Abstract
Adeno-associated viral (AAV) vectors are an extensively studied and highly used vector platform for gene therapy applications. We hypothesize that in the first clinical trial using AAV to treat hemophilia B, AAV capsid proteins were presented on the surface of transduced hepatocytes, resulting in clearance by antigen-specific CD8+ T cells and consequent loss of therapeutic transgene expression. It has been previously shown that proteasome inhibitors can have a dramatic effect on AAV transduction in vitro and in vivo. Here, we describe using the US Food and Drug Administration-approved proteasome inhibitor, bortezomib, to decrease capsid antigen presentation on hepatocytes in vitro, whereas at the same time, enhancing gene expression in vivo. Using an AAV capsid-specific T-cell reporter (TCR) line to analyze the effect of proteasome inhibitors on antigen presentation, we demonstrate capsid antigen presentation at low multiplicities of infection (MOIs), and inhibition of antigen presentation at pharmacologic levels of bortezomib. We also demonstrate that bortezomib can enhance Factor IX (FIX) expression from an AAV2 vector in mice, although the same effect was not observed for AAV8 vectors. A pharmacological agent that can enhance AAV transduction, decrease T-cell activation/proliferation, and decrease capsid antigen presentation would be a promising solution to obstacles to successful AAV-mediated, liver-directed gene transfer in humans.
Introduction
Adeno-associated viral (AAV) vectors are one of the most extensively studied and highly used vector platforms for gene therapy applications. Low immunogenicity, ease of production, lack of pathogenicity, and simplicity of design have made these vectors attractive to clinical investigators. The safety profile of AAV vectors in the clinic has been excellent, and the first studies using AAV to treat Leber's congenital amaurosis demonstrated significant efficacy.1,2,3 In the first clinical study using AAV to deliver the human Factor IX (hFIX) gene to the liver in subjects with hemophilia B, therapeutic levels of FIX expression were observed. However, 4–6 weeks after gene transfer an AAV capsid-specific T cell response was documented that coincided with a rise in liver transaminases and a drop in FIX transgene expression to baseline levels.4 This CD8+ T cell-mediated immune response was unexpected, as this had not been observed in any animal models. These data suggested that strategies to avoid capsid-specific immune responses would be required to achieve long-term liver-directed gene transfer in the clinic.
Major histocompatibility complex class I (MHCI) molecules carrying short peptides are displayed on the surface of the majority of cells. The primary purpose of these surface protein complexes is to present representative peptides from the interior of the cell to circulating T cells. Specialized protein complexes, proteasomes, in the cytoplasm and nucleus are responsible for degrading proteins into short peptides (9-15aa), which are then transported into the endoplasmic reticulum via the transporter associated with antigen processing and loaded onto MHCI. If the circulating T cells recognize a peptide–MHCI complex that is not a part of the self-repertoire (e.g., viral protein or mutated tumor antigen), that T cell will become activated, targeting the cell for destruction either directly (via cytotoxic lymphocytes) or indirectly (CD4+ help). We recently provided evidence for capsid-derived antigen presentation through MHCI on the surface of AAV-transduced cells,5 supporting the hypothesis that in the hemophilia B trial, AAV peptide epitopes from capsid proteins are presented on the surface of transduced hepatocytes, resulting in clearance by antigen-specific CD8+ T cells and consequent loss of therapeutic transgene expression.
Proteasome inhibitors are small molecule compounds that are able to specifically inhibit the activity of the proteasome, resulting in a buildup of ubiquitinated proteins, increased intracellular reactive oxygen species,6,7,8 and a general decrease in presentation of MHCI–peptide complexes.9 It has been previously shown that proteasome inhibitors can have a dramatic effect on AAV transduction in vitro and in vivo. Studies using polarized lung airway epithelia demonstrated >200-fold enhancement of expression levels when tri-peptide proteasome inhibitors (LLnL) were coapplied with AAV2 to the apical surface. Significant enhancement of expression was also observed when tri-peptide proteasome inhibitors (LLnL or z-LLL) were coadministered in vivo to the lung or liver; however, no such enhancement was observed for skeletal or cardiac muscle.10 The proteasome inhibitor enhancement effect on AAV transduction has been observed with multiple serotypes and cell types.10,11,12,13,14 Whereas the mechanism of the enhancement is unclear, it is hypothesized that modulation of the ubiquitin–proteasome system might alter AAV intracellular processing at the level of endosomal trafficking, endosomal escape, nuclear transport, or vector uncoating. Recent data have demonstrated that proteasome inhibitors can increase the rate at which intact capsids enter the nucleus and can significantly increase their nucleolar accumulation.15 Whether this is due to decreasing the degradation of capsids in the cell, or to some other effect of the proteasome inhibitor is currently unclear.
Based on this information, we hypothesized that inhibiting the proteasome using small molecule compounds would prevent or reduce the presentation of AAV capsid epitopes on the surface of transduced cells, potentially avoiding T cell-mediated destruction, while at the same time increasing the levels of gene expression. With this aim in mind, the clinically approved proteasome inhibitor bortezomib (Velcade) was used to examine gene expression and antigen presentation after AAV transduction of a human hepatocyte cell line in vitro as well as to determine the kinetics of its effect on gene expression in vivo. Hepatocytes were chosen as our target cell of interest due to the large number of diseases, including Hunter's syndrome, mucopolysaccharidosis I and VII, ornithine transcarbamylase deficiency, and hemophilia A and B, that have been shown to be treatable by AAV-mediated, liver-directed gene transfer in animal models.
Results
Proteasome inhibitors increase AAV transduction in vitro and decrease peptide: MHCI surface expression
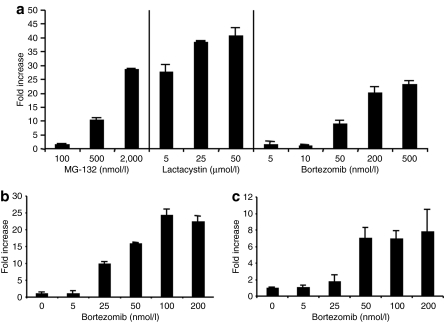
We first sought to determine the effect of proteasome inhibitors on AAV2 transduction in a variety of cell lines in vitro. For this purpose the human hepatocyte cell line HHL5 was treated with three structurally unrelated proteasome inhibitors, MG-132, lactacystin, and bortezomib and then transduced with an AAV2-CMV-LacZ vector. As can be seen in Figure 1a, all proteasome inhibitors significantly increased gene expression following AAV transduction in a dose-dependent manner. These effects were not limited to HHL5 cells as similar results were observed with human embryonic kidney-293 and murine Hepa 1-6 (murine hepatocyte) cell lines that had been treated with various concentrations of bortezomib at the time of AAV transduction (Figure 1b,c). These results confirm the previous reports that proteasome inhibitors can enhance AAV-mediated gene expression levels, and more specifically, show that proteasome inhibitors are able to enhance transduction of both human and murine hepatocyte, as well as nonhepatocyte, cell lines.
Figure 1.
Proteasome inhibitor mediated enhancement of gene expression following AAV2 transduction in vitro. (a) HHL5 cells were transduced with AAV2-CMV-LacZ (MOI 10,000) with the proteasome inhibitors MG-132, lactacystin, or bortezomib at the concentrations indicated. LacZ expression was measured 24 hours after transduction. (b) 293 or (c) Hepa 1-6 cells were transduced with AAV2-CMV-LacZ (MOI 10,000) in the presence of increasing amounts of bortezomib. LacZ expression was measured 24 hours after transduction. All experiments are performed in triplicate and SEM is shown. AAV, Adeno-associated virus; CMV, cytomegalovirus; MOI, multiplicity of infection.
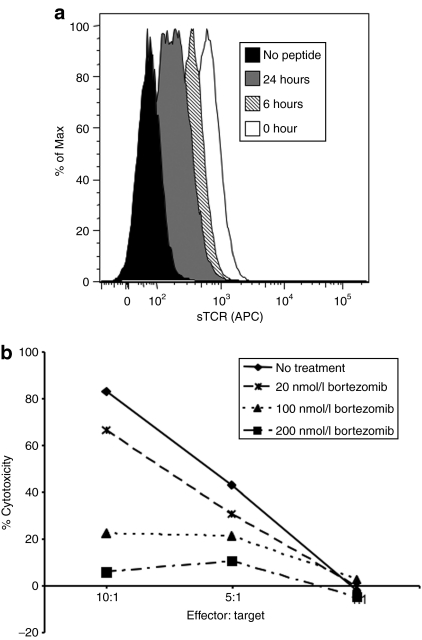
Having established that bortezomib, a drug approved for human use, was able to augment AAV transduction in the human hepatocyte cell line HHL5, we wished to determine the effect of proteasome inhibitors on antigen presentation. The proteasome is an essential part of the cellular antigen processing machinery, responsible for the generation of peptides to be loaded onto MHCI molecules in the endoplasmic reticulum before transport and presentation on the cell surface. MHCI molecules are unstable and are not presented on the cell surface in the absence of a peptide and β2-microglobulin light chain.16 Therefore we hypothesized that inhibiting the proteasome would lead to a decrease in AAV capsid-derived peptide:MHCI complexes on the cell surface. To test this hypothesis, we used a derivative of the HHL5 cell line that was stably expressing the human HLA B*0702 MHCI gene (HHL5-B7).5 HHL5-B7 cells were incubated with bortezomib for 0, 6, or 24 hours before being loaded with the AAV2 capsid epitope peptide VPQYGYLTL for 2 hours at 37 °C. A fluorescently labeled soluble T-cell receptor (sTCR) specific for the VPQYGYLTL:HLA B*0702 complexes, recently described by our group,5 was used to determine the level of peptide:MHCI complexes on the cell surface. As seen in Figure 2a, the longer the cells were exposed to proteasome inhibitor, the lower the levels of peptide:MHCI complexes that were detected by flow cytometry on the cell surface.
Figure 2.
Bortezomib treatment decreases peptide:MHCI complexes on the cell surface and decreases CTL killing. (a) HHL5-B7 cells were incubated with 75 nmol/l bortezomib for 0, 6, or 24 hours. During the last 2 hours the cells were loaded with the capsid-derived VPQYGYLTL peptide epitope (10 µg/ml). After loading, cells were washed and stained with a PE-labeled sTCR specific for the VPQYGYLTL peptide and analyzed by flow cytometry. (b) HHL5-B7 cells were transduced with AAV2 (3 × 105 MOI) for 24 hours in the presence of a range of doses of bortezomib. After 24 hours, the cells were washed and HLA-matched human PBMCs expanded against AAV2 capsid were added at various effector:target ratios (E:T). The percentage of specific lysis was calculated from the release of intracellular lactate dehydrogenase and all samples were normalized to untransduced cells incubated with the appropriate concentration of bortezomib and E:T ratio. CTL assay was performed in triplicate wells and SEM is shown. CTL, cytotoxic T lymphocyte; MHCI, major histocompatibility complex class I; MOI, multiplicity of infection; PBMC, peripheral blood mononuclear cell; PE, phycoerythrin; sTCR, soluble T-cell receptor.
Proteasome inhibitors decrease CTL-mediated killing of AAV-transduced cells
AAV capsid-specific cytotoxic T lymphocyte (CTL)-mediated killing of transduced hepatocytes was hypothesized as the causative factor for the loss of hFIX expression observed in the phase 1 clinical study using AAV2 vectors in subjects with severe hemophilia B.4,17 We hypothesized that proteasome inhibitors could be used to reduce the production of antigenic peptides from AAV capsid proteins, and thus reduce recognition and killing of AAV-transduced cells by capsid-specific CTLs. In order to test this hypothesis, a CTL assay was performed using HHL5-B7 cells transduced with AAV2-hFIX-16 vectors as target cells. Effector cells were generated from HLA-B*0702 human donor peripheral blood mononuclear cells or splenocytes that were expanded in culture with either intact AAV capsids or AAV capsid-derived peptides. Bortezomib prevented CTL-mediated lysis of AAV-transduced target cells in a dose-dependent manner (Figure 2b). This was observed for effector:target (E:T) cell ratios of 5:1 and the protection from CTL killing is even more pronounced at an E:T of 10:1.
Development of TCR line to analyze effect of proteasome inhibitor on antigen presentation
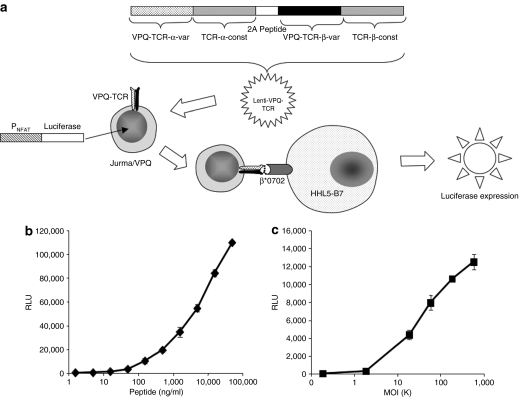
Previous attempts from our group to use a capsid-specific sTCR to measure antigen presentation after cell transduction directly using flow cytometry were not successful; although the sTCR easily detects complexes on peptide-loaded cells, with a lower limit of detection of ~1,000 peptide:MHC complexes per cell, it is not sensitive enough to detect antigen presentation directly following AAV transduction unless techniques more sensitive than flow cytometry are used.5 We therefore sought to develop a more sensitive system for measurement of antigen presentation. The Jurkat/MA reporter cell line is a CD8+ Jurkat T cell that is devoid of endogenous TCR β-chain expression and has been engineered to express luciferase upon engagement of the TCR.18,19 We used a lentiviral system to make a stable Jurkat/MA cell line expressing a TCR that is specific for the AAV capsid peptide epitope VPQYGYLTL presented by HLA B*0702 (Jurma-VPQ). Thus, upon recognition by the TCR of its cognate antigen (AAV capsid peptide VPQYGYLTL presented by HLA B*0702), the cells will produce luciferase in amounts proportional to the amount of VPQYGYLTL:B0702 that the TCR engaged (Figure 3a). Studies using peptide-loaded HHL5-B7 cells demonstrate that these cells are sensitive reporters of antigen presentation, able to detect peptide at concentrations as low as 1.6 ng/ml (Figure 3b). HHL5-B7 cells loaded with an irrelevant peptide (HLA B*0702-binding peptide from HIV, IPRRIRQGL) gave rise to background levels of luciferase expression (data not shown). We used this reporter cell line to measure antigen presentation following AAV transduction. HHL5-B7 cells were transduced at a range of multiplicities of infection (MOIs) with an AAV2 vector expressing hFIX under control of the liver-specific human α1 antitrypsin promoter.4 As can be seen in Figure 3c, antigen presentation was detected in a dose-dependent manner with the lower limit of detection at an MOI of 2,000 (P < 0.005), with increasing MOIs (up to 6 × 105) giving rise to increasing antigen presentation.
Figure 3.
Capsid-specific TCR cell line. (a) Jurkat/MA cells were transduced with a lentivirus expressing the α and β chains of the TCR specific for the AAV-capsid peptide VPQYGYLTL. Upon engagement of the TCR with the VPQYGYLTL peptide associated with HLA B*0702, the T cell becomes activated and expresses luciferase under control of the NFAT promoter that can be measured as relative light units on a standard luminometer. HHL5-B7 cells were either (b) loaded with VPQYGYLTL peptide for 4 hours or (c) transduced with AAV2 hAAT-hFIX for 24 hours before being washed and incubated with Jurma/VPQ reporter cells at an E:T ratio of 10:1. Cells were harvested after 24-hours incubation and assayed for luciferase expression. All RLU values are normalized by subtracting background Jurma/VPQ values. Experiments were performed in triplicate and (SD) is shown for each data point. AAV, adeno-associated virus; E:T, effector:target; hAAT, human α1-antitrypsin; hFIX, human Factor IX; NFAT, nuclear factor of activated T cells; RLU, relative light unit; TCR, T-cell receptor.
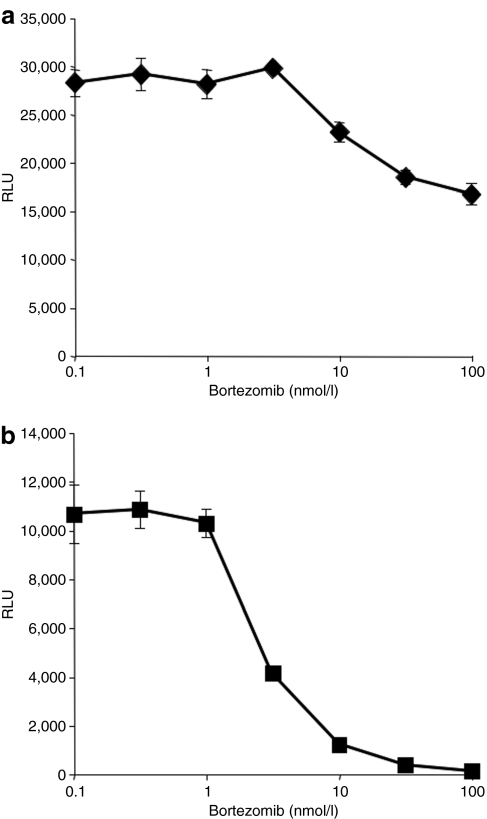
To determine whether treatment with proteasome inhibitors has any effect on antigen presentation following AAV transduction, we treated either peptide-loaded or AAV-transduced cells with bortezomib. Not surprisingly, bortezomib had only a modest effect on antigen presentation in peptide-loaded cells (Figure 4a). The small decrease in antigen presentation at high concentrations of bortezomib is most likely due to a global decrease in MHCI expression on the cell surface as seen in Figure 2a. In contrast, following AAV transduction, antigen presentation is decreased in a dose-dependent fashion (Figure 4b), with >95% inhibition at a concentration of 30 nmol/l bortezomib.
Figure 4.
Proteasome inhibitor blocks antigen presentation following AAV transduction. HHL5-B7 cells were either (a) incubated with increasing concentrations of the proteasome inhibitor bortezomib for 24 hours and peptide loaded with VPQYGYLTL peptide for the final 4 hours or (b) transduced with AAV2-hAAT-hFIX (2.5 × 105 MOI) in the presence of increasing amounts of bortezomib for 24 hours. Jurma/VPQ reporter cells were added at an E:T ratio of 10:1 and incubated for 24 hours before being harvested and assayed for luciferase expression. All RLU values are normalized by subtracting background Jurma/VPQ luciferase expression levels. Experiments were performed in triplicate and (SD) is shown for each data point. AAV, adeno-associated virus; E:T, effector:target; hAAT, human α1-antitrypsin; hFIX, human Factor IX; MOI, multiplicity of infection.
Proteasome inhibitors increase transduction in vivo by AAV2 but not by AAV8
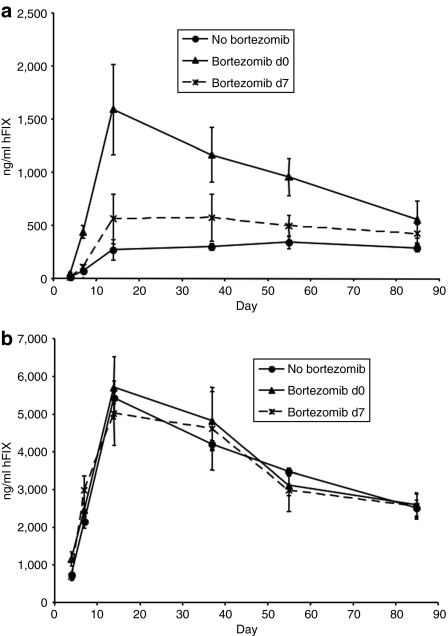
We wished to determine whether administration of bortezomib would give rise to enhancement of gene expression following in vivo AAV administration. Therefore an AAV2 vector encoding hFIX under control of the human α1 antitrypsin promoter [2.5 × 1010 vector genomes (vg)/animal] was coadministered with bortezomib (25 µg) in 200 µl of phosphate-buffered saline (PBS) intravenously to 8–10-week-old male C57Bl/6 mice. As seen in Figure 5a, we observed a sixfold increase in gene-expression levels that peaked 2 weeks after gene transfer (P < 0.05), followed by a slow decrease over time. By 8 weeks after treatment there was a 2.5-fold difference in expression between bortezomib-treated and untreated groups (P < 0.05), dropping to a twofold increase by 12 weeks (P < 0.05).
Figure 5.
Proteasome inhibitors increase hFIX expression in vivo following transduction by AAV2 but not AAV8. C57Bl/6 mice (n = 4) were administered 25 µg of bortezomib at different time points (d0, d7) and transduced with (a) AAV2 (2.5 ×1010 vg) or (b) AAV8 (2.5 × 109 vg) expressing the hFIX gene under control of the hAAT promoter (hFIX16) on d0. hFIX levels were assayed by ELISA at time-points indicated. SEM is indicated. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay; hAAT, human α1-antitrypsin; hFIX, human Factor IX; vg, vector genomes.
Next we examined the effect of bortezomib on AAV8 transduction. C57Bl/6 mice were injected with 2.5 × 109 vg/animal of an AAV8 vector, with the same hFIX expression cassette as the AAV2 vector described above, mixed with 25 µg bortezomib. In contrast to our observations with AAV2, there was no difference between bortezomib-treated groups and control groups when using AAV8 (Figure 5b).
We then determined the effect of the timing of bortezomib administration with respect to the AAV injection. Bortezomib (25 µg) was administered on day 0 or day 7 with respect to AAV administration (on day 0). Optimal expression levels were obtained when bortezomib was administered at the same time as AAV2, whereas administration of bortezomib on day 7 did not have a significant effect (Figure 5). These results are interesting as previous work from others using polarized airway epithelia had shown that proteasome inhibitors can have a dramatic effect on AAV expression in vitro when added up to 2 weeks after vector.20
Discussion
The potential for using proteasome inhibitors to increase AAV-mediated gene expression has been investigated since the first report of enhanced AAV-mediated gene expression using proteasome inhibitors in 2000.10 The recent approval by the US Food and Drug Administration of bortezomib, a first-in-class proteasome inhibitor, for clinical use has heightened interest in this finding, and others have indeed confirmed that this clinically relevant proteasome inhibitor increases AAV transduction in a mouse model.21 The mechanism by which this enhancement occurs is currently unclear; however, there is evidence that proteasome inhibitors can increase the accumulation of vector particles in the nucleolus of transduced cells.15
Our interest in the effect of proteasome inhibitors on AAV transduction arises from a different hypothesis, and is related to findings in a previous clinical trial of AAV-FIX in subjects with severe hemophilia B. At the highest dose tested, AAV2-FIX administration resulted in therapeutic levels of circulating FIX, which began to drop starting 4 weeks after vector administration, and slowly declined to the baseline of <1% by 12 weeks after vector administration.4 This was accompanied by a self-limited, asymptomatic rise in liver enzymes that resolved over the same time course as the loss of FIX expression. Subsequent studies demonstrated expansion of a population of capsid-specific CD8+ T cells in the same time course, suggesting that CD8+ T cell-mediated destruction of the transduced hepatocytes accounted for the rise in liver enzymes and the loss of FIX expression.17 We hypothesized, given the known role of the proteasome in capsid processing and presentation, that coadministration of an AAV vector with the proteasome inhibitor bortezomib would result in reduced presentation of AAV capsid-derived peptides on the surface of the transduced cell, making it less vulnerable to destruction by CD8+ T cells that recognize capsid-derived peptide/MHC complexes. In our initial experiments, we showed that bortezomib treatment of human hepatocytes results in a dose-dependent decrease in surface expression of MHCI molecules, not surprising as other published work has demonstrated downregulation of MHCI on the surface of both murine and human cells in culture22 and multiple myeloma cells in vitro and in vivo23 after proteasome inhibitor treatment.
We next showed that treatment of AAV-transduced hepatocytes with bortezomib results in a dose-dependent inhibition of killing in a CTL assay; this is consistent with lower levels of presentation of peptide/MHC on the cell surface. Using a human T cell line modified to express a TCR that recognizes an AAV-derived capsid peptide presented on the B*0702 molecule, we have shown that activation of these cognate T cells on exposure to AAV-transduced hepatocytes is dramatically reduced in the presence of bortezomib, again in a dose-dependent manner, consistent with reduced antigen presentation in the presence of the drug. It should be noted that the pharmacologic concentrations of bortezomib required to inhibit antigen presentation (30–100 nmol/l), are well below the concentrations used in the setting of multiple myeloma in the clinic (~300 nmol/l). The fate of capsid proteins after proteasome inhibition is currently unknown. Possibilities include the capsid proteins being put “on hold”, until the proteasome inhibition has subsided and then all capsids being processed and degraded en masse, or more likely, the capsids being eliminated by some other cellular degradation mechanism when proteasomal degradation is blocked. Given data in the literature that when the proteasome is inhibited, there is a reciprocal upregulation of other degradation pathways such as autophagy,24 we speculate that capsid proteins might be eliminated by other mechanisms. This is an ongoing area of research in our laboratory.
Our lab has previously attempted to measure capsid antigen presentation using a fluorescently labeled sTCR and found that the threshold for measuring peptide-loaded cells was ~25 ng/ml (~1,000 molecules/cell) and that this threshold was too high to measure capsid epitope presentation directly by flow cytometry following AAV transduction.5 In this study, we use the identical capsid epitope-specific TCR in a T cell-based assay and observe an increase in the sensitivity of the system by 15-fold (1.6 ng/ml). This system is sensitive enough to directly measure antigen presentation following AAV transduction of a human hepatocyte cell line, with a lower limit of detection down to cells transduced at an MOI of 2,000 vg/cell. Note that this is a therapeutically relevant MOI: the dose of vector that gave a therapeutic level of FIX in the clinical trial was 2 × 1012 vg/kg or 2 × 1014 vg total. The human liver contains ~1.5 × 1011 hepatocytes,25 yielding an average MOI of ~1,000, close to the range detected in this assay.
Proteasome inhibitors can affect virtually all aspects of normal cellular processes, and there has therefore been interest in using these compounds as immunosuppressive agents.26 There is considerable evidence that proteasome inhibitors can induce apoptosis in activated and proliferating cells, but not resting T cells,27,28 suppress immune functions of human CD4+ T cells,29 and as prevent the activation of human dendritic cells.30,31 In mouse models of heart and islet transplants proteasome inhibitors have shown efficacy at prolonging allograft function and inducing tolerance.32,33 Thus, it is not unlikely to envision the use of proteasome inhibitors in the context of gene transfer as immunosuppressants, an approach currently proposed to escape immune responses to transgene and capsid.34,35
Our data confirm previously published studies demonstrating that proteasome inhibitors are able to enhance AAV transduction both in vitro and in vivo.10,15,20,21 We showed enhanced transgene expression following administration of an AAV-hFIX vector and proteasome inhibitors to C57Bl/6 mice at clinically relevant doses of bortezomib. We observed a significant (approximately sixfold) increase in expression at 2 weeks after transduction; however, this enhancement decreases to about twofold enhancement by 12 weeks. This transient increase was also observed when proteasome inhibitors were used to enhance transduction by AAV from the basolateral side, but not from the apical side, of airway epithelia in vitro. Interestingly, the blood side of a hepatocyte is also the basolateral side. How differences in spatial distribution of functional properties within the cell may influence differences in transduction efficiency, or persistence of response to proteasome inhibitors, is not well understood. One possible explanation for the transient nature of the response to proteasome inhibitors after transduction from the basolateral side is that there is a gradual loss of nonstabilized genomes after transduction. Previous work has shown that single-stranded genomes from AAV2 are continuously uncoated and synthesized into double-stranded genomes for weeks after administration to the liver of mice in vivo; however, the majority of these genomes are not stabilized and are lost.36 Thus one possibility may be that proteasome inhibitors enhance the efficiency of double-stranded genome synthesis (either through enhanced vector trafficking, avoiding proteasomal degradation, or increased vector genome uncoating) from the basolateral side; however, the capacity of the cell to stabilize vector-derived genomes is eventually overwhelmed, resulting in a transient increase in transgene expression followed by a gradual loss of expression as the double-stranded genomes are lost over time. Interestingly, we did not observe any increased hFIX expression between bortezomib-treated or control groups after administration of an AAV8 vector. This is in contrast to a recent paper published by Nathwani et al.,21 where a small but significant increase in transduction was demonstrated using self-complementary AAV8 vectors with bortezomib in mice. Differences between our study and the one published include the nature of the AAV genome (single stranded versus self-complementary, the AAV dose (1 × 1011 vg/kg versus 4 × 1010 vg/kg), the dose and administration schedule of bortezomib (1.25 µg/g at time of vector administration versus 1 µg/mg 24 hours before AAV), and the number of time points analyzed (time course versus single-time point). It is unclear what determined the different result in the two studies. Because the transduction efficiency of AAV8 in mouse hepatocytes is greater than other serotypes, a saturation effect at higher vector doses could prevent any further increase in expression with proteasome inhibitors. Alternately, this could be indicative of differences in the intracellular trafficking of the two serotypes. It is well known that proteasome inhibitors have a whole host of effects on the cell, not the least of which is increasing intracellular reactive oxygen species (see refs. 7,37 and our own unpublished data). Earlier work has demonstrated that reactive oxygen species are important for recombinant AAV2 transduction38 and we hypothesize that this side effect of proteasome inhibition might play a role in enhancing AAV2 expression. This may help to explain why bortezomib did not enhance AAV8 transduction; it constitutes additional evidence suggesting that AAV2 and AAV8 may differ in critical ways in terms of intracellular trafficking/processing.39,40 We are currently investigating this further.
In this report, we also find that the timing of proteasome inhibitor administration can have an effect on the efficacy of AAV-mediated gene transfer. We found that adding bortezomib at the time of vector administration resulted in the highest levels of gene expression, whereas delivery on day 7 showed no statistically significant increase in expression. This is interesting as earlier work using polarized airway epithelia showed that proteasome inhibitors can enhance transduction in vitro when added up to 2 weeks after vector.10 This difference highlights the observations that the type of target cell can influence the effect of proteasome inhibitors on AAV transduction. For example, proteasome inhibitors only transiently increase AAV transduction when added from the basolateral side of a polarized airway epithelium, but the effect is much more sustained when added from the apical side; or can enhance transduction from the liver, but not muscle.10 The mechanisms underlying these differences in response to proteasome inhibitors among different cell types are currently unclear but are of obvious interest; further investigation may shed light on the biology of AAV vector trafficking or other determinants of gene expression.
Based on the data presented here, it appears as if future studies using proteasome inhibitors in large animal models may be warranted. A pharmacological agent that can enhance AAV transduction, decrease T cell activation/proliferation, and decrease antigen presentation would be a promising solution to obstacles to successful translation of AAV-mediated, liver-directed gene transfer in the clinic.
Materials and Methods
AAV vectors, peptides, sTCR. AAV vectors were produced using previously described triple transfection methods into human embryonic kidney-293 cells and subsequent CsCl density gradient purification.41 AAV2 capsid-specific sTCR monomers were generated as previously described.5 Multimerization was performed by conjugation with streptavidin-APC (Invitrogen, Carlsbad, CA) in a 1:1 molar ratio of biotin:streptavidin.
Cell lines. Human embryonic kidney derived human embryonic kidney-293 (ATCC CRL-1573) and human hepatocyte cell line HHL542 were grown in minimal essential media + 10% fetal bovine serum (Invitrogen), 1% L-glutamine (Gibco, Carlsbad, CA), and 1% antibiotic/antimycotic (Invitrogen) at 37 °C in 5% CO2. The murine hepatocyte cell line Hepa 1-6 (ATCC CRL-1830) was propagated in Dulbecco's modified Eagle's media + 10% fetal bovine serum, 1% L-glutamine, and 1% antibiotic/antimycotic. HHL5-B7 is a derivative of HHL5 stably expressing the human HLA-B*0702.5 The Jurma-VPQ cell line was derived by transducing the Jurkat/MA cell line [a kind gift of Eriik Hooijberg (VU University Medical Center, Amsterdam, the Netherlands) and Aviva Joseph (Albert Einstein College of Medicine, New York, NY)] with a lentivirus expressing a human TCR specific for the AAV2 capsid class I epitope (VPQYGYLTL). Briefly, a VSV-pseudotyped lentivirus containing a cytomegalovirus-based human VPQ-TCR (see Pien et al. for detailed information regarding isolation of TCR sequence5) was generated using the ViraPower HiPerform lentiviral expression system (Invitrogen). Jurkat/MA cells (Jurkat cells engineered to expression firefly luciferase upon TCR activation18) were transduced with the lentivirus by spinoculation in RPMI supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% antibiotic/antimycotic (2 ml of 5 × 104 cells/ml + 500 µl virus + 6 µg/ml polybrene (final concentration) centrifuged for 30 minutes at 800 g at room temperature). Cells were expanded to a T-75 flask and then TCR-expressing cells were stained with fluorescein isothiocyanate–conjugated CD8 (ProImmune, Oxford, UK) and a phycoerythrin -conjugated VPQ-TCR pentamer (ProImmune) and cells expressing high levels of VPQ-TCR were sorted by flow cytometry.
In vitro transductions and LacZ assays. Cells were plated in 96-well plates at a density of 30,000 cells/well in 100 µl of complete media. The following day cells were treated with proteasome inhibitors as indicated and transduced with the indicated amount of rAAV. Twenty-fours hours after transduction, media was removed and cells were washed 1× with 200 µl of PBS. PBS was removed and 100 µl of lysis buffer (60 mmol/l Na2HPO4, 40 mmol/l NaH2PO4, 10 mmol/l KCl, 1 mmol/l MgSO4, 0.27% β-mercaptoethanol, 0.005% sodium dodecyl sulfate) was added. Plates were incubated for 5 minutes with shaking before adding 30 µl ONPG. Plates were then incubated for 30–60 minutes at 37 °C with shaking and A420 measurements were taken using a SpectraMax 190 (Molecular Devices, Sunnyvale, CA).
Jurma/VPQ antigen presentation assays. HHL5-B7 cells were plated into 96-wells at 2.5 × 104 cells/well. AAV or bortezomib at a range of doses was added to the cells before overnight incubation. The following day, the supernatant was removed and Jurma/VPQ cells were added at an E:T ratio of 10:1 in a final volume of 200 µl cRPMI media (RPMI + 10% fetal bovine serum, 1% L-glutamine, 1% antibiotic/antimycotic). For peptide loading studies, peptide was added during the final 4 hours of incubation. After 24 hours the plate was centrifuged 1,400 r.p.m. for 5 minutes and the supernatant was removed and discarded. Passive lysis buffer (Promega, Madison, WI) was added (150 µl) and plates were shaken for 10 minutes at room temperature. Twenty-five microliters of each well was transferred to an assay plate (get plate info) and was assayed using the Promega luciferase assay system and read on a Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA).
MHC assay. HHL5-B7 cells were plated into 6-well plates at 2.5 × 105 cells/well. Bortezomib was added to a final concentration of 75 nmol/l to different wells at various time points. During the final 2 hours of incubation, VPQYGYLTL peptide was added to a final concentration of 10 µg/ml. Cells were then washed, trypsinized, and stained with a phycoerythrin-labeled sTCR specific for VPQYGYLTL and analyzed by flow cytometry as previously described.5
In vivo studies. C57Bl/6 mice (6–8 weeks) were administered 200 µl of bortezomib (25 µg diluted in PBS) intravenously on day 0 or day 7. On day 0, AAV was diluted in PBS and administered intravenously in a total volume of 200 µl. For day 0 bortezomib administration, bortezomib and AAV were mixed to give a final volume of 200 µl (bortezomib + AAV). At time points indicated mice were bled retro-orbitally and blood was assayed for hFIX expression by enzyme-linked immunosorbent assay as previously described.43
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (to K.A.H.) and by NIH grant P01 HL078810 (to K.A.H.).
REFERENCES
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling YH, Liebes L, Zou Y., and , Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- Alexandrova A, Petrov L, Georgieva A, Kirkova M., and , Kukan M. Effects of proteasome inhibitor, MG132, on proteasome activity and oxidative status of rat liver. Cell Biochem Funct. 2008;26:392–398. doi: 10.1002/cbf.1459. [DOI] [PubMed] [Google Scholar]
- Fribley A, Zeng Q., and , Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M., and , Schmidtke G. Selective proteasome inhibitors: modulators of antigen presentation. Drug Discov Today. 1999;4:63–71. doi: 10.1016/s1359-6446(98)01292-6. [DOI] [PubMed] [Google Scholar]
- Duan D, Yue Y, Yan Z, Yang J., and , Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denby L, Nicklin SA., and , Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Jennings K, Miyamae T, Traister R, Marinov A, Katakura S, Sowders D, et al. Proteasome inhibition enhances AAV-mediated transgene expression in human synoviocytes in vitro and in vivo. Mol Ther. 2005;11:600–607. doi: 10.1016/j.ymthe.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U., and , Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douar AM, Poulard K, Stockholm D., and , Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS., and , Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL., and , Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Scholten KB, Schreurs MW, Ruizendaal JJ, Kueter EW, Kramer D, Veenbergen S, et al. Preservation and redirection of HPV16E7-specific T cell receptors for immunotherapy of cervical cancer. Clin Immunol. 2005;114:119–129. doi: 10.1016/j.clim.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, et al. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai A., and , Forman J. The effect of the proteasome inhibitor lactacystin on the presentation of transporter associated with antigen processing (TAP)-dependent and TAP-independent peptide epitopes by class I molecules. J Immunol. 1997;159:2139–2146. [PubMed] [Google Scholar]
- Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias IM, Jakoby WB, Popper H, Schachter D., and , Shafritz DA.1988The Liver: Biology and Pathology2nd edn. Raven Press: New York; p 1405 [Google Scholar]
- Wu J. On the role of proteasomes in cell biology and proteasome inhibition as a novel frontier in the development of immunosuppressants. Am J Transplant. 2002;2:904–912. doi: 10.1034/j.1600-6143.2002.21006.x. [DOI] [PubMed] [Google Scholar]
- Cui H, Matsui K, Omura S, Schauer SL, Matulka RA, Sonenshein GE, et al. Proteasome regulation of activation-induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515–7520. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo H, Chen H, Duguid W., and , Wu J. Role of proteasomes in T cell activation and proliferation. J Immunol. 1998;160:788–801. [PubMed] [Google Scholar]
- Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, et al. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008;124:234–246. doi: 10.1111/j.1365-2567.2007.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni A, Garuti A, Schwarzenberg K, Cirmena G, Dal Bello G, Rocco I, et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol. 2006;36:681–689. doi: 10.1002/eji.200535298. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Grunebach F, Patrone F, Ballestrero A., and , Brossart P. The proteasome and its inhibitors in immune regulation and immune disorders. Crit Rev Immunol. 2006;26:487–498. doi: 10.1615/critrevimmunol.v26.i6.20. [DOI] [PubMed] [Google Scholar]
- Luo H, Wu Y, Qi S, Wan X, Chen H., and , Wu J. A proteasome inhibitor effectively prevents mouse heart allograft rejection. Transplantation. 2001;72:196–202. doi: 10.1097/00007890-200107270-00005. [DOI] [PubMed] [Google Scholar]
- Wu Y, Han B, Luo H, Shi G., and , Wu J. Dipeptide boronic acid, a novel proteasome inhibitor, prevents islet-allograft rejection. Transplantation. 2004;78:360–366. doi: 10.1097/01.tp.0000128855.10397.db. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., and , High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Wang J, Xie J, Lu H, Chen L, Hauck B, Samulski RJ, et al. Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc Natl Acad Sci USA. 2007;104:13104–13109. doi: 10.1073/pnas.0702778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Gomes E., and , Rockwell P. Reactive oxygen species induced by proteasome inhibition in neuronal cells mediate mitochondrial dysfunction and a caspase-independent cell death. Apoptosis. 2007;12:1389–1405. doi: 10.1007/s10495-007-0069-5. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S., and , Engelhardt JF. Cellular redox state alters recombinant adeno-associated virus transduction through tyrosine phosphatase pathways. Gene Ther. 1999;6:1427–1437. doi: 10.1038/sj.gt.3300967. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Bhagwat A, Edmonson S, Zhou S., and , High KA. High-throughput screening and biophysical interrogation of hepatotropic AAV. Mol Ther. 2008;16:1960–1967. doi: 10.1038/mt.2008.210. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and , Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Clayton RF, Rinaldi A, Kandyba EE, Edward M, Willberg C, Klenerman P, et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005;25:389–402. doi: 10.1111/j.1478-3231.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- Walter J, You Q, Hagstrom JN, Sands M., and , High KA. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc Natl Acad Sci USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]