtained from five patients than those detected in newly diagnosed tumor tissues from the same patients.203 The reverse transcription-polymerase chain reaction analyses of gene expression profile for CD133-positive cancer progenitor cells found in three primary cultured cell lines established from GBM patients have also indicated that these cancer progenitor cells express higher levels of diverse stemness genes, including certain gene products related to drug resistance, than the autologous CD133-negative cells.203 Among the stem cell-like gene products are Oct-4 and CD44 biomarkers, BCRP/ABCG2 transporter, the DNA repair protein O6-methylguanine-DNA methyltransferase, antiapoptotic factors, including Bcl-2, survivin, inhibitor of apoptosis proteins, survival signaling elements SHH/PTCH/GLI, and the transcriptional repressor of the E-cadherin snail.203 Additionally, the CD133-positive cancer progenitor cells from GBM tissues were significantly more resistant to chemotherapeutic agents, including oral DNA alkylating agent, temozolomide, carboplatin, etoposide, and PTX, than the autologous CD133-negative cancer cells.203 This suggests that the persistence of these CD133-positive cancer progenitor cells may partly contribute, to the recurrence of highly aggressive GBMs. Importantly, it has recently been reported that the ABC transporters did not appear to contribute to the resistance of GBM stem cells to several chemotherapeutic drugs. In fact, other MDR mechanisms, such as the alterations in apoptotic signaling cascades, including the overexpression of antiapoptotic factors, could represent a more important factor that confers on them their chemoresistance.210 It is noteworthy that the GBMs, which appear to derive from the malignant transformation of neural stem cells that acquire mesenchymal properties like mesenchymal stem cells, are frequently accompanied by the overexpression of EGFR.44,45,52 The enhanced EGFR expression may then contribute, at least in part, to their survival, acquisition of more aggressive behavior, and chemoresistance. Interestingly, a subset of tumorigenic progenitor cells designated as tumorospheres and possessing an angiogenic potential and MDR phenotype, including the expression of MRP1 and -3, has also been identified in human glioblastoma. This suggests that this cancer cell population may contribute to drug resistance.211 In addition, the resistance of CD133-positive glioma progenitor cells to radiation may also be due to the activation of DNA damage checkpoint response and DNA repair mechanisms.205 In contrast, it has been reported that the BCR-ABL kinase inhibitor, imatinib mesylate, can also act as an inhibitor of BCRP/ABCG2 transporter, which is over-expressed in chronic myelogenous leukemia (CML) CD34+ hematopoietic progenitor cells.133 However, it appears that non-proliferative CML BCR-ABL+ CD34+ progenitor cells may be more resistant to cytotoxic effects induced by diverse agents, including imatinib mesylate, cytosine arabinoside, and arsenic trioxide, than the dividing BCR-ABL+ CD34+ progenitor cells.165,206,209 Similarly, it has been observed that the mammospheres of non-adherent CD44+/CD24−/low breast cancer stem cells established after their isolation from MCF-7 breast cancer cell line were more resistant to radiation than adherent cancer cells grown as monolayer cultures, and their number was enhanced after treatment with the fractionated doses of irradiation.208 Hence, the persistence and increase of the number of cancer-initiating cell population after treatment with these types of anticarcinogenic treatment may partly contribute to disease relapse.
Additionally, the increased expression levels and activity of diverse ABC transporters and survival factors have also been observed in the SP fraction relative non-SP cell sub-population. For instance, the expression of MDR1/P-gp, BCRP/ABCG2, and CEACAM6, which are associated with multidrug chemoresistance to anticancer agents, was significantly increased in SP cells versus non-SP cells isolated by the drug efflux Hoechst dye technique from hepatoma HuH7 and colorectal SW480 cancer cell lines.199 HuH7 SP cells also displayed a higher resistance to doxorubicin, 5-fluorouracil, and gemcitabine than non-SP cells.199 Interestingly, a SP cell population has also been detected in neuroblastoma cells from 15 of 23 patients (65%), which expressed high levels of BCRP/ABCG2 and ABCA3 transporter genes and a greater capacity to efflux cytotoxic drugs such as mitoxantrone than a non-SP cell sub-population.50 The ABCB5 transporter, which is expressed in human malignant melanoma cells with a CD133+ stem cell-like phenotype, may also mediate the enhanced doxorubicin efflux transport and chemoresistance. 207 In addition, the results from recent studies have revealed that EGF may induce the upregulation of ABC transporters in certain normal cells and enhanced SP cell fraction in human metastatic head and neck squamous cell carcinoma cell lines, UMSCC10B and HN12 cells expressing BCRP/ABCG2 transporter.201,212 Importantly, it has also been observed that in the treatment of head and neck squamous cell carcinoma cell lines by EGFR inhibitor, gefitinib decreased the cell number in the SP population, which was associated with a decrease of the ABC transporter on the cell surface.201 This suggests that the activation of EGF–EGFR pathway in cancer progenitor cells could contribute to the enhancement of membrane ABC transporter levels and thereby contribute to MDR phenomenon. Further studies on the regulatory mechanisms of expression and activity of ABC transporters in MDR cancer progenitor cells versus parental drug-sensitive cancer cells should allow us to confirm the implication of growth factors, including the EGF–EGFR system, in the control of ABC pump expression in other cancer progenitor cell types.
Targeting of cancer progenitor cells
The developmental signaling cascades (EGFR, hedgehog, and Wnt/β-catenin), oncogenic signaling elements (telomerase, Scr, Bcl-2, nuclear factor-κB, PI3K/AKT, and COX-2), and ABC multidrug efflux pumps may assume a critical function in regulating the stem cell self-renewal, differentiation, survival, and drug resistance of cancer stem/progenitor cells.2,13,15,21,24,30,31,39,49,50,92,152,153,203,213–217 Therefore, the molecular targeting of these signaling elements, which are activated in certain leukemia subtypes, multiple myeloma, and numerous solid cancers, is of particular therapeutic interest (Table 2).1 Regardless, the recent development of diverse enhanced delivery techniques for administration of anticarcinogenic drugs may constitute promising approaches for targeting cancer progenitor cells, which express specific biomarkers and thereby reverse MDR in vivo. Among them are the targeted delivery of therapeutic agents into tumors by using the conjugation/fusion of drugs to tumor-specific antibodies, encapsulation of chemotherapeutic drugs in liposomes or other carriers such as nanoparticules, and the use of genetically engineered stem cells as vehicles.114,218–220 These delivery techniques may enhance drug bioavailability, improve selectivity, and continue delivering drugs into particular tumoral tissues, thereby decreasing the systemic toxicity.114,218–220
Certain investigations have also revealed that the use of combined pharmacological agent that are able to induce the differentiation and apoptotic death of leukemic or tumorigenic cancer progenitor cells may constitute effective therapeutic treatment or adjuvant therapy.221–223 For instance, the use of a highly effective therapeutic agent, imatinib mesylate, a TKI-active against BCR-ABL oncoprotein, generally leads to a clinical remission in the early chronic phase of CML.224–226 Although BCR-ABL inhibitors, including imatinib mesylate and dasatinib (BMS-354825), may be effective in eradicating the dividing BCR-ABL+ CML cells, the persistence of non-proliferative BCR-ABL+ CML cells expressing lower level of BCR-ABL oncoprotein, which may be more resistant to apoptotic effect of these agents, may subsequently lead to disease relapse.165,206,209,224,227 Additionally, the progression to the advanced- and late-stage disease, accelerated phase, or terminal blast crisis phase of CML disease is often accompanied by acquisition of a more malignant phenotype and a lack of response to BCR-ABL inhibitors concomitant with the leukemia recurrence.224–226 The inhibition of hedgehog and Wnt/β-catenin cascades, antiapoptotic factor Bcl-2, and Scr-related LYN kinase, which are activated in certain patients during CML progression and the cellular organic cation transporter OCT-1 involved in the intracellular uptake of imatinib, may represent promising adjuvant strategies (Table 2).227–230 Of particular therapeutic interest, recent in vitro studies revealed that the exposure of primitive quiescent BCR-ABL+ CML progenitor cells to growth factors such as granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor at clinically achievable concentrations may reduce the number of non-dividing BCR-ABL+ CML progenitor cells.231,232 This effect may thereby overcome the resistance to imatinib mesylate and promote the elimination of residual malignant CML cells.231,232 Interferon-α also appears to be more effective than imatinib mesylate in eliminating the primitive BCR-ABL+ CML progenitor cells, which are responsible for the maintenance of long-term cultures, whereas imatinib mesylate could be more active against the further differentiated CML progenitor cells.233 In fact, the antileukemic activity of interferon-α may be mediated, in part, by inducing a differentiating effect on primitive CML progenitors.233 Thus, despite the fact that clinical therapeutic effects by using interferon-α plus stem cell transplantation are generally less rapid and effective after the initiation treatment than imatinib mesylate, the response could be more stable after discontinuation of the drug. Therefore, this therapy could represent certain clinical advantages for long-term care of certain patients who did not respond to treatment with BCR-ABL inhibitors. Similarly, the use of differentiating agent, all-trans retinoic acid with chemotherapeutic drugs such as anthracyclines has resulted in a successful treatment of acute promyelocytic leukemia, which is associated with the expression of the PML-RAR-α+ oncoprotein produced from the fusion of the promyelocytic leukemia (PML) gene to retinoic acid receptor α (RAR-α) gene.234,235 The combination of all-trans retinoic acid-based therapy with other anticancer drugs, including arsenic trioxide, which can induce the antiproliferative and proapoptotic effect on PML-RAR-α+ acute promyelocytic leukemia cells, including leukemic stem cells, may represent a more promising approach to treat acute promyelocytic leukemia. The advanced or refractory/relapsed acute promyelocytic leukemia or other cancer types such as multiple myeloma are also sensitive to these agent types.222,234–236
CONCLUSIONS AND PERSPECTIVES
Together, these recent investigations revealed that high resistance to current therapeutic treatments may result from several different structural and functional properties between the cancer progenitor cells and their further differentiated progeny, including certain molecular mechanisms that may contribute to their high resistance to current therapeutic treatments. Further characterization of cancer progenitor cells versus their more differentiated progeny and normal counterpart adult stem cells should lead to the identification of new drug targets for the development of more effective strategies for the prevention and curative treatment of aggressive cancers. Additional analyses of oncogenic gene expression profiles and drug resistance properties specific to tumorigenic and migrating cancer progenitor cells should allow us to selectively target this minority of cancer-initiating cells for improving the current clinical therapies against the most metastatic and incurable cancers. The molecular targeting of ABC multidrug efflux transporters, EGFR, hedgehog, and Wnt/β-catenin, and deregulated apoptotic signaling that provide a critical function for the sustained growth, survival, metastasis, and development of chemoresistance of cancer progenitor cells should lead to improved treatments for highly aggressive and metastatic cancers. In this regard, it will be important to more precisely establish the molecular mechanisms associated with the MDR inhibitory effect induced by specific or broad ABC multidrug transporter inhibitors, EGFR inhibitor such as gefitinib and ceramide metabolism inhibitors, alone or in combination, on isolated leukemic or tumorigenic cancer progenitor cells. The studies carried out with the cancer progenitor cells made resistant to drugs of clinical interest in vitro and in vivo, and subsequently in clinical settings, merit further investigations. Hence, further works should lead to development of novel strategies for counteracting the MDR phenomenon that occurs specifically in leukemic or tumorigenic cancer progenitor cells and thereby improve current therapeutic treatments against recurrent cancers and long-term survival of patients.
Acknowledgments
This paper was supported by grants from the US Department of Defense (PC040493, PC04502, and OC04110) and the National Institutes of Health (CA78590, CA72712, and CA111294).
Footnotes
CONFLICT OF INTEREST The authors declared no conflict of interest.
References
- 1.Brawley OW, Kramer BS. Cancer screening in theory and in practice. J Clin Oncol. 2005;23:293–300. doi: 10.1200/JCO.2005.06.107. [DOI] [PubMed] [Google Scholar]
- 2.Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 3.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 4.Lock-Andersen J, Horn J, Sjostrand H. Prognosis after sentinel node biopsy in malignant melanoma. Ugeskr Laeger. 2006;168:2457–2462. [PubMed] [Google Scholar]
- 5.Shiraishi J, et al. Computer-aided diagnosis for the detection and classification of lung cancers on chest radiographs ROC analysis of radiologists’ performance. Acad Radiol. 2006;13:995–1003. doi: 10.1016/j.acra.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Chatterton K, Ray E, O’Brien TS. Fluorescence diagnosis of bladder cancer. Br J Nurs. 2006;15:595–597. doi: 10.12968/bjon.2006.15.11.21226. [DOI] [PubMed] [Google Scholar]
- 7.Alexander A, et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med. 2006;29:301–311. [PubMed] [Google Scholar]
- 8.Calza S, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petignat P, Du Bois A, Bruchim I, Fink D, Provencher DM. Should intraperitoneal chemotherapy be considered as standard first-line treatment in advanced stage ovarian cancer? Crit Rev Oncol Hematol. 2006;62:137–147. doi: 10.1016/j.critrevonc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 10.van der Merwe DE, Oikonomopoulou K, Marshall J, Diamandis EP. Mass spectrometry: uncovering the cancer proteome for diagnostics. Adv Cancer Res. 2006;96:23–50. doi: 10.1016/S0065-230X(06)96002-3. [DOI] [PubMed] [Google Scholar]
- 11.Nicum S, Midgley R, Kerr DJ. Colorectal cancer. Acta Oncol. 2003;42:263–275. doi: 10.1080/02841860310000412. [DOI] [PubMed] [Google Scholar]
- 12.Mimeault M, Batra S. Interplay of distinct growth factors during epithelial–mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007 doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 13.Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 14.Pantel K, Woelfle U. Micrometastasis in breast cancer and other solid tumors. J Biol Regul Homeost Agents. 2004;18:120–125. [PubMed] [Google Scholar]
- 15.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 16.Beckman M. New technologies aim to find cancer in the blood. J Natl Cancer Inst. 2006;98:1180–1181. doi: 10.1093/jnci/djj383. [DOI] [PubMed] [Google Scholar]
- 17.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 18.Ceschel S, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer. 2006;47:560–566. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 19.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 20.Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH. Extensive contribution of the multidrug transporters Pglycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000;60:5761–5766. [PubMed] [Google Scholar]
- 21.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 22.Milas L, Raju U, Liao Z, Ajani J. Targeting molecular determinants of tumor chemo-radioresistance. Semin Oncol. 2005;32:S78–S81. doi: 10.1053/j.seminoncol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Roberg K, Jonsson AC, Grenman R, Norberg-Spaak L. Radiotherapy response in oral squamous carcinoma cell lines: evaluation of apoptotic proteins as prognostic factors. Head Neck. 2007;29:325–334. doi: 10.1002/hed.20520. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 26.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 28.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004;51:1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Fang D, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 30.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 31.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 32.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 33.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 35.Trosko JE, Tai MH. Adult stem cell theory of the multi-stage, multi-mechanism theory of carcinogenesis: role of inflammation on the promotion of initiated stem cells. Contrib Microbiol. 2006;13:45–65. doi: 10.1159/000092965. [DOI] [PubMed] [Google Scholar]
- 36.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 37.Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620–1624. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- 38.Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007;26:203–214. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- 40.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 41.Al-Hajj M, Wicha MS, Ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 43.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 44.Yuan X, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 45.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 46.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 48.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 49.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 50.Hirschmann-Jax C, et al. A distinct “side population”of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Tso CL, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res. 2006;4:607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 55.van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol. 2001;195:563–570. doi: 10.1002/path.993. [DOI] [PubMed] [Google Scholar]
- 56.Kurbel S. Selective reduction of estrogen receptor (ER) positive breast cancer occurrence by estrogen receptor modulators supports etiological distinction between ER positive and ER negative breast cancers. Med Hypotheses. 2005;64:1182–1187. doi: 10.1016/j.mehy.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5:1799–1807. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White DE, Rayment JH, Muller WJ. Addressing the role of cell adhesion in tumor cell dormancy. Cell Cycle. 2006;5:1756–1759. doi: 10.4161/cc.5.16.2993. [DOI] [PubMed] [Google Scholar]
- 59.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–659. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 60.Bregni M, Bernardi M, Ciceri F, Peccatori J. Allogeneic stem cell transplantation for the treatment of advanced solid tumors. Springer Semin Immunopathol. 2004;26:95–108. doi: 10.1007/s00281-004-0164-4. [DOI] [PubMed] [Google Scholar]
- 61.Small TN, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA-matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biol Blood Marrow Transplant. 2007;13:235–244. doi: 10.1016/j.bbmt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Di Bona E, et al. Transplant-finalized salvage of adult acute lymphoblastic leukemia: results of a mitoxantrone- and methotrexate-based regimen in 36 patients. Leuk Lymphoma. 2005;46:879–884. doi: 10.1080/10428190500080801. [DOI] [PubMed] [Google Scholar]
- 63.Waheed F, et al. High dose chemotherapy with thiotepa, mitoxantrone and carboplatin (TMJ) followed by autologous stem cell support in 100 consecutive lymphoma patients in a single centre: analysis of efficacy, toxicity and prognostic factors. Leuk Lymphoma. 2004;45:2253–2259. doi: 10.1080/10428190410001723250. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Martinez A, et al. High-dose chemotherapy with autologous stem cell rescue for children with high risk and recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors. J Neurooncol. 2005;71:33–38. doi: 10.1007/s11060-004-4527-4. [DOI] [PubMed] [Google Scholar]
- 65.Corradini P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–1538. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 66.Oyan B, et al. High dose sequential chemotherapy and autologous stem cell transplantation in patients with relapsed/refractory lymphoma. Leuk Lymphoma. 2006;47:1545–1552. doi: 10.1080/10428190600570958. [DOI] [PubMed] [Google Scholar]
- 67.von Allmen D, et al. Aggressive surgical therapy and radiotherapy for patients with high-risk neuroblastoma treated with rapid sequence tandem transplant. J Pediatr Surg. 2005;40:936–941. doi: 10.1016/j.jpedsurg.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 68.George RE, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 69.Dillman RO, et al. High-dose chemotherapy and autologous stem cell rescue for metastatic breast cancer: superior survival for tandem compared with single transplants. Am J Clin Oncol. 2005;28:281–288. doi: 10.1097/01.coc.0000156917.43490.65. [DOI] [PubMed] [Google Scholar]
- 70.Carella AM, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005;366:318–320. doi: 10.1016/S0140-6736(05)66989-9. [DOI] [PubMed] [Google Scholar]
- 71.Stiff PJ, et al. Randomized phase II trial of two high-dose chemotherapy regimens with stem cell transplantation for the treatment of advanced ovarian cancer in first remission or chemosensitive relapse: a Southwest Oncology Group study. Gynecol Oncol. 2004;94:98–106. doi: 10.1016/j.ygyno.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 72.Avramova B, et al. Myeloablative chemotherapy with autologous peripheral blood stem cell transplantation in patients with poor-prognosis solid tumors—Bulgarian experience. J BUON. 2006;11:433–438. [PubMed] [Google Scholar]
- 73.Efferth T, Volm M. Pharmacogenetics for individualized cancer chemotherapy. Pharmacol Ther. 2005;107:155–176. doi: 10.1016/j.pharmthera.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 75.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Brandt B, Meyer-Staeckling S, Schmidt H, Agelopoulos K, Buerger H. Mechanisms of egfr gene transcription modulation: relationship to cancer risk and therapy response. Clin Cancer Res. 2006;12:7252–7260. doi: 10.1158/1078-0432.CCR-06-0626. [DOI] [PubMed] [Google Scholar]
- 77.Miller CR, McLeod HL. Pharmacogenomics of cancer chemotherapy-induced toxicity. J Support Oncol. 2007;5:9–14. [PubMed] [Google Scholar]
- 78.Berndt SI, et al. Genetic variation in base excision repair genes and the prevalence of advanced colorectal adenoma. Cancer Res. 2007;67:1395–1404. doi: 10.1158/0008-5472.CAN-06-1390. [DOI] [PubMed] [Google Scholar]
- 79.Sohn JW, et al. MDR1 polymorphisms predict the response to etoposide–cisplatin combination chemotherapy in small cell lung cancer. Jpn J Clin Oncol. 2006;36:137–141. doi: 10.1093/jjco/hyi231. [DOI] [PubMed] [Google Scholar]
- 80.Su D, et al. Genetic polymorphisms and treatment response in advanced non-small cell lung cancer. Lung Cancer. 2007;56:281–288. doi: 10.1016/j.lungcan.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Laakso M, et al. Basoluminal carcinoma: a new biologically and prognostically distinct entity between basal and luminal breast cancer. Clin Cancer Res. 2006;12:4185–4191. doi: 10.1158/1078-0432.CCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 82.Tang P, et al. Expression patterns of ER-alpha PR HER-2/neu, and EGFR in different cell origin subtypes of high grade and non-high grade ductal carcinoma in situ. Ann Clin Lab Sci. 2006;36:137–143. [PubMed] [Google Scholar]
- 83.Buyse M, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 84.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 85.Hynes RO. Metastatic potential: generic predisposition of the primary tumor or rare, metastatic variants—or both? Cell. 2003;113:821–823. doi: 10.1016/s0092-8674(03)00468-9. [DOI] [PubMed] [Google Scholar]
- 86.Park S, et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99:9–17. doi: 10.1007/s10549-006-9175-2. [DOI] [PubMed] [Google Scholar]
- 87.L’Esperance S, et al. Gene expression profiling of paired ovarian tumors obtained prior to and following adjuvant chemotherapy: molecular signatures of chemoresistant tumors. Int J Oncol. 2006;29:5–24. [PubMed] [Google Scholar]
- 88.Yeoh EJ, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 89.Holleman A, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 90.Yasui K, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 91.Filipits M, et al. Clinical role of multidrug resistance protein 1 expression in chemotherapy resistance in early-stage breast cancer: the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2005;23:1161–1168. doi: 10.1200/JCO.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 92.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 94.Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 95.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 96.Giaccone G, Rodriguez JA. EGFR inhibitors: what have we learned from the treatment of lung cancer? Nat Clin Pract Oncol. 2005;2:554–561. doi: 10.1038/ncponc0341. [DOI] [PubMed] [Google Scholar]
- 97.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 98.Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25:231–259. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- 99.Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: structure, function and disease. Biochim Biophys Acta. 2006;1762:510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR) Curr Drug Targets. 2006;7:893–909. doi: 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- 102.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 104.Abbott BL. ABCG2 (BCRP): a cytoprotectant in normal and malignant stem cells. Clin Adv Hematol Oncol. 2006;4:63–72. [PubMed] [Google Scholar]
- 105.David-Beabes GL, et al. Doxorubicin-resistant variants of human prostate cancer cell lines DU145, PC-3, PPC-1, and TSU-PR1: characterization of biochemical determinants of antineoplastic drug sensitivity. Int J Oncol. 2000;17:1077–1086. doi: 10.3892/ijo.17.6.1077. [DOI] [PubMed] [Google Scholar]
- 106.Diestra JE, et al. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 107.Bessho Y, et al. Role of ABCG2 as a biomarker for predicting resistance to CPT-11/SN-38 in lung cancer. Cancer Sci. 2006;97:192–198. doi: 10.1111/j.1349-7006.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boonstra R, et al. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 upregulation. Br J Cancer. 2004;90:2411–2417. doi: 10.1038/sj.bjc.6601863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mack JT, Beljanski V, Tew KD, Townsend DM. The ATP-binding cassette transporter ABCA2 as a mediator of intracellular trafficking. Biomed Pharmacother. 2006;60:587–592. doi: 10.1016/j.biopha.2006.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Efferth T, et al. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2006;5:1986–1994. doi: 10.1158/1535-7163.MCT-06-0086. [DOI] [PubMed] [Google Scholar]
- 111.Steinbach D, et al. ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. Clin Cancer Res. 2006;12:4357–4363. doi: 10.1158/1078-0432.CCR-05-2587. [DOI] [PubMed] [Google Scholar]
- 112.Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–1519. [PubMed] [Google Scholar]
- 113.Haus-Cohen M, Assaraf YG, Binyamin L, Benhar I, Reiter Y. Disruption of P-glycoprotein anticancer drug efflux activity by a small recombinant single-chain Fv antibody fragment targeted to an extracellular epitope. Int J Cancer. 2004;109:750–758. doi: 10.1002/ijc.20037. [DOI] [PubMed] [Google Scholar]
- 114.Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 115.Modok S, Mellor HR, Callaghan R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol. 2006;6:350–354. doi: 10.1016/j.coph.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–2909. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 117.Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di PA. Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev. 2005;25:453–472. doi: 10.1002/med.20032. [DOI] [PubMed] [Google Scholar]
- 118.Ahmed-Belkacem A, et al. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anticancer Drugs. 2006;17:239–243. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 119.Cnubben NH, Wortelboer HM, van Zanden JJ, Rietjens IM, van Bladeren PJ. Metabolism of ATP-binding cassette drug transporter inhibitors: complicating factor for multidrug resistance. Expert Opin Drug Metab Toxicol. 2005;1:219–232. doi: 10.1517/17425255.1.2.219. [DOI] [PubMed] [Google Scholar]
- 120.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–1876. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 121.Aouali N, Eddabra L, Macadre J, Morjani H. Immunosuppressors and reversion of multidrug-resistance. Crit Rev Oncol Hematol. 2005;56:61–70. doi: 10.1016/j.critrevonc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 122.Goda K, et al. Complete inhibition of P-glycoprotein by simultaneous treatment with a distinct class of modulators and the UIC2 monoclonal antibody. J Pharmacol Exp Ther. 2007;320:81–88. doi: 10.1124/jpet.106.110155. [DOI] [PubMed] [Google Scholar]
- 123.Saeki T, et al. Dofequidar fumarate (MS-209) in combination with cyclophosphamide, doxorubicin, and fluorouracil for patients with advanced or recurrent breast cancer. J Clin Oncol. 2007;25:411–417. doi: 10.1200/JCO.2006.08.1646. [DOI] [PubMed] [Google Scholar]
- 124.Robert J. MS-209 Schering. Curr Opin Investig Drugs. 2004;5:1340–1347. [PubMed] [Google Scholar]
- 125.Dieras V, et al. Phase I combining a P-glycoprotein inhibitor MS209, in combination with docetaxel in patients with advanced malignancies. Clin Cancer Res. 2005;11:6256–6260. doi: 10.1158/1078-0432.CCR-04-2316. [DOI] [PubMed] [Google Scholar]
- 126.Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 127.van Zanden JJ, et al. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69:699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 129.Shen LZ, et al. Tamoxifen can reverse multidrug resistance of colorectal carcinoma in vivo. World J Gastroenterol. 2005;11:1060–1064. doi: 10.3748/wjg.v11.i7.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saeki T, Tsuruo T, Sato W, Nishikawsa K. Drug resistance in chemotherapy for breast cancer. Cancer Chemother Pharmacol. 2005;56 (suppl 1):84–89. doi: 10.1007/s00280-005-0106-4. [DOI] [PubMed] [Google Scholar]
- 131.Sugimoto Y, et al. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol Cancer Ther. 2003;2:105–112. [PubMed] [Google Scholar]
- 132.Gao L, et al. STI571 combined with vincristine greatly suppressed the tumor formation of multidrug-resistant K562 cells in a human-nude mice xenograft model. Chin Med J (Engl) 2006;119:911–918. [PubMed] [Google Scholar]
- 133.Jordanides NE, Jorgensen HG, Holyoake TL, Mountford JC. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2006;108:1370–1373. doi: 10.1182/blood-2006-02-003145. [DOI] [PubMed] [Google Scholar]
- 134.Erlichman C, et al. The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res. 2001;61:739–748. [PubMed] [Google Scholar]
- 135.Ozvegy-Laczka C, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 136.Yanase K, et al. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Mol Cancer Ther. 2004;3:1119–1125. [PubMed] [Google Scholar]
- 137.Yang CH, et al. Gefitinib reverses chemotherapy resistance in gefitinib-insensitive multidrug resistant cancer cells expressing ATP-binding cassette family protein. Cancer Res. 2005;65:6943–6949. doi: 10.1158/0008-5472.CAN-05-0641. [DOI] [PubMed] [Google Scholar]
- 138.Kitazaki T, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer. 2005;49:337–343. doi: 10.1016/j.lungcan.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 139.Leggas M, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–4807. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- 140.Stewart CF, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura Y, et al. Gefitinib (‘‘Iressa’’ ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res. 2005;65:1541–1546. doi: 10.1158/0008-5472.CAN-03-2417. [DOI] [PubMed] [Google Scholar]
- 142.Burger H, Nooter K. Pharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinib. Cell Cycle. 2004;3:1502–1505. doi: 10.4161/cc.3.12.1331. [DOI] [PubMed] [Google Scholar]
- 143.Cusatis G, et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 2006;98:1739–1742. doi: 10.1093/jnci/djj469. [DOI] [PubMed] [Google Scholar]
- 144.Festuccia C, et al. Additive antitumor effects of the epidermal growth factor receptor tyrosine kinase inhibitor, gefitinib (Iressa), and the nonsteroidal antiandrogen, bicalutamide (Casodex), in prostate cancer cells in vitro. Int J Cancer. 2005;115:630–640. doi: 10.1002/ijc.20917. [DOI] [PubMed] [Google Scholar]
- 145.Mimeault M, Bonenfant D, Batra SK. New advances on the functions of epidermal growth factor receptor and ceramides in skin cell differentiation, disorders and cancers. Skin Pharmacol Physiol. 2004;17:153–166. doi: 10.1159/000078818. [DOI] [PubMed] [Google Scholar]
- 146.Penne K, Bohlin C, Schneider S, Allen D. Gefitinib (Iressa ZD1839) and tyrosine kinase inhibitors: the wave of the future in cancer therapy. Cancer Nurs. 2005;28:481–486. doi: 10.1097/00002820-200511000-00012. [DOI] [PubMed] [Google Scholar]
- 147.Freeman BB, III, Daw NC, Geyer JR, Furman WL, Stewart CF. Evaluation of gefitinib for treatment of refractory solid tumors and central nervous system malignancies in pediatric patients. Cancer Invest. 2006;24:310–317. doi: 10.1080/07357900600632058. [DOI] [PubMed] [Google Scholar]
- 148.Peng XH, et al. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnston JB, et al. Targeting the EGFR pathway for cancer therapy. Curr Med Chem. 2006;13:3483–3492. doi: 10.2174/092986706779026174. [DOI] [PubMed] [Google Scholar]
- 150.Herbst RS, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- 151.Mimeault M, et al. Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer. 2006;118:1022–1031. doi: 10.1002/ijc.21440. [DOI] [PubMed] [Google Scholar]
- 152.Mimeault M, et al. Novel combination therapy against metastatic and androgen-independent prostate cancer by using a combination of gefitinib, tamoxifen and etoposide. Int J Cancer. 2006;120:160–169. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- 153.Mimeault M, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6:967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 154.Sugita S, et al. Effect of type I growth factor receptor tyrosine kinase inhibitors on phosphorylation and transactivation activity of the androgen receptor in prostate cancer cells: ligand-independent activation of the N-terminal domain of the androgen receptor. Oncol Rep. 2004;11:1273–1279. [PubMed] [Google Scholar]
- 155.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 156.Ciardiello F, et al. ZD1839 (IRESSA), an EGFR-selective tyrosine kinase inhibitor, enhances taxane activity in bcl-2 overexpressing, multidrug-resistant MCF-7 ADR human breast cancer cells. Int J Cancer. 2002;98:463–469. doi: 10.1002/ijc.10230. [DOI] [PubMed] [Google Scholar]
- 157.Jones HE, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 158.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 159.Massarweh S, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 160.Qiu L, et al. Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int J Oncol. 2005;27:823–830. [PubMed] [Google Scholar]
- 161.Qiu L, et al. Targeted inhibition of transient activation of the EGFR-mediated cell survival pathway enhances paclitaxel-induced ovarian cancer cell death. Int J Oncol. 2005;27:1441–1448. [PubMed] [Google Scholar]
- 162.Xia W, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006;66:1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 163.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 164.Mimeault M, Pommery N, Henichart JP. New advances on prostate carcinogenesis and therapies: involvement of EGF–EGFR transduction system. Growth Factors. 2003;21:1–14. doi: 10.1080/0897719031000094921. [DOI] [PubMed] [Google Scholar]
- 165.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 166.Gouaze-Andersson V, Cabot MC. Glycosphingolipids and drug resistance. Biochim Biophys Acta. 2006;1758:2096–2103. doi: 10.1016/j.bbamem.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 167.Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 2006;5:200–208. doi: 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- 168.Pchejetski D, et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–11675. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- 169.Eto M, Bennouna J, Hunter OC, Lotze MT, Amoscato AA. Importance of C16 ceramide accumulation during apoptosis in prostate cancer cells. Int J Urol. 2006;13:148–156. doi: 10.1111/j.1442-2042.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 170.Thon L, et al. Ceramide mediates caspase-independent programmed cell death. FASEB J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- 171.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, Levade T. Sphingolipids as modulators of cancer cell death: potential therapeutic targets. Biochim Biophys Acta. 2006;1758:2104–2120. doi: 10.1016/j.bbamem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 173.Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sawai H, Domae N, Okazaki T. Current status and perspectives in ceramide-targeting molecular medicine. Curr Pharm Des. 2005;11:2479–2487. doi: 10.2174/1381612054367463. [DOI] [PubMed] [Google Scholar]
- 175.Prinetti A, et al. Lack of ceramide generation and altered sphingolipid composition are associated with drug resistance in human ovarian carcinoma cells. Biochem J. 2006;395:311–318. doi: 10.1042/BJ20051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zheng W, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 177.Huwiler A, Pfeilschifter J. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des. 2006;12:4625–4635. doi: 10.2174/138161206779010422. [DOI] [PubMed] [Google Scholar]
- 178.Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 179.Gouaze V, et al. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 180.Beier UH, Gorogh T. Implications of galactocerebrosidase and galactosylcerebroside metabolism in cancer cells. Int J Cancer. 2005;115:6–10. doi: 10.1002/ijc.20851. [DOI] [PubMed] [Google Scholar]
- 181.Liu X, et al. Modulation of ceramide metabolism enhances viral protein apoptin’s cytotoxicity in prostate cancer. Mol Ther. 2006;14:637–646. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 182.Bonhoure E, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- 183.Gouaze V, et al. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- 184.Sun YL, et al. Suppression of glucosylceramide synthase by RNA interference reverses multidrug resistance in human breast cancer cells. Neoplasma. 2006;53:1–8. [PubMed] [Google Scholar]
- 185.Lavie Y, et al. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem. 1997;272:1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- 186.Wang H, Charles AG, Frankel AJ, Cabot MC. Increasing intracellular ceramide: an approach that enhances the cytotoxic response in prostate cancer cells. Urology. 2003;61:1047–1052. doi: 10.1016/s0090-4295(02)02511-6. [DOI] [PubMed] [Google Scholar]
- 187.Yang Z, Khoury C, Jean-Baptiste G, Greenwood MT. Identification of mouse sphingomyelin synthase 1 as a suppressor of Bax-mediated cell death in yeast. FEMS Yeast Res. 2006;6:751–762. doi: 10.1111/j.1567-1364.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 188.Patrawala L, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 189.Patrawala L, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2_ cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 190.Tai MH, et al. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 191.Kopfstein L, Christofori G. Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci. 2006;63:449–468. doi: 10.1007/s00018-005-5296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- 193.Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev. 2006;16:60–64. doi: 10.1016/j.gde.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 194.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 195.Setoguchi T, Taga T, Kondo T. Cancer stem cells persist in many cancer cell lines. Cell Cycle. 2004;3:414–415. doi: 10.4161/cc.3.4.799. [DOI] [PubMed] [Google Scholar]
- 196.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Szotek PP, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haraguchi N, et al. Cancer stem cells in human gastrointestinal cancers. Hum Cell. 2006;19:24–29. doi: 10.1111/j.1749-0774.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 199.Haraguchi N, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 200.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- 201.Chen JS, et al. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–406. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 202.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 203.Liu G, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 205.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 206.Ravandi F, Estrov Z. Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res. 2006;12:340–344. doi: 10.1158/1078-0432.CCR-05-1879. [DOI] [PubMed] [Google Scholar]
- 207.Frank NY, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–4333. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 208.Phillips TM, McBride WH, Pajonk F. The response of CD24(_/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 209.Goldman J, Gordon M. Why do chronic myelogenous leukemia stem cells survive allogeneic stem cell transplantation or imatinib: does it really matter? Leuk Lymphoma. 2006;47:1–7. doi: 10.1080/10428190500407996. [DOI] [PubMed] [Google Scholar]
- 210.Eramo A, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 211.Salmaggi A, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- 212.Meyer zu Schwabedissen HE, et al. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP) Drug Metab Dispos. 2006;34:524–533. doi: 10.1124/dmd.105.007591. [DOI] [PubMed] [Google Scholar]
- 213.Romer J, Curran T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 2005;65:4975–4978. doi: 10.1158/0008-5472.CAN-05-0481. [DOI] [PubMed] [Google Scholar]
- 214.Galmozzi E, Facchetti F, La Porta CA. Cancer stem cells and therapeutic perspectives. Curr Med Chem. 2006;13:603–607. doi: 10.2174/092986706776055661. [DOI] [PubMed] [Google Scholar]
- 215.Ysebaert L, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20:1211–1216. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]
- 216.Feldmann G, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sengupta A, et al. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- 218.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 219.Schatzlein AG. Delivering cancer stem cell therapies—a role for nanomedicines? Eur J Cancer. 2006;42:1309–1315. doi: 10.1016/j.ejca.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 220.Thorne SH. Strategies to achieve systemic delivery of therapeutic cells and microbes to tumors. Expert Opin Biol Ther. 2007;7:41–51. doi: 10.1517/14712598.7.1.41. [DOI] [PubMed] [Google Scholar]
- 221.Sell S. Leukemia: stem cells, maturation arrest, and differentiation therapy. Stem Cell Rev. 2005;1:197–205. doi: 10.1385/SCR:1:3:197. [DOI] [PubMed] [Google Scholar]
- 222.Mathieu J, Besancon F. Arsenic trioxide represses NF-kappaB activation and increases apoptosis in ATRA-treated APL cells. Ann NY Acad Sci. 2006;1090:203–208. doi: 10.1196/annals.1378.022. [DOI] [PubMed] [Google Scholar]
- 223.Stevenson GT. CD38 as a therapeutic target. Mol Med. 2006;12:345–346. doi: 10.2119/2006-00082.Stevenson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Copland M, Jorgensen HG, Holyoake TL. Evolving molecular therapy for chronic myeloid leukaemia—are we on target? Hematology. 2005;10:349–359. doi: 10.1080/10245330500234195. [DOI] [PubMed] [Google Scholar]
- 225.Mauro MJ. Defining and managing imatinib resistance. Hematology Am Soc Hematol Educ Program. 2006:219–225. doi: 10.1182/asheducation-2006.1.219. [DOI] [PubMed] [Google Scholar]
- 226.Mughal T, Goldman JM. Optimal management of patients with newly diagnosed chronic phase chronic myeloid leukemia in 2007. Clin Lymphoma Myeloma. 2007;7 (suppl 3):S95–S101. doi: 10.3816/clm.2007.s.008. [DOI] [PubMed] [Google Scholar]
- 227.Copland M, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 228.Donato NJ, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 229.White DL, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 230.Konopleva M, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 231.Jorgensen HG, et al. Intermittent exposure of primitive quiescent chronic myeloid leukemia cells to granulocyte-colony stimulating factor in vitro promotes their elimination by imatinib mesylate. Clin Cancer Res. 2006;12:626–633. doi: 10.1158/1078-0432.CCR-05-0429. [DOI] [PubMed] [Google Scholar]
- 232.Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–1120. doi: 10.1158/0008-5472.CAN-06-2014. [DOI] [PubMed] [Google Scholar]
- 233.Angstreich GR, et al. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 234.Zhou GB, Zhang J, Wang ZY, Chen SJ, Chen Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci. 2007;362:959–971. doi: 10.1098/rstb.2007.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zheng X, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica. 2007;92:323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 236.Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65. doi: 10.1016/j.beha.2006.11.002. [DOI] [PubMed] [Google Scholar]