Abstract
Fabry disease is an X-linked lysosomal storage disorder caused by a deficiency in α-galactosidase A (α-Gal A) activity and subsequent accumulation of the substrate globotriaosylceramide (GL-3), which contributes to disease pathology. The pharmacological chaperone (PC) DGJ (1-deoxygalactonojirimycin) binds and stabilizes α-Gal A, increasing enzyme levels in cultured cells and in vivo. The ability of DGJ to reduce GL-3 in vivo was investigated using transgenic (Tg) mice that express a mutant form of human α-Gal A (R301Q) on a knockout background (Tg/KO), which leads to GL-3 accumulation in disease-relevant tissues. Four-week daily oral administration of DGJ to Tg/KO mice resulted in significant and dose-dependent increases in α-Gal A activity, with concomitant GL-3 reduction in skin, heart, kidney, brain, and plasma; 24-week administration resulted in even greater reductions. Compared to daily administration, less frequent DGJ administration, including repeated cycles of 4 days with DGJ followed by 3 days without or every other day with DGJ, resulted in even greater GL-3 reductions that were comparable to those obtained with Fabrazyme. Collectively, these data indicate that oral administration of DGJ increases mutant α-Gal A activity and reduces GL-3 in disease-relevant tissues in Tg/KO mice, and thus merits further evaluation as a treatment for Fabry disease.
Introduction
Fabry disease is an X-linked lysosomal storage disorder caused by inherited mutations in the gene (GLA) that encodes the lysosomal hydrolase α-galactosidase A (α-Gal A). More than 400 GLA mutations have been reported, ~60% of which are missense (Human Gene Mutation Database; http://www.hgmd.org).1 These mutations lead to reduced cellular α-Gal A activity as a result of lowered catalytic efficiency, improper or less stable folding, and/or inefficient lysosomal trafficking.2,3,4 Deficiency of α-Gal A results in progressive accumulation and deposition of glycosphingolipids with a terminal α-galactose, primarily globotriaosylceramide (GL-3, Gb3, and ceramide trihexoside), in lysosomal and nonlysosomal compartments of cells of the skin, heart, kidney, brain, and other tissues,5,6 which contributes to disease pathology.7
Fabry disease has been classified into two major forms according to the age of onset of clinical symptoms: early (or “classic”) and late.6,8 Classic Fabry disease patients are typically males with little-to-no detectable α-Gal A activity who present with severe clinical symptoms during adolescence, including acroparesthesia, angiokeratomas, hypohidrosis, and corneal dystrophy. If untreated, progressive vascular disease affects the heart, kidney, and central nervous system (CNS), resulting in death by the fourth or fifth decade.8,9 Late-onset patients tend to have higher residual α-Gal A activity and are usually asymptomatic until the third or fourth decade.2,6,10 Ultimately, disease progression leads to heart or kidney failure, or cerebrovascular complications such as stroke, resulting in death by the fifth or sixth decade.6,11 Recent studies suggest that this late-onset form may be more prevalent than originally thought.10,12,13,14 Furthermore, female Fabry patients may be mildly symptomatic or as severely affected as classic males.15,16,17 Enzyme replacement therapy is currently the only treatment available for Fabry patients, with two approved products: Fabrazyme (agalsidase beta; Genzyme, Cambridge, MA) and Replagal (agalsidase alfa; Shire Pharmaceuticals, Cambridge, MA). These treatments are well tolerated, reduce plasma, urine, and microvascular endothelial GL-3 levels, and may alleviate neuropathic pain, improve hypertrophic cardiomyopathy, stabilize kidney function, and increase the ability to sweat.18,19,20,21,22,23 However, enzyme replacement therapy is not a cure and delivery of infused enzyme to some disease-relevant cells, tissues, and organs may be insufficient in certain cases.15,18
Pharmacological chaperone (PC) therapy has been proposed as a treatment for Fabry and other lysosomal diseases.24,25,26,27,28 PCs selectively bind and stabilize mutant forms of α-Gal A in the endoplasmic reticulum, facilitating proper protein folding and trafficking, and increasing lysosomal enzyme activity.24,29,30 Because PCs are low molecular weight molecules, they may be orally available with broad biodistribution, including the CNS. The iminosugar, 1-deoxygalactonojirimycin (DGJ, migalastat HCl, AT1001), is an analog of the terminal galactose of GL-3, acts as a reversible, competitive inhibitor of α-Gal A,31 and improves the folding, stability, and lysosomal trafficking of multiple mutant forms of α-Gal A.29,30,31,32,33,34
To study the effects of DGJ in vivo, we used a new mouse model of Fabry disease [hR301Q α-Gal A transgenic (Tg)/knockout (KO)] that lacks endogenous GLA but expresses a human R301Q GLA transgene transcriptionally regulated by the human GLA promoter. Importantly, R301Q α-Gal A has been identified in patients with both classic and late-onset Fabry disease.35,36,37,38 hR301Q α-Gal A Tg/KO mice show age-dependent GL-3 accumulation in disease-relevant tissues including skin, heart, kidney, and brain. Previous studies using a different Tg mouse homozygous for the expression of hR301Q (TgM/KO) failed to show GL-3 accumulation due to high expression of the transgene by the β-actin promoter.39 More recently, it was shown that heterozygous female TgM/KO mice do accumulate modest levels of GL-3 in kidney that was reduced after 2-week DGJ administration.40
In the current study, daily 4-, 12-, and 24-week oral administration of DGJ to hR301Q α-Gal A Tg/KO mice resulted in significant increases in α-Gal A activity in skin, heart, kidney, and brain, with concomitant reductions in GL-3. Dose optimization revealed that an even greater reduction in GL-3 could be achieved using less frequent DGJ administration. Importantly, these results provide the first clear in vivo demonstration of PC-mediated increases in cellular enzyme activity as measured by reduction of endogenous substrate in multiple tissues including the CNS. Furthermore, the less frequent administration studies demonstrate that a larger net gain in cellular activity can be achieved with lower overall drug exposure, consistent with the mechanism of action for a reversible competitive inhibitor that acts as a PC. Collectively, these data indicate that DGJ warrants further evaluation as a treatment for Fabry disease.
Results
hR301Q α-Gal A Tg/KO mice have reduced α-Gal A activity and elevated GL-3
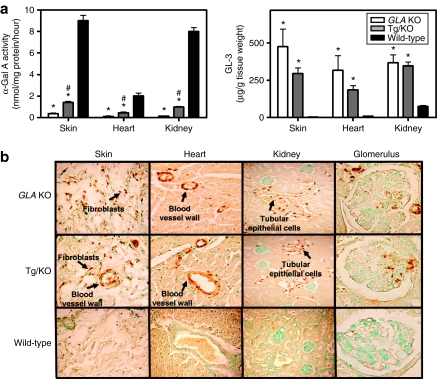
Tissue α-Gal A and GL-3 levels were measured in 12-week-old male hR301Q α-Gal A Tg/KO, GLA KO, and age-matched wild-type (WT) C57BL/6 mice. α-Gal A activities in skin, heart, and kidney of hR301Q α-Gal A Tg/KO mice were 16 ± 0.6, 22 ± 2.0, and 12 ± 0.4%, respectively, of those measured in WT mouse tissues (Figure 1a). Correspondingly, GL-3 levels (assessed by LC-MS/MS) in skin, heart, and kidney of hR301Q α-Gal A Tg/KO mice were significantly elevated compared to WT mice, and were 68 ± 10, 63 ± 10, and 95 ± 7%, respectively, of those measured in GLA KO mouse tissues (Figure 1a). By immunohistochemistry, the GL-3 staining pattern was similar in hR301Q α-Gal A Tg/KO and GLA KO mice, with no GL-3 seen in WT mice (Figure 1b). Most GL-3 staining was seen in dermal fibroblasts, smooth muscle cells of blood vessel walls of the skin and heart (cardiac ventricles), and distal tubular epithelial cells of the kidney cortex (brown spots indicated by black arrows, Figure 1b). The presence of GL-3 in distal tubules of the kidney was confirmed using peanut agglutinin lectin, which stains the apical surface of the distal tubular epithelial cells (data not shown). In addition, tissue GL-3 levels increased with age in both the hR301Q α-Gal A Tg/KO and GLA KO mice (Supplementary Figure S1). Importantly, GL-3 staining in cells of the glomeruli was evident in aged (at least 6 months) hR301Q α-Gal A Tg/KO and GLA KO mice (Figure 1b), although these levels were only 10–20% of those seen in distal tubular epithelial cells. Collectively, these results indicate that hR301Q α-Gal A Tg/KO mice have reduced α-Gal A activity and elevated GL-3 levels in the cells of Fabry disease–relevant tissues including skin, heart, and kidney.
Figure 1.
hR301Q α-Gal A Tg/KO mice have reduced α-Gal A and elevated GL-3 levels. (a) α-Gal A and GL-3 levels were assessed in tissue lysates prepared from skin, heart, and kidney of 12-week-old male GLA KO (open bars), hR301Q α-Gal A Tg/KO (gray bars), and wild-type C57BL/6 (black bars) mice. α-Gal A activity in hR301Q α-Gal A Tg/KO and GLA KO mice was significantly lower than that of wild-type mice (*P < 0.05, t-test); α-Gal A activity in hR301Q α-Gal A Tg/KO mice was significantly higher than that of GLA KO mice (#P < 0.05, t-test). GL-3 levels in hR301Q α-Gal A Tg/KO and GLA KO mice were significantly higher than those of wild-type mice (*P < 0.05, t-test). Each bar represents the mean ± SEM from four mice per group analyzed in triplicate. (b) Cell-type specific GL-3 staining was assessed by immunohistochemistry in GLA KO, hR301Q α-Gal A Tg/KO, and wild-type C57BL/6 mouse tissues. GL-3 staining is represented as brown spots (black arrows); nuclei are represented as green spots (stained with methyl green). The data shown are representative photomicrographs from four 12-week-old male mice using ×20 magnification, except for the glomeruli, which are representative of four 7-month-old male mice using ×40 magnification. α-Gal A, α-galactosidase A; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
DGJ increases α-Gal A activity and decreases GL-3 in hR301Q α-Gal A Tg/KO mice
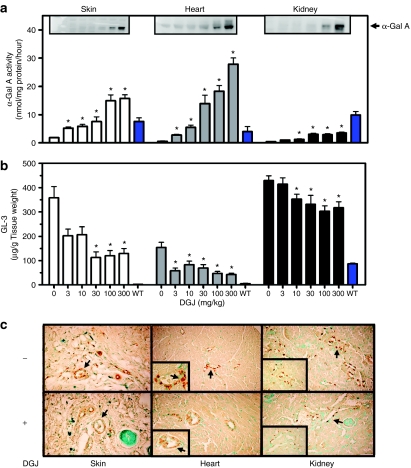
Significant levels of DGJ were measured in plasma, skin, heart, kidney, and brain upon ad libitum administration in drinking water to 8-week-old male C57BL/6 mice, indicating that DGJ is orally available, has a broad tissue distribution profile including the CNS, and attains tissue concentrations that are in excess of the Ki value for α-Gal A (30 ± 2 nmol/l), thus confirming the potential for interaction with the target enzyme in vivo (Supplementary Figure S2). DGJ was then administered to 8-week-old male hR301Q α-Gal A Tg/KO mice (3, 10, 30, 100, and 300 mg/kg per day) ad libitum in drinking water for 4 weeks. A dose-dependent and significant increase in α-Gal A activity was seen in lysates from all tissues examined after DGJ administration. Tissue α-Gal A activity showed maximal increases of 9 ± 2.0-fold, 50 ± 7.0-fold, and 8 ± 1.0-fold in skin, heart, and kidney, respectively, after administration of 300 mg/kg DGJ (Figure 2a). In skin and heart, α-Gal A activity reached age-matched, WT levels after 4-week administration of 30 and 10 mg/kg DGJ, respectively. In kidney, α-Gal A activity reached ~40% of age-matched, WT levels after administration of 300 mg/kg DGJ. Importantly, the increase in α-Gal A activity in vivo was selective, as DGJ administration to hR301Q α-Gal A Tg/KO mice did not affect tissue activity of three other lysosomal hydrolases, acid α-glucosidase, β-glucocerebrosidase, and β-galactosidase (data not shown). Western blotting revealed that DGJ administration also results in a dose-dependent increase in the 46 kd mature form of α-Gal A in these tissues (Figure 2a, insets). These data are consistent with the observed increase in α-Gal A activity and suggest that DGJ stabilizes the mutant α-Gal A protein in vivo, thereby increasing overall protein levels and enzyme activity in the cells of various tissues.
Figure 2.
DGJ administration increases α-Gal A activity and reduces GL-3 in hR301Q α-Gal A Tg/KO mice. Eight-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ ad libitum in drinking water for 4 weeks at the indicated doses. Lysates from skin, heart, and kidney were subsequently tested for (a) α-Gal A activity and (b,c) GL-3 levels. (a) Significant increases in α-Gal A activity were seen in all three tissues (*P < 0.05 versus untreated, t-test). The effect was also significant for a linear trend, indicating a dose-dependent increase in α-Gal A activity (post hoc analysis, P < 0.05). Insets: Tissue α-Gal A protein levels were determined by western blotting. A dose-dependent increase in the 46 kd mature form of α-Gal A was seen in all tissues. Each lane on the blot contains tissue lysate from a single animal. The western blot shown is representative of two experiments. (b) Significant reductions in GL-3 levels were seen in all three tissues as measured by LC-MS/MS (*P < 0.05 versus untreated, t-test). Blue bars (a,b) represent α-Gal A and GL-3 levels from age-matched untreated wild-type C57BL/6 mice. Each bar represents pooled data from three independent studies with the mean ± SEM of 21 hR301Q α-Gal A Tg/KO mice/group or four untreated wild-type mice, all analyzed in triplicate. (c) Cell type–specific reduction of GL-3 in hR301Q α-Gal A Tg/KO mice administered DGJ (100 mg/kg per day) ad libitum in drinking water was assessed by immunohistochemistry. GL-3 staining is represented as brown spots denoted with black arrows. The data shown are representative photomicrographs from 7–8 mice/group (magnification: ×20, insets: ×40). α-Gal A, α-galactosidase A; DGJ, 1-deoxygalactonojirimycin; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
Importantly, concomitant with the elevation in α-Gal A activity, DGJ administration also reduced tissue GL-3 levels in hR301Q α-Gal A Tg/KO mice. Maximal GL-3 reductions of 69 ± 4, 72 ± 4, and 30 ± 5% were seen in skin, heart, and kidney of the 30, 300, and 100 mg/kg dose groups, respectively (Figure 2b). Similar effects were seen when DGJ was administered by oral gavage (Supplementary Table S1). Immunohistochemical analyses of the 100 mg/kg dose group revealed that GL-3 was markedly reduced in both quantity and intensity in skin fibroblasts, smooth muscle cells of blood vessel walls of skin and heart, and distal tubular epithelial cells of the kidney (Figure 2c). Similar results were seen after administration of 300 mg/kg DGJ (data not shown); lower doses were not assessed.
In separate studies, the specificity of the effects of DGJ was further evaluated. Eight-week-old male GLA KO and hR301Q α-Gal A Tg/KO mice were administered DGJ (100 mg/kg per day) ad libitum in drinking water for 4 weeks. Significant increases in tissue α-Gal A activity and decreases in GL-3 levels were seen in hR301Q α-Gal A Tg/KO mice. Importantly, no effect was seen in GLA KO mice (Supplementary Figure S3).
Long-term DGJ administration leads to greater reduction of GL-3
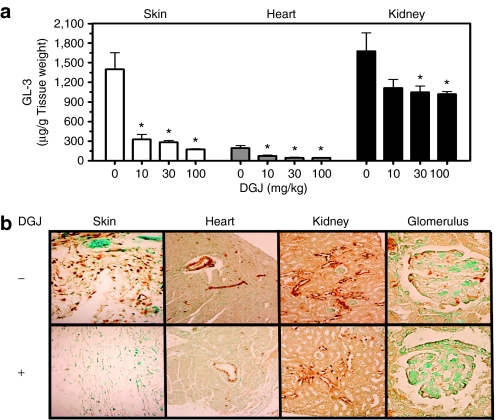
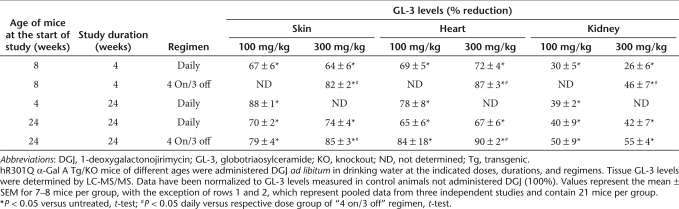
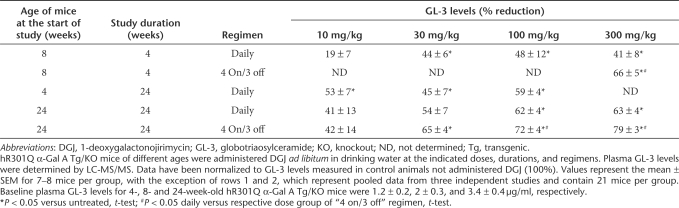
Four-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ (10, 30, and 100 mg/kg per day) ad libitum in drinking water for 24 weeks. A dose-dependent and significant reduction in tissue GL-3 was seen, with maximal reductions of 88 ± 1, 78 ± 8, and 39 ± 2% in skin, heart, and kidney, respectively (Figure 3a). Compared to 4-week DGJ administration to young mice (12-week-old at the end of study), 24-week DGJ administration to young mice (28-week-old at the end of study) resulted in a more pronounced reduction of GL-3 at all doses tested (100 mg/kg shown in Table 1; data not shown for 10 and 30 mg/kg). Immunohistochemical analyses also showed a dose-dependent reduction of GL-3 in specific cell types of skin, heart, and kidney, with maximal reduction at 100 mg/kg (Figure 3b). Importantly, GL-3 staining in cells of the glomeruli was also significantly reduced after 24-week administration of 100 mg/kg DGJ (Figure 3b). Lastly, no apparent adverse events were observed in these mice after 24-week DGJ administration, indicating that DGJ is well tolerated, consistent with an earlier report.40
Figure 3.
Long-term (24-week) administration of DGJ results in greater GL-3 reduction compared to 4-week administration. (a) Four-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ ad libitum in drinking water for 24 weeks at the indicated doses. GL-3 levels were subsequently measured in skin, heart, and kidney lysates by LC-MS/MS. Significant reductions in GL-3 were seen in all three tissues (*P < 0.05 versus untreated, t-test). The effect was also significant for a linear trend, indicating a dose-dependent decrease in GL-3 levels (post hoc analysis, P < 0.05). Each bar represents the mean ± SEM of 7–8 mice/dose group analyzed in triplicate. (b) Cell type–specific GL-3 reductions were assessed by immunohistochemistry. GL-3 staining is represented as brown spots. Data shown are representative photomicrographs from 7–8 mice/group (magnification: ×20, except glomeruli: ×40). DGJ, 1-deoxygalactonojirimycin; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
Table 1.
Effect of 4- and 24-week DGJ administration on tissue GL-3 levels in hR301Q α-Gal A Tg/KO mice
Less frequent DGJ administration results in greater GL-3 reduction
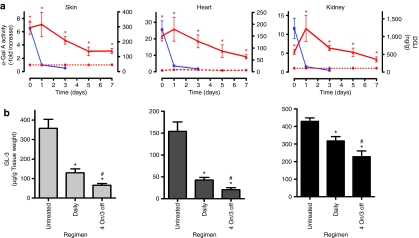
To determine the duration of elevated hR301Q α-Gal A activity in vivo, 8-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ (100 mg/kg per day) ad libitum in drinking water. After 4 weeks, DGJ was withdrawn and mice were provided access to drinking water only. Groups of mice were then sacrificed and tissue α-Gal A activity was measured 0, 1, 3, 5, and 7 days after DGJ withdrawal (Figure 4a). On day 0 (4-week DGJ administration), α-Gal A activity was significantly increased in skin, heart, and kidney. Importantly, α-Gal A levels remained elevated for up to 7 days after DGJ withdrawal. The half-life of elevated hR301Q α-Gal A was estimated to be 2.4, 2.2, and 2.0 days in skin, heart, and kidney, respectively. Compared to the activities measured on day 0, tissue α-Gal A activity was higher 1 day after DGJ withdrawal, suggesting that some DGJ may be present in day 0 tissue samples leading to inhibition of α-Gal A in the enzyme activity assays.
Figure 4.
Less frequent DGJ administration results in greater GL-3 reduction. (a) Eight-week-old male hR301Q α-Gal A Tg/KO mice were administered drinking water (red dotted lines) or DGJ [100 mg/kg per day (red solid lines) ad libitum in drinking water] for 4 weeks, followed by a washout period (drinking water only) of up to 7 days. Groups of mice were then euthanized on days 0, 1, 3, 5, or 7 after DGJ withdrawal. Skin, heart, and kidney were collected at each time point, and α-Gal A activity and DGJ levels (blue lines) were measured in tissue lysates. Significantly increased α-Gal A activity was sustained in all three tissues for up to 7 days (*P < 0.05 versus untreated, t-test). For the α-Gal A activity, each data point represents the mean ± SEM of 7–8 mice/time point analyzed in triplicate; data were normalized to untreated levels. For tissue DGJ levels, each data point represents the mean ± SEM for five mice/time point. The limit of quantitation for DGJ in skin, heart, and kidney was 6, 4, and 10 ng/g, respectively. (b) Eight-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ (300 mg/kg per day) ad libitum in drinking water for 4 weeks either daily or less frequently using four cycles of a “4 on/3 off” regimen. GL-3 levels were subsequently measured in lysates from skin, heart, and kidney by LC-MS/MS. Significant reductions in GL-3 levels were seen in all tissues (*P < 0.05 versus untreated, t-test). GL-3 levels in skin, heart, and kidney also showed significantly greater reductions with less frequent compared to daily administration (#P < 0.05 daily versus “4 on/3 off”, t-test). Each bar represents pooled data from three independent studies with the mean ± SEM of 21 mice/group analyzed in triplicate. DGJ, 1-deoxygalactonojirimycin; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
To this end, tissue concentrations of DGJ were measured using LC-MS/MS (Figure 4a). On the last day of administration (day 0), DGJ levels in plasma, skin, heart, and kidney were 2.4 ± 0.4 µmol/l, 1.8 ± 0.2 µmol/l, 1.1 ± 0.2 µmol/l, and 7.5 ± 1.8 µmol/l, respectively. DGJ levels dropped ~90% by day 1 and were even lower by day 3. In separate studies, the tissue half-life of DGJ after single oral administration by gavage to C57BL/6 mice was calculated to be 2–3 hours in skin, heart, and kidney (data not shown). Collectively, these data indicate that the tissue half-life of DGJ is significantly shorter (hours) than the half-life of α-Gal A (days), suggesting that lysosomal hR301Q α-Gal A is stable in the absence of the PC.
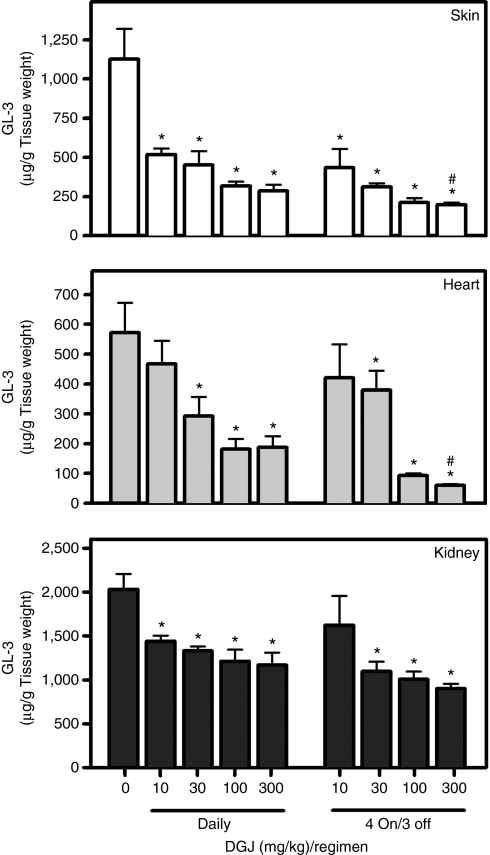
We hypothesized that this difference in half-lives could be exploited by using a less frequent administration regimen (i.e., periods of DGJ administration that stabilize α-Gal A in the endoplasmic reticulum and promote lysosomal trafficking, followed by periods without DGJ to maximize lysosomal α-Gal A activity and substrate turnover). To test this hypothesis, 8-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ (300 mg/kg per day) ad libitum in drinking water for 4 consecutive days followed by 3 consecutive days with drinking water only (“4 on/3 off”) for 4 weeks (Figure 4b). In skin, heart, and kidney, a significantly greater reduction in GL-3 was seen with the “4 on/3 off” regimen compared to daily administration. GL-3 levels were reduced by 82 ± 2, 87 ± 3, and 46 ± 7% in skin, heart, and kidney, respectively, with the less frequent regimen, and by 64 ± 6, 72 ± 4, and 26 ± 6%, respectively, with daily DGJ administration (Table 1). Similar results were seen by immunohistochemistry (data not shown). Compared to daily administration, a generally greater GL-3 reduction was also seen using an “every other day” DGJ administration regimen, which is currently under evaluation in a Fabry phase 2 clinical trial; importantly, these less frequent regimens were equally effective in reducing tissue GL-3 as compared to a once-weekly tail vein injection of Fabrazyme (1 mg/kg for 4 weeks) (Supplementary Figure S4).
Long-term DGJ administration reduces tissue GL-3 in aged hR301Q α-Gal A Tg/KO mice
We next determined whether DGJ administration can reduce accumulated tissue GL-3 in aged mice (Figure 5). Twenty-four-week-old male mice were administered DGJ (10, 30, 100, and 300 mg/kg per day) ad libitum in drinking water for 24 weeks either daily or less frequently (“4 on/3 off”). A dose-dependent and significant reduction in GL-3 was seen after daily administration, with maximal reductions of 74 ± 4, 67 ± 6, and 42 ± 7%, in skin, heart, and kidney, respectively (Table 1). Similar to young mice, less frequent DGJ administration resulted in even greater GL-3 reduction in aged mice (85 ± 3, 90 ± 2, and 55 ± 4% in skin, heart, and kidney, respectively) (Table 1).
Figure 5.
Long-term DGJ administration reduces accumulated tissue GL-3 in aged hR301Q α-Gal A Tg/KO mice. Twenty-four-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ ad libitum in drinking water for 24 weeks either daily or less frequently using a “4 on/3 off” regimen at the indicated doses. GL-3 levels were measured in lysates of skin, heart, and kidney by LC-MS/MS. Dose-dependent and significant reductions in GL-3 were seen in all three tissues (*P < 0.05 versus untreated, t-test). Reduction of GL-3 in skin and heart was significantly greater after less frequent compared to daily administration of 300 mg/kg DGJ (#P < 0.05 daily versus “4 on/3 off”, t-test). Each bar represents the mean ± SEM of 7–8 mice/group analyzed in triplicate. DGJ, 1-deoxygalactonojirimycin; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
DGJ administration reduces plasma GL-3
The effect of DGJ on plasma GL-3 was assessed after 4- and 24-week administration ad libitum in drinking water to male hR301Q α-Gal A Tg/KO mice (Table 2). Statistically significant reductions were observed in both young (4- and 8-week) and aged (24-week) mice, again with less frequent administration (“4 on/3 off”) resulting in greater GL-3 reductions compared to the daily administration in the respective age groups.
Table 2.
Effect of DGJ on plasma GL-3 levels in hR301Q α-Gal A Tg/KO mice
DGJ administration increases α-Gal A activity and reduces GL-3 in the brain of aged mice
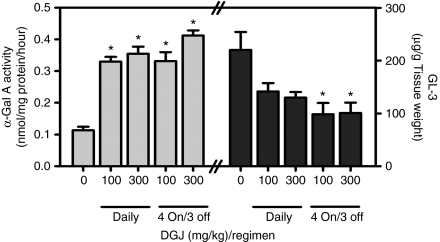
The effect of 12-week oral ad libitum DGJ administration on brain α-Gal A and GL-3 was assessed in 24-week-old male hR301Q α-Gal A Tg/KO mice (Figure 6). Statistically significant increases in α-Gal A activity were seen, with maximal increases of three- to fourfold after daily and less frequent administration of 300 mg/kg. In addition, decreases in brain GL-3 were seen, with maximal reductions of 41 ± 5 and 54 ± 9% after daily and less frequent administration of 300 mg/kg, respectively. These results indicate that DGJ can lead to reduction of GL-3 in the CNS.
Figure 6.
DGJ increases α-Gal A and decreases GL-3 levels in brain of aged hR301Q α-Gal A Tg/KO mice. Twenty-four-week-old male hR301Q α-Gal A Tg/KO mice were administered DGJ ad libitum in drinking water for 12 weeks either daily or less frequently using a “4 on/3 off” regimen at the indicated doses. Brain α-Gal A and GL-3 levels were subsequently measured. Significant increases in α-Gal A and concomitant reductions in GL-3 were seen with DGJ administration (*P < 0.05 versus untreated, t-test). Each bar represents the mean ± SEM of 7–8 mice/group analyzed in triplicate. DGJ, 1-deoxygalactonojirimycin; GL-3, globotriaosylceramide; KO, knockout; Tg, transgenic.
Discussion
We and others have shown that the PC DGJ binds and stabilizes mutant forms of the lysosomal hydrolase α-Gal A, promoting lysosomal trafficking and increasing total enzyme levels in vitro and in vivo.29,30,31,34,39,41 However, the ability of elevated mutant α-Gal A to turnover GL-3, the accumulated substrate that contributes to Fabry disease pathology, has not been extensively investigated in vivo, largely due to the lack of a suitable animal model. Previously described TgM/KO mice homozygous for the hR301Q α-Gal A transgene do not accumulate GL-3 due to high transgene expression by the β-actin promoter.39 However, a recent study showed that female heterozygous TgM/KO mice do accumulate some GL-3 in kidney, which was reduced by ~46% after 4-week administration of DGJ.40 As these mice do not accumulate GL-3 in other tissues, the effects of DGJ were not further evaluated. In the current study, we characterized the effects of DGJ on α-Gal A activity and GL-3 levels in a new mouse model of Fabry disease (hR301Q α-Gal A Tg/KO mice). Unlike TgM/KO mice, the hR301Q transgene in α-Gal A Tg/KO mice is driven by the human GLA promoter, leading to approximately tenfold lower levels of α-Gal A activity compared to wild-type mice and resulting in accumulation of GL-3 in disease-relevant tissues. The levels and cell-type specific accumulation of GL-3 in these mice are similar to those previously reported in GLA KO mice.15,42,43 Using immunohistochemistry, we show that GL-3 accumulation in 12-week-old hR301Q α-Gal A Tg/KO and GLA KO mice is heterogeneous and confined largely to fibroblasts in skin, smooth muscle cells of blood vessel walls in skin and cardiac ventricles, and distal tubular epithelial cells in renal cortex. Age-dependent accumulation of GL-3 is evident in these mice, with some GL-3 apparent in cells of the glomeruli by 28 weeks. Similar GL-3 staining patterns have been reported in tissue biopsies from Fabry patients. In contrast, however, accumulation of GL-3 in Fabry patients is also seen in cardiomyocytes, renal capillary endothelial cells, podocytes, and peritubular cells.7,18,44,45 Although hR301Q α-Gal A Tg/KO mice lack GL-3 accumulation in some clinically relevant cell types, they serve as a good biochemical model to investigate the effects of PCs on mutant enzyme-mediated substrate reduction in vivo.
Daily oral administration of DGJ for 4 weeks to hR301Q α-Gal A Tg/KO mice resulted in dose-dependent and significant increases in α-Gal A activity and protein levels in skin, heart, and kidney. The increases in activity were even greater than the increases in protein levels, suggesting that DGJ may increase the absolute levels and the specific activity of the mutant enzyme in vivo, as has been reported previously for DGJ and other PCs.29,40,46 Most importantly, however, we show that daily oral administration of DGJ to hR301Q α-Gal A Tg/KO mice for 4 weeks also leads to significant decreases in GL-3 in skin, heart, kidney, and plasma (maximal reductions of 69, 72, 30, and 48%, respectively). These reductions are similar to those reported in heart and kidney of GLA KO mice after a single administration of 3 mg/kg recombinant human α-Gal A.15 Furthermore, daily long-term (24-week) administration of DGJ to young hR301Q α-Gal A Tg/KO mice showed greater GL-3 reduction than 4-week administration to young mice. Notably, 24-week administration of DGJ reduced GL-3 levels close to (kidney) or lower than (skin, heart, and plasma) those found in untreated 3-month-old mice, indicating that long-term DGJ administration to young mice can prevent the accumulation of tissue GL-3. Clinical trials with Fabrazyme have shown reduction of GL-3 in the vascular endothelium of skin and heart, as well as the vascular endothelium, glomerular mesangial cells, and cortical interstitial cells of the kidney. However, GL-3 levels were not significantly reduced in other cell types (e.g., pericytes, histiocytes, fibrocytes, and arteriole muscle cells of the skin; cardiomyocytes, pericytes, and vascular smooth muscle of the heart; distal convoluted tubules, collecting ducts, and podocytes of the kidney), potentially due to inefficient delivery and/or uptake of the enzyme.18 In contrast, DGJ was recently shown to increase α-Gal A activity in cardiomyocytes and distal convoluted tubules,40 indicating penetration into these clinically relevant cell types. Our immunohistochemical studies show that GL-3 levels are reduced in distal tubular epithelial cells as well as in cells of the glomeruli, consistent with distribution into these cell types. Future electron microscopy and colocalization studies will assess the glomerular cell types that showed reduced GL-3.
The magnitude of GL-3 reduction was different in different tissues, potentially due to a number of factors including baseline α-Gal A and GL-3 levels, the physical form and location of GL-3, the rates of GL-3 accumulation, cell turnover, and DGJ uptake/efflux within different tissues and cell types. The efficient GL-3 reduction seen in skin and heart may be due to the high turnover rates of the affected cell types in these tissues (fibroblasts and smooth muscle cells).12,47 In contrast, GL-3 reduction in kidney was less efficient, potentially due to a lower turnover rate of tubular epithelial cells.45,48 It is also possible that tubular epithelial cells are exposed to high GL-3 levels in concentrated urine, facilitating reuptake.45 Lastly, kidney α-Gal A activity was only modestly elevated, even though high DGJ levels were present in this tissue. These modest elevations in kidney enzyme levels in the presence of high DGJ concentrations underscore the need for a balance between the presence of chaperone to maximize α-Gal A levels and its subsequent clearance to maximize in situ enzyme activity and GL-3 reduction.
In order to maximize cellular α-Gal A activity and GL-3 reduction, we investigated less frequent DGJ administration in hR301Q α-Gal A Tg/KO mice. The rationale was based on the observation that DGJ has a significantly shorter tissue half-life (hours) compared to elevated α-Gal A (days). We hypothesized that this difference could be exploited to produce a larger net gain in lysosomal enzyme activity with less total drug administered. In this case, 4-day DGJ administration would provide a period of enhanced protein stabilization and trafficking to lysosomes (maximal chaperone effect) followed by 3-day DGJ withdrawal to allow for dissociation and tissue clearance of the PC, thus providing maximal in situ enzyme activity. Four cycles of this “4 on/3 off” regimen showed superior substrate reduction compared to 4-week daily administration, and importantly utilized ~60% less drug. Furthermore, the GL-3 reduction seen with this regimen over a 4-week period was equivalent to, or better than, 24-week daily administration of DGJ, suggesting that dose regimen optimization can result in more rapid and robust substrate reduction. These observations were extended in two ways. First, we compared 24-week daily and “4 on/3 off” DGJ administration to aged (6-month-old) mice. Here, maximal GL-3 reductions of 85, 90, and 55% were seen in skin, heart, and kidney, respectively, similar to reductions reported in GLA KO mice after eight injections of recombinant human α-Gal A over a 2-week period.15 Long-term DGJ administration reduced GL-3 levels close to (kidney), or lower than (skin, heart, and plasma), those of untreated 3-month-old mice, indicating that DGJ can reduce accumulated GL-3 to baseline levels or even lower. Second, we investigated an “every other day” administration regimen that is currently being evaluated in phase 2 clinical trials. Again, GL-3 reduction was comparable to, or better than, that seen with daily administration. In addition, the effects of DGJ were directly compared to those of Fabrazyme; importantly, both drugs were equally effective at reducing total GL-3 in skin, heart, and kidney.
DGJ was also found to cross the blood-brain barrier in mice. Importantly, 12-week DGJ administration significantly increased brain α-Gal A activity. In contrast, multiple administrations of recombinant human α-Gal A to GLA KO mice had no effect on brain α-Gal A activity, underscoring the challenges associated with brain penetration of exogenous enzyme.15 Furthermore, brain GL-3 levels were reduced by 41 and 54% using daily and “4 on/3 off” administration regimens, respectively. These data suggest that DGJ may offer an advantage over enzyme replacement therapy for treating the CNS manifestations of Fabry disease.
In conclusion, our data indicate that DGJ is orally available and has broad tissue distribution that includes the CNS. Furthermore, oral administration of DGJ not only increases α-Gal A activity in hR301Q α-Gal A Tg/KO mice, but also significantly decreases accumulated GL-3 in disease-relevant tissues including skin, heart, kidney, and brain. Importantly, these results provide the first clear in vivo demonstration of increased cellular enzyme activity and biodistribution as measured by reduction of endogenous substrate. Dose optimization studies revealed that even greater GL-3 reduction could be achieved using less frequent DGJ administration regimens, resulting in a larger net gain in cellular activity with reduced overall drug exposure. Collectively, these in vivo data support further evaluation of the PC DGJ for therapeutic efficacy in patients with Fabry disease who have responsive mutant forms of α-Gal A.29
Materials and Methods
Materials. Homozygous Tg/KO mice that express a mutant form of human α-Gal A (R301Q) on a GLA KO mixed background of C57BL/6 and B129Sve (hR301Q α-Gal A Tg/KO) were obtained from Mount Sinai School of Medicine. Wild-type C57BL/6, B129Sve, and B6/129 (50:50) mice were purchased from Taconic Farms (Germantown, NY). Animal husbandry and all experiments were conducted under Institutional Animal Care and Use Committee approved protocols. The hydrochloride salt of DGJ HCl was synthesized by Cambridge Major Laboratories (Germantown, WI). Rabbit antihuman α-Gal A polyclonal antibody was provided by Raphael Schiffmann (Baylor Institute, Dallas, TX). Antihuman GL-3 monoclonal antibody was purchased from Seikagaku (Tokyo, Japan). Fabrazyme was purchased from Genzyme. All other reagents were purchased from Sigma-Aldrich (St Louis, MO) unless noted otherwise.
Genotyping of hR301Q α-Gal A Tg/KO mice. The presence of Tg and KO alleles in hR301Q α-Gal A Tg/KO mice was confirmed by PCR genotyping of genomic DNA isolated from mouse tail. Briefly, for confirmation of the KO allele, a 500 base-pair fragment was amplified using 5′-TTCTTTTTGTCAAGACCGA-3′ forward and 5′-TCGATGAATCCAGAAAA-3′ reverse primers. The PCR amplification consisted of 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 50 °C for 30 seconds, and elongation at 72 °C for 60 seconds. For confirmation of the Tg allele, a 231 base-pair fragment was amplified using 5′-GTCCTTGGCCCTGAATAG-3′ forward and 5′-TCATTCCAACCC CCTGGT-3′ reverse primers. The PCR amplification consisted of 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 60 °C for 30 seconds, and elongation at 72 °C for 30 seconds. Homozygosity for the transgene in hR301Q α-Gal A Tg/KO mice was confirmed (data not shown) using primers and PCR conditions described previously.39
Oral administration of DGJ. DGJ HCl (referred to as DGJ) was administered orally to mice ad libitum in drinking water or by gavage. For ad libitum administration, the appropriate concentrations of DGJ in drinking water were determined based on the average daily water consumption of hR301Q α-Gal A Tg/KO mice (~5 ml/day per mouse) (all doses represent the free-base equivalent of the salt form). Dosing solutions were made fresh weekly. At study completion, mice were euthanized with CO2 and body weights were recorded. Whole blood was drawn into lithium heparin tubes from the inferior vena cava and plasma was collected by centrifuging blood at 2,700 g for 10 minutes at 4 °C. Heart, kidney, brain, and skin (shaved and removed from the lower ventral side of the neck) were quickly removed, rinsed in cold phosphate-buffered saline (PBS), blotted dry, and stored on dry ice. Samples of skin, heart, and kidney were stored in 4% paraformaldehyde for immunohistochemical analysis.
Measurement of tissue α-Gal A activity. Tissue lysates from mutant and wild-type mice were prepared by homogenization of ~50 mg tissue for 3–5 seconds on ice with a microhomogenizer (Pro Scientific, Thorofare, NJ) in 200 µl lysis buffer (27 mmol/l sodium citrate, 46 mmol/l sodium phosphate dibasic, 0.1% Triton X-100, pH 4.6). Lysates (20 µl) were added to 50 µl assay buffer (27 mmol/l sodium citrate, 46 mmol/l sodium phosphate dibasic, 6 mmol/l 4-MU-α-D-galactopyranoside, 90 mmol/l N-acetyl-D-galactosamine, pH 4.6) and incubated for 1 hour at 37 °C. Reactions were stopped by addition of 70 µl 0.4 mol/l glycine, pH 10.8. Fluorescence at 460 nm was read on a Victor3 plate reader (Perkin Elmer, Waltham, MA) after excitation at 355 nm. Raw fluorescence counts were background subtracted (defined by assay buffer only). A Micro BCA protein assay (Pierce, Rockford, IL) was used to determine total protein concentration in tissue lysates according to the manufacturer's instructions. A 4-methylumbelliferone (4-MU) standard curve ranging from 1.3 nmol/l to 30 mmol/l was run each day for conversion of fluorescence counts to absolute α-Gal A activity, expressed as nanomoles of released 4-MU per milligram of total protein per hour (nmol/mg protein/hr). No significant differences in tissue α-Gal A levels were seen between the three wild-type strains investigated (C57BL/6, B129Sve, and 50:50 B6/129; data not shown). For selectivity measurements, substrates specific for the lysosomal hydrolases acid α-glucosidase, β-glucocerebrosidase, and β-galactosidase (4-methylumbelliferryl α-D-glucopyranoside, 4-methylumbelliferryl β-D-glucopyranoside, and 4-methylumbelliferryl β-D-galactopyranoside, respectively) were used according to the methods described previously.49
Western blotting of tissue α-Gal A. Tissue lysates (50 µg total protein) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis on 12% polyacrylamide gels (Bio-Rad, Hercules, CA), transferred to polyvinylidene fluoride membranes (Bio-Rad), and immunoblotted with rabbit antihuman α-Gal A primary antibody (1:1,000 dilution). Protein bands were detected using peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoSearch Labs, West Grove, PA) in combination with enhanced chemiluminescence (Pierce). Blots were scanned on an Image Station 4000R (Kodak, Rochester, NY).
Tissue DGJ quantitation. Mouse tissue was homogenized and extracted (300 µl water per 100 mg kidney, heart, and brain tissue; 500 µl 1:1 water:methanol per 100 mg skin tissue) using a Fast Prep homogenizer (MP Biomedicals, Irvine, CA) followed by centrifugation at 10,600 g for 5 minutes at 4 °C. An equal volume of acetonitrile (ACN):water (95:5) was added to 25 µl of tissue homogenate or plasma, and was then spiked with 50 ng/ml d2-DGJ 13C HCl internal standard (manufactured by MDS Pharma Services, Lincoln, NE). Each sample was vortexed and centrifuged at 10,000 g for 5 minutes at room temperature. DGJ levels in 15 µl supernatant from each sample were determined by LC-MS/MS (LC: Tosoh Bioscience, Cincinnati, OH; MS/MS: Sciex API 3000 MS/MS; AME BioSciences, Toten, Norway). The liquid chromatography was conducted using an ACN:water:formate binary mobile phase system (mobile phase A: 5 mmol/l ammonium formate, 0.05% formic acid in 95:5 ACN:water; mobile phase B: 5 mmol/l ammonium formate, 0.05% formic acid in 55:45 ACN:water) with a flow rate of 0.6 ml/minute on an amide-80 column (50 × 2 mm, 5 µm) (Tosoh Bioscience). MS/MS analysis was carried out under APCI+ ion mode. The following transitions were monitored: mass/charge (m/z) 164.2→m/z 80.10 for DGJ and m/z 167.2→m/z 80.10 for the internal standard. An 11-point calibration curve and quality control samples were prepared in the same manner as the samples. The ratio of the area under the curve for DGJ to that of the internal standard was determined and final concentrations of DGJ in each sample were calculated using the linear least squares fit equation applied to the calibration curve. To derive approximate molar concentrations, one gram of tissue was estimated as 1 ml of volume.
Tissue GL-3 quantitation. Mouse tissues were homogenized in water (1:16.7 tissue:water) using a Fast Prep homogenizer. Acetone:methanol (50:50) was added at 5× the volume of the homogenate. Samples were vortexed, rehomogenized in the Fast Prep system, and centrifuged at 10,600 g for 10 minutes at room temperature. Each supernatant (200 µl of the upper organic portion) was prepared for solid phase extraction by addition of 200 µl dimethyl sulfoxide, 50 µl lactosylceramide internal standard (from bovine buttermilk; Matreya, Pleasant Gap, PA) at a final concentration of 1.4 µg/ml, and 600 µl of water:methanol (13:87) followed by vortexing. Solid phase extraction was conducted on a preconditioned Bond Elut 40 µm, 100 mg C-18 column (Varian, Palo Alto, CA) by washing with 67:23:10 methanol:acetone:water and elution with 1 ml of 9:1 acetone:methanol into silanized glass culture tubes containing 200 µl of 0.2 mmol/l sodium acetate in dimethyl sulfoxide. Samples were evaporated to the dimethyl sulfoxide layer at 45 °C for 10 minutes and vortexed. Total GL-3 levels were determined from 20 µl of each sample extract by LC-MS/MS. LC was conducted using an acetone:methanol:ACN with sodium acetate binary mobile phase system (mobile phase A: 40:60 methanol:water and 0.1 mmol/l sodium acetate; mobile phase B: 75:15:10 acetone:methanol:acetonitrile with 0.1 mmol/l sodium acetate) with a flow rate of 0.5 ml/min on a C18 column (Aqua 3 µm 100 x 3.0 mm, 125A; Phenomenex, Torrance, CA). The final GL-3 elution condition was 90% mobile phase B. MS/MS analysis was carried out under positive ion mode (ESI+) and 12 different isoforms of GL-3 [C16:0, C18:0, C20:0, C22:1, C22:0, C23:0, C22:0(2OH), C24:2, C24:1, C24:0, C24:1(2OH), C24:0(2OH)] as well as internal standard were identified in each sample. The following transitions were monitored: m/z 1,046.70→m/z 884.7 for C16:0; m/z 1,074.8→m/z 912.8 for C18:0; m/z 1,102.8→m/z 940.8 for C20:0; m/z 1,128.8→m/z 966.8 for C22:1; m/z 1,130.9→m/z 968.8 for C22:0; m/z 1,144.9→m/z 982.8 for C23:0; m/z 1,146.9→m/z 984.8 for C22:0(2OH); m/z 1,154.9→m/z 992.8 for C24:2; m/z 1,156.9→m/z 994.8 for C24:1; m/z 1,158.9→m/z 996.9 for C24:0; m/z 1,172.9→m/z 1,010.8 for C24:1(2OH); m/z 1,174.9→m/z 1,012.8 for C24:0(2OH); and m/z 982.9→m/z 820.8 for the lactosyl ceramide internal standard. For quantitation, the area counts for each isoform were determined and then summed to obtain the total GL-3 area counts. The ratio of the total GL-3 area counts to that of the internal standard was determined and used to calculate the final concentration of GL-3 in each sample based on a linear least squares fit equation applied to an 11-point calibration curve prepared in dimethyl sulfoxide. Total GL-3 measurements were normalized to the wet tissue weight of each sample. No significant differences in tissue GL-3 levels were seen among the three wild-type strains investigated (C57BL/6, B129Sve, and 50:50 B6/129; data not shown).
Immunohistochemical detection of GL-3. Paraformaldeyde-fixed tissue samples were processed through a graded ethanol series (Fisher Scientific, Pittsburgh, PA) using a Tissue-Tek VIP 5 (Sakura Finetek, Torrance, CA) and paraffin embedded using an embedding station (Tissue-Tek TEC 5; Sakura Finetek). Samples were sectioned (5 µm for heart and kidney; 10 µm for skin) using a microtome HM 325 (Microm International, Walldorf, Germany). Sections were then dewaxed in xylene (Fisher Scientific), rehydrated through a graded ethanol series, and post-fixed overnight in Z-Fix (Anatech, Battle Creek, MI). Endogenous tissue horseradish peroxidase was blocked in 3% H2O2/PBS for 60 minutes followed by a PBS wash (20 minutes). Endogenous mouse IgG was blocked in rodent block M (Biocare Medical, Concord, CA) for 30 minutes followed by a PBS wash (10 minutes). Sections were then incubated overnight at 4 °C with anti-GL-3 antibody (Seikagaku) diluted 1:200 with Da Vinci Green (Biocare Medical). Unbound antibody was removed by washing in PBS (5 × 5 minutes). Sections were stained with mouse-on-mouse horseradish peroxidase polymer (Biocare Medical) for 20 minutes and then washed in PBS (5 × 5 minutes). Sections were developed using a di-amino benzoid chromogen kit (Biocare Medical) for 5 minutes, dehydrated through a graded ethanol series, and cleared in xylene (Fisher Scientific). Nuclei were counterstained with methyl green (Vector Laboratories, Burlingame, CA) for 5 minutes at room temperature. Finally, samples were mounted in cytoseal mounting media (Richard-Allan Scientific, Kalamazoo, MI) and analyzed on a Nikon microscope Eclipse 90i using NIS Element software (Micron Optics, Cedar Knolls, NJ). The presence of GL-3 in distal tubules of the kidney was confirmed using a specific stain (peanut agglutinin lectin) for the apical surface of distal tubular epithelial cells, according to methodologies described previously.50
Data analysis. The enzyme half-life of α-Gal A after DGJ withdrawal was calculated using a nonlinear one-phase exponential decay curve fitting function in GraphPad Prism, version 4.02 (GraphPad Software, San Diego, CA). Determinations of statistical significance were conducted using Microsoft Excel 2003 (Microsoft, Redmond, WA) or GraphPad Prism as defined in the figure and table legends. Linear trends for dose-dependence were calculated using a one-way analysis of variance in GraphPad Prism. Unless stated otherwise, all values represent mean ± SEM.
SUPPLEMENTARY MATERIALFigure S1. Age-dependent accumulation of tissue GL-3 in hR301Q α-Gal A Tg/KO mice. GL-3 levels in four-, 12-, 24-, and 48-week old male hR301Q α-Gal A Tg/KO and GLA KO mice were determined by (a) immunohistochemistry and (b) LC-MS/MS in skin, heart, and kidney. (a) GL-3 staining (represented as dark red spots) increases with age (in both quantity and intensity) in skin fibroblasts, smooth muscle cells of blood vessel walls of skin and heart, and distal tubular epithelial cells of the kidney cortex. The data shown are representative photomicrographs from 4-5 hR301Q α-Gal A Tg/KO and GLA KO mice/age group (magnification: 20X). (b) Quantitative increases in tissue GL-3 levels were seen with age in both hR301Q α-Gal A Tg/KO and GLA KO mice. Each bar represents the mean±SEM of 4-5 mice/age group.Figure S2. Pharmacokinetic profile of DGJ in C57BL/6 mice. DGJ was administered ad libitum in drinking water to wild-type C57BL/6 mice for seven days (100 mg/kg per day). Beginning on day 7, mice were euthanized at the indicated times with plasma, skin, heart, kidney, and brain collected. DGJ levels were determined by LC-MS/MS. DGJ levels tracked with the light-dark cycle over the 24-hour monitoring period, showing higher levels during the dark cycle when the mice were active; maximal DGJ concentrations of 23±4.3 μM, 7±1.0 μM, 7.7±1.0 μM, 47±9 μM, and 3.1±0.2 μM were attained in plasma, skin, heart, kidney, and brain, respectively. These data indicate that DGJ is orally available, has a broad tissue distribution profile, crosses the blood-brain barrier, and attains tissue concentrations that are in excess of the Ki value for α-Gal A (30±2 nM), confirming the potential for interaction with the target enzyme in vivo. Each bar represents the mean±SEM of 5 mice/time point.Figure S3. DGJ does not affect tissue α-Gal A or GL-3 levels in GLA KO mice. Eight-week old male GLA KO and hR301Q α-Gal A Tg/KO mice were administered DGJ (100 mg/kg per day) ad libitum in drinking water for four weeks. α-Gal A activity and GL-3 levels were subsequently measured in lysates of skin, heart, and kidney. No effects on α-Gal A or GL-3 were seen in GLA KO mice. Significant increases in hR301Q α-Gal A activity and decreases in GL-3 levels were seen in all three tissues of hR301Q α-Gal A Tg/KO mice (*p<0.05 vs. untreated, t-test). Each bar represents the mean±SEM of 7-8 mice/group.Figure S4. Multiple less-frequent DGJ administration regimens are as effective as Fabrazyme in reducing tissue GL-3 in hR301Q α-Gal A Tg/KO mice. Eight-week old male hR301Q α-Gal A Tg/KO mice were orally administered DGJ (300 mg/kg per day) ad libitum in drinking water or by gavage for four weeks. DGJ was administered either daily or less-frequently using four cycles of a '4 on/3 off' regimen or an 'every other day' regimen. A separate group of mice was given a once per week tail vein injection of 1 mg/kg Fabrazyme® for four weeks and tissue GL-3 levels were subsequently measured. Significant reductions in GL-3 were seen with DGJ in the daily and less-frequent regimens and were comparable to those seen with Fabrazyme®. (*p<0.05 vs. untreated, t-test; #p<0.05 daily vs. less-frequent in respective ad libitum or gavage administration) The data presented have been normalized to untreated levels, with each bar representing the mean±SEM of 7-8 hR301Q α-Gal A Tg/KO mice/group analyzed in triplicate.Table S1. Comparison of oral gavage and ad libitum administration of DGJ on α-Gal A and GL-3 levels in male hR301Q α-Gal A Tg/KO mice.
Supplementary Material
Age-dependent accumulation of tissue GL-3 in hR301Q α-Gal A Tg/KO mice. GL-3 levels in four-, 12-, 24-, and 48-week old male hR301Q α-Gal A Tg/KO and GLA KO mice were determined by (a) immunohistochemistry and (b) LC-MS/MS in skin, heart, and kidney. (a) GL-3 staining (represented as dark red spots) increases with age (in both quantity and intensity) in skin fibroblasts, smooth muscle cells of blood vessel walls of skin and heart, and distal tubular epithelial cells of the kidney cortex. The data shown are representative photomicrographs from 4-5 hR301Q α-Gal A Tg/KO and GLA KO mice/age group (magnification: 20X). (b) Quantitative increases in tissue GL-3 levels were seen with age in both hR301Q α-Gal A Tg/KO and GLA KO mice. Each bar represents the mean±SEM of 4-5 mice/age group.
Pharmacokinetic profile of DGJ in C57BL/6 mice. DGJ was administered ad libitum in drinking water to wild-type C57BL/6 mice for seven days (100 mg/kg per day). Beginning on day 7, mice were euthanized at the indicated times with plasma, skin, heart, kidney, and brain collected. DGJ levels were determined by LC-MS/MS. DGJ levels tracked with the light-dark cycle over the 24-hour monitoring period, showing higher levels during the dark cycle when the mice were active; maximal DGJ concentrations of 23±4.3 μM, 7±1.0 μM, 7.7±1.0 μM, 47±9 μM, and 3.1±0.2 μM were attained in plasma, skin, heart, kidney, and brain, respectively. These data indicate that DGJ is orally available, has a broad tissue distribution profile, crosses the blood-brain barrier, and attains tissue concentrations that are in excess of the Ki value for α-Gal A (30±2 nM), confirming the potential for interaction with the target enzyme in vivo. Each bar represents the mean±SEM of 5 mice/time point.
DGJ does not affect tissue α-Gal A or GL-3 levels in GLA KO mice. Eight-week old male GLA KO and hR301Q α-Gal A Tg/KO mice were administered DGJ (100 mg/kg per day) ad libitum in drinking water for four weeks. α-Gal A activity and GL-3 levels were subsequently measured in lysates of skin, heart, and kidney. No effects on α-Gal A or GL-3 were seen in GLA KO mice. Significant increases in hR301Q α-Gal A activity and decreases in GL-3 levels were seen in all three tissues of hR301Q α-Gal A Tg/KO mice (*p<0.05 vs. untreated, t-test). Each bar represents the mean±SEM of 7-8 mice/group.
Multiple less-frequent DGJ administration regimens are as effective as Fabrazyme in reducing tissue GL-3 in hR301Q α-Gal A Tg/KO mice. Eight-week old male hR301Q α-Gal A Tg/KO mice were orally administered DGJ (300 mg/kg per day) ad libitum in drinking water or by gavage for four weeks. DGJ was administered either daily or less-frequently using four cycles of a '4 on/3 off' regimen or an 'every other day' regimen. A separate group of mice was given a once per week tail vein injection of 1 mg/kg Fabrazyme® for four weeks and tissue GL-3 levels were subsequently measured. Significant reductions in GL-3 were seen with DGJ in the daily and less-frequent regimens and were comparable to those seen with Fabrazyme®. (*p<0.05 vs. untreated, t-test; #p<0.05 daily vs. less-frequent in respective ad libitum or gavage administration) The data presented have been normalized to untreated levels, with each bar representing the mean±SEM of 7-8 hR301Q α-Gal A Tg/KO mice/group analyzed in triplicate.
Comparison of oral gavage and ad libitum administration of DGJ on α-Gal A and GL-3 levels in male hR301Q α-Gal A Tg/KO mice.
Acknowledgments
We thank Jim Fan (Mount Sinai School of Medicine, New York, NY) and Hui Hua Chang for initial development of hR301Q α-Gal A Tg/KO mice. Sincere thanks are also due to Eurofins Product Safety Labs (Dayton, NJ) for conducting the in-life portion of the studies highlighted in Supplementary Table S1, and to PPDI (Middleton, WI) for LC-MS/MS measurements of tissue GL-3 presented in Figures 3,5 and 6. We also thank Matthew J. Toth (Barth Syndrome Foundation) and Hung V. Do for helpful discussions during these studies. All authors of Amicus Therapeutics are shareholders. R.J.D. is a consultant and shareholder of Amicus Therapeutics. This work was supported, in part, by funding from American Heart Association, National Institutes of Health research grant R37 DK34045, and Shire Pharmaceuticals, Cambridge, MA.
REFERENCES
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- Bishop DF, Grabowski GA., and , Desnick RJ. Fabry disease: an asymptomatic hemizygote with significant residual α-galactosidase A activity. Am J Hum Genet. 1981;33:71A. [Google Scholar]
- Lemansky P, Bishop DF, Desnick RJ, Hasilik A., and , von Figura K. Synthesis and processing of alpha-galactosidase A in human fibroblasts. Evidence for different mutations in Fabry disease. J Biol Chem. 1987;262:2062–2065. [PubMed] [Google Scholar]
- von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hübner G, Olsen EG, et al. An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991;324:395–399. doi: 10.1056/NEJM199102073240607. [DOI] [PubMed] [Google Scholar]
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL., and , Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA., and , Eng CM.2001α-galactosidase A deficiency: Fabry diseaseIn: Scriver, CR, Beaudet, AL, Sly, WS and Valle, D (eds). The Metabolic and Molecular Bases of Inherited Disease McGraw-Hill: New York; 3733–3774. [Google Scholar]
- Askari H, Kaneski CR, Semino-Mora C, Desai P, Ang A, Kleiner DE, et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007;451:823–834. doi: 10.1007/s00428-007-0468-6. [DOI] [PubMed] [Google Scholar]
- Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81:122–138. doi: 10.1097/00005792-200203000-00003. [DOI] [PubMed] [Google Scholar]
- Shah JS., and , Elliott PM. Fabry disease and the heart: an overview of the natural history and the effect of enzyme replacement therapy. Acta Paediatr Suppl. 2005;94:11–4; discussion 9. doi: 10.1111/j.1651-2227.2005.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- Grewal RP. Stroke in Fabry's disease. J Neurol. 1994;241:153–156. doi: 10.1007/BF00868342. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004;110:1047–1053. doi: 10.1161/01.CIR.0000139847.74101.03. [DOI] [PubMed] [Google Scholar]
- Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, et al. High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou YA, Zeidner KM, Gordon RE., and , Desnick RJ. Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploos van Amstel JK, Jansen RP, de Jong JG, Hamel BC., and , Wevers RA. Six novel mutations in the alpha-galactosidase A gene in families with Fabry disease. Hum Mol Genet. 1994;3:503–505. doi: 10.1093/hmg/3.3.503. [DOI] [PubMed] [Google Scholar]
- Redonnet-Vernhet I, Ploos van Amstel JK, Jansen RP, Wevers RA, Salvayre R., and , Levade T. Uneven X inactivation in a female monozygotic twin pair with Fabry disease and discordant expression of a novel mutation in the alpha-galactosidase A gene. J Med Genet. 1996;33:682–688. doi: 10.1136/jmg.33.8.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CM, Banikazemi M, Gordon RE, Goldman M, Phelps R, Kim L, et al. A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet. 2001;68:711–722. doi: 10.1086/318809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- Hughes DA. Early therapeutic intervention in females with Fabry disease. Acta Paediatr Suppl. 2008;97:41–47. doi: 10.1111/j.1651-2227.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28:703–710. doi: 10.1002/mus.10497. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, et al. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24:2102–2111. doi: 10.1093/ndt/gfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009;20:1132–1139. doi: 10.1681/ASN.2008080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JQ., and , Ishii S. Cell-based screening of active-site specific chaperone for the treatment of Fabry disease. Meth Enzymol. 2003;363:412–420. doi: 10.1016/S0076-6879(03)01069-3. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE., and , Kelly JW. Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropak MB, Reid SP, Guiral M, Withers SG., and , Mahuran D. Pharmacological enhancement of beta-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff Patients. J Biol Chem. 2004;279:13478–13487. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, et al. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci USA. 2003;100:15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapace G, Waheed A, Shah GN., and , Sly WS. Chemical chaperones protect from effects of apoptosis-inducing mutation in carbonic anhydrase IV identified in retinitis pigmentosa 17. Proc Natl Acad Sci USA. 2004;101:12300–12305. doi: 10.1073/pnas.0404764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin ER, Flanagan JJ, Schilling A, Chang HH, Agarwal L, Katz E, et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis. 2009;32:424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- Fan J-Q, Nakao S, Kaneski CR, Brady RO, Desnick RJ, Suzuki S, et al. Intracellular enhancement of α-galactosidase A activity in 31 Fabry lymphoblasts and fibroblasts by 1-deoxy-galactonojirimycin. Am J Hum Genet. 1999;65:A308. [Google Scholar]
- Asano N, Ishii S, Kizu H, Ikeda K, Yasuda K, Kato A, et al. In vitro inhibition and intracellular enhancement of lysosomal alpha-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur J Biochem. 2000;267:4179–4186. doi: 10.1046/j.1432-1327.2000.01457.x. [DOI] [PubMed] [Google Scholar]
- Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci. 2003;24:355–360. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Shin SH, Murray GJ, Kluepfel-Stahl S, Cooney AM, Quirk JM, Schiffmann R, et al. Screening for pharmacological chaperones in Fabry disease. Biochem Biophys Res Commun. 2007;359:168–173. doi: 10.1016/j.bbrc.2007.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam GH, Zuber C., and , Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19:12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- Ashton-Prolla P, Tong B, Shabbeer J, Astrin KH, Eng CM., and , Desnick RJ. Fabry disease: twenty-two novel mutations in the alpha-galactosidase A gene and genotype/phenotype correlations in severely and mildly affected hemizygotes and heterozygotes. J Investig Med. 2000;48:227–235. [PubMed] [Google Scholar]
- Germain DP., and , Poenaru L. Fabry disease: identification of novel alpha-galactosidase A mutations and molecular carrier detection by use of fluorescent chemical cleavage of mismatches. Biochem Biophys Res Commun. 1999;257:708–713. doi: 10.1006/bbrc.1999.0310. [DOI] [PubMed] [Google Scholar]
- Germain DP, Shabbeer J, Cotigny S., and , Desnick RJ. Fabry disease: twenty novel alpha-galactosidase A mutations and genotype-phenotype correlations in classical and variant phenotypes. Mol Med. 2002;8:306–312. [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Sakuraba H., and , Suzuki Y. Point mutations in the upstream region of the alpha-galactosidase A gene exon 6 in an atypical variant of Fabry disease. Hum Genet. 1992;89:29–32. doi: 10.1007/BF00207037. [DOI] [PubMed] [Google Scholar]
- Ishii S, Yoshioka H, Mannen K, Kulkarni AB., and , Fan JQ. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690:250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ishii S, Chang HH, Yoshioka H, Shimada T, Mannen K, Higuchi Y, et al. Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J Pharmacol Exp Ther. 2009;328:723–731. doi: 10.1124/jpet.108.149054. [DOI] [PubMed] [Google Scholar]
- Yam GH, Bosshard N, Zuber C, Steinmann B., and , Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol, Cell Physiol. 2006;290:C1076–C1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, et al. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000;105:1563–1571. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, et al. alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human alpha-galactosidase A–replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Rennke H, Colvin RB, Dikman S, Gordon RE, Collins AB, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- Steet RA, Chung S, Wustman B, Powe A, Do H., and , Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci USA. 2006;103:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh EA, Chai CM, de Lumen BO, Neese RA., and , Hellerstein MK. Dynamics of keratinocytes in vivo using HO labeling: a sensitive marker of epidermal proliferation state. J Invest Dermatol. 2004;123:530–536. doi: 10.1111/j.0022-202X.2004.23303.x. [DOI] [PubMed] [Google Scholar]
- Vogetseder A, Karadeniz A, Kaissling B., and , Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005;124:97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, et al. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Truong LD, Phung VT, Yoshikawa Y., and , Mattioli CA. Glycoconjugates in normal human kidney. A histochemical study using 13 biotinylated lectins. Histochemistry. 1988;90:51–60. doi: 10.1007/BF00495707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Age-dependent accumulation of tissue GL-3 in hR301Q α-Gal A Tg/KO mice. GL-3 levels in four-, 12-, 24-, and 48-week old male hR301Q α-Gal A Tg/KO and GLA KO mice were determined by (a) immunohistochemistry and (b) LC-MS/MS in skin, heart, and kidney. (a) GL-3 staining (represented as dark red spots) increases with age (in both quantity and intensity) in skin fibroblasts, smooth muscle cells of blood vessel walls of skin and heart, and distal tubular epithelial cells of the kidney cortex. The data shown are representative photomicrographs from 4-5 hR301Q α-Gal A Tg/KO and GLA KO mice/age group (magnification: 20X). (b) Quantitative increases in tissue GL-3 levels were seen with age in both hR301Q α-Gal A Tg/KO and GLA KO mice. Each bar represents the mean±SEM of 4-5 mice/age group.
Pharmacokinetic profile of DGJ in C57BL/6 mice. DGJ was administered ad libitum in drinking water to wild-type C57BL/6 mice for seven days (100 mg/kg per day). Beginning on day 7, mice were euthanized at the indicated times with plasma, skin, heart, kidney, and brain collected. DGJ levels were determined by LC-MS/MS. DGJ levels tracked with the light-dark cycle over the 24-hour monitoring period, showing higher levels during the dark cycle when the mice were active; maximal DGJ concentrations of 23±4.3 μM, 7±1.0 μM, 7.7±1.0 μM, 47±9 μM, and 3.1±0.2 μM were attained in plasma, skin, heart, kidney, and brain, respectively. These data indicate that DGJ is orally available, has a broad tissue distribution profile, crosses the blood-brain barrier, and attains tissue concentrations that are in excess of the Ki value for α-Gal A (30±2 nM), confirming the potential for interaction with the target enzyme in vivo. Each bar represents the mean±SEM of 5 mice/time point.
DGJ does not affect tissue α-Gal A or GL-3 levels in GLA KO mice. Eight-week old male GLA KO and hR301Q α-Gal A Tg/KO mice were administered DGJ (100 mg/kg per day) ad libitum in drinking water for four weeks. α-Gal A activity and GL-3 levels were subsequently measured in lysates of skin, heart, and kidney. No effects on α-Gal A or GL-3 were seen in GLA KO mice. Significant increases in hR301Q α-Gal A activity and decreases in GL-3 levels were seen in all three tissues of hR301Q α-Gal A Tg/KO mice (*p<0.05 vs. untreated, t-test). Each bar represents the mean±SEM of 7-8 mice/group.
Multiple less-frequent DGJ administration regimens are as effective as Fabrazyme in reducing tissue GL-3 in hR301Q α-Gal A Tg/KO mice. Eight-week old male hR301Q α-Gal A Tg/KO mice were orally administered DGJ (300 mg/kg per day) ad libitum in drinking water or by gavage for four weeks. DGJ was administered either daily or less-frequently using four cycles of a '4 on/3 off' regimen or an 'every other day' regimen. A separate group of mice was given a once per week tail vein injection of 1 mg/kg Fabrazyme® for four weeks and tissue GL-3 levels were subsequently measured. Significant reductions in GL-3 were seen with DGJ in the daily and less-frequent regimens and were comparable to those seen with Fabrazyme®. (*p<0.05 vs. untreated, t-test; #p<0.05 daily vs. less-frequent in respective ad libitum or gavage administration) The data presented have been normalized to untreated levels, with each bar representing the mean±SEM of 7-8 hR301Q α-Gal A Tg/KO mice/group analyzed in triplicate.
Comparison of oral gavage and ad libitum administration of DGJ on α-Gal A and GL-3 levels in male hR301Q α-Gal A Tg/KO mice.