Abstract
In the absence of an immune response from the host, intramuscular (IM) injection of recombinant adeno-associated virus (rAAV) results in the permanent expression of the transgene from mouse to primate models. However, recent gene transfer studies into animal models and humans indicate that the risk of transgene and/or capsid-specific immune responses occurs and depends on multiple factors. Among these factors, the route of delivery is important, although poorly addressed in large animal models. Here, we compare the IM and the drug-free regional intravenous (RI) deliveries of rAAV in nonhuman primate (NHP) skeletal muscle monitoring the host immune response toward the transgene. We show that IM is consistently associated with immunotoxicity and the destruction of the genetically modified myofibers, whereas RI allows the stable expression of the transgene. This has important implications for the design of clinical trials for gene transfer in skeletal muscle.
Introduction
The most popular route of delivery of a recombinant adeno-associated virus (rAAV) vector in the skeletal muscle remains the direct intramuscular (IM) injection. This has been extensively used in a wide variety of mammals, including humans affected with factor IX (FIX),1,2,3 α1-antitrypsin,4,5 lipoprotein lipase (LPL),6 or α-sarcoglycan7 deficiencies. In the absence of an immune response from the host, IM administration of rAAV results in the permanent expression of the transgene from mouse to primate models.3,8,9,10,11,12 However, recent gene transfer studies into animal models and humans indicate that the risk of transgene and/or capsid-specific immune responses depends on multiple factors including the dose and type of rAAV, the expression cassette and tissue specificity of the promoter, the level of transgene expression, the mode of delivery, the transduced cell type, the underlying mutation of the model, and the pre-existing immune status of the host with respect to rAAV.13,14,15,16,17,18,19 There is also a species issue whereby certain mouse strains are less prone to develop a cytotoxic T-lymphocyte (CTL) response to the transgene product than are nonhuman primates (NHPs). Indeed, after rAAV-mediated gene delivery by IM injection in immune-competent mice, β-galactosidase,20 human FIX,21 human LPL,22 or the doxycycline (Dox)-sensitive reverse tetracycline transactivator (rtTA)8,23 remain permanently expressed, whereas in the large animal models (cats, dogs, and NHP), they often trigger host immune responses including both the generation of neutralizing antibodies and CTL against the transgene.10,24,25,26 In that respect, immunotoxicity data in NHP are both rare and extremely relevant for gene therapy.
We previously showed that 12 out of 14 macaques (85%) injected with AAV IM vectors from various serotypes, which express the cynomolgus macaque erythropoietin (cmEpo) and the transactivator rtTA, developed antibodies and a cytotoxic T-cell response to the latter product (refs. 10,27 and P. Chenuaud and P. Moullier, unpublished results). This adverse effect provides an interesting immune reaction model against the transgene after rAAV IM delivery in the macaque.
We hypothesized that the expression of the immunosuppressive molecule LEA29Y (Belatacept), a mutant of CTLA4-Ig with more potency for its immunosuppressive actions, could prevent the development of an immune response against the rtTA transactivator allowing long-term Epo regulation. Indeed, LEA29Y both blocks the co-stimulation signal required for the complete T-cell activation28 and also induces the suppressive molecule indoleamine-2,3-dioxygenase expression in dendritic cells.29 LEA29Y is currently in a Phase III clinical trial and is part of a new generation of immunosuppressive drugs.30
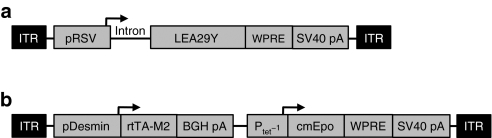
To avoid the challenge of achieving reliable daily intakes of LEA29Y to our NHP cohort, we cloned the corresponding complementary DNA into an AAV2 vector backbone (Figure 1a). To obtain a wide range of circulating levels of LEA29Y, we explored two different modes of vector delivery, IM and regional intravenous (RI) injection, and two different rAAV serotypes (1 and 8) (ref. 31). Once the levels of LEA29Y achieved a therapeutic window, animals were injected in the opposite limb with an AAV-rtTA/Epo vector (Figure 1b) injected IM, and the lack of immune reaction against rtTA was evaluated by the long-term ability to regulate the recombinant cmEpo secretion upon Dox administration.
Figure 1.
Adeno-associated virus (AAV) vectors used in this study. (a) The first vector used in this study consists of the Rous sarcoma virus promoter (pRSV), a chimeric intron, the coding sequence of the immunosuppressive molecule LEA29Y, the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) and the SV40 polyadenylation signal (SV40 pA). (b) The second vector used in this study encodes the cynomolgus macaque erythropoietin (cmEpo) under the control of the doxycycline-inducible Ptet−1 promoter and the rtTA-M2 chimeric protein under the control of the muscle-specific 1-kb human desmin promoter (pDesmin). Arrows indicate the transcription start sites. BGH pA, bovine growth hormone polyadenylation signal; ITR, inverted terminal repeat sequence of AAV2.
We found that although all macaques expressed therapeutic LEA29Y serum levels, this molecule was not sufficient to avoid the host immune response against rtTA. Indeed, 4/4 animals receiving the rAAV-LEA29Y by IM injection showed antibodies against rtTA associated with the rapid loss of cmEpo regulation. On the contrary, 3/4 of those who received the rAAV-LEA29Y vector by RI injection subsequently did not develop an immune response toward the rtTA transgene, and exhibited long-term cmEpo regulation. To further investigate the impact of the RI delivery on the lack of immunotoxicity against the transgene product, we injected three additional naive and fully immunocompetent macaques with the AAV-rtTA/Epo vector delivered by RI injection. None of the primates developed an immune reaction against the transgene indicating that RI delivery, in addition to be clinically well tolerated,31 is an efficient and safe procedure to achieve widespread and permanent skeletal muscle transduction.
Results
First vector administration using rAAV-LEA29Y
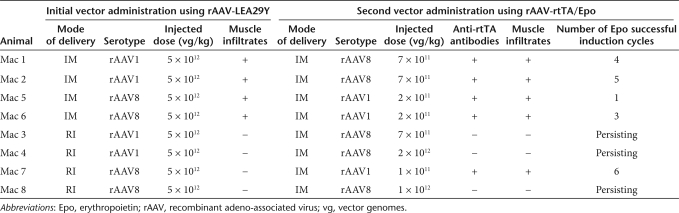
Our first aim was to achieve immunosuppressive levels of LEA29Y in the serum of our eight-macaque cohort, already described.31 Two different modes of delivery (im and ri) and two different rAAV serotypes (rAAV1 and rAAV8) were used to determine the optimal protocol (Table 1 and ref. 31). Transgene expression was monitored in the serum of each animal at different time points. More than a year postinjection (p.i.), the two macaques that received the rAAV1 vector by IM injection (Mac 1 and 2) had an average of 15 µg of LEA29Y/ml of serum, whereas all the other animals (Mac 3–8) expressed between 50 and 100 µg of LEA29Y/ml of serum.31 These LEA29Y levels are considered to be well above the therapeutical range of the molecule in macaques.32
Table 1.
Characteristics of the rAAV doses injected, muscle infiltrates, and Epo regulation outputs in the first cohort of macaques
To assess the in vivo efficacy of the secreted LEA29Y to block humoral immune responses, mock and rAAV-injected macaques (except Mac 5) were immunized with sheep red blood cells. Three weeks later, antibodies against sheep red blood cells were detected in the serum of the control animals (n = 3), whereas none or low levels were detected in serum of rAAV-LEA29Y-injected animals (Supplementary Figure S1 and Supplementary Materials and Methods). To explore the cellular immune status of the cohort, all macaques received a histoincompatible skin allograft. All rAAV-LEA29Y-injected macaques rejected the graft, however, with a small but significant delay in comparison with the control group (P = 0.03, analysis of variance) (Supplementary Figure S2 and Supplementary Materials and Methods). Altogether, these results confirmed that LEA29Y produced after vector delivery was able to suppress the humoral response and to a lesser extent the time to a cellular immune reaction.
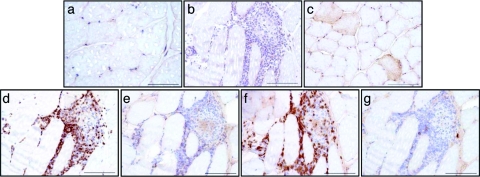
To characterize the transduction pattern and tissue lesions of each group, muscle biopsies were obtained 5 weeks p.i. In the IM group, one biopsy was carried out at one of the pretattooed sites of rAAV injection. In the RI group, biopsies were obtained from the gastrocnemius, the tibialis anterior, the biceps femoris, and the sartorius. As previously reported, the presence of transgene DNA was confirmed by quantitative PCR in all biopsies from all animals.31 Surprisingly, and despite the lack of detection of proinflammatory cytokines interleukin-6 and tumor necrosis factor-α in their serum31 and the lack of significant variation of creatine phosphokinase levels (data not shown), we found that, regardless of the rAAV serotype, all animals in the IM group exhibited inflammatory infiltrates frequently but not exclusively around transgene-expressing myofibers (Figure 2b). Conversely, in the RI group, none of the biopsies showed inflammatory infiltrates even in areas where LEA29Y was detected (Figure 2c). Using CD3, CD4, CD8 (T cells), and CD79a (B cells) markers, we found that the infiltrates were primarily made of CD8+ T cells (Figure 2d–g). The same observations were made at 11 and 22 months p.i. (data not shown).
Figure 2.
Immunostaining of rAAV-LEA29Y-injected muscles 5 weeks after recombinant adeno-associated virus (rAAV) delivery. Samples obtained from muscle biopsies. CTLA4 staining of (a) noninjected muscle, (b) rAAV1-im-injected muscle (cryosection obtained at the injection site from Mac 2) and of (c) rAAV1-ri-injected muscle (cryosection obtained in the gastrocnemius from Mac 3). Phenotypic characterization of the cellular infiltrates of the rAAV1-im-injected muscle: (d) anti-CD3; (e) anti-CD4; (f) anti-CD8; (g) anti-CD79a. Bar = 100 µm.
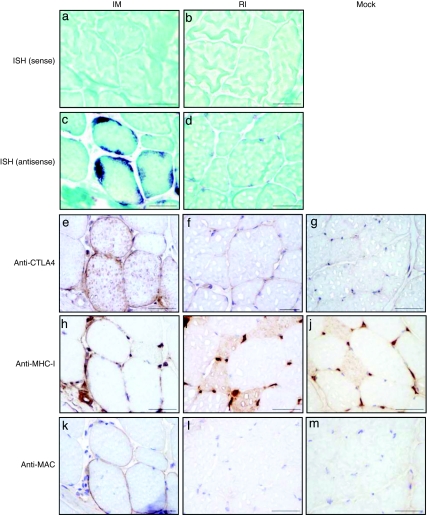
To further characterize the muscle transduction pattern 5 weeks p.i., we performed RNA in situ hybridization using an antisense probe specific for the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) sequence. Figure 3c shows strong subsarcolemmal accumulations of WPRE mRNA in rAAV1-im-transduced myofibers (see also Supplementary Figure S3). As a control, no signal was found with the sense probe (Figure 3a). Accordingly, immunohistochemical staining using an anti-CTLA4 antibody showed strong sarcolemmal LEA29Y expression colocalizing with the in situ hybridization–positive cells (compare Figure 3c and e). Interestingly, the same fibers expressed high levels of major histocompatibility complex class I (MHC-I) molecules on the cell surface (Figure 3h) and deposition of the complement-derived membrane attack complex (MAC) (Figure 3k). Conversely, after RI delivery, the signal for the WPRE messengers using the antisense probe was dramatically weaker and was restricted to the perinuclear cytoplasmic area, but was detected in all fibers in the biopsy sample (Figure 3d and Supplementary Figure S3). No signal was found after incubation with the sense probe (Figure 3b). However, according to our previous work,31 this expression level was not sufficient to allow the immunohistochemical detection of LEA29Y-positive fibers (Figure 3f), and was correlated with the lack of detection of MHC-I expression and MAC deposition on the surface of the transduced myofibers (Figure 3i,l). The same expression patterns were found after IM and RI delivery of rAAV8 (data not shown). These data indicate that IM, and not RI injection, results in: (i) high local concentration of the transgene product; (ii) overexpression of MHC-I molecules at the cell surface of the genetically modified myofibers; and (iii) colocalization of the MAC with the transgene and MHC-I overexpressing myofibers.
Figure 3.
In situ hybridization and immunostaining of rAAV-LEA29Y-injected muscles 5 weeks after recombinant adeno-associated virus (rAAV) delivery. (a,c,e,h,k) rAAV1-im-injected muscle (cryosection obtained at the injection site from Mac 1); (b,d,f,i,l) rAAV1-ri-injected muscle (cryosection obtained in the gastrocnemius from Mac 3); and (g,j,m) noninjected muscle. In situ hybridization with (a,b) a sense and (c,d) an antisense woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) probe. (e–g) CTLA4 staining; (h–j). major histocompatibility complex class I (MHC-I) staining; (k–m) membrane attack complex (MAC) staining. Bar = 100 µm. IM, intramuscular; RI, regional intravenous.
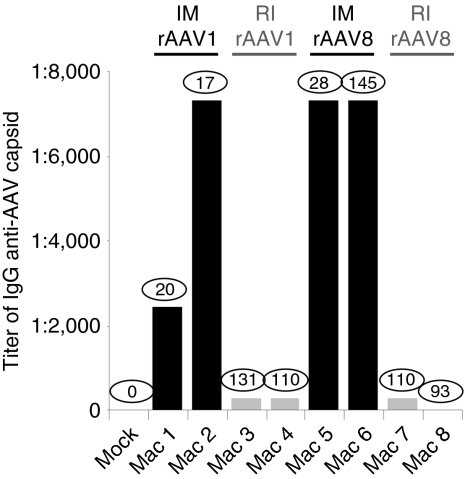
Because muscle lesions with respect to the host immune reaction were so impressively different between the RI and the IM groups, we further analyzed by enzyme-linked immunosorbent assay for the presence of anti-rAAV capsid IgG antibodies in the serum of all eight NHPs. Three months p.i., we found that the im-injected animals produced high titers of anticapsid antibodies, regardless of the serotype. In contrast, the RI group showed an insignificant humoral response (Figure 4). Importantly, these results were independent of the serum LEA29Y levels. Furthermore, the same difference in humoral response was obtained when we looked for the presence of neutralizing antibodies against the rAAV capsid, i.e. high levels of neutralizing antibodies were found in the IM group sera and low-to-undetectable levels in the RI group (data not shown). These results are consistent with the pathology findings and strengthen the observation that IM, as opposed to RI, is an efficient route of delivery to trigger the host immune system despite immunosuppression.
Figure 4.
Serum antibody titers for recombinant adeno-associated virus (rAAV1) or rAAV8 3 months after gene transfer. Serum samples are obtained from noninjected or rAAV-LEA29Y-injected macaques. Serum LEA29Y concentration values (µg/ml) at this time point are shown within circles for each animal. Sample anti-IgG antibody titers were assigned at the highest dilution where the average of the duplicates was higher than the average of serum before rAAV-injection ± 2SD. IM, intramuscular; RI, regional intravenous.
Second vector administration using rAAV-rtTA/Epo
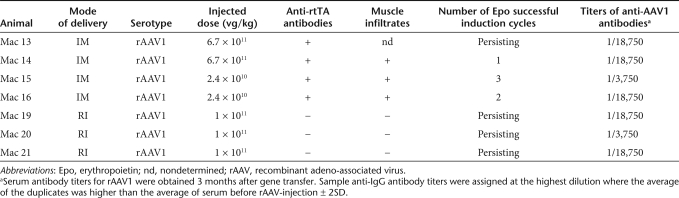
We then tested whether the levels of LEA29Y achieved could impact the rtTA/Epo antigenic model (refs. 10,27 and unpublished results) using the same administration protocol that we previously described.10,27 Briefly, when serum LEA29Y levels stabilized (between 3 and 9 months p.i.), a serotype 1 or alternatively a serotype 8 AAV-rtTA/Epo vector (depending on the presence of pre-existing neutralizing antibodies) was administered by IM injection in the opposite limb at doses ranging from 2 × 1011 to 1 × 1012 vector genomes/kg (Table 1).
Two months p.i., Dox-induction cycles were initiated for 3 days every month and cmEpo secretion was monitored by enzyme-linked immunosorbent assay.10,27,33 As shown in Table 1 and Figure 5a, Mac 1, 2, 5, and 6 had lost cmEpo secretion ability after only 1–5 Dox-induction cycles, concomitant with the detection of anti-rtTA antibodies 1 month before the decline of cmEpo expression. On the contrary, Mac 7 remained inducible until the 6th Dox-induction cycle, and the remaining primates, Mac 3, 4, and 8 showed persisting cmEpo-induction capacity, without detectable anti-rtTA antibodies (over at least 11, 6, and 7 inductions, respectively) (Table 1 and Figure 5a). To complete the study, 6–11 months p.i., all eight NHPs were subjected to a muscle biopsy at the site of rAAV-rtTA/Epo injection. As previously described,10 we found lymphomonocytic infiltrates with destruction of myofibers and significant architectural tissue rearrangements only in the animals that had lost Epo regulation (Table 1).
Figure 5.
Serum erythropoietin (Epo) level in rAAV-rtTA/Epo-injected animals. (a) Serum Epo level in representative animals Mac 3 and 5. (b) Serum Epo level in Mac 19–21, injected via regional intravenous (RI) with an rAAV-rtTA/Epo vector. Solid arrows represent doxycycline (Dox) administration. Ab(rtTA), detection of anti-rtTA antibodies in successive serum samples by western blot analysis; C+, positive control consisting in a commercial-specific MAb against rtTA; C−, negative control obtained from sera before rAAV administration.
In conclusion, despite therapeutic levels of circulating LEA29Y, an immune response against the rtTA transactivator was observed in several animals independent of the LEA29Y level and the AAV-rtTA/Epo vector dose (Table 1). In addition and unexpectedly, the mode of delivery of the first injected AAV vector seems to have an impact on the development of the anti-rtTA immune response following the IM delivery of the second AAV vector. Indeed, the four animals, which received the AAV-LEA29Y vector by IM mounted a destructive immune response against the rtTA. In contrast, only one out of the four animals, which received the AAV-LEA29Y vector by RI, developed such an immune response.
RI delivery of an rAAV-rtTA/Epo, without immunosuppression
To further explore whether RI delivery of rAAV can promote a nonresponding status of the host immune system to the rtTA transgene, three naive macaques (Mac 19–21) received the AAV1-rtTA/Epo vector by RI injection, in the absence of any immunosuppressive regiment.
As in our first cohort, the protocol consisted of measuring cmEpo concentration after repeated Dox inductions every month. Interestingly, 100% of animals (n = 3) showed a persistent ability to secrete cmEpo after Dox exposure for at least 10 months and 7 inductions following vector administration, and none of the NHP showed detectable anti-rtTA antibodies (Figure 5b and Table 2). In contrast, when the same AAV1-rtTA/Epo vector was administered by IM injection without immunosuppression in a previous study,27 only one individual out four showed a persisting cmEpo-induction capacity. Indeed, the other three macaques lost cmEpo expression following the first or the second induction cycle. Here too, this decline was concomitant with the presence of anti-rtTA antibodies in the serum and the presence of cellular infiltrates at the site of vector injection (Table 2). Accordingly, biopsies performed on several muscles on Mac 19–21 at 7 months p.i. neither showed any sign of inflammation nor myofiber destruction (Table 2).
Table 2.
Characteristics of the rAAV doses injected and Epo regulation outputs in the macaques injected with the rAAV-rtTA/Epo vector without immunosuppression
Altogether, these results confirm that RI delivery is able to avoid the host immune reaction against the transgene, at least in our rtTA antigenic model, whereas IM injection results predominantly in antigen recognition and loss of transgene expression.
Given the fact that expression of a transgene from an AAV vector in liver can promote tolerance to the transgene product,34 we tested whether the vector was distributed to the liver and expressed the transgene product. For this, we performed PCR and reverse transcriptase-PCR analysis from liver biopsies obtained between 15 and 18 months after RI or IM delivery of the AAV1-rtTA/Epo vector. We found that vector DNA was detectable in both groups, whereas rtTA transcript was only detectable in the RI group (Figure 6).
Figure 6.
PCR and reverse transcriptase (RT)-PCR analysis on liver biopsies from nonhuman primates injected by intramuscular (Mac 14, 15, and 16) or by regional intravenous (Mac 19, 20, 21) with the AAV1-rtTA/Epo vector. Liver biopsies were performed between 15 and 18 months after rAAV injection. (a) Genomic DNA was extracted from liver biopsies and analyzed by PCR using primers specific for the rtTA sequence (269-bp amplicon) or for the endogenous GAPDH sequence as a positive control (219-bp amplicon). A liver biopsy obtained from a naive macaque was used as a negative control. A range of the pAAV-rtTA/Epo plasmid was used to determine the sensitivity of the rtTA-PCR reaction. (b) Total RNA was extracted from liver biopsies and analyzed by RT-PCR with (+RT) or without (−RT) reverse transcription using primers specific for the rtTA sequence (269-bp amplicon) or for the hypoxanthine guanine phosphoribosyltransferase (HPRT) sequence as a positive control (250-bp amplicon). A liver biopsy obtained from a naive macaque was used as a negative control, and 293 cells transfected with the pAAV-rtTA/Epo plasmid were used as positive controls (C+). A range of the pAAV-rtTA/Epo plasmid was used to determine the sensitivity of the rtTA-PCR reaction.
Discussion
At a time when the rAAV IM delivery is being used in clinical trials to express therapeutic transgenes,4,5,6,7 controlling the host immune response against the transgene is a major goal. Here, in NHP, we tested whether the immunosuppressive molecule LEA29Y, expressed from an AAV vector, could allow long-term expression of the rtTA immunogenic transgene after IM delivery of a second rAAV. Our results showed that although LEA29Y alone was able to promote partial immunosuppression, it was not sufficient per se to avoid the host immune response against rtTA. However, we confirmed that IM injections of AAV vector were always associated with the presence of local inflammatory infiltrates for several weeks or even months p.i. despite the in situ deposition of ~2 × 1012 vector genomes per IM site.17 These infiltrates were primarily made of CD8+ T cells. In the same way, studies done in outbred immune-competent mice,17 rabbits,35 normal dogs,25,26 hemophilia B dogs,15 LPL-deficient patients,36 and α-sarcoglycan-deficient patients7 demonstrated dose-dependent immune response against the transgene or the capsid after IM administration of either AAV2 or AAV1 vectors.
The first result of our study was the complete inhibition in most cases or the delayed production, of antibodies against the rtTA transgene product carried by the second AAV vector, when the first rAAV delivery was made by RI injection. The opposite situation was obtained when the first rAAV delivery by IM injection led to the systematic presence of an anti-rtTA response. To date, we are not able to explain why the mode of delivery of the first injected AAV vector seems to have an impact on the development of an anti-rtTA immune response following the IM delivery of the second AAV vector. Nevertheless our data showed, as in previous studies made in our laboratory10 and elsewhere, that IM injection is frequently associated with severe adverse effects, i.e. immunotoxicity toward the transgene product. Indeed, this was observed against the heterologous proteins β-galactosidase in normal dogs,26 canine FIX in hemophilia dogs,37 or human LPL in LPL-deficient cats.24 In some instances, when high amounts of rAAV1 or rAAV2 were delivered by IM injection, we and others even described the outcome of several cases of autoimmune anemia due to the development of antibodies against the homologous Epo transgene, which in turn also neutralized the endogenous Epo.38,39 More strikingly in this study, among the rAAV-LEA29Y injected animals, Mac 5, an im-injected animal in which the LEA29Y serum level was high (>100 µg/ml; ref. 31), developed neutralizing antibodies against the transgene product LEA29Y itself 2 months after IM injection of rAAV8 (Supplementary Figure S4 and Supplementary Materials and Methods). As for some anti-Epo38,39 and some anti-canine FIX15 responses described earlier, the humoral response against the LEA29Y was also transient.
The most striking result of this study was the ability to achieve persisting Epo regulation with no detectable antibodies against the rtTA transgene product after rAAV RI delivery in naive immunocompetent animals. Obtaining such systematic outcome in outbred NHP is highly significant with respect to both modes of delivery, i.e. antibodies after IM and none after RI injection (Table 2). These results also suggest that an immunosuppressive treatment to circumvent an immune response against the transgene product could be simply unnecessary if the RI route is used to administer the AAV vector.
Whether the relationship between the mode of delivery to the skeletal muscle and the immunotoxicity toward the transgene also applies to the rAAV capsid remains unclear at this stage. Indeed, we showed in Figure 4 that NHP mounted anticapsid antibodies after IM delivery, whereas they remained essentially unresponsive after RI delivery, regardless of the levels of circulating LEA29Y. However, the immunosuppression context generated in these animals makes it difficult to draw any relevant conclusion regarding the host immune response against the rAAV capsid. One strong argument against the potential beneficial effect of RI delivery with respect to the anticapsid response is the fact that the naive immunocompetent ri-administered Mac 19–21 exhibited anti-rAAV1 capsid antibodies detected by enzyme-linked immunosorbent assay at similar concentrations as im-administered Mac 15 and 16 (Table 2).
Several factors can be implicated to explain such a different outcome between IM versus RI rAAV administration. Among them, the vector biodistribution within the target organ as well as distant sites is likely critical. As we recently described, both IM and RI protocols led to similar systemic biodistribution patterns regardless of the serotype.31 In particular, vector genome was detected in all lymphoid organs (spleen, draining and nondraining lymph nodes) and in peripheral blood mononuclear cells.31 Moreover, at euthanasia (between 14 and 34 months p.i.), genome was transcriptionally active in the draining lymph nodes of all animals (data not shown). Regarding the biodistribution of the vector in the liver, we found vector DNA in all animals injected with the AAV1-rtTA/Epo vector. As the expression of rtTA was under the control of a desmin promoter, which can be active in hepatic stellate cells, we further analyzed the liver samples by reverse transcriptase-PCR. We found rtTA transcripts in liver samples after RI delivery and not after IM It is important to note that at the time of liver biopsies (between 15 and 18 months p.i.), the im-injected animals had already developed a destructive immune response that may had targeted the transduced hepatocytes in addition to the genetically modified myocytes. However, with these results and given the fact that expression of a transgene from an AAV vector in liver can promote tolerance to the transgene product,34 we cannot rule out that some hepatic expression contributed to the lack of an immune response in the RI group.
The local in situ distribution of the vector within the skeletal muscle is also likely an important factor. Indeed, we observed that IM delivery resulted in high local vector genome copies per cell at the site of rAAV injection with undetectable levels in the area immediately surrounding the needle track. In contrast, RI delivery resulted in a homogenous vector distribution involving large areas of the muscle and strikingly weaker vector genome copies per cell.31 A similar finding was previously reported in canine skeletal muscle by Arruda et al. for FIX.40
The presence of high local vector concentration following IM administration might increase the number of virions captured by the lymphatic system, and in turn, increase vector input to the draining lymph nodes.16,17 resulting in a facilitated antigen-presenting cell and lymphocyte transduction, and antigen presentation.10,35,41 There are two nonexclusive possibilities for antigen presentation that might initiate the antitransgene immune response: (i) direct transgene product presentation by rAAV-transduced antigen-presenting cell; (ii) indirect cross-presentation following antigen capture by antigen-presenting cell from transgene-overexpressing myofibers.42,43 The cross-presentation pathway has been a favored hypothesis following in vivo rAAV administration because of low efficiency of dendritic cell transduction.44 That being said, in both pathways, high local myofiber transduction following IM delivery might increase antigen recognition and subsequent destruction of myofibers by CD8+ CTL.44,45 At the same time, high local transduction following IM injection might favor antigen recognition by increasing the likelihood of peptide presentation to antigen-presenting cell,45 while promoting misfolding and/or aberrant post-translation modifications.46,47
Another mechanistic scenario could relate to the detection of MHC-I molecules restricted to the surface of overexpressing transgene myofibers after IM delivery, whereas in the ri-transduced muscles, we were not able to detect any MHC-I expression. Our data show that in NHPs overexpressing transgene myofibers exhibit detectable MHC-I molecules on their cell surface. Similarly, in a recent clinical trial in which an AAV1 vector was injected intramuscularly in LGMD2D patients, the expression of MHC-I molecules on sarcolemma of most muscle fibers on the side of upregulated transgene expression was shown.7 MHC-I is a prerequisite for antigen-specific T-cell-mediated cytotoxicity.48 Accordingly, we found a significant number of CD8+ T cells restricted to the transduced area after IM injection. Another hypothesis raised by MHC-I detection on myofibers is the possible involvement of these myofibers themselves in antigen presentation to CD8+ T cells. This hypothesis still needs to be documented, but could provide an additional insight into the mechanisms involved in the induction of the host immune response following rAAV IM delivery.
Another original finding in this study is the detection of the complement-derived MAC restricted to the overexpressing transgene myofibers after rAAV IM delivery, whereas it remained undetectable in the ri-transduced muscles. This also provides an additional insight into the mechanisms involved in the host immune response after rAAV IM delivery. Indeed, this finding strongly suggests that the destruction of transduced myofibers after IM delivery might be due in part to a MAC-dependent lysis. A recent study reported the interaction of iC3b with rAAV capsids, without subsequent complement activation,49 which also showed in mice that complement had a stimulatory effect on anti-rAAV B-cell responses.49 In our study, the deposition of MAC at the surface of rAAV-LEA29Y im-transduced myofibers revealed a complement activation. We do not know whether the classical and/or the alternative pathways are implicated in this activation; however, it is likely that the classical pathway is involved. As this pathway depends on the presence of antigen–antibody immune complexes, the deposition of MAC at the surface of rAAV-LEA29Y-transduced myofibers in macaques that did not show antibodies against the LEA29Y transgene product (data not shown) could suggest that the antigen involved is the rAAV capsid.
Altogether, high local vector delivery and high local transgene product concentration appear to be key factors in triggering the host immune response against the transgene after rAAV IM delivery. This involves MHC-I expression as well as complement-derived MAC complex deposition strictly restricted to the overexpressing transgene product myofibers. In contrast, when transgene overexpression is avoided and homogeneously distributed within the skeletal muscle, which is the case after RI delivery, the rtTA transgene product is not recognized by the host, resulting in persisting Dox-mediated regulation. Although immune tolerance or ignorance with respect to the rtTA antigen in this context still needs to be analyzed, this study demonstrates that RI delivery is not associated with immunotoxicity. It is critical to investigate the primary mechanism(s) involved, but yet the lack of immunotoxicity against the transgene product, combined with the muscle transduction pattern previously published,31 make the RI route of administration an attractive procedure for rAAV-mediated gene transfer to patients skeletal muscle.
Materials and Methods
Vector production. The LEA29Y vector plasmid was generated as described recently.31 Briefly, this vector contained the constitutive Rous sarcoma virus promoter (pRSV), a chimeric intron, the coding sequence of the immunosuppressive molecule LEA29Y, the WPRE, and the SV40 polyadenylation signal (SV40 pA) (Figure 1a). The rtTA/Epo vector plasmid contained (i) the cynomolgus macaque Epo (cmEpo) complementary DNA under the control of the Dox-inducible TetO-cytomegalovirus promoter, and (ii) an expression cassette encoding the reverse transactivator protein (rtTA-M2) under the control of the muscle-specific 1-kb human desmin promoter.50 The two cassettes were cloned in the same direction (forward orientation)27 (Figure 1b). Recombinant AAV were manufactured as described elsewhere27 and purified by cesium chloride density gradients followed by extensive dialysis against phosphate-buffered saline.
Animals. Experiments were conducted on 3–5 kg male captive-bred cynomolgus macaques purchased from BioPrim (Baziège, France). The Institutional Animal Care and Use Committee of the University of Nantes (France) approved the protocol. We selected primates that had no detectable neutralizing antibody against AAV serotypes 1 and 8 in the serum. All blood samples were collected under ketamine-induced anesthesia (10 mg/kg). Surgical biopsies were performed under ketamine (8 mg/kg)/metedomidine (40 µg/kg)–induced anesthesia, and marbofloxacin and meloxicam were administered orally for the following 3–5 days to avoid discomfort for the animal.
Vectors administration. The IM and RI delivery of rAAV were conducted as described previously.31 Eight animals, corresponding to Mac 1–8 (described in ref. 31), were studied first. They received either the rAAV1-LEA29Y vector or the rAAV8-LEA29Y vector, administered by IM or RI injection (Table 1). To prevent an eventual immune response against the human LEA29Y, each macaque was immunosuppressed with 40 mg/kg/day of mycophenolate mofetil and 5 mg/day of prednisone, both orally administered, during the first 3 weeks after rAAV administration. These eight macaques received an AAV-rtTA/Epo vector by IM injection 9 months (Mac 1 and 2), 4 months (Mac 3–6 and 8), or 3 months (Mac 7) after the injection of the rAAV-LEA29Y (Table 1). In the last part of the study, seven animals, corresponding to Mac 13–16 (described in ref. 27), and to Mac 19–21 were studied. They received the rAAV1-rtTA/Epo vector administered either by IM or RI injection (Table 2).
Detection of circulating LEA29Y. Serum LEA29Y levels were measured using a sandwich enzyme-linked immunosorbent assay, as recently described.31
Immunohistochemical analysis. This analysis was carried out as described recently,31 using antibodies directed against CTLA4 (Santa Cruz Biotechnology, Heidelberg, Germany), CD3 (University of California, Berkeley, CA), CD4 (Abcam, Cambridge, UK), CD8 (Abd Serotec, Dusseldorf, Germany), CD79a (Dako, Glostrup, Denmark), MHC-I (Abcam, Paris, France), or MAC (anti-C5b9; Abcam, Paris, France).
In situ hybridization. The 480-bp WPRE sequence was subcloned in pSPT-18 vector (Roche Applied Science, Meylan, France). Antisense and sense riboprobes were obtained after plasmid linearization with either HindIII or EcoRI (New England Biolab's, Saint Quentin Yvelines, France), respectively, and labeled according to the manufacturer's instructions after in vitro transcription with either T7 or SP6 polymerase using a digoxigenin labeling kit (DIG RNA Labelling Kit; Roche Applied Science). All steps before and during hybridization were conducted under RNase-free conditions. Muscle samples were frozen in isopentane cooled with liquid nitrogen and serially sectioned to 12-µm thick. After drying, sections were postfixed in 4% paraformaldehyde, pretreated at 98 °C for 10 minutes in a 1× sodium citrate buffer (Dako). Following prehybridization for 60 minutes at 42 °C in a hybridization buffer containing 50% deioned formamide, 10% (w/v) dextran sulfate, 4× standard saline citrate, 1× Denhardt's solution, 10× blocking solution (Roche Applied Science) and 425 µg/ml salmon fish DNA; sections were hybridized overnight at 42 °C with 1 µg/ml of antisense probes diluted in hybridization buffer. Negative controls were conducted by the substitution of sense for antisense probes or by the omission of antisense probes in the hybridization solution. Then slides were washed with 2× standard saline citrate, treated 20 minutes at 37 °C with 5 µg/ml RNaseA in NaCl–Tris–EDTA buffer (0.5 mol/l NaCl, 10 mmol/l Tris–HCl, 1 mmol/l EDTA, pH 8.0). After stringent washing for 30 minutes with 50% (v/v) formamide/0.2× standard saline citrate at 37 °C, sequential washes were performed with 2× standard saline citrate (10 minutes × 2 times), buffer 1 (0.1 mol/l Tris, 0.15 NaCl, pH 7.5) containing 5% blocking reagent (Roche Applied Science) for 30 minutes, and buffer 1 containing 1% bovine serum albumin and 0.3% Triton X-100 for 30 minutes. Following incubation during 2 hours at room temperature with sheep anti-DIG antibody conjugated with alkaline phosphatase (Roche Applied Science) diluted to 1:1,000 in buffer 1, slides were sequentially washed with buffer 1 (5 minutes × 3 times) and buffer 2 (0.1 mmol/l Tris–HCl, 0.1 mol/l NaCl, 50 mmol/l MgCl2, pH 9.5) for 10 minutes. Alkaline phosphatase substrate (NBT/BCIP; Roche Applied Science) was then applied for 2 hours. Finally, after washing, slides were counterstained with fast green for 10 seconds, dehydrated, and mounted.
Anti-AAV IgG-specific antibody detection by enzyme-linked immunosorbent assay. Circulating IgG antibodies against AAV capsid were detected using enzyme-linked immunosorbent assay. Plates were coated overnight at 4 °C with purified particles of rAAV1 or 8 (109 vector genomes/well); blocked for 2 hours at 37 °C with phosphate-buffered saline containing 0.1% Tween and 1% gelatin; incubated for 2 hours at 37 °C with six serial threefold dilutions of thawed macaque sera (a positive control serum known to contain anti-AAV-neutralizing antibodies was assayed); incubated for 1 hour at 37 °C with biotin-conjugated antihuman IgG (γ-chain-specific) at 0.1 µg/ml (Jackson ImmunoResearch Laboratories, West Grove, PA); incubated for 1 hour at 37 °C with horseradish peroxidase–conjugated streptavidin (Vector Laboratories, Burligame, CA) at 1 µg/ml. The reaction was developed using 2.2-azino-di-3-ethylbenthiazolinsulfunat-6 (Roche Applied Science) and absorbance of duplicate samples was read at 405 nm (MRX; Dynatech, Chantilly, VA). Sample anti-IgG antibody titers were assigned at the highest dilution where the average of the duplicates was higher than the average of serum before rAAV-injection ± 2SD.
In vivo cmEpo regulation analysis. Dox (Ratiopharm, Ulm, Germany) was given intravenously (20 mg/kg the first day, then 10 mg/kg). The induction protocol started 2 months after rAAV-rtTA/Epo delivery and consisted of 3-day Dox administration repeated once every month, as described in ref. 33. Serum cmEpo levels were measured by enzyme-linked immunosorbent assay (Quantikine IVD kit; R&D Systems, Lille, France). Detection of anti-rtTA-M2 antibodies was conducted as previously described10 by western blot analysis.
PCR analysis on liver biopsies. Total DNA was extracted from liver biopsies as previously described.10 DNA (750 ng) was used as a template for the PCR reactions. The 5′ primer (5′-AGGAGCATCAAGTAGCAAAAGAG-3′) and the 3′ primer (5′-AAGAGCGTCAGCAGGCAGCA-3′) were both within the rtTA sequence. We used Ampli Tag Gold DNA polymerase (Applied Biosystems, Courtaboeuf, France) and a GeneAmp PCR System 9700 thermocycler (Applied Biosystems). The PCR cycle was as follows: initial denaturation at 95 °C for 5 minutes followed by 40 cycles at 94 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds, and final extension at 72 °C for 10 minutes. A control reaction was performed to detect the cynomolgus glyceraldehyde-3-phosphate deshydrogenase sequence, as previously described.31 Amplified products were analyzed using agarose gel electrophoresis and ethidium bromide staining.
Reverse transcriptase-PCR analysis on liver biopsies. Total RNA was extracted from liver biopsies with TRIzol reagent (Invitrogen, Cergy Pontoise, France) and treated with RNAse-free DNaseI from the TURBO DNA-free kit (Ambion, Applied Biosystems) according to the manufacturer's instructions. Reverse transcription was performed on 1 µg of RNA using an oligo dT primer (Roche Applied Science) and an M-MLV Reverse transcriptase (Invitrogen, Cergy Pontoise, France), for 1 hour at 37 °C followed by an inactivation step at 65 °C for 30 minutes. For each sample, the DNA contamination was checked by performing the same reaction without reverse transcriptase. A 2 µl of complementary DNA was used as a template for the PCR. The PCR specific for the rtTA sequence was conducted as described above. A control reaction was performed to detect the hypoxanthine guanine phosphoribosyltransferase complementary DNA (5′-CACCAGCAAGCTTGCGACC-3′/5′-TGCTGGATTACATCAAAGCAC-3′). The PCR cycle was as follows: initial denaturation at 95 °C for 5 minutes followed by 40 cycles at 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 1 minute, and a final extension at 72 °C for 10 minutes. Amplified products were analyzed using agarose gel electrophoresis and ethidium bromide staining.
SUPPLEMENTARY MATERIALFigure S1. Effect of LEA29Y on T-cell-dependent humoral immune response in cynomolgus monkeys. SRBC-specific IgG antibody response analyzed by flow cytometry 3 weeks after IV immunization. The fold change in titer was calculated using the mean of serum at each time-point divided by the mean of serum obtained just before immunization. Mac 1-8: rAAV-LEA29Y-injected macaques. Controls: average (+/− SD) of 3 healthy immunized macaques.Figure S2. Effect of LEA29Y on cellular immune responses in cynomolgus monkeys. Mac1-8 and 4 healthy macaques received skin allografts from MHC-II histoincompatible healthy donors. Kaplan-Meier analysis of graft survival showed a significant prolongation in rAAV-LEA29Y injected vs control macaques (p<0.03).Figure S3. In situ hybridization of rAAV-LEA29Y-injected muscles 5 weeks after rAAV delivery. (a, c) rAAV1-IM-injected muscle (cryosection obtained at the injection site from Mac 1) and (b, d) rAAV1-RI–injected muscle (cryosection obtained in the gastrocnemius from Mac 4). (a, b) In situ hybridization with an antisense WPRE probe and (c, d) in situ hybridization with a sense WPRE probe. Scale bar = 100μm.Figure S4. Kinetics of serum LEA29Y and anti-LEA29Y antibody levels in rAAV8-IM-injected Mac 5. Black curve represent LEA29Y levels. Anti-LEA29Y antibodies are shown with a grey bar at the time-point they were determined.Materials and Methods.
Supplementary Material
Effect of LEA29Y on T-cell-dependent humoral immune response in cynomolgus monkeys. SRBC-specific IgG antibody response analyzed by flow cytometry 3 weeks after IV immunization. The fold change in titer was calculated using the mean of serum at each time-point divided by the mean of serum obtained just before immunization. Mac 1-8: rAAV-LEA29Y-injected macaques. Controls: average (+/− SD) of 3 healthy immunized macaques.
Effect of LEA29Y on cellular immune responses in cynomolgus monkeys. Mac1-8 and 4 healthy macaques received skin allografts from MHC-II histoincompatible healthy donors. Kaplan-Meier analysis of graft survival showed a significant prolongation in rAAV-LEA29Y injected vs control macaques (p<0.03).
In situ hybridization of rAAV-LEA29Y-injected muscles 5 weeks after rAAV delivery. (a, c) rAAV1-IM-injected muscle (cryosection obtained at the injection site from Mac 1) and (b, d) rAAV1-RI–injected muscle (cryosection obtained in the gastrocnemius from Mac 4). (a, b) In situ hybridization with an antisense WPRE probe and (c, d) in situ hybridization with a sense WPRE probe. Scale bar = 100μm.
Kinetics of serum LEA29Y and anti-LEA29Y antibody levels in rAAV8-IM-injected Mac 5. Black curve represent LEA29Y levels. Anti-LEA29Y antibodies are shown with a grey bar at the time-point they were determined.
Acknowledgments
We thank the personnel at the Boisbonne Centre (large animal facility) in Nantes, the Vector Core (www.vectors.nantes.inserm.fr) at the University Hospital of Nantes for providing the rAAV1 and rAAV8 stocks, Hermann Bujard for providing the purified tTA2-His6 recombinant protein, and Mark E. Haskins for manuscript review. Financial supports came from the INSERM, the INRA, the University Hospital of Nantes, the Fondation pour la Thérapie Génique en Pays de Loire, CLINIGENE (a European Network of Excellence) and the AFM (Association Française contre les Myopathies) Award #13063. This work was done in Nantes, France.
REFERENCES
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 αl-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores α-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl D, Salvetti A, Moullier P., and , Heard JM. Control of erythropoietin delivery by doxycycline in mice after intramuscular injection of adeno-associated vector. Blood. 1998;92:1512–1517. [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Chérel Y, Chenuaud P, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- Wu H, Reding M, Qian J, Okita DK, Parker E, Lollar P, et al. Mechanism of the immune response to human factor VIII in murine hemophilia A. Thromb Haemost. 2001;85:125–133. [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F., and , Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Wang L, Dobrzynski E, Schlachterman A, Cao O., and , Herzog RW. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Lin J, Calcedo R, Vandenberghe LH, Figueredo JM., and , Wilson JM. Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum Gene Ther. 2008;19:663–669. doi: 10.1089/hum.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J., and , Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CJ, Twisk J, Meulenberg JM, Liu G, van den Oever K, Moraal E, et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL(S447X) beneficial mutation. Hum Gene Ther. 2004;15:906–919. doi: 10.1089/hum.2004.15.906. [DOI] [PubMed] [Google Scholar]
- Bohl D, Bosch A, Cardona A, Salvetti A., and , Heard JM. Improvement of erythropoiesis in β-thalassemic mice by continuous erythropoietin delivery from muscle. Blood. 2000;95:2793–2798. [PubMed] [Google Scholar]
- Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Joussemet B, Bujard H, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL., and , Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Belatacept Study Group et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- Toromanoff A, Chérel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME, et al. Safety and efficacy of regional intravenous (RI) versus intramuscular (IM) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobertm E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Loduca PA, Hoffman BE., and , Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Conlon TJ, Poirier A, Campbell-Thompson M., and , Byrne BJ. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum Gene Ther. 2007;18:245–256. doi: 10.1089/hum.2006.113. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA., and , Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N, et al. Autoimmune anemia in macaques following erythropoietin gene therapy. Blood. 2004;103:3303–3304. doi: 10.1182/blood-2003-11-3845. [DOI] [PubMed] [Google Scholar]
- Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B, et al. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood. 2004;103:3300–3302. doi: 10.1182/blood-2003-11-3852. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Provost N, Blouin V, Blancho G, Chérel Y, Salvetti A, et al. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol Ther. 2001;4:559–566. doi: 10.1006/mthe.2001.0494. [DOI] [PubMed] [Google Scholar]
- Kurts C, Miller JF, Subramaniam RM, Carbone FR., and , Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR., and , Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Jooss K, Yang Y, Fisher KJ., and , Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O., and , Jooss K. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J Virol. 2001;75:269–277. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Hagstrom JN, Deitch J, Heiman-Patterson T, Camire RM, Chu K, et al. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001;97:130–138. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, de Ceaurriz J, Larcher T, Moullier P., and , Chenuaud P. “Genetic Doping” with erythropoietin cDNA in primate muscle is detectable. Mol Ther. 2004;10:409–410. doi: 10.1016/j.ymthe.2004.07.024. [DOI] [PubMed] [Google Scholar]
- McMichael AJ. HLA restriction of human cytotoxic T cells. Springer Semin Immunopathol. 1980;3:3–22. doi: 10.1007/BF00199923. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM, et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZL., and , Paulin D. High level desmin expression depends on a muscle-specific enhancer. J Biol Chem. 1991;266:6562–6570. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of LEA29Y on T-cell-dependent humoral immune response in cynomolgus monkeys. SRBC-specific IgG antibody response analyzed by flow cytometry 3 weeks after IV immunization. The fold change in titer was calculated using the mean of serum at each time-point divided by the mean of serum obtained just before immunization. Mac 1-8: rAAV-LEA29Y-injected macaques. Controls: average (+/− SD) of 3 healthy immunized macaques.
Effect of LEA29Y on cellular immune responses in cynomolgus monkeys. Mac1-8 and 4 healthy macaques received skin allografts from MHC-II histoincompatible healthy donors. Kaplan-Meier analysis of graft survival showed a significant prolongation in rAAV-LEA29Y injected vs control macaques (p<0.03).
In situ hybridization of rAAV-LEA29Y-injected muscles 5 weeks after rAAV delivery. (a, c) rAAV1-IM-injected muscle (cryosection obtained at the injection site from Mac 1) and (b, d) rAAV1-RI–injected muscle (cryosection obtained in the gastrocnemius from Mac 4). (a, b) In situ hybridization with an antisense WPRE probe and (c, d) in situ hybridization with a sense WPRE probe. Scale bar = 100μm.
Kinetics of serum LEA29Y and anti-LEA29Y antibody levels in rAAV8-IM-injected Mac 5. Black curve represent LEA29Y levels. Anti-LEA29Y antibodies are shown with a grey bar at the time-point they were determined.