Abstract
Short hairpin RNAs (shRNAs) have emerged as a novel therapeutic modality, but there is increasing concern over nonspecific effects in vivo. Here, we used viral vectors to express shRNAs against endogenous p53 in livers of conditional MYC-transgenic mice. As expected, the shRNAs silenced hepatic p53 and accelerated liver tumorigenesis when MYC was concurrently expressed. Surprisingly, various irrelevant control shRNAs similarly induced a rapid onset of tumorigenesis, comparable to carbon tetrachloride (CCl4), a potent carcinogen. We found that even marginal shRNA doses can already trigger histologically detectable hepatoxicity and increased hepatocyte apoptosis. Moreover, we noted that shRNA expression globally dysregulated hepatic microRNA (miRNA) expression, and that shRNA levels and activity further increased in the presence of MYC. In MYC-expressing transgenic mice, the marginal shRNA-induced liver injury sufficed to further stimulate hepatocellular division that was in turn associated with markedly increased expression of the mitotic cyclin B1. Hence, even at low doses, shRNAs can cause low-level hepatoxicity that can facilitate the ability of the MYC oncogene to induce liver tumorigenesis. Our data warrant caution regarding the possible carcinogenic potential of shRNAs when used as clinical agent, particularly in circumstances where tissues are genetically predisposed to cellular transformation and proliferation.
Introduction
The MYC oncogene is known to play an important role in the pathogenesis of hepatocellular carcinoma (HCC).1,2 MYC overexpression is thought to exert its neoplastic function by inducing autonomous cellular proliferation and growth, blocking differentiation, and causing genomic destabilization.3,4,5,6,7 Previously, we have described the use of the tetracycline regulatory system to develop a conditional transgenic mouse model of MYC-induced HCC.8,9,10,11 Using this model system, we have found evidence suggesting that MYC overexpression is restrained from inducing tumorigenesis in adult—but not in embryonic or neonatal—mice, in part, through a p53-regulated mechanism.8,12
Here, we explored the role of p53 in liver tumorigenesis by using RNA interference (RNAi) to suppress hepatic p53 expression in adult MYC-transgenic mice. We therefore engineered potent adeno-associated virus serotype-8 (AAV-8) vectors that are known to be highly efficient in murine liver13,14,15,16,17,18,19 to encode and deliver anti-p53 short hairpin RNAs (shRNAs). To maximize efficacy, we used self-complementary AAVs carrying double-stranded (ds) DNA genomes. During vector production, their replication arrests after a single cycle, leading to insert duplication and packaging as an inverted tandem. In transduced cells, the two copies efficiently and rapidly self-anneal, yielding markedly increased transgene expression as compared to standard single-stranded AAV vectors. The specific vector plasmid backbone we used here has been optimized with respect to stability in bacteria, by flanking the recombinant genome with packaging signals derived from two distinct AAV genotypes (AAV-2/-4; D. Grimm, L.S. Wang, J.S. Lee, T.A. Storm, and M.A. Kay, unpublished results). All vectors used in this study were bicistronic, with one cistron encoding the shRNA under a strong U6 promoter and the other a gfp transduction marker.
We previously had used our new dsAAV vector backbone to express 49 distinct shRNAs against numerous targets in murine livers, from reporter genes to hepatitis B virus.16 The in vivo vector efficacy was very high, yielding complete liver transduction from single peripheral infusion of medium AAV doses (2 × 1011/mouse). Notably, shRNA expression frequently resulted in hepatoxicity and, at high doses (1 × 1012), in mortality. We had concluded that high-level shRNA expression can saturate the cellular RNAi machinery [especially exportin-5 (ref. 16)], disrupting microRNA (miRNA) functions, causing cytotoxicity and ultimately liver failure. Survival rates were higher at lower doses, but RNAi was often only transient. In these cases, we found that local cell death had induced division of adjacent cells, causing liver repopulation with shRNA-negative hepatocytes (AAV vectors persist episomally and are lost in mitosis). Still, judicious shRNA selection, coupled with low-vector doses (up to 2 × 1011) and use of a minimal liver-specific promoter allowed us to stably block viral gene expression in hepatitis B virus–transgenic mice, in the absence of phenotypically detectable hepatoxicity.16,20 We also recently used our dsAAVs to achieve safe, efficient, and stable suppression of various endogenous genes encoding fatty acid transporters in livers of adult mice.21
Here, we report that even marginal, peripherally delivered shRNA doses can yield drastic phenotypes in the adult liver. In MYC-expressing mice, various unrelated shRNAs caused global miRNA dysregulation and low-level cytotoxicity, that in turn promoted unrestrained hepatocyte proliferation and accelerated liver tumorigenesis. Our data imply a dual functional role for MYC, by transactivating U6-shRNA expression in AAV-transduced hepatocytes, and stimulating proliferation of adjacent cells. Our study reveals a previously unknown general carcinogenic property of shRNAs in vivo. Thus they warrant further caution in the use of these promising biotherapeutics in humans.
Results
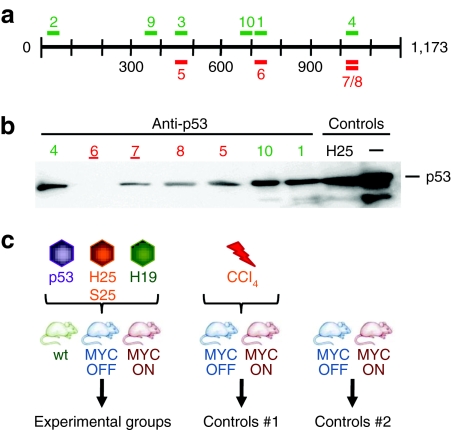
We designed and screened a panel of 10 different shRNAs for efficacy at p53 knockdown in vitro, before engineering the best two candidates into dsAAV-8 vectors (Figure 1a,b).13,14,15,16,17,18,19 For their in vivo evaluation, we then injected the following cohorts of mice (Figure 1c): first, we infused either normal or MYC-transgenic mice with each vector at three distinct particle doses, 1012 (high), 2 × 1011 (mid), or 2 × 1010 (low). The two higher doses previously had been found to be sufficient to yield complete liver transduction.16 Among the infected MYC-transgenic mice, MYC was induced in one cohort and kept inactive in controls (MYC ON or OFF, respectively). To account for nonspecific shRNA effects, we also infused three AAV/shRNA vectors targeting genes irrelevant for HCC. Two of the vectors served as positive controls for adverse side effects, by expressing shRNAs known from our prior work to be highly toxic [25 mer against human α-1-antitrypsin (hAAT-25) or against hepatitis B virus surface antigen (sAg-25)], whereas the third expressed a nontoxic anti-hAAT 19 mer.16 As a positive control for liver tumorigenesis, we injected MYC ON or OFF mice with carbon tetrachloride (CCl4), a known and potent carcinogen. Finally, we also monitored noninjected control MYC transgenics in which MYC was either overexpressed or kept inactive (Figure 1c).
Figure 1.
Selection of anti-p53 shRNAs. (a) Illustration of the murine p53 mRNA (1,173 nt) and the location of the 10 different anti-p53 shRNAs used in this study. Colors indicate 19 mers (light) or 21 mers (dark). (b) Representative p53 western blot analysis from Huh-7 cells co-transfected with a p53 expression plasmid and a subset of the anti-p53 constructs (shRNAs 2, 3, and 9 were comparable to the other shown 19 mers). The two best shRNAs (6 and 7, both 21 mers) are underlined; they were later packaged into AAV-8 for analyses in mice. Control cells were co-transfected with the p53 plasmid and an unrelated shRNA (hAAT-25) or pBlueScript (last lane). (c) Injection schemes (see text for details). H19, hAAT-19 shRNA; H25, hAAT-25 shRNA; hAAT, human α-1-antitrypsin; nt, nucleotide; p53, anti-p53 shRNAs #6/7; S25, sAg-25 shRNA; sAg, surface antigen; shRNA, short hairpin RNA.
We observed that the high- and mid-anti-p53 vector doses were lethal in all mice across all cohorts, regardless of MYC status. This was consistent with our previous findings of frequent morbidity from shRNA overdosing in murine livers in our recent study,16 where about 50% of all tested shRNAs had killed the animals within a few weeks after injection. Gross and histological analyses of all major organs in ailing mice injected with the high- or mid-p53 shRNA dose revealed that the main changes were in the liver, with chronic hepatoxicity characterized by lobular collapse and multifocal necrosis, likewise consistent with our previous observations.16 At necropsy, there was no evidence for liver tumorigenesis in these mice (data not shown).
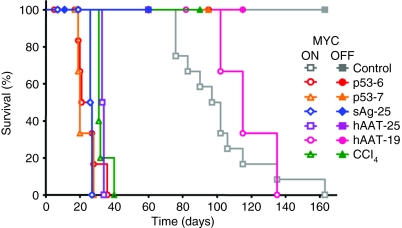
The outcome of infusion at the low shRNA vector dose (2 × 1010) was markedly different, depending on both shRNA as well as MYC status (comprehensively summarized in Supplementary Table S1). In MYC OFF mice, AAV/anti-p53 infusion was not associated with evidence of toxicity or lethality. We also observed no morbidity in MYC OFF mice treated with any of the controls (hAAT/HBV-shRNAs or CCl4) (Figure 2). In contrast, infusion of MYC ON mice with the anti-p53 shRNAs (#6 and 7 gave similar results) induced the development of liver cancers and mortality at a substantially accelerated onset, comparable to CCl4 (Figure 2). At first, our results seemed to support the predicted role for p53 in MYC-induced HCC formation. Yet, we also noted identical phenotypes in two other MYC ON groups, namely, the controls infused with low doses of the p53-unrelated toxic sAg-25 or hAAT-25 shRNA vectors. Thus, four independent shRNAs (only two against p53) and one chemical carcinogen all induced comparable accelerated tumorigenesis and high mortality in the presence of MYC. In contrast, MYC ON animals treated with the nontoxic hAAT-19 shRNA developed tumors at a slow rate and survived longer, similar to naive MYC ON mice (gray/pink open symbols in Figure 2). The data discussed below are based on the lowest dsAAV vector dose.
Figure 2.
shRNAs accelerate the onset of MYC-induced HCC. Shown are Kaplan–Meier survival curves of adult MYC ON or OFF mice injected through their tail vein with AAV-8 encoding the indicated shRNAs. Also shown are survival curves of noninjected or CCl4-treated adult MYC ON or OFF mice. Cohorts consisted of 5–10 mice. Mice were euthanized when moribund with tumor burden. MYC transgene expression was activated by doxycycline removal from the drinking water on the day of AAV-8/shRNA injection. AAV-8, adeno-associated virus serotype-8; CCl4, carbon tetrachloride; hAAT, human α-1-antitrypsin; HCC, hepatocellular carcinoma; sAg, surface antigen; shRNA, short hairpin RNA.
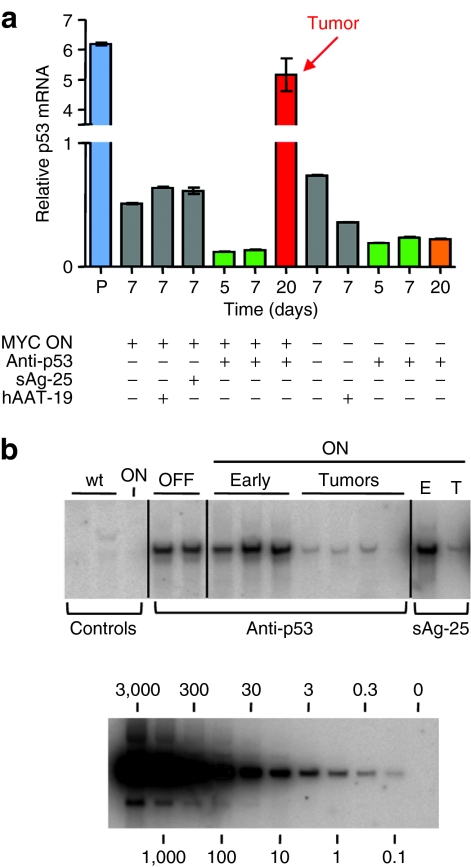
Next, we characterized our various cohorts on a molecular, gross, and histological level. First, we assayed p53 expression and AAV vector copy numbers (Figure 3a,b). We noted an expected rapid and substantial p53 suppression following infusion of the anti-p53 vectors, compared to untreated mice or control shRNAs (Figure 3a). Yet, in the presence of MYC, p53 mRNA levels consistently increased again at later timepoints (arrow in Figure 3a), concurrent with HCC formation and morbidity. We inferred that this loss of p53 suppression resulted from elimination of the episomal anti-p53 shRNA-encoding AAV vectors from the rapidly dividing tumor cells. Our results were confirmed by Southern blot analyses of AAV vector copy numbers in total liver DNA (Figure 3b). An identical gradual loss of vector DNA was also seen with the toxic control shRNAs (sAg-25 and hAAT-25; Figure 3b and data not shown).
Figure 3.
Molecular analyses of AAV/shRNA-injected livers. (a) SYBR Green qRT-PCR analysis of p53 mRNA expression (mean ± SD), demonstrating reduction of p53 mRNA in both MYC ON and MYC OFF livers 5 and 7 days after p53 shRNA injection. Levels of p53 remained low in MYC OFF/p53 shRNA livers even 20 days after injection, whereas tumorigenesis in the MYC ON/p53 shRNA livers was associated with a significant p53 mRNA elevation. Lane P is a p53 plasmid-injected mouse liver used as positive control. (b) Southern blot analysis using a probe against a region conserved in all vectors. AAV DNA copy numbers (top panel) were high after initial injection of anti-p53 shRNA in MYC OFF livers (lanes “OFF”, each lane is an individual mouse) and MYC ON livers (lanes “ON Early”), as well as in MYC ON/sAg-25 livers (lane “ON E”). MYC ON/anti-p53 shRNA liver tumors (lanes “ON Tumors”) contained very little if any AAV DNA copies, identical to MYC ON/sAg-25 tumors (lane “ON T”). Lanes “Controls wt” are age-matched wild-type murine livers, and lane “Controls ON” is a noninjected MYC ON liver. The bottom panel shows a standard curve obtained using serial dilutions of the parental AAV/shRNA vector plasmid. AAV, adeno-associated virus; hAAT, human α-1-antitrypsin; qRT, quantitative real-time; sAg, surface antigen; shRNA, short hairpin RNA.
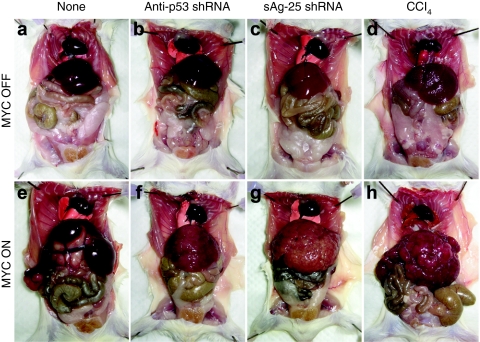
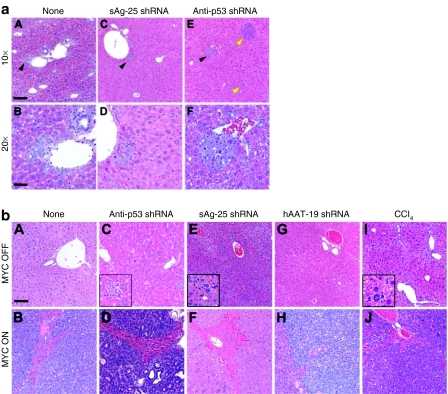
On a gross level, we could distinguish three distinct phenotypes among the different cohorts of mice. A first group comprised MYC ON mice treated with the anti-p53 shRNA vectors: 3–4 weeks after injection, these animals developed 2.5–3.1-fold enlarged livers that exhibited diffuse carcinoma consisting of a multitude of coalescing tumor nodules (Figure 4f, compare to panels a,b). Similarly, MYC ON mice injected with the toxic control shRNAs also rapidly developed tumors that strikingly resembled those in the anti-p53 groups [Figure 4g, compare to panel f; results for hAAT-25 were similar (data not shown)]. Moreover, MYC ON/CCl4 tumors were also grossly similar to shRNA-induced HCC (Figure 4h, compare to panels f,g). All these tumors were distinct in appearance from the focal tumors that were instead typical for the second group, which comprised naive and hAAT-19-injected MYC ON mice. Tumors in this second category also developed with a much greater latency of up to 5 months (Figure 4e and data not shown). Finally, a third group of unique phenotypes was seen in MYC OFF mice, in which neither CCl4, nor any of the vectors caused tumors (Figure 4b,c,d and data not shown). Untreated control MYC OFF mice also remained entirely tumor-free and their livers appeared grossly normal during the duration of the experiment (5 months) (Figure 4a).
Figure 4.
Cooperation of shRNAs with MYC to induce HCC. Top: representative MYC OFF mice that were (a) untreated (control for normal liver morphology), (b) injected with AAV/p53 shRNA, (c) injected with AAV/sAg-25 shRNA, or (d) repeatedly treated with CCl4. Bottom: MYC ON mice under the same conditions. Note the similar formation of diffuse HCC in the (f,g) AAV/shRNA and (h) CCl4 control groups. MYC induction alone did not cause tumors during this timeframe (3–4 weeks for all other groups); instead, tumors developed with a latency of up to 5 months and moreover had a (e) unique and distinct focal appearance. AAV, adeno-associated virus; CCl4, carbon tetrachloride; HCC, hepatocellular carcinoma; sAg, surface antigen; shRNA, short hairpin RNA.
Similarly, phenotypes specific for MYC, shRNA, and time postinjection were observed upon histological examination of all livers. MYC ON mice injected with anti-p53 or sAg-25 shRNAs were again comparable and exhibited rapid onset of hepatic microcarcinomas as early as 1 week after vector delivery (Figure 5a, panels C–F). Importantly, 46% found in MYC ON/anti-p53, and 70% in MYC ON/sAg-25 livers were located adjacent to periportal areas of the liver lobule (Figure 5a, panels C–F). At the later onset of morbidity, both cohorts developed diffuse HCC (Figure 5b, panels D, F) similar to the MYC ON/CCl4 control group (Figure 5b, panel J). In contrast, untreated MYC ON mice also developed 93% of microcarcinomas near the portal triad region (Figure 5a, panels A, B), but only after a much longer latency period, as did MYC ON mice injected with the nontoxic hAAT-19 shRNA (data not shown). Moreover, tumors in naive and hAAT-19-treated MYC ON mice were multifocal and thus histologically distinct from the diffuse HCC in the other groups (anti-p53, sAg-25, CCl4) (Figure 5b, panels B, H versus D, F, J). Collectively, our results illustrated that several unrelated shRNAs cooperated with MYC to induce the rapid onset of tumorigenesis with similar pathology and histology, comparable to a potent carcinogen (see also Supplementary Table S1).
Figure 5.
AAV/shRNA treatment induces characteristic histological phenotypes in mice. (a) HCCs generally arise from the periportal region of the liver. Shown are representative examples for microcarcinomas that developed in periportal liver regions (as opposed to a central vein region) in untreated MYC ON mice (black arrowhead, panel A), in sAg-25 shRNA-injected mice (panel C), or in anti-p53 shRNA-treated mice (panel E). The yellow arrows in panel E highlight sporadic HCC development in other regions of the liver. Higher magnifications of panels A, C, and E (bar = 50 µm) are shown in panels B, D, and F, respectively (bar = 100 µm). (b) Toxic shRNAs and CCl4 promote characteristic diffuse HCC. Top: representative liver histologies from MYC OFF mice that were (A) untreated (control), (C) injected with AAV/anti-p53 shRNA (inner panel shows an enlarged view of pleocellular inflammation), (E) injected with AAV/sAg-25 shRNA (inner panel shows an enlarged view of centrilobular microvesicular lipidosis), (G) injected with AAV/hAAT-19 shRNA (normal liver structure), or (I) repeatedly treated with CCl4 (inner panel shows an enlarged view of microvesicular lipidosis). Bottom: MYC ON mice under the same conditions. Note the multifocal liver tumors in (B) nontreated adult mice as well as in the (H) group injected with hAAT-19. In contrast, infusion of AAV expressing the (D) anti-p53 or (F) sAg-25 shRNA caused diffuse liver tumors, identical to treatment with (J) CCl4. Bar = 80 µm. AAV, adeno-associated virus; CCl4, carbon tetrachloride; HCC, hepatocellular carcinoma; sAg, surface antigen; shRNA, short hairpin RNA.
Like in MYC ON mice, we also could distinguish the effects of toxic and nontoxic shRNAs in the vector-treated MYC OFF cohorts. Mice injected with the nontoxic hAAT-19 shRNA maintained a histologically normal liver, as did untreated control MYC OFF mice (Figure 5b, panels A, G). However, injection of the anti-p53 shRNAs induced multiple histological changes suggestive of hepatoxicity, including multifocal pleocellular inflammation, scattered individual cell degeneration and necrosis, and anisokaryosis (Figure 5b, panel C; and data not shown). Likewise, MYC OFF/sAg-25 mice showed liver injury associated with small numbers of individual necrotic cells and mitoses throughout the parenchyma, and occasional extramedullary hematopoiesis in the sinusoids (Figure 5b, panel E). Moreover, their hepatocytes had small cytoplasmic vacuoles consistent with microvesicular lipidosis (Figure 5b, panel E). Note, these changes were similar to CCl4-treated MYC OFF mice that exhibited diffuse liver damage consistent with lobular postnecrotic collapse (Figure 5b, panel I). Also note that despite these distinct histological abnormalities in the liver, all MYC OFF mice remained comparable on a gross level (tumor-free, see above and Supplementary Table S1).
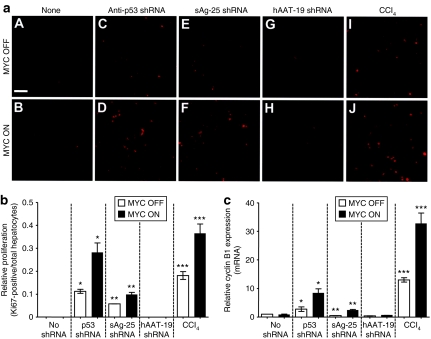
Previously, we have shown that MYC-induced tumorigenesis in the adult murine liver is associated with MYC acquiring the ability to induce mitotic division of hepatocytes.8 We speculated that shRNAs, by causing hepatoxicity, were inducing compensatory proliferation and mitotic cellular division. Indeed, we found a marked increase in cell proliferation in anti-p53- or sAg25-shRNA-injected MYC OFF mice as compared to untreated MYC OFF controls (Figure 6a, compare panels C/E to A, and Figure 6b). MYC induction resulted in a further four- and twofold increase in hepatocyte proliferation, respectively (Figure 6a, compare panels D/F to C/E; and Figure 6b). Similarly, CCl4-treated MYC OFF mice also showed higher percentages of Ki67-positive hepatocytes compared to untreated controls (Figure 6a, compare panel I to A) that even further increased upon induction of MYC (Figure 6a, compare panel J to B; also Figure 6b). However, MYC expression alone or in the presence of nontoxic hAAT-19 shRNA did not enhance proliferation (Figure 6a, compare panels B/H to A/G; and Figure 6b). Thus, only MYC overexpression in specific combination with toxic shRNAs, or a known liver toxin/carcinogen, potentiated the mitotic proliferation of adult hepatocytes.
Figure 6.
MYC and shRNAs cooperate to induce hepatocyte proliferation associated with increased cyclin B1 expression. (a) Representative Ki67 immunofluorescence analyses of hepatocytes from livers treated with the indicated vectors in the absence (A, C, E, G, I) or presence (B, D, F, H, J) of MYC. Bar = 80 µm. (b) Quantitation of Ki67-positive hepatocytes in livers (treated as indicated) relative to total hepatocytes. (c) qRT-PCR analysis of cyclin B1 mRNA expression in the same livers. Cyclin B1 mRNA expression levels were normalized to ubiquitin expression levels and depicted relative (mean ± SD) to levels in a normal liver. Statistical significance was measured using a Mann–Whitney test (*P = 0.03, **P = 0.03, ***P = 0.03). AAV, adeno-associated virus; CCl4, carbon tetrachloride; HCC, hepatocellular carcinoma; qRT, quantitative real-time; sAg, surface antigen; shRNA, short hairpin RNA.
MYC is thought to contribute to tumorigenesis by functioning as a transcription factor and inducing genes involved in cellular proliferation and growth.3,22,23,24,25,26 Cyclin B1, a key gene involved in the initiation of mitotic entry, is a direct transcription target of c-MYC.24,26 To determine whether MYC's ability to promote proliferation and tumorigenesis is related to its ability to induce cyclin B1, we used quantitative real-time PCR to measure cyclin B1 expression in our cohorts (Figure 6c). Compared to the corresponding MYC OFF groups, anti-p53- and sAg-25-shRNA-injected MYC ON mice exhibited a three- to fivefold increase in cyclin B1 mRNA, respectively (Figure 6c), that corresponded to the similar changes in cellular proliferation (Figure 6a,b). Note that CCl4 treatment similarly induced an increase in Ki67 and cyclin B1 mRNA levels that was potentiated in MYC ON mice. In contrast, cyclin B1 mRNA levels were neither elevated in MYC ON mice treated with the nontoxic hAAT-19 shRNA, nor in untreated mice, compared to the respective MYC OFF groups (Figure 6c). Notably, we did not observe the induction of the S phase regulator cyclin D1 (data not shown). Our results suggest that when MYC is overexpressed in conjunction with toxic shRNAs, it is more capable of transactivating genes involved in mitotic regulation in the adult liver. Accordingly, differences in cyclin B1 expression could, in part, explain the increased hepatocyte proliferation observed upon shRNA treatment, and particularly in the presence of MYC overexpression.
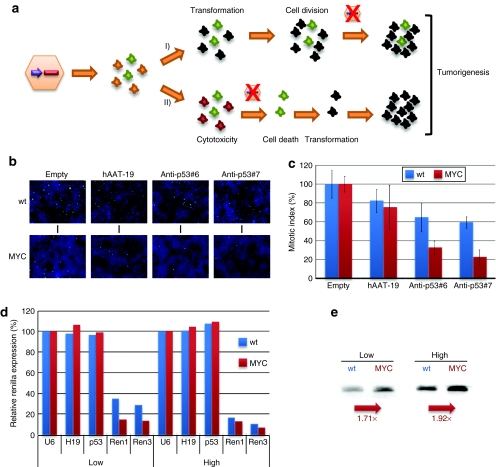
We considered there were two possible mechanisms by which hepatic shRNA/MYC coexpression had caused the observed accelerated liver tumorigenesis, as depicted in Figure 7a: either the shRNAs had functionally cooperated with MYC in the same cell and enhanced MYC's proliferative potential, or, alternatively, they were inducing tumorigenesis indirectly, most likely through general cytotoxicity that nonspecifically increased hepatocyte proliferation. Accordingly, the tumors would have originated either from cells initially transduced with the AAV vectors, or nontransduced cells that started to proliferate following local hepatocyte death. Both possibilities were compatible with our observation that there was a loss of AAV/shRNA vector genomes in the tumors (Figure 3b) that would have resulted from either cell division (model 1) or cell death (model 2).
Figure 7.
Identification of intracellular MYC–shRNA interactions. (a) Two alternative models for MYC–shRNA interactions in cells/tissues (scheme). In model 1, shRNAs enhance MYC's ability to transform cells, resulting in proliferation and loss of AAV vector genomes. In model 2, MYC increases shRNA activity and thus cytotoxicity, causing local cell death (and thus AAV loss) and transformation/proliferation of adjacent cells. (b) PH3 staining to determine mitotic indexes under various combinations of MYC and shRNA expression (mean ± SD from three independent experiments in c). Mitotic indexes were normalized to cells transfected with an empty U6 vector. (d) Renilla luciferase knockdown with specific shRNAs in the presence or absence of MYC (means from two independent experiments). The shRNAs were transfected at two different doses (low/high = 10/100 µg per well in 24-well plates). (e) MYC increases shRNA expression from U6 promoters (northern blot analyses, numbers indicate representative values). AAV, adeno-associated virus; hAAT, human α-1-antitrypsin; shRNA, short hairpin RNA.
To experimentally distinguish the two options, we first directly examined whether shRNAs could cooperate with MYC to enhance the proliferative state of liver cells. In cultured human cells stably expressing MYC, we found that the two anti-p53 shRNAs yielded a 70–80% drop in mitotic indexes, compared to about 40% in MYC-negative control cells (Figure 7b,c). Our results thus suggested that toxic shRNAs negatively impact the ability of MYC to induce cellular proliferation, arguing against the first model in Figure 7a.
We next studied whether MYC vice versa also interacted with the shRNAs and potentially increased their cytotoxicity, in line with our alternative second model. In fact, when we transfected MYC-negative or -positive cells with Renilla luciferase and either prevalidated specific or irrelevant control shRNAs, we observed an increase in target silencing with the anti-Renilla shRNAs in the presence of MYC (Figure 7d), most notably at the low shRNA dose. MYC is known to transactivate RNA pol III promoters.27,28,29,30 Indeed, we found that the increase in target knockdown was related to a boost in shRNA expression from our U6-shRNA plasmids (Figure 7e). We concluded that MYC expression can enhance shRNA levels and activity and thereby directly increase shRNA cytotoxicity, which in turn accelerated tumorigenesis in our mice (Figure 7a, model 2).
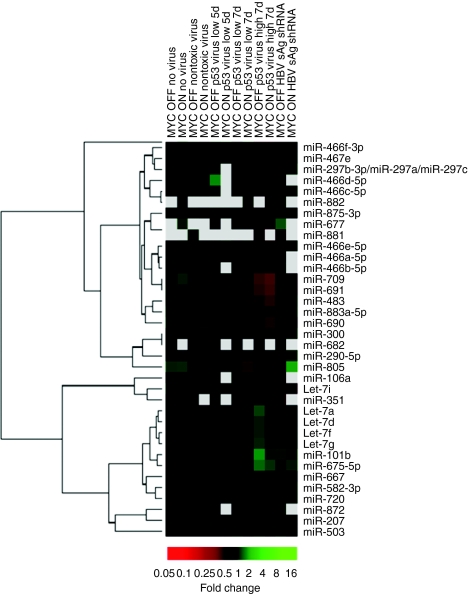
Based on our prior findings that shRNA overexpression can saturate components of the cellular RNAi machinery and cause global miRNA dysregulation,16 we examined the miRNA expression profiles in the various livers. The representative data in Figure 8 confirm that multiple miRNAs were dysregulated following anti-p53 vector infusion, in a dose-, time-, and MYC-dependent manner. One notable example included several let7 miRNA family members whose expression transiently increased following low dose treatment. At the same shRNA dose, other miRNAs, such as miR-875-3p, -677 and -690, were specifically downregulated in MYC ON/anti-p53 mice at day 7, but not at earlier timepoints, nor in MYC OFF mice, nor in any controls. Our observation of miRNA dysregulation particularly in the presence of MYC further supports our model that MYC potentiated adverse shRNAs effects on cell survival (Figure 7a, model 2). Note that the same miRNAs were also downregulated in mice treated with the higher (lethal) shRNA vector dose (2 × 1011) regardless of MYC, further supporting the synergistic cytotoxic effect of low-dose shRNA and MYC coexpression. Interestingly, at the nonlethal shRNA dose, an additional large miRNA set was concurrently upregulated in mice receiving anti-p53 or sAg-25 shRNA (Figure 8, four right lanes, let7a and below). In contrast, numerous other miRNAs were exclusively downregulated in mice treated with the same dose of anti-p53 vector (Figure 8, lanes 3/4 from right, miR-805 and up), perhaps suggesting a direct or indirect effect of p53 knockdown on their expression. The observation that miR-805 was downregulated in nearly all mice treated with the anti-p53 shRNA, regardless of dose, time point, or MYC, may imply a previously unknown key role of this particular miRNA in the p53 pathway, that will require further investigation.
Figure 8.
shRNA acceleration of MYC-induced HCC corresponds with distinct changes in miRNA profiles. Microarray analysis was performed to detect changes in miRNA expression due to shRNA and/or MYC in livers of transgenic mice. Note the multiple changes in mice treated with high titer of the anti-p53 shRNA vector, that are not represented in animals treated with a nontoxic shRNA. Several of these miRNAs (especially upregulators) demonstrated comparable altered expression in livers treated with the toxic sAg-25 shRNA, that induced similar phenotypes and also accelerated tumorigenesis (see text and Supplementary Table S1). Also note the many differences between the MYC ON and OFF groups for a given shRNA, as well as the specific and MYC-independent response (decrease) of miR-805 to p53 RNAi (see text for details). HCC, hepatocellular carcinoma; miRNA, microRNA; RNAi, RNA interference; sAg, surface antigen; shRNA, short hairpin RNA.
Discussion
Our study is the first to reveal the potential carcinogenic properties that intracellularly expressed shRNAs can exert in the adult liver. The most likely explanation for our unexpected findings is that shRNAs, even at low doses not previously associated with morbidity or mortality in normal mice, can have subtle albeit significant hepatoxic effects that facilitate the ability of a potent oncogene, such as MYC, to induce cellular proliferation and thereby neoplasia.
Our conclusion is supported by a variety of new observations in this study, as well as by previously reported data (Supplementary Figure S1). First, four independent shRNAs against three different targets accelerated the onset of MYC-dependent HCC, as evidenced by typical pathological and histological changes. Two of the shRNAs (sAg-25, hAAT-25) were irrelevant to HCC, the murine liver or p53, strongly implying a common and general carcinogenic shRNA effect. Second, under conditions of MYC overexpression, they were highly comparable to CCl4 (a potent liver carcinogen causing centrilobular necrosis and liver regeneration31,32,33) in their ability to accelerate HCC. In fact, CCl4-treated MYC ON mice developed tumors with a latency of onset that was identical to the toxic shRNAs. Also, both CCl4 and shRNAs induced an increase in Ki67-positive hepatocytes and cyclin B1 expression that was even more pronounced during MYC overexpression. We conclude that shRNAs can create a hepatic context of constant mitotic division that promotes MYC-induced HCC, similar to a known carcinogen. Third, our observations are consistent with prior models that hepatocytes gain susceptibility to neoplastic transformation under circumstances causing organ regeneration and cellular proliferation, in particular liver damage.34,35,36,37,38,39,40 Moreover, our data are congruent with our previous report that MYC overexpression induces HCC only when the hepatocytes are already rapidly proliferating, such as during early mouse development or after partial hepatectomy.8
MYC is well known to cooperate with carcinogens to induce HCC.41,42 Generally, it is thought that these agents cause liver damage, and that the resulting compensatory hepatocyte proliferation cooperates with MYC to induce tumorigenesis. Similarly, we think it is likely that toxic shRNAs are cooperating with MYC by inducing liver cell death and thereby promoting a cellular state more permissive to MYC for inducing malignant transformation. Hence, we propose that shRNAs initially exert a toxic effect in the liver, killing numerous dispersed transduced cells, and stimulating mitotic division of adjacent hepatocytes (model 2 in Figure 7a). Even if some of the liver cells originally still contained AAV vectors, the viral DNA was then lost during cell division due to the episomal nature of AAV genomes in the liver. Our explanation is consistent with our previous report that sublethal shRNA doses can trigger liver damage and repopulation in normal mice.16 Yet, a crucial difference from what we describe here is that the present livers were additionally expressing a potent oncogene. A critical combination of repopulation signal (shRNA-induced cell death and liver injury) and high proliferation potential (MYC overexpression) conceivably promoted the observed accelerated tumorigenesis.
We recognize that, as an alternative possibility, the vector-expressed shRNAs could have interacted directly with MYC within the same cells to promote their malignant transformation (model 1 in Figure 7a). We thus attempted to determine whether the introduction of shRNAs into MYC overexpressing cells increased their proliferation. Instead, we observed a significant decrease in the mitotic index suggesting that they are toxic to cells and generally inhibit cellular proliferation. Along this line, we noted marked global changes in hepatic miRNA expression profiles upon microarray analyses of shRNA vector-treated mouse livers that were even more pronounced in animals coexpressing MYC. Although we cannot experimentally rule out here that this miRNA dysregulation directly influenced the ability of MYC to induce tumorigenesis, we consider it more likely that it resulted in more general hepatocyte toxicity and death. The diverse pattern of miRNA up- or downregulation we observed appears to indicate a global interference of shRNA expression with the hepatic miRNA machinery, that could result in toxicity, as we have proposed before.16 Of note, more specific modulation of miRNAs that does not induce hepatoxicity could have therapeutic benefit in liver tumorigenesis, as for example has been shown recently for the AAV-8-mediated delivery of mir-26a.43,44 Altogether, our data thus strongly support model 2 in Figure 7a, according to which shRNA-induced hepatoxicity and subsequent changes in the hepatic microenvironment indirectly cooperated with MYC overexpression to induce tumorigenesis.
Intriguingly, our data suggest that shRNA-induced hepatoxicity may have concurrently stimulated oval cell expansion and proliferation. Histological analyses yielded evidence for the development of neoplastic foci near the periportal areas, implying a critical role for less mature hepatocytes or oval cells in shRNA/MYC-induced tumorigenesis, as described previously.38,45 These less mature hepatocytes are probably poor AAV-8 targets and were thus initially likely not infected by our shRNA vectors. Collectively, poor transduction of proliferative oval cells, death of initially well transduced hepatocytes, and propagation of, and subsequent liver repopulation with, less or untransduced other hepatocytes can readily explain our observation of carcinogenic shRNA effects.
The findings and model reported here have important implications for the future development and use of shRNAs as human therapeutics. Multiple prior reports have documented dose- and sequence-dependent adverse shRNA effects on normal cells, and had suggested diverse underlying mechanisms, including saturation of the miRNA machinery and off-targeting.16,46,47,48 Yet, we are the first to demonstrate that even marginal shRNA expression can have a marked effect, in particular on the susceptibility to tumorigenesis. In a normal adult liver, the low shRNA doses used here would induce mild hepatic damage, followed by compensatory hepatocyte proliferation, without a clinical phenotype. In contrast, as shown here, the combination with an active oncogene can drastically alter the outcome, by accelerating tumorigenesis and causing mortality. Intriguingly, the carcinogenic effects of shRNA-induced liver toxicity were only uncovered in the presence of MYC oncogene overexpression in our mice, making them highly susceptible to even minimal cell damage. A particularly interesting finding in this context was that MYC can stimulate shRNA expression from U6 promoters, as used in our vectors. We conclude that MYC actually exerted a synergistic dual effect in our mice, both boosting shRNA expression and thus concurrently increased cytotoxicity and death of hepatocytes, and then stimulating proliferation of adjacent nontransduced liver cells (perhaps including oval cells, as discussed above) and thereby ultimately accelerated tumorigenesis. In this respect, our mouse model system represents a highly sensitive and straightforward in vivo assay for future screens of toxic or tumorigenic effects of new shRNAs. Concurrently, our mice provide a new small animal model to further define the underlying molecular and cellular mechanisms of shRNA-induced cellular toxicity. It will also be interesting to determine whether shRNAs will have carcinogenic effects in other tissues, and in combination with further oncogenes.
Our study dramatically illustrates the necessity to design and use shRNA sequences and vectors with the lowest possible degree of toxicity or carcinogenicity. We and others have recently made marked efforts in this direction through the development of tissue-specific (liver) shRNA expression cassettes20 or our selection of more efficient and specific viral vector capsids for RNAi delivery.49 We thus believe that the safe introduction of RNAi therapeutics into humans remains a highly realistic goal.
Materials and Methods
Transgenic mice. The TRE-MYC transgenic line has been described previously.9 The LAP-tTA transgenic line was kindly provided by Hermann Bujard.10 MYC expression was activated by removing doxycycline treatment (100 µg/ml) from the drinking water of mice transgenic for both TRE-MYC and LAP-tTA.
Tumorigenicity assays. MYC was activated in the liver by removing doxycycline from the water. Mice were injected with AAV vectors at three different particle doses, 1 × 1012, 2 × 1011, or 2 × 1010 [all in 300 µl phosphate-buffered saline (PBS)], via the tail vein. The 1 × 1012 and 2 × 1011 doses were lethal in all mice, consistent with our previously reported findings that some shRNAs can be highly toxic in the liver.16 All further analyses were thus performed on mice injected with the lowest (2 × 1010) vector dose. Mice were injected intraperitoneally with CCl4 (diluted in mineral oil, 1 µl/g of mouse) (Sigma-Aldrich, St Louis, MO) two times a week every week until killed. Mice were monitored daily and killed when moribund with tumor burden. During necropsy, liver tissues were saved by snap-freezing in liquid nitrogen. Liver tissues for histological analyses were fixed in 10% buffered formalin for 24 hours and then transferred to 70% ethanol until paraffin embedding. Tissue sections 4-µm thick were cut from paraffin-embedded blocks, placed on glass slides and stained with hematoxylin and eosin. Paraffin embedding and staining were performed by the Stanford Histology Core (Stanford, CA). All procedures were approved by the Animal Care Committee at Stanford University (Stanford, CA).
Design and analyses of plasmids for shRNA expression. A set of 10 different shRNAs directed against murine p53 was selected using the Invivogen (San Diego, CA) “siRNA Wizard” online tool (www.sirnawizard.com). Design criteria were: (i) 19 or 21 nucleotide (nt) stem lengths, (ii) sense strand (beginning with a G) located before the antisense strand, (iii) both strands separated by a seven nt loop (5′ TCA AGA G 3′), and (iv) no significant homology to the mouse genome. The following sequences were chosen (shown are the sense strands, with target region and stem length in brackets; see also Figure 1a): P1 5′ GCC GAC CTA TCC TTA CCA T 3′ (nt 734–753, 19 mer), P2 5′ GAG TCA CAG TCG GAT ATC A 3′ (nt 16–34, 19 mer), P3 5′ GGC CAT CTA CAA GAA GTC A 3′ (nt 471–489, 19 mer), P4 5′ GAA TGA GGC CTT AGA GTT A 3′ (nt 1,023–1,041, 19 mer), P5 5′ GGC CAT CTA CAA GAA GTC ACA 3′ (nt 471–491, 21 mer), P6 5′ GAC CTA TCC TTA CCA TCA TCA 3′ (nt 737–757, 21 mer), P7 5′ GGA GCT GAA TGA GGC CTT AGA 3′ (nt 1,017–1,037, 21 mer), P8 5′ GCT GAA TGA GGC CTT AGA GTT 3′ (nt 1,020–1,040, 21 mer), P9 5′ GTA CTC TCC TCC CCT CAA T 3′ (nt 366–384, 19 mer), P10 5′ GTA CAT GTG TAA TAG CTC C 3′ (nt 696–714, 19 mer). All shRNAs were purchased as complementary oligonucleotides (IDT-DNA, Coralville, IA), annealed, cloned into our AAV vector plasmid,16 and eventually verified by DNA sequencing. In the resulting constructs, the shRNAs were expressed from a human U6 promoter and terminated by a stretch of five Ts. All other shRNAs used in this study (hAAT-19, hAAT-25, sAg-25) have been reported before16 and were expressed from an identical backbone. An exception are the shRNAs against Renilla luciferase that will be reported elsewhere (N. Schürmann, L.S. Wang, E. Wiedtke, and D. Grimm, personal communication). To evaluate shRNA efficacy, murine p53 complementary DNA was first PCR-amplified from a commercial mouse liver complementary DNA library (BD Clontech, Palo Alto, CA), using as primers 5′ GAC CT TCTAGA ATG ACT GCC ATG GAG GAG TCA CAG 3′ and 5′ CTT GG GTCGAC TCA GTC TGA GTC AGG CCC CAC TTT C 3′. The primers contained XbaI or SalI restriction sites (underlined), allowing subcloning into plasmid pTRUF2 (bringing the p53 complementary DNA under the control of a cytomegalovirus promoter).50 Human Huh-7 liver cells were then co-transfected with equal amounts of p53 expression plasmid and each shRNA construct. Three days later, total protein extracts were prepared and analyzed via western blotting, using a rabbit polyclonal antibody (ab2433-1; Abcam, Cambridge, MA) for p53 detection. The psiCheck-2 plasmid expressing Renilla luciferase (plus firefly luciferase for normalization) and reagents for luciferase assays were purchased from Promega (Mannheim, Germany). Assays to determine MYC/shRNA interactions were performed in human wild-type HaCat or stably MYC-transfected HaCat cells.
Production of shRNA-expressing AAV vectors. All vector genomes were packaged into capsids from AAV-8, based on this particular isolate's superior performance in mouse liver.18 Recombinant viruses were generated via a standard triple-transfection method, purified using cesium chloride density gradient centrifugation and titered by dot blot, as described before.16 Typical vector particle yields were 5 × 1012/ml or higher.
Southern and northern blot analysis. Extraction of genomic DNA from mouse liver and analysis of persisting AAV vector genomes were performed as described previously,16 using a 1.7-kb probe against a sequence conserved in all shRNA vector genomes. Northern blots for shRNA detection were performed as described previously.16
RNA isolation and complementary DNA preparation. Total cellular RNA was isolated from snap-frozen liver tissue using the RNeasy kit (Qiagen, Valencia, CA) following the manufacturer's protocol. RNA was quantified using spectrophotometric OD260 measurements and reverse transcribed using Oligo(dT)12–18 primers and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA).
Quantitative real-time-PCR. Quantitative real-time-PCR was performed on the ABI Prism 7700 Sequence detection system (Applied Biosystems, Foster City, CA) using the SYBR Green PCR Master Mix (Applied Biosystems). PCRs were performed in triplicate in a final volume of 20 µl. Primer sequences were as follows: p53 forward 5′ TCA CCT CAC TGC ATG GAC GA 3′, p53 reverse 5′ ACT CGG AGG GCT TCA CTT GG 3′, cyclin B1 forward 5′ ACT TCC TCC GTA GAG CAT C 3′, cyclin B1 reverse 5′ GCA GAG TTG GTG TCC ATT C 3′, ubiquitin forward 5′ AGC CCA GTG TTA CCA CCA AG 3′, ubiquitin reverse 5′ ACC CAA GAA CAA GCA CAA GG 3′. Thermal cycling conditions were: 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds, 57 °C for 30 seconds, 72 °C for 30 seconds, and a dissociation stage consisting of 95 °C for 15 seconds, 60 °C for 15 seconds, and 95 °C for 15 seconds. p53 mRNA and cyclin B1 mRNA expression levels were normalized to ubiquitin expression levels and expressed relative to normal livers.
Proliferation assay. Ki67 expression was examined by immunofluorescence using a mouse antihuman Ki67 monoclonal antibody (BD Biosciences, San Jose, CA) and the vector mouse on mouse (M.O.M.) basic kit (Vector Laboratories, Burlingame, CA). Slides were deparaffinized in xylene and rehydrated in a graded series of ethanols, followed by antigen retrieval in a microwave for 14 minutes in vector antigen unmasking solution (H-3300; Vector Laboratories, Burlingame, CA), and incubated in 100 mmol/l glycine two times for 8 minutes to reduce fluorescent background. They were then incubated in avidin followed by biotin for 10 minutes each using the Dako biotin blocking system (DAKO, Carpinteria, CA), and subsequently incubated for 1 hour in M.O.M. Immunoglobin G–blocking reagent diluted 1:4 in PBS. Slides were then incubated for 1 hour in mouse antihuman Ki67 monoclonal antibody diluted 1:100 in M.O.M. diluent, washed in Tris-buffered saline Tween-20 three times for 5 minutes to reduce background, and then treated with M.O.M. biotin-labeled anti-mouse immunoglobin G, diluted 1:250 in M.O.M. diluent. Following another three washes of Tris-buffered saline Tween-20 for 5 minutes, slides were incubated for exactly 45 minutes in Cy3-conjugated streptavidin (Amersham Biosciences, Piscataway, NJ) diluted 1:800 in PBS in the dark. To visualize nuclei, slides were counterstained with 0.2 µg/ml 4′-6-diamidino-2-phenylindole. Ki67-positive cells were visualized by fluorescence microscopy.
Quantitation of mitotic indexes. Wild-type or stably MYC-expressing HaCat cells were transfected with shRNA expression plasmids and 48 hours later fixed with 4% paraformaldehyde at room temperature for 30 minutes. They were then permeabilized using 0.2% triton (30 minutes at room temperature), washed three times with 1× PBS and subsequently incubated for 30 minutes with 1× PBS/5% fetal bovine serum, to block unspecific binding sites. This solution was aspirated and the cells were incubated overnight at 4 ºC with a mouse monoclonal antibody against histone 3 phospho-serine 10 (PH3, diluted 1:500 in 1× PBS/5% fetal bovine serum) (New England Biolabs, Frankfurt, Germany). PH3 is a marker for dividing nuclei, as it correlates with chromosome condensation during mitosis. Unbound antibody was then removed by washing the cells three times with 1× PBS, prior to staining with secondary Alexa Fluor 488-coupled anti-mouse immunoglobin G antibody (diluted 1:500) for 2 hours at room temperature. Moreover, Hoechst fluorescent DNA binding dye was added to identify individual nuclei/cells. The cells were next washed again three times in 1× PBS and left in wash buffer for subsequent automatic data acquisition. Fluorescence was measured in an Olympus microscope (Olympus, Center Valley, PA), using appropriate excitation and emission filters, and numbers of total nuclei and mitotic cells were recorded for each individual well. Automated image segmentation was then performed via voronoi-like watershed transformation, using image analysis software kindly provided by Christoph Sommer (Interdisciplinary Center for Scientific Computing, Heidelberg University, Heidelberg, Germany). Finally, the fraction of cells in mitosis (mitotic index) was calculated by dividing numbers of PH3-positive cells by total nuclei counts.
Microarray analysis of miRNA expression profiles. MiRNA was extracted using a miRNeasy mini kit (Qiagen) from livers of mice in which transgenic MYC was expressed together with various shRNAs. In addition, miRNA was extracted from livers of mice that were AAV-treated but in which transgenic MYC was silent, to determine MYC- versus shRNA-dependent effects. MiRNA was Cy3-labeled and hybridized to miRCURY LNA miRNA arrays according to manufacturer's instructions (Exiqon, Woburn, MA). Cy5-labeled miRNA from wild-type, untreated liver was hybridized together with experimental miRNA for normalization. Arrays were scanned and images acquired at 556 nm and 656 nm to detect Cy3- and Cy5-labeled miRNA, respectively. Resulting data were analyzed by linear regression [Log(base 2) of R/G normalized ratio], and genes were subsequently clustered by Pearson's correlation.
SUPPLEMENTARY MATERIALFigure S1. In vivo outcomes of AAV/shRNA infusion into adult mice (Scheme). This figure depicts an overview over our results with shRNA-expressing AAV vectors that we have injected (peripherally) into adult mice in our previous or the present work: Depending on vector and dose, AAV/shRNA infusion resulted in rapid lethality, transient target knockdown, or stable-partial or complete-RNAi. As shown in this paper, injection of low doses of inherently toxic shRNAs can also accelerate tumorigenesis in the presence of MYC expression. Finally, in some cases (low doses), we detected transient or stable transduction of AAV/shRNA vector genomes, but RNAi was not quantifiable due to the lack of an appropriate target in these mice.Table S1. Summary of in vivo results obtained with various AAV/shRNA vectors or control treatments. This table provides on overview over the outcomes of infusion of toxic or non-toxic shRNAs into MYC ON or OFF mice, respectively. Categories are survival, tumorigenesis, p53 RNAi, pathology, histology, proliferation and cyclin B1 expression.
Supplementary Material
In vivo outcomes of AAV/shRNA infusion into adult mice (Scheme). This figure depicts an overview over our results with shRNA-expressing AAV vectors that we have injected (peripherally) into adult mice in our previous or the present work: Depending on vector and dose, AAV/shRNA infusion resulted in rapid lethality, transient target knockdown, or stable-partial or complete-RNAi. As shown in this paper, injection of low doses of inherently toxic shRNAs can also accelerate tumorigenesis in the presence of MYC expression. Finally, in some cases (low doses), we detected transient or stable transduction of AAV/shRNA vector genomes, but RNAi was not quantifiable due to the lack of an appropriate target in these mice.
Summary of in vivo results obtained with various AAV/shRNA vectors or control treatments. This table provides on overview over the outcomes of infusion of toxic or non-toxic shRNAs into MYC ON or OFF mice, respectively. Categories are survival, tumorigenesis, p53 RNAi, pathology, histology, proliferation and cyclin B1 expression.
Acknowledgments
S.B., K.K., H.K., D.I.B., and D.W.F. were supported by the NIH (grant numbers R01-CA105102, R01-CA89305, ICMIC-P50-CA114747, P01-CA03423; and Fellowship F32-CA132312 for D.I.B.), Burroughs Wellcome Fund, Damon Runyon Foundation and the Leukemia and Lymphoma Society; D.G., J.S.L., L.S.W., T.A.S., and M.A.K. were supported by DK078424 (M.A.K.). D.G. was additionally supported by the Cluster of Excellence CellNetworks, as well as the Chica and Heinz Schaller Foundation (CHS). K.B. was supported by the German Federal Ministry of Education and Research (BMBF), as well as ViroQuant. We thank Petra Boukamp (DKFZ, Heidelberg, Germany) for the kind gift of human HaCat and stably MYC-transfected HaCat cells. We are grateful to Christoph Sommer (Interdisciplinary Center for Scientific Computing, Heidelberg University, Germany) for providing his image analyses software. The authors declared no conflict of interest.
REFERENCES
- Calvisi DF., and , Thorgeirsson SS. Molecular mechanisms of hepatocarcinogenesis in transgenic mouse models of liver cancer. Toxicol Pathol. 2005;33:181–184. doi: 10.1080/01926230590522095. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS., and , Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW., and , Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP., and , Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- Oster SK, Ho CS, Soucie EL., and , Penn LZ. The myc oncogene: MarvelouslY Complex. Adv Cancer Res. 2002;84:81–154. doi: 10.1016/s0065-230x(02)84004-0. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M., and , Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- Beer S, Zetterberg A, Ihrie RA, McTaggart RA, Yang Q, Bradon N, et al. Developmental context determines latency of MYC-induced tumorigenesis. PLoS Biol. 2004;2:e332. doi: 10.1371/journal.pbio.0020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsher DW., and , Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Felsher DW. Putting oncogenes into a developmental context. Cancer Biol Ther. 2004;3:942–944. doi: 10.4161/cbt.3.10.1307. [DOI] [PubMed] [Google Scholar]
- Grimm D., and , Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Grimm D., and , Kay MA. Therapeutic short hairpin RNA expression in the liver: viral targets and vectors. Gene Ther. 2006;13:563–575. doi: 10.1038/sj.gt.3302727. [DOI] [PubMed] [Google Scholar]
- Grimm D, Pandey K., and , Kay MA. Adeno-associated virus vectors for short hairpin RNA expression. Meth Enzymol. 2005;392:381–405. doi: 10.1016/S0076-6879(04)92023-X. [DOI] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE., and , Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y., and , Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and , Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giering JC, Grimm D, Storm TA., and , Kay MA. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther. 2008;16:1630–1636. doi: 10.1038/mt.2008.144. [DOI] [PubMed] [Google Scholar]
- Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283:22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati B, Alevizopoulos K., and , Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menssen A., and , Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proc Natl Acad Sci USA. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaya AJ, Mateyak MK., and , Sedivy JM. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene. 1999;18:2934–2941. doi: 10.1038/sj.onc.1202749. [DOI] [PubMed] [Google Scholar]
- Yin XY, Grove L, Datta NS, Katula K, Long MW., and , Prochownik EV. Inverse regulation of cyclin B1 by c-Myc and p53 and induction of tetraploidy by cyclin B1 overexpression. Cancer Res. 2001;61:6487–6493. [PubMed] [Google Scholar]
- Cole MD., and , Cowling VH. Transcription-independent functions of MYC: regulation of translation and DNA replication. Nat Rev Mol Cell Biol. 2008;9:810–815. doi: 10.1038/nrm2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman N, Grandori C, Eisenman RN., and , White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Dubeau L., and , Johnson DL. Enhanced RNA polymerase III-dependent transcription is required for oncogenic transformation. J Biol Chem. 2008;283:19184–19191. doi: 10.1074/jbc.M802872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneth NS, Ramsbottom BA, Gomez-Roman N, Marshall L, Cole PA., and , White RJ. TRRAP and GCN5 are used by c-Myc to activate RNA polymerase III transcription. Proc Natl Acad Sci USA. 2007;104:14917–14922. doi: 10.1073/pnas.0702909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N. Biochemical and cellular mechanisms of toxic liver injury. Semin Liver Dis. 2002;22:137–144. doi: 10.1055/s-2002-30100. [DOI] [PubMed] [Google Scholar]
- Plaa GL. Chlorinated methanes and liver injury: highlights of the past 50 years. Annu Rev Pharmacol Toxicol. 2000;40:42–65. doi: 10.1146/annurev.pharmtox.40.1.43. [DOI] [PubMed] [Google Scholar]
- Recknagel RO, Glende EA Jr, Dolak JA., and , Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Chisari FV.1995Hepatitis B virus transgenic mice: insights into the virus and the disease Hepatology 224 Pt 1): 1316–1325. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- Dunsford HA, Sell S., and , Chisari FV. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990;50:3400–3407. [PubMed] [Google Scholar]
- Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS., and , Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor α natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- Fausto N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis. 1999;19:243–252. doi: 10.1055/s-2007-1007114. [DOI] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Sanders S., and , Thorgeirsson SS. Phenobarbital promotes liver growth in c-myc/TGF-α transgenic mice by inducing hypertrophy and inhibiting apoptosis. Carcinogenesis. 1999;20:41–49. doi: 10.1093/carcin/20.1.41. [DOI] [PubMed] [Google Scholar]
- Sanders S., and , Thorgeirsson SS. Promotion of hepatocarcinogenesis by phenobarbital in c-myc/TGF-α transgenic mice. Mol Carcinog. 2000;28:168–173. doi: 10.1002/1098-2744(200007)28:3<168::aid-mc5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- Calin GA., and , Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- Snøve O., Jr, and , Rossi JJ. Toxicity in mice expressing short hairpin RNAs gives new insight into RNAi. Genome Biol. 2006;7:231. doi: 10.1186/gb-2006-7-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snøve O., Jr, and , Rossi JJ. Expressing short hairpin RNAs in vivo. Nat Methods. 2006;3:689–695. doi: 10.1038/nmeth927. [DOI] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Hauswirth WW, Guy J., and , Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo outcomes of AAV/shRNA infusion into adult mice (Scheme). This figure depicts an overview over our results with shRNA-expressing AAV vectors that we have injected (peripherally) into adult mice in our previous or the present work: Depending on vector and dose, AAV/shRNA infusion resulted in rapid lethality, transient target knockdown, or stable-partial or complete-RNAi. As shown in this paper, injection of low doses of inherently toxic shRNAs can also accelerate tumorigenesis in the presence of MYC expression. Finally, in some cases (low doses), we detected transient or stable transduction of AAV/shRNA vector genomes, but RNAi was not quantifiable due to the lack of an appropriate target in these mice.
Summary of in vivo results obtained with various AAV/shRNA vectors or control treatments. This table provides on overview over the outcomes of infusion of toxic or non-toxic shRNAs into MYC ON or OFF mice, respectively. Categories are survival, tumorigenesis, p53 RNAi, pathology, histology, proliferation and cyclin B1 expression.