Abstract
The ability of human adipose tissue–derived mesenchymal stem cells (AT-MSCs), engineered to express the suicide gene cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT), to convert the relatively nontoxic 5-fluorocytosine (5-FC) into the highly toxic antitumor 5-fluorouracil (5-FU) together with their ability to track and engraft into tumors and micrometastases makes these cells an attractive tool to activate prodrugs directly within the tumor mass. In this study, we tested the feasibility and efficacy of these therapeutic cells to function as cellular vehicles of prodrug-activating enzymes in prostate cancer (PC) therapy. In in vitro migration experiments we have shown that therapeutic AT-MSCs migrated to all the prostate cell lines tested. In a pilot preclinical study, we observed that coinjections of human bone metastatic PC cells along with the transduced AT-MSCs into nude mice treated with 5-FC induced a complete tumor regression in a dose dependent manner or did not even allow the establishment of the tumor. More importantly, we also demonstrated that the therapeutic cells were effective in significantly inhibiting PC tumor growth after intravenous administration that is a key requisite for any clinical application of gene-directed enzyme prodrug therapies.
Introduction
Stem and progenitor cells engineered to express therapeutic gene products are becoming more and more promising in cancer therapy for their ability to selectively target tumor cells. Different therapeutic genes have been so far successfully inserted into stem cells including prodrug-activating enzymes,1,2,3,4,5 interleukins,6,7,8 and apoptosis-promoting genes.9 Their administration in experimental setups resulted in successful inhibition of growth of variety of tumors, regardless of tumor size and anatomical location. An overview on this topic can be found in Aboody et al.10 Mesenchymal stem cells (MSCs) have a great potential in regenerative medicine as well (for a review see ref. 11).
MSCs can be isolated as a fraction of bone marrow cells or other adult tissues. They posses an extensive proliferative potential and the capacity to differentiate into various cell types.12 Unlike embryonal stem cells, the use of MSC does not involve ethical conflicts preserving on the other hand the characteristic to be easily expanded in culture and efficiently transduced with viral vectors containing therapeutic genes. Importantly, MSC also maintain an inherent capacity to track the tumor and engraft into it after systemic application. For these reasons, MSCs have been lately exploited as cellular vehicles in targeted-gene therapy.10,11,12,13 Interestingly, MSCs can be successfully isolated also from adipose tissue (AT) that is more easily obtained and with a less invasive and painful clinical procedure compared to the bone marrow or umbilical cord–derived MSC. The success rate of isolation of MSC from human AT is 100% and they can be easily expanded for a prolonged time.14,15
Human AT–derived MSCs (AT-MSCs) have been recently engineered, by retrovirus transduction, to express the suicide gene cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT). The ability of yeast cytosine deaminase (CD)-expressing AT-MSCs (CDy-AT-MSC) to convert the relatively nontoxic 5-fluorocytosine (5-FC) into the highly toxic antitumor drug 5-fluorouracil (5-FU) along with their ability to target tumor sites and micrometastases and to have a low immunogenic potential, makes these cells a unique tool to convert prodrugs to cytotoxic drugs directly within the tumor mass.1 Previous results from in vivo experiments showed that CDy-AT-MSCs, administered subcutaneously (s.c.) as a mixture with tumor cells, or intravenously significantly inhibited the growth of human colon adenocarcinoma (HT-29)16 and human melanoma xenografts in nude mice treated with 5-FC.17
In this study, we explored, by in vitro and in vivo investigations, the feasibility and efficacy of CDy-AT-MSCs as cellular vehicles of the therapeutic gene CD::UPRT in the treatment of human prostate cancer (PC). PC became the second cause of tumor-related deaths of men in the western world, due also to the longer life expectancy. Clinically localized PC is successfully treated by radical prostatectomy or radiotherapy. In contrast, advanced stages of the tumor, initially responsive to androgen ablation therapy, develop in a few years a phenotype refractory to therapy.18,19 All the attempts to arrest this stage of the disease, in the most successful cases, result in only a few months prolongation of survival.20
In the present study, we demonstrate that AT-MSCs expressing fusion yeast CD::UPRT gene, when systemically administered in combination with the prodrug 5-FC to human prostate tumor-bearing mice, are able to inhibit the tumor growth.
Results
Sensitivity of human prostate cell lines to the cytotoxic effect of 5-FU in vitro
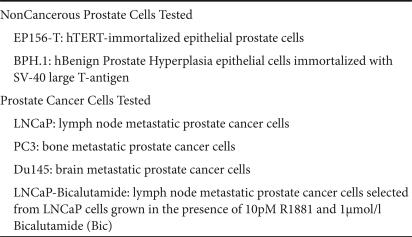
Our objective was to verify the feasibility and efficacy of CDy::UPRT-expressing AT-MSCs as vehicles for gene-directed enzyme prodrug conversion in PC therapy. To this aim, we initially tested the sensitivity of several prostate cell lines to the cytotoxic effect of 5-FU. In particular, we analyzed a panel of PC cells chosen between those that are still responsive to androgen ablation therapy (LNCaP), those that became resistant after prolonged antiandrogen treatment [LNCaP-Bicalutamide (LNCaP-Bic)],21 and those that are androgen-independent (PC3, Du145). The noncancerous cell lines BPH.122 and EP-156T23 were also included as representative of benign prostate hyperplasia epithelial cells and epithelial prostate cells, respectively (for more details see Table 1).
Table 1.
Description of the prostate cell lines used in this study
Cytotoxicity was quantified by MTS assay after direct exposure of the cells to increasing doses of 5-FU. The 5-FU dose that decreased cell viability by 50% (IC50) (compared to untreated cells) was calculated on the base of logarithmic functions obtained for each cell line.
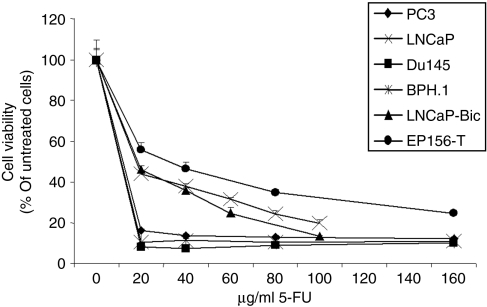
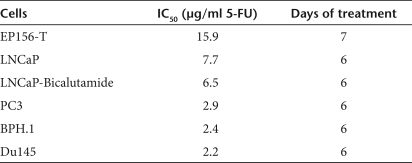
As expected, results showed that all the cell lines tested are sensitive to the cytotoxic drug 5-FU, although to a different extent (Figure 1 and Table 2). In particular, the noncancerous prostate epithelial cells proved to be the least sensitive ones with an IC50 of 15.9 µg/ml despite exposure to the drug was 1 day longer. The brain and bone metastatic PC cells were more sensitive than the lymph node metastatic cells and of their derivated LNCaP-Bic. Interestingly, BPH.1 cells were comparable to PC3 and DU145 cells regarding their IC50 for 5-FU. In general, it seems that the androgen-independent PC cells are more sensitive to the drug, as expected on the base of their higher proliferation rate. CDy-AT-MSCs and, to a lesser extent, AT-MSCs have been previously shown to be also sensitive to the cytotoxic effect of 5-FU in that 5 days of exposure to 50 µg/ml of the drug decreased the cell viability of both cell lines by about 60%.17
Figure 1.
Sensitivity of prostatic cell lines to 5-FU. Prostate cell lines were exposed for 6–7 days to increasing concentrations of 5-FU. Cell viability was quantified by MTS assay. For each prostate cell line, mean values ± SD are expressed as a percentage of cell viability measured in the absence of the drug. 5-FU, 5-fluorouracil.
Table 2.
Dose of 5-FU that decreased cell viability by 50% compared to untreated cells
Sensitivity of human prostate cell lines to the bystander effect of CDy-AT-MSCs in vitro
Toxicity of CDy-AT-MSCs relies not only on the capacity of these cells to convert the prodrug in situ but also on the ability of the drug to pass from the therapeutic to the surrounding target cells (bystander effect). Direct coculture experiments were therefore performed in order to test the cytotoxic bystander effect of CDy-AT-MSCs on the PC cells.
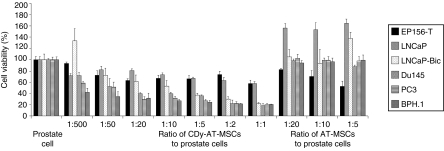
In detail, a fixed number of prostate cells were cultured alone or together with increasing numbers of the therapeutic AT-MSCs. Ratios up to 1:1 were tested. Cell viability was measured by MTS assay after 6–7 days of exposure to 100 µg/ml 5-FC (Figure 2). Cocultures of prostate cells with AT-MSCs were also performed to rule out a possible cytotoxic effect of the CDy-AT-MSCs independent on the conversion of the prodrug.
Figure 2.
Cytotoxic effect mediated by CDy-AT-MSCs in the presence of 5-FC on prostatic cell lines. In direct coculture experiments therapeutic cells were seeded in the same wells containing prostate cells in ratios from 1:500 to 1:1. As a negative control for the transgene, not transduced AT-MSCs were also seeded with the prostate cells, in ratios from 1:20 to 1:5. Cell viability was quantified by MTS assay after 6–7 days of coculture. For each prostate cell line, mean values ± SD are expressed as a percentage of the cell viability measured in the absence of transduced or untransduced AT-MSCs. AT-MSCs, adipose tissue–derived mesenchymal stem cells.
Results show that, EP156-T cells are the least sensitive being only slightly affected by the presence of the CDy-AT-MSCs/5-FC; indeed, despite the duration of the coculture was purposely prolonged, the ratio of 1:1 cells was not sufficient to decrease cell viability by 50%. This seems to be a nonspecific effect because a decrease in cell viability was observed also when cells were cocultured with the AT-MSCs.
Again, PC3, Du145, and BPH.1 cells proved to be the most affected by this approach whereas, not even a ratio of 1:1 between CDy-AT-MSC/5-FC and LNCaP cells was able to reduce the cell viability by 50%. However, when the coculture was prolonged for 2 more days, also LNCaP cells were highly affected by the treatment (data not shown). Interestingly, the androgen-independent LNCaP-Bic cells were more sensitive than the parental cells to the presence of the therapeutic CDy-AT-MSCs.
Prostate cell viability in the presence of AT-MSCs was similar to that of PC not in coculture and therefore higher compared to cell viability in the presence of the therapeutic CDy-AT-MSCs, at equivalent ratios; this is consistent with the inability of the AT-MSCs to convert the prodrug. Only exceptions were the cocultures with EP156-T (see above), LNCaP, and its derivated LNCaP-Bic cells for which the presence of AT-MSCs for 6 days increased cell viability.
CDy-AT-MSCs tropism to prostate cells in vitro
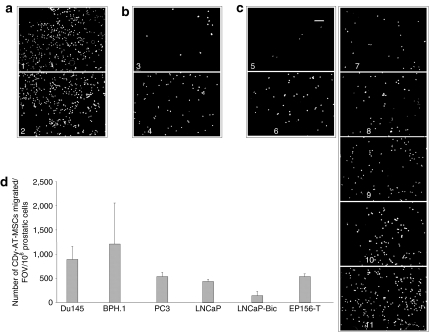
In order to efficiently transport converting enzymes to the tumor site, the CDy-AT-MSCs must maintain their tropism for the tumor. We verified, in in vitro migration experiments, the homing of CDy-AT-MSCs to the different prostatic cell lines (Figure 3). In particular, we observed an impressive migration of CDy-AT-MSCs in the presence of the growth medium of EP156-T cells regardless the presence of the cells (Figure 3a). This is due to the rich composition of such medium which contains, indeed, also synthetic androgen, epidermal growth factor, and pituitary extract. A slight migration was noted also in the presence of the only growth medium for LNCaP-Bic cells (containing synthetic androgen and bicalutamide) (Figure 3b). Therefore, in order to exclude any interference due to the media's components, all prostate cell lines were seeded in RPMI (Roswell Park Memorial Institute) containing 10% fetal calf serum which has been shown not to induce any migration (Figure 3c). Additionally, to exclude also interference due to the cell density, the experiment was performed by seeding onto the lower chamber a different number of the prostatic cells (so to have a comparable number of cells at the end of the experiments). Indeed, the extent of migration depends also on the number of prostate cells seeded (data not shown). Equal number of CDy-AT-MSCs were instead seeded onto the upper chamber, as described in Materials and Methods. Under these experimental conditions, the number of CDy-AT-MSCs migrated, per fields of view, normalized to 1 million of prostatic cells, seems to be similar for all the cell lines with the exception of LNCaP-Bic cells to which migration of the therapeutic cells is reduced (Figure 3c,d).
Figure 3.
Tropism of therapeutic cells to prostate cell lines. Tropism of CDy-AT-MSCs to prostate cancerous and noncancerous cells was tested in vitro. Different numbers of prostate cells were seeded onto 24-well plates [in appropriate growth medium or in RPMI (Roswell Park Memorial Institute) containing 10% fetal calf serum (FCS)]. After 1 day, 8 µm pore-size cell culture inserts were added to the wells and 20,000 CDy-AT-MSCs per insert were seeded. Two days later, cells were processed as described in Materials and Methods. (a-c) Representatives fields of views (FOVs) from different inserts showing migrated CDy-AT-MSCs after staining with DAPI. In detail, fotos represent therapeutic cells migrated to (i) growth medium for EP156-T, (ii) EP156-T cells seeded in the appropriate growth medium, (iii) growth medium for LNCaP-Bicalutamide (LNCaP-Bic), (iv) LNCaP-Bic seeded in the appropriate growth medium, (v) RPMI containing 10% FCS, (vi) PC3, (vii) LNCaP-Bic, (viii) LNCaP, (ix) EP156-T, (x) Du145, (xi) BPH.1, all seeded in RPMI containing 10% FCS. (d) Quantification of the CDy-AT-MSCs migrated per each FOV, normalized to 106 of prostatic cells. 5 FOVs per insert were considered. Columns represent the mean values of CDy-AT-MSCs counted (i.e., migrated to each prostatic cell line) in 3–5 different wells ± SD. AT-MSCs, adipose tissue–derived mesenchymal stem cells.
CDy-AT-MSC-mediated tumor growth inhibition in vivo
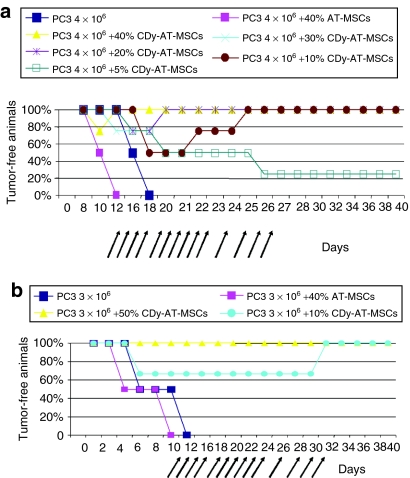
To test the described experimental approach in a pilot preclinical study, mixtures of 4 × 106 of PC3 tumor cells either with 40% of AT-MSCs or with various ratios of the therapeutic CDy-AT-MSCs were injected s.c. into flank of each nude mouse (n = 4 in each group) (Figure 4a). Animals were treated with daily dose of 500 mg/kg of 5-FC intraperitoneally starting on second day after tumor appearance. By day 40 the experiments were ended and animals were inspected for tumors by biopsy. The entire animal control group, inoculated with PC3 cells alone, developed a palpable tumor between the 12th and 16th day and died by day 18 post-tumor implantation. The mixture of PC cells with 40% of AT-MSCs accelerated the appearance of tumors by 4 days. All animals in these control groups died within 12 days. Within the therapeutic group 40% and 30%, one animal developed tumor by day 10 and another one by day 12, respectively, but they were both regressed by day 20. In the group of 20% one animal developed tumor at day 16 that regressed by day 20. In the group 10%, two animals developed tumor at day 18 but, also in this case, both tumors regressed by day 25. Three animals receiving PC3 plus 5% CDy-AT-MSCs developed palpable tumors at day 16, 20, and 26 whereas one animal of the same group remained tumor free until the end of the experiment. Importantly, all animals in the therapeutic groups 40%, 30%, 20%, and 10% were tumor-free at the experimental endpoint.
Figure 4.
Local 5-FC conversion in tumors leads to tumor regression in vivo. (a) PC3 tumor cells (4 × 106) alone or mixtures of PC3 cells with AT-MSCs or with various ratio of therapeutic CDy-AT-MSCs were injected s.c. into flank of each nude mouse (n = 4 in each group). All cells were mixed with the same volume of Matrigel before inoculation. All animals were treated with daily dose of 500 mg/kg of 5-FC intraperitoneally (i.p.) starting on second day after tumor appearance in control group and up to the 27th day. Animals were inspected for tumors every 2 days. At day 40 the experiments were ended. (b) PC3 cells (3 × 106) or mixtures of 3 × 106 of PC3 tumor cells either with 40% of AT-MSCs or with 50% and 10% of therapeutic cells were injected into flank of each nude mouse (n = 4 in each group). All animals were treated with daily dose of 500 mg/kg of 5-FC i.p. as indicated by arrows. Animals were inspected by palpation for tumors every 2 days. At day 40 the experiments were ended and animals were inspected for tumors by biopsy. AT-MSCs, adipose tissue–derived mesenchymal stem cells; 5-FU, 5-fluorouracil.
In order to confirm these results, proportions of 10% and 50% of CDy-AT-MSCs were selected for a second ex vivo pilot study. In particular, PC3 cells (3 × 106) and mixtures of 3 × 106 of PC3 tumor cells either with 40% of AT-MSCs or with 50% and 10% of therapeutic cells were injected s.c. into flank of each nude mouse (n = 4 in each treatment group). All animals were treated with daily dose of 500 mg/kg of 5-FC intraperitoneally starting on second day after tumor appearance until the 30th day. At day 40, the experiments were ended and animals were inspected for tumors (Figure 4b).
Control group of animals inoculated with PC3 cells alone or with mixture of 40% AT-MSCs died within 14 days. Again, addition of 40% of AT-MSCs to tumor cells decreased the time for tumor formation by 2 days. Both groups of CDy-AT-MSCs coinjected animals responded to therapy by complete regression. In particular, in the experimental group coinjected with 10% CDy-AT-MSCs, tumors started to significantly regress few days after administration of 5-FC whereas, the animal group injected with 50% PC3 plus 50% CDy-AT-MSCs did not develop tumor at all. Together, these data show that the local 5-FC conversion leads to tumor regression in vivo or does not allow the development of the tumor when higher numbers of therapeutic cells are coinjected.
Antitumor effect of systemically administered CDy AT-MSCs in the presence of 5-FC in vivo
The applicability of such a therapeutic approach depends on the capability of CDy-AT-MSCs to be effective after systemic administration. In order to test this prerequisite separate in vivo studies were performed in which therapeutic cells were injected into the lateral tail vein of each mice, after tumor induction (Figure 5a).
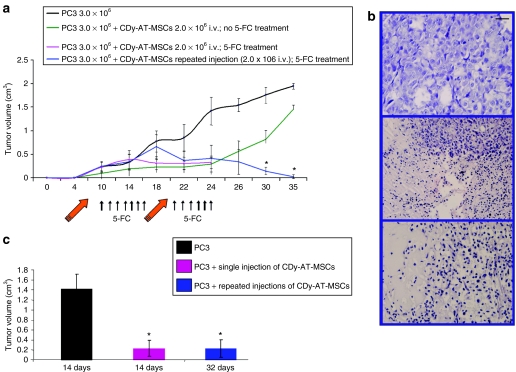
Figure 5.
Systemically administered CDy-AT-MSCs exert antitumor effect in the presence of 5-FC (a) Tumors were induced with 3.0 ×106 PC3 cells (without Matrigel) by subcutaneous inoculation into flank of each nude mouse in all four animal groups. Control group (n = 6) received tumor cells without any treatment. Animals in the second control group (n = 3) received equal number of tumor cells and were successively inoculated intravenously (i.v.) with 2.0 × 106 of CDy-AT-MSCs and not treated with 5-FC. The third group of animals (n = 8) received 3.0 ×106 PC3 cells, were inoculated i.v. with a single dose of 2.0 × 106 of CDy-AT-MSCs, and were treated daily with 500 mg/kg of 5-FC intraperitoneally starting on fourth day after therapeutic cells inoculation. On day 24, animals were killed and tumors examined by histopathology. The fourth group of animals (n = 6) received two doses of therapeutic cells, as indicated in the figure by the orange arrows. On day 36 animals were killed. Tumor volumes were measured at the indicated time points and expressed as mean ± SD. *P < 0.05. (b) Hematoxylin-eosin staining of PC3 tumors from control animals (upper panel), from animals treated with single dose of CDy-AT-MSCs (middle panel), and from animals treated with two doses of CDy-AT-MSCs (lower panel). (c) Nude mice were inoculated with 3.0 × 106 PC3 cells mixed with the same volume of Matrigel. Control group animals (n = 6) received tumor cells only. Animals in the second group (n = 9) were inoculated i.v. with one dose of 2.0 × 106 of CDy-AT-MSCs. These two groups of animals were killed on day 14, tumor volume was estimated and histopathologic examinations performed by hematoxylin-eosin staining. Mice of the third group (n = 9) received a second injection of 1 × 106 of CDy-AT-MSCs on day 18. Tumor volumes were measured on day 32 and expressed as mean ± SD. *P < 0.05. AT-MSCs, adipose tissue–derived mesenchymal stem cells; 5-FU, 5-fluorouracil.
In detail, PC3 tumors were induced with 3.0 × 106 PC3 cells (without MaxGel extracellular matrix) in all four experimental animal groups. The control group (n = 6) did not receive 5-FC. Animals in the second control group (n = 3) were inoculated intravenously with a dose of 2 × 106 of CDy-AT-MSCs and were not treated with 5-FC. Animals that received a single dose of 2 × 106 of CDy-AT-MSCs (n = 8) and animals that were injected twice with the same amounts of therapeutic cells (n = 6) were daily treated with 500 mg/kg of 5-FC intraperitoneally starting on the fourth day after inoculation of therapeutic cells. On day 24, animals that were injected once were killed. Average tumor volume decreased in all treated animals compared to the first control group. Tumors exhibited signs of regression being soft differently from the solid consistence of tumors in the control group. Hematoxylin-eosin staining confirmed necrotic character in all tumors of treated animals in comparison to viable character of tumors in control animals (Figure 5b). In the second group of treated mice, the injection of therapeutic cells was repeated on day 20, as indicated in the figure. On day 36, the experiment was ended. In three out of six mice a complete regression was observed. Tumor volume in the other three mice was 0.1, 0.06, and 0.03 cm3. Thus systemic application of therapeutic cells significantly decreased tumor burden and led to partial tumor regression. Importantly, a complete tumor regression was observed in 50% of the animals receiving two injections of therapeutic cells.
In order to clarify whether fast growing tumors can be influenced by systemic inoculation of CDy-AT-MSCs, we induced PC3 xenografts by mixing tumor cells with MaxGel extracellular matrix. We found in previous experiments that tumor onset and its growth were accelerated by this type of injection. Significant difference in tumor burden was found both in the group that received one single injection (n = 9) and in the group that received repeated injections (n = 9) in comparison with the control untreated group (n = 6), as indicated in Figure 5c. Tumor regression in the treated groups was confirmed by histological examination with hematoxylin-eosin staining (data not shown). We conclude that fast growing PC tumors can be inhibited by stem cell targeted-gene therapy.
Discussion
In this study, we tested the feasibility and efficacy of yeast CD::UPRT expressing AT-MSCs to target and inhibit prostate tumors in vitro and in vivo. PC is one of the most common cancers in men in the western world. Despite significant efforts to cure this disease, mortality because of PC became one of the highest among patients with solid tumors. Median survival of men with metastatic therapy-resistant PC is limited to only 1–2 years. The current standard therapy for the treatment of these late stage patients is a docetaxel-based chemotherapy that however prolongs life expectancy by only a few months.20,24 Moreover, despite the improved safety features of some new therapeutic agents, for some patients therapy must be suspended because of the serious side effects. An elegant way to improve the response of tumor cells to chemotherapeutics and, at the same time, to focus their activation at the tumor site, sparing the healthy tissues and limiting therefore the side effects, is the employment of prodrug enzyme gene therapy. Prodrugs are relatively nontoxic, inactive compounds delivered systemically and converted into biologically active cytotoxic agents only after reaching the target region. Introduction of prodrug-converting enzymes into cells able to track and infiltrate into the tumor represents one of the emerging gene therapy approaches.1,10 In this study, we applied, as cellular vehicle of a prodrug-converting enzyme, human AT-MSCs engineered by retrovirus transduction to express the yeast suicide gene CD::UPRT. This prodrug system relies on the ability of yeast CD enzyme to convert the less toxic antifungal substrate 5-FC into the highly toxic antineoplastic 5-FU able to exert a strong bystander effect for its ability to diffuse across the cell membrane. 5-FU is then processed to cytotoxic metabolites that inhibit DNA and RNA synthesis, thus targeting both proliferating and nonproliferating cells.25 We and others have previously shown, in in vitro and in vivo studies, that the bystander effect mediated by CD::UPRT gene directed enzyme prodrug therapy does not require a direct cell to cell contact or functional gap junctions,1,26 thus further enhancing the strength of this therapeutic approach. It was reported indeed that expression of the bifunctional suicide gene CD::UPRT increases radiosensitization and bystander effect of 5-FC in PC cells.27 In mammalian cells, which appear to lack UPRT, orotate phosphoribosyl transferase (OPRT) is the initial enzyme of 5-FU activation. It is also the rate-limiting enzyme in the de novo process of DNA and RNA synthesis. Other key enzymes for 5-FU metabolism are thymidylate synthase, dihydropyrimidine dehydrogenase, which produces inactive 5-FU metabolites, and thymidine phosphorylase. We found that cell viability of the prostate cell lines tested, both cancerous and noncancerous, is impaired by 5-FU. Brain and bone metastatic PC cell lines that represent a very aggressive phenotype of the tumor not being responsive to the first-line therapy (androgen ablation) are more sensitive to the cytotoxic effect of the drug compared to the metastatic androgen receptor positive LNCaP cells that, vice versa, represent an earlier stage of the disease. This different drug sensitivity may reflect the fact that the proliferation rate of PC3 and Du145 cells is much higher than that of LNCaP cells and even higher than that of the noncancerous epithelial prostate cell line EP156-T. These latter's are indeed the cells less affected by the treatment. The BPH.1 cells, although noncancerous, have also a very high proliferation rate, comparable to that of PC3 and Du145, and also a very similar IC50. A further reason for the different extent of response to the cytotoxic effect of 5-FU observed between cancerous and noncancerous prostatic cells and between hormone sensitive and hormone-refractory PC cells may be due to the different expression levels of the enzymes involved in 5-FU metabolism. Indeed, Tanaka et al. have recently demonstrated that the expression of OPRT mRNA (i.e., the capability to activate 5-FU) is significantly higher in PC specimens compared to normal prostate specimens.28 Between PC specimens, the hormone sensitive PC had lower levels of OPRT mRNA compared to hormone-refractory PC with a significant correlation between OPRT mRNA expression levels and the tumor pathological grade. Additionally, the OPRT/dihydropyrimidine dehydrogenase expression ratio, a powerful predictive factor to evaluate 5-FU sensitivity, was significantly higher in the hormone-refractory PC group than in the low grade hormone sensitive PC group. Finally, consistent with our results, in vitro sensitivity to 5-FU was shown to be higher for Du145 cells compared to LNCaP cells.29 Also the enzyme thymidylate synthase is expressed at greater levels in the PC specimens than in the normal prostatic tissue specimens. Mizutani et al. have shown that the levels of OPRT activity increases in rapidly growing cells, including tumor cells.30 Based on this finding, and considering that OPRT is the principal de novo DNA and RNA synthetic enzyme associated with cell division and proliferation, they assume that clones of cells that overexpress OPRT may have a growth advantage compared to clones that do not express the enzyme. This correlation may be valid also for PC and noncancer cells, as discussed above. Finally, the effect of 5-FU on cell growth arrest and apoptosis has been attributed to the ability of its metabolites to induce the level and activity of the tumor suppressor p53.31 However, we observed a minor cytotoxicity of 5-FU on LNCaP cells which express the wild-type p53 compared to PC3 cells which do not express it, or Du145 cells which have a mutated p53. Therefore, in our cellular models, the effect mediated by the drug through cell cycle arrest and/or apoptosis might be less relevant compared to the effect mediated through inhibition of DNA synthesis.
When compared to other malignances, prostate tumor is considered a slow growing one, containing about only 3% of cycling cells.32 Indeed, 5-FU has not been successfully applied to PC patients due to its mechanism of action that affects highly proliferating rather than slow growing cells; low efficiency of conversion of 5-FU into toxic metabolites may also explain the partial resistance of these cells. The transduction of the bifunctional fusion gene CD::UPRT increases the sensitivity of target cells to the 5-FU because of the catalytic action of the UPRT gene in the conversion of the drug.33 Besides, the construction of such a bifunctional yeast fusion gene has been shown to further enhance the bystander effect.34,35,36,37 This can explain the strong inhibition of tumor growth that we observed when therapeutic cells were coinjected s.c. together with PC3 cells into nude mice receiving the prodrug. The number of tumor-free animals was proportional to the percentage of the therapeutic cells coinjected showing that the outcome is not casual. Interestingly, 10–40% of therapeutic cells were able to induce a total tumor regression by the end of the experiment differently from what was observed in animals injected with the only PC3 cells.
More importantly, a 50% mixture of therapeutic cells with PC3 cells did not allow the establishment of tumor in any mouse. So, the bystander effect we observed in vitro was confirmed also in the in vivo studies. The acceleration of tumor formation in animals coinjected with AT-MSCs (as well as the higher cell viability of some PC cells observed in the direct cocultures with AT-MSCs) should not be a surprise. It is known that MSC cells may support subcutaneous tumor growth when coinjected with tumor cells.38,39 Tumor growth support by AT-MSCs is caused by several factors like growth factors production, immunosuppressive character of MSCs, but also by formation of favorable microenviroment for cancer cells. On the contrary, other studies observed that MSCs may inhibit tumor growth in animal models.40,41 and posses antitumorigenic effects on a model of Kaposi's sarcoma.42 CDy-AT-MSCs used in the study have the advantage to be safe because of suicide gene presence. The administration of 5-FC eliminates not only tumor cells by also therapeutic cells as we have previously reported.16
Several reports26,43,44 documented efficacy of such a prodrug converting enzyme gene therapy applied to mice bearing androgen-dependent or -independent PC xenografts. They were able to control and inhibit to some extent the growth of tumor. However, in all those studies, therapeutic genes were injected intraprostatically. Another way to concentrate the effect of virus vectors containing therapeutic genes to prostate carcinoma cells was obtained with the use of promoter-enhancers that may be activated only in the prostate.1,45 In our in vivo studies, in order to target and deliver the therapeutic genes to the tumor site, we exploited the characteristic of mesenchymal stromal stem cells to home to primary tumors and metastases. Stem cells have been previously shown to be capable to migrate to the tumor site in prostate xenografs bearing mice,10,46 however these stem cells were of different origins or carrying different therapeutic genes. Novelty of our approach is based on the choice of the transgene, as above discussed, and on the source of the MSCs. AT is indeed an easily obtainable abundant source of adult multipotent cells, also for autologous use, with no ethical concerns. Most importantly, we demonstrated that CDy-AT-MSCs were effective in significantly inhibiting the growth of a human androgen-independent tumor in vivo, also after systemic administration; this is a key requisite for any clinical application of this gene-directed enzyme prodrug therapy. Regarding the choice of the transgene, it is relevant to point out that CD::UPRT expressing AT-MSCs, with the increasing number of passages, become more sensitive to the effect of the suicide gene they carry,17 thus self-limiting their survival; this is ideal for chemotherapy applications because delivery cells need to survive only sufficiently long to mediate effective therapy. Of note, the cell viability of the noncancerous epithelial prostate cells were only slightly affected by the presence of the CDy-AT-MSCs in the in vitro experiments. Their higher resistance is promising when considering a possible future therapy aimed at targeting primary prostate tumors in that noncancerous epithelial prostate cells could be spared from the local bystander effect of 5-FU produced by the CDy-AT-MSCs.
We have previously demonstrated the efficacy of CDy-AT-MSCs to target and inhibit the growth of human colon adenocarcinoma HT-2916 and human melanoma xenografts-bearing nude mice treated with 5-FC.17 In this study, we applied for the first time this gene therapy approach to mice bearing human PC tumors. In particular, we demonstrated the feasibility of this prodrug gene-directed enzyme therapy against PC3 cells that represent the most common metastases in PC patients. However, due to their capability to cross the blood-brain barrier, MSCs may serve as a platform for therapy in PC patients with brain metastases or in patients with primary brain tumors. A phase 1 neural stem cells mediated glioma therapy clinical trial is already under development.10 It has been demonstrated that MSCs can target and deliver therapeutic genes to metastases in a mouse PC lung metastases model46 and recently human neural stem cells were shown to be able to target and deliver therapeutic genes to breast cancer brain metastases.47
Similarly to our previous studies, a total regression of all tumors was not achieved when CDy-AT-MSCs were administered systemic. This is probably due to fewer number of therapeutic cells reaching the tumor with this delivery route48 and to the fact that therapeutic cells are administered at a later time-point. We believe that appropriate multiple injections of CDy-AT-MSCs could bring more striking results in terms of growth arrest as well as an earlier beginning of the therapy. Indeed only with two injections of therapeutic cells, we were able to arrest the tumor growth in animals receiving the prodrug for the entire duration of the experiments. It is likely that saturation of bifunctional yeast CD/UPRT enzyme by 5-FC at the doses applied per kg of body weight play a role. Kievit et al.49 reported that % of conversion of 0.5 mmol/l 5-FC by 0.2 µg CDy in 2 hours is 77.9 Kilometer under these conditions is 0.8 ± 0.2 mmol/l. Similarly, it has been recently reported that km value for CD is 0.51 mmol/l.50 Furthermore, 5-FC as an antimycoticum drug is known from human studies for its high clearance rate. Additional pharmacokinetics studies will be needed to clarify this point.
We demonstrated that stem cell targeted tumor therapy is promising in heterogenous tumors like the PC, encouraging further investments to refine and better characterize this therapy. It might offer a hope in the treatment of late-stage tumor patients but it may also be applied to prevent formation of metastases in patients with organ-confined disease. Eradication of microscopic metastases that evade current detection techniques would be translated in a longer survival of PC patients with a better quality of life. Importantly, AT-MSCs could be easily derived from the same patient.
Materials and Methods
Cell lines and chemicals. PC cells Du145, PC3, and LNCaP were obtained from American Type Culture Collection (Rockville, MD) and cultured in RPMI 1640 medium (HyClone, Logan, UT) supplemented with 10% fetal calf serum, 1% glutamine, and penicillin/streptomycin. BPH.1 cells, maintained under the same conditions, were a kind gift of Prof. Schalken (Radboud University Nijmegen Medical Centre, the Netherlands). The androgen-independent LNCaP-Bic subline were derived from LNCaP cells grown in the presence of charcoal-stripped serum supplemented with 10 pM R1881 and 1 µmol/l Bic.21 EP156-T cells were maintained as previously described.22 For a description of cell lines, see Table 1. All chemicals were purchased from Sigma if not stated otherwise. The synthetic androgen methyltrienolone R1881 was purchased from New England Nuclear (Dreieichenhein, Germany). The nonsteroidal antiandrogen Bic was a gift from Astra Zeneca (Macclesfield, UK).
Construction of retroviral vector containing CD and preparation of virus producing cells. Construction of retroviral vector containing CD::UPRT and preparation of virus from helper cells were performed as previously described.17
MSC isolation from human AT, culture, and retrovirus transduction. AT-MSCs were isolated from lipoaspirate using a collagenase type VIII digestion and plastic adherence technique as described17. Material was obtained from healthy persons undergoing elective lipoaspiration, who provided an informed consent. Cells were plated in low-glucose (1 g/l) Dulbecco's Modified Eagle Medium supplemented with 10% MSC-stimulatory supplement (human; StemCell Technologies) and antibiotic–antimycotic at a density of 2 × 105–5 × 105 nucleated cells/cm2. Adherent cells were split after reaching confluence and AT-MSC were used for the experiments up to passage 5. To prepare MSCs expressing CDy (CDy-AT-MSCs), subconfluent cultures of AT-MSCs were transduced thrice in three consecutive days with virus-containing medium from GP+envAM12/pST2 cells supplemented with 100 µg/ml protamine sulfate.
Toxicicity assay. Prostate cells were seeded in quadruplicates in 96-well plates, in appropriate growth media. On day 1, medium was replaced with fresh medium containing 20–160 µg/ml of 5-FU. Six days later (7 days for the EP156-T cells), cell viability was quantified by MTS test according to the manufacturer's protocol.
In vitro bystander effect experiments. Prostate cells were seeded in quadruplicate in 96-well plates, in their appropriate growth medium. Increasing amounts of either CDy-AT-MSCs or AT-MSCs were added to the tumor cells on day 1.5-FC (100 µg/ml) was added to the mixed cultures on day 2. Cells were incubated for 6–7 days and cell viability was measured afterwards by MTS assay according to the manufacturer's protocol.
Cell migration assay. Different numbers of prostatic cells were seeded onto 24-well plates in their appropriate growth medium. Additionally, EP156-T and LNCaP-Bic cells were also seeded in RPMI containing 10% fetal calf serum. After 1 day, 8 µm pore-size cell culture inserts (Becton Dickinson, GMBH, Schwechat, Austria) were added to the wells followed by addition of 20,000 CDy-AT-MSCs per insert. Medium was removed 48 hours later and cells washed with PBS and detached mechanically with a cotton swab. Cells migrated through the insert were fixed with cold methanol for 10 minutes at room temperature. Inserts were thereafter cut and mounted on Vectashield Mounting Medium with DAPI (Szabo Scandic, Vienna, Austria). Tissue-Quest and A-Quest programs (TissueGnostics GmbH, Wien, Austria) were used for acquisition and quantification of the migrated cells.
Experiments in vivo. Six- to 8-week-old athymic nude mice (Balb/c-nu/nu) were used in accordance with institutional guidelines under the approved protocols. The following cell suspensions were injected in coinjection studies: 4 × 106 PC3 plus 40%, 30%, 20%, 10%, or 5% of CDy-AT-MSCs; 3 × 106 PC3 plus 50% or 10% of CDy-AT-MSCs; 4 × 106 PC3 and 3 × 106 PC3 alone, 4 × 106 PC3 or 3 × 106 PC3 plus 40% of AT-MSCs. Cells were delivered s.c. into the flank of each mouse in 100 µl of PBS mixed with the same volume of MaxGel extracellular matrix (Matrigel). For experiments with systemic applications of the therapeutic cells, PC3 xenografts were induced either with cells alone or mixed with MaxGel extracellular matrix. Suspension of 2 × 106 CDy-AT-MSC, or 106 AT-MSCs in 200 µl of PBS per each animal was injected intravenously into the lateral tail vein. Animals were treated intraperitoneally with 500 mg/kg/day of 5-FC diluted in PBS as indicated in figures. Tumors were measured by caliper and volume was calculated according to the formula: volume = length × width2/2. Animals were killed at the point when the tumors penetrated skin within the control group.
Statistics. Statistical comparisons of groups were performed using the Student's t-test. P values < 0.05 were considered statistically significant.
Acknowledgments
The study was supported by the ICRETT fellowship No. ICR/07/142 awarded to Ilaria Cavarretta from the International Union Against Cancer. We thank Helmut Klocker and Frédéric R. Santer, Department of Urology, Innsbruck Medical University, Innsbruck, Austria for allowing the use of EP156-T cells and for technical assistance, Dusan Guba, Institute of Medical Cosmetics, Bratislava, Slovakia for providing us with material for AT-MSCs isolation, Maria Dubrovcakova and Viera Frivalska for technical assistance. The study was supported by APVV grant 0260/07; financial support from SPP Foundation, FIDURA Capital Consult GmbH, Munich, Germany; and grant awarded by the League Against Cancer. The authors indicate no potential conflict of interest.
REFERENCES
- Altaner C. Prodrug cancer gene therapy. Cancer Lett. 2008;270:191–201. doi: 10.1016/j.canlet.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- Shimato S, Natsume A, Takeuchi H, Wakabayashi T, Fujii M, Ito M, et al. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene Ther. 2007;14:1132–1142. doi: 10.1038/sj.gt.3302932. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro-oncology. 2006;8:119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL., and , Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- Yuan X, Hu J, Belladonna ML, Black KL., and , Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ., and , Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Shah K, Tung CH, Breakefield XO., and , Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Najbauer J., and , Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and , Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Bobis S, Jarocha D., and , Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, et al. Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Klüter H., and , Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S., and , Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- Culig Z, Steiner H, Bartsch G., and , Hobisch A. Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endocr Relat Cancer. 2005;12:229–244. doi: 10.1677/erc.1.00775a. [DOI] [PubMed] [Google Scholar]
- Yuan X., and , Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi K., and , Dawson NA. Emerging therapies in castrate-resistant prostate cancer. Curr Opin Oncol. 2009;21:260–265. doi: 10.1097/CCO.0b013e32832a1868. [DOI] [PubMed] [Google Scholar]
- Hobisch A, Fritzer A, Comuzzi B, Fiechtl M, Malinowska K, Steiner H, et al. The androgen receptor pathway is by-passed in prostate cancer cells generated after prolonged treatment with bicalutamide. Prostate. 2006;66:413–420. doi: 10.1002/pros.20365. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N., and , Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Kogan I, Goldfinger N, Milyavsky M, Cohen M, Shats I, Dobler G, et al. hTERT-immortalized prostate epithelial and stromal-derived cells: an authentic in vitro model for differentiation and carcinogenesis. Cancer Res. 2006;66:3531–3540. doi: 10.1158/0008-5472.CAN-05-2183. [DOI] [PubMed] [Google Scholar]
- Friedman J, Dunn RL, Wood D, Vaishampayan U, Wu A, Bradley D, et al. Neoadjuvant docetaxel and capecitabine in patients with high risk prostate cancer. J Urol. 2008;179:911–5; discussion 915. doi: 10.1016/j.juro.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath P., and , Ghosh SS. Implication of functional activity for determining therapeutic efficacy of suicide genes in vitro. Biotechnol Lett. 2008;30:1913–1921. doi: 10.1007/s10529-008-9787-1. [DOI] [PubMed] [Google Scholar]
- Khatri A, Zhang B, Doherty E, Chapman J, Ow K, Pwint H, et al. Combination of cytosine deaminase with uracil phosphoribosyl transferase leads to local and distant bystander effects against RM1 prostate cancer in mice. J Gene Med. 2006;8:1086–1096. doi: 10.1002/jgm.944. [DOI] [PubMed] [Google Scholar]
- Xing L, Sun X, Deng X, Kotedia K, Urano M, Koutcher JA, et al. Expression of the bifunctional suicide gene CDUPRT increases radiosensitization and bystander effect of 5-FC in prostate cancer cells. Radiother Oncol. 2009;92:345–352. doi: 10.1016/j.radonc.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kawashima H, Matsumura K, Yamashita-Hosono T, Yoshimura R, Kuratsukuri K, et al. Overexpression of orotate phosphoribosyl transferase in hormone-refractory prostate cancer. Oncol Rep. 2009;21:33–37. [PubMed] [Google Scholar]
- Li Y, Mizutani Y, Shiraishi T, Okihara K, Ukimura O, Kawauchi A, et al. Prognostic significance of thymidylate synthase expression in patients with prostate cancer undergoing radical prostatectomy. Urology. 2007;69:988–995. doi: 10.1016/j.urology.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Wada H, Fukushima M, Yoshida O, Nakanishi H, Li YN, et al. Prognostic significance of orotate phosphoribosyltransferase activity in bladder carcinoma. Cancer. 2004;100:723–731. doi: 10.1002/cncr.11955. [DOI] [PubMed] [Google Scholar]
- Sun XX, Dai MS., and , Lu H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J Biol Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- Cher ML, Chew K, Rosenau W., and , Carroll PR. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate. 1995;26:87–93. doi: 10.1002/pros.2990260205. [DOI] [PubMed] [Google Scholar]
- Tiraby M, Cazaux C, Baron M, Drocourt D, Reynes JP., and , Tiraby G. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Lett. 1998;167:41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- Ramnaraine M, Pan W, Goblirsch M, Lynch C, Lewis V, Orchard P, et al. Direct and bystander killing of sarcomas by novel cytosine deaminase fusion gene. Cancer Res. 2003;63:6847–6854. [PubMed] [Google Scholar]
- Chung-Faye GA, Chen MJ, Green NK, Burton A, Anderson D, Mautner V, et al. In vivo gene therapy for colon cancer using adenovirus-mediated, transfer of the fusion gene cytosine deaminase and uracil phosphoribosyltransferase. Gene Ther. 2001;8:1547–1554. doi: 10.1038/sj.gt.3301557. [DOI] [PubMed] [Google Scholar]
- Kanai F, Kawakami T, Hamada H, Sadata A, Yoshida Y, Tanaka T, et al. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998;58:1946–1951. [PubMed] [Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hall B, Andreeff M., and , Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007;180:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D., and , Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- Ohlsson LB, Varas L, Kjellman C, Edvardsen K., and , Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T, Koshida K, Hori O, Konaka H, Katoh H, Kitagawa Y, et al. Gene therapy for prostate cancer using the cytosine deaminase/uracil phosphoribosyltransferase suicide system. J Gene Med. 2003;5:30–37. doi: 10.1002/jgm.317. [DOI] [PubMed] [Google Scholar]
- Khatri A, Husaini Y, Ow K, Chapman J., and , Russell PJ. Cytosine deaminase-uracil phosphoribosyltransferase and interleukin (IL)-12 and IL-18: a multimodal anticancer interface marked by specific modulation in serum cytokines. Clin Cancer Res. 2009;15:2323–2334. doi: 10.1158/1078-0432.CCR-08-2039. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Zhang S, Yu ZM, Xia SJ., and , Li H. Specific targeting of prostate cancer cells in vitro by the suicide gene/prodrug system, uracil phosphoribosyltransferase/5-fluorouracil, under the control of prostate-specific membrane antigen promoter/enhancer. Prostate Cancer Prostatic Dis. 2009;12:166–171. doi: 10.1038/pcan.2008.39. [DOI] [PubMed] [Google Scholar]
- Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Park IH, Shin JY, Jin J, Kang BG, Kim MH, et al. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol Ther. 2009;17:570–575. doi: 10.1038/mt.2008.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- Park JI, Cao L, Platt VM, Huang Z, Stull RA, Dy EE, et al. Antitumor therapy mediated by 5-fluorocytosine and a recombinant fusion protein containing TSG-6 hyaluronan binding domain and yeast cytosine deaminase. Mol Pharm. 2009;6:801–812. doi: 10.1021/mp800013c. [DOI] [PMC free article] [PubMed] [Google Scholar]