Abstract
To explore whether stable transduction of myogenic stem cells using lentiviral vectors could be of benefit for treating dystrophic muscles, we generated vectors expressing a functional microdystrophin/enhanced green fluorescence protein fusion (µDys/eGFP) gene. Lentiviral vector injection into neonatal mdx4cv muscles resulted in widespread and stable expression of dystrophin for at least 2 years. This expression resulted in a significant amelioration of muscle pathophysiology as assessed by a variety of histological and functional assays. To assess whether this long-term expression was accompanied by stable transduction of satellite cells, we harvested muscle mononuclear cells 1 year after vector injection. Up to 20% of the cultured myoblast colonies expressed the µDys/eGFP transgene following myotube formation. Furthermore, transplantation of the muscle mononuclear cells into secondary mdx4cv recipients showed their ability to regenerate dystrophin-expressing myofibers in vivo. The ability to isolate myogenic cells able to form dystrophin-positive myotubes or myofibers in vitro and in vivo >1 year postinjection indicates that the vectors stably transduced muscle satellite cells, or a progenitor of such cells, in neonatal mdx4cv muscles. These studies suggest that integrating lentiviral vectors have potential utility for gene therapy of muscular dystrophy.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked, lethal muscle disorder caused by mutation of the dystrophin gene.1 Affected boys are usually diagnosed between 3 and 5 years of age.2 Early symptoms of delayed walking and gait disturbance rapidly progress to general muscle, especially proximal, weakness. By age 12, almost all patients use a wheelchair and most develop severe scoliosis. Even though improved clinical management has extended the life expectancy of DMD patients in recent years, most of the patients still die by age 30 due to respiratory and/or cardiac failure.2,3 Current treatment for DMD patients focuses primarily on relief of symptoms, as there are no major treatment options.
Transgenic replacement of dystrophin in the mdx mouse model for DMD restores normal expression of the dystrophin–glycoprotein complex and also prevents development of the dystrophic phenotype in striated muscles.4,5 Recombinant adenoviral or adeno-associated viral (AAV) vector–mediated transfer of full-length, mini-, and microdystrophins to adult dystrophic muscles has also been shown to result in a dramatic amelioration of the dystrophic pathology.6,7,8,9,10,11 Therefore, gene therapy is viewed as one of the most promising approaches for clinical application.4,12,13,14 Despite the promise shown by these vectors, especially AAV which can systemically deliver genes to striated muscles,13 neither has been shown to integrate into myonuclear genomic DNA to a significant degree.15 In contrast, lentiviral vectors derived from the human immunodeficiency virus-1 can integrate into the host genome and achieve long-term transgene expression in a wide variety of dividing and nondividing cells including skeletal muscle.16,17,18,19,20 We and others have shown that VSV-G-pseudotyped lentiviral vectors transduce adult mouse skeletal muscle with a relatively low efficiency.20,21 Nonetheless, myofibers transduced with a fully functional mini-dystrophin gene were partially protected from degeneration for at least 6 months in mdx mice.16,21 Proliferating myoblasts, postmitotic myocytes and myotubes, freshly isolated primary myoblasts, and several different type of stem cell sources such as side population cells, marrow stromal cells, mesoangioblasts, pericytes, and dermal fibroblasts have been shown to be efficiently transduced by lentiviral vectors in vitro.21,22,23,24,25,26,27
In dystrophic muscles of DMD patients as well as the mdx4cv mouse model, activated-satellite cells proliferate and terminally differentiate to form new muscle fibers during cycles of myofiber necrosis and regeneration.28,29 Although most of those cells terminally differentiate to form muscle fibers, some return to quiescence adjacent to myofibers as satellite cells for future cycles of muscle regeneration.30,31,32 These satellite cells and other types of muscle “stem cells” are considered an attractive target for genetic modification by lentiviral vectors, as these vectors enable stable gene expression of transgenes following integration into the genomic DNA of a host cell.
Here, we report that intramuscular injection of lentiviral vectors expressing a microdystrophin/enhanced green fluorescent protein (µDys/eGFP) fusion gene led to successful transduction of myogenic stem cells as well as myofibers in neonatal mdx4cv mice. Expression was maintained for at least 2 years, and was supported by a pool of stably transduced satellite cells that were able to participate in muscle regeneration to form dystrophin-expressing myofibers in vivo. These results suggest that integrating vectors systems could be of use for long-term genetic correction of myogenic stem cells in vivo in various muscle disorders.
Results
Long-term µDys/eGFP expression in mdx4cv mouse muscle following lentiviral vector–mediated gene delivery
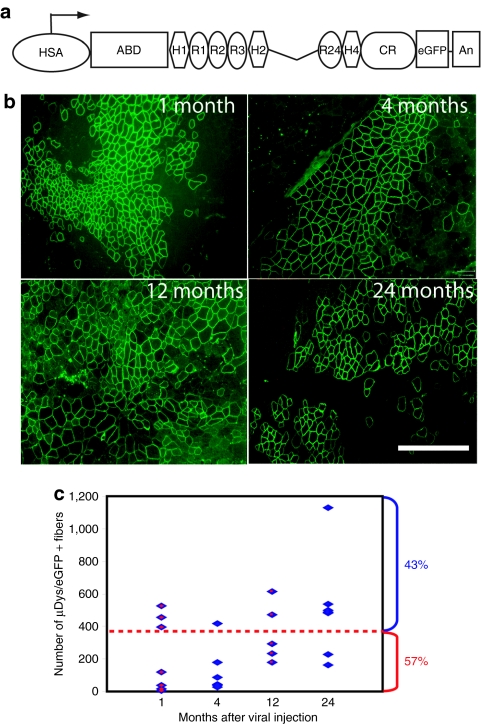
Our previous studies revealed an age-dependent loss of transduction by lentiviral vectors when injected into mouse muscles.21 To determine whether lentiviral-mediated gene transfer could lead to phenotypic improvement in a mouse model for DMD, we tested injection of a novel dystrophin-expressing vector (Lv-HSA-µDys/eGFP; Figure 1a) into muscles of neonatal mdx4cv mice. We reasoned that injection into neonatal mice might avoid potential immune responses against the vector and might allow for wider dissemination into a variety of myofibers and muscle progenitor cells, potentially leading to long-term expression. The vector utilized a powerful, skeletal muscle–specific promoter/enhancer element derived from the human α-skeletal actin gene (HSA). This HSA promoter has previously been shown to generate high-level muscle-specific gene expression in the context of transgenic mice.33 The dystrophin transgene was based on our previously described ΔR4-R23 µDys, which has been shown to be highly functional and able to ameliorate dystrophic pathology when tested in transgenic mdx mice or when delivered systemically to mdx mice via AAV vectors.5,7 Finally, to allow efficient tracking of transduced myofibers or myotubes, we replaced the C-terminal domain of dystrophin with sequences encoding the eGFP. Such a substitution was previously shown not to affect the functionality of otherwise full-length or mini-dystrophin fusion proteins.33,34
Figure 1.
Persistent expression of microdystrophin (µDys) in mdx4cv muscles injected with Lv-HSA-µDys/eGFP. Tibialis anterior muscles of 2-day-old mdx4cv mice were injected with 5.0 × 105 TU (in 5 µl) of the lentiviral vector. Muscles were examined by fluorescence microscopy at 1, 4, 12, and 24 months post-treatment for green fluorescence. (a) Schematic structure of the µDys/eGFP transgene. (b) Representative images of treated muscles harvested at various time points postinjection as indicated. The examples shown here were immunostained with an antibody against the N-terminal domain of dystrophin.49 Bar = 800 µm. (c) Summary of the number of µDys/eGFP-expressing myofibers in each treated muscle harvested at various time points postinjection. Each data point is from an individual muscle. N = 7 for each group except the 12 month time point, where N = 6. More than 40% of the injected muscles contained at least 400 positive myofibers. ABD, dystrophin actin-binding domain; An, SV40 polyA addition site; CR, dystrophin cysteine-rich domain; eGFP, enhanced green fluorescence protein; H, dystrophin hinge region; HSA, promoter derived from the human α-skeletal actin gene;33 R, dystrophin spectrin-like repeat.
Tibialis anterior (TA) muscles from mdx4cv mice injected with this Lv-HSA-µDys/eGFP vector at postnatal day 2 were harvested 4 weeks, 4 months, 1 year, and 2 years later to examine dystrophin expression and effects on the dystrophic histopathology. The µDys/eGFP fusion protein was expressed correctly along the sarcolemma of numerous myofibers in injected muscles at all time points examined (Figure 1b). Dystrophin-positive fibers were directly observed using fluorescence microscopy, and were also visualized and quantitated using indirect immunostaining with anti-GFP and/or dystrophin N-terminal antibodies. The numbers of positive fibers reached a maximum of between ~400 and 1,200 in each TA muscle at all four time points (covering on average between 20 and 25% of the muscle cross-sectional area; Figure 1c). There was a relatively wide variation in the absolute number of positive fibers in each muscle section, likely owing to variability in the injection protocol.
Lentiviral vector–mediated µDys/eGFP expression ameliorated dystrophic histopathology
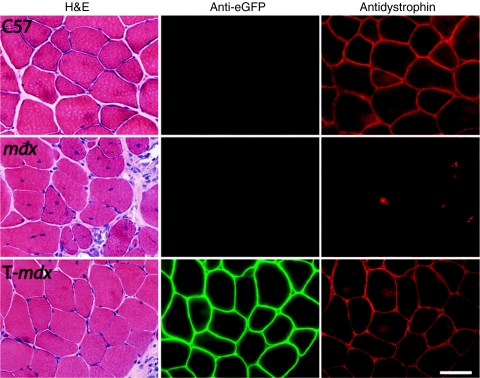
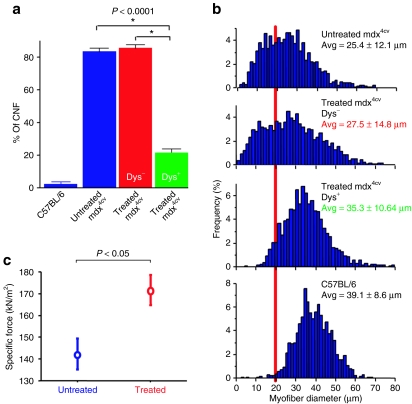
Examination of myofiber morphology 1 year after vector injection revealed that most of the µDys/eGFP-positive fibers were peripherally nucleated, whereas the majority of dystrophin-negative myofibers in both injected and uninjected mdx4cv muscles were centrally nucleated, a hallmark of myofiber regeneration (Figures 2 and 3a). The percentage of centrally nucleated myofibers in various samples is shown graphically in Figure 3a. In µDys/eGFP-positive fibers observed in treated muscles, only 20.2 ± 2.4% of the fibers carried centrally located nuclei. In contrast, 84.9 ± 1.7% of the dystrophin-negative fibers in treated muscles, and 83.4 ± 1.5% of the myofibers in mdx4cv control muscles were centrally nucleated at 1 year of age. Regions of injected muscles with extensive dystrophin-positivity also displayed few mononuclear cell infiltrates, necrotic fibers, or fibrotic lesions (Figures 2 and 4).
Figure 2.
Preservation of normal morphology in mdx4cv skeletal muscles expressing µDys/eGFP. TA muscles of 2-day-old mdx4cv mice were injected with Lv-HSA-µDys/eGFP vector, and 1 year later both treated and untreated muscles were harvested. Serial cryosections were stained with H&E, or were immunostained with antibodies against either eGFP or the N-terminal domain of dystrophin. Areas with widespread dystrophin expression showed normal morphology. Bar = 50 µm. H&E, hematoxylin and eosin; µDys/eGFP, microdystrophin/enhanced green fluorescent protein.
Figure 3.
Functional properties of µDys/eGFP expressing mdx4cv TA muscles. Mice injected 1 year earlier with Lv-HSA-µDys/eGFP were killed and the injected TA muscles were analyzed for morphological and physiologic properties. (a) Shown are the percentage of myofibers displaying centrally located nuclei in age-matched wild-type muscles (C57BL/6), untreated mdx4cv muscles, and treated mdx4cv muscles. For the treated mouse group, myofibers were separately scored based on whether the fibers were µDys/eGFP-positive (red) or negative (blue). Shown is the mean ± SEM; N = 6 for each group; *P < 0.0001. (b) Histogram showing the distribution of myofiber size in the same groups as in a. The red line highlights the number of fibers smaller than 20 µm in diameter, which primarily represent regenerating fibers. N = 6 for each group. (c) Specific force-generating levels in treated mdx4cv TA muscles 1 year after lentiviral injection, and in TA muscles from age matched untreated mdx4cv controls (Shown is the mean ± SEM; N = 4 for each group). CNF, centrally nucleated myofibers; µDys/eGFP, microdystrophin/enhanced green fluorescent protein.
Figure 4.
Morphology of an mdx4cv tibialis anterior (TA) muscle 2 years after injection with a lentiviral vector expressing Dys/eGFP. TA muscles of 2-day-old mdx4cv mice were injected with the µDys/eGFP lentiviral vector, harvested 2 years later and analyzed for Dys/eGFP expression and position of nuclei. Cryosections were immunostained using antisera against dystrophin (red staining) and counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride to reveal nuclei (blue). (a) Mosaic image of an entire tibias anterior muscle showing widespread but variable µDys expression. Bar = 160 µm. (b) High-power images of two regions from a (in green boxes). Left: An area with a large number of contiguous dystrophin-positive myofibers and which contained predominantly peripherally nucleated myofibers; right: an area with relatively few dystrophin-positive myofibers that had mostly centrally nucleated myofibers. Bar = 50 µm. µDys/eGFP, microdystrophin/enhanced green fluorescent protein.
To explore whether this level of dystrophin expression was conferring a functional benefit on the treated muscles, we examined myofiber cross-sectional area and muscle mechanical properties (Figure 3). A wide distribution of myofiber diameters was observed in both uninjected mdx4cv muscles as well as in dystrophin-negative myofibers from injected mdx4cv muscles (Figure 3b). In contrast, µDys/eGFP-positive myofibers from injected mdx4cv muscles displayed a much narrower range of average fiber CSA, closer to that in age-matched wild-type control muscles. We also measured the specific force-generating capacity and susceptibility to eccentric contraction-induced injury in injected and uninjected 1-year-old mdx4cv muscles. As a wide range of overall dystrophin-positivity was observed in injected muscles, we limited our analysis of contractile properties to those muscles that displayed a relatively high percentage of dystrophin-positive myofibers. These muscles were observed to develop specific forces of 172 ± 7 kN/cm2, a highly significant improvement over untreated mdx4cv controls 141 ± 7 kN/cm2, although still lower than wild-type controls (241 ± 10 kN/cm2), likely due to the mosaic expression pattern (Figure 3c; P < 0.05). These values remained lower than in control muscles. In contrast, we did not observe a significant protection in the susceptibility of the injected muscles to contraction-induced injury following either 1 or 2 lengthening contractions (Table 1).
Long-term maintenance of integrated lentiviral vectors in muscle satellite cells
Previous studies in transgenic mdx mice with low or mosaic expression of dystrophin suggested that dystrophin needs to be expressed in >50% of myofibers to prevent ongoing myofiber necrosis and regeneration.35,36 It was therefore of interest that the lentiviral-injected muscles in this study typically displayed dystrophin expression in only 20–25% of the myofibers, yet that level of expression was maintained for at least 2 years (Figure 1b). Also, the presence of dystrophin-positive, centrally nucleated myofibers both 1 and 2 years after vector injection (Figures 3 and 4) suggested that some myogenic progenitor cells that were contributing to ongoing muscle regeneration might harbor integrated copies of the lentiviral vector. To explore this issue, we asked whether the intramuscular injection protocol used here could lead to stable transduction of satellite cells. TA muscles from two mdx4cv mice were harvested 1 year after injection with the Lv-HSA-µDys/eGFP vector and used to isolate mononuclear cells. Some of the isolated mononuclear cells were used to establish primary myoblast cultures by plating onto six gelatin-coated culture dishes and growing for 7 days before being switched to myoblast differentiation media for an additional 7 days (ref. 37). Aliquots of the remaining cells were injected intramuscularly into secondary mdx4cv recipient TA muscles.
In the primary cultures, we observed both µDys/eGFP-positive and µDys/eGFP-negative myotube clones (e.g., Figure 5). Counting six different plates established from each of the two injected mice revealed that 10.2 ± 3.8% of the myotube clones were µDys/eGFP-positive (one plate had >20% positive clones). The percentage of positive clones was always lower than the percentage of positive myofibers in the muscles from which they were derived, suggesting that myogenic progenitor cells were transduced at a lower efficiency than were myofibers, or that a significant fraction of the progenitors that are transduced in 2-day muscles go on to form myofibers without first becoming satellite cells. For the cell transplantation studies, 2 × 105 mononuclear cells isolated from Lv-injected TA muscles were transplanted by intramuscular injection into TA muscles of 3-week-old mdx4cv mice. Two weeks after transplantation, the injected muscles were harvested, cryosectioned, and analyzed for the presence of µDys/eGFP-positive myofibers. Analysis of three different injected muscles revealed the presence of between 3 and 10 µDys/eGFP-positive myofibers in each serial 10-µm cryosection spanning an ~200-µm section in the center of each transplanted muscle. No µDys/eGFP-positive myofibers were observed in muscles injected with primary myoblasts from a wild-type mouse or in uninjected controls. These two different assays therefore show that the injected lentiviral vectors stably transduced a myogenic progenitor cell population and could be found a year later in the satellite cell pool. Finally, these transduced satellite cells were able to (i) give rise to proliferating myoblasts capable of fusing into µDys/eGFP-positive myotubes in vitro and (ii) contribute to muscle regeneration and provide therapeutic gene products in dystrophic muscles in vivo.
Figure 5.
Stable retention in satellite cells of a µDys/eGFP lentiviral vector. Tibialis anterior (TA) muscles from mdx4cv mice injected 1 year earlier with Lv-HSA-µDys/eGFP were harvested and used to prepare muscle mononuclear cells. (a–d): Mononuclear cells from these primary cultures were plated at clonal density, grown for 7 days in myoblast proliferation medium, then switched to differentiation media for an additional 7 days before fixation of the cultures, immunostained with anti-GFP antibodies, and counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride to visualize nuclei. (a,c) A µDys/eGFP-positive clone from Lv-injected mdx TA muscle. (b,d) A clone generated from untreated age-matched wild-type mouse TA muscle as a control. (a,b) Brightfield photography; (c,d) fluorescence image of clones shown in a and b. The frequency of µDys/eGFP-positive clones was 10.2 ± 3.8% (n = 6 from each of two harvested muscles). (e–h) Immunostaining for µDys/eGFP-positive myofibers in mdx4cv TA muscles injected with 2 × 105 cells from (e) a wild type mouse TA muscle, or (f–h): mice 1 year post-lentiviral-injection into 2-day-old mdx4cv mice. Muscles in e–h were harvested 2-weeks postinjection with freshly isolated mononuclear cells. Bar = 50 µm. µDys/eGFP, microdystrophin/enhanced green fluorescent protein.
Discussion
A number of recent developments have raised the prospects for successful gene therapy of the muscular dystrophies.14 Important among these is the growing availability of different types of viral vectors with which to transfer genes to muscle. Recombinant AAV (rAAV) vectors have shown great promise for systemic gene delivery to mature, striated muscle fibers.38 However, rAAV vectors have a limited cloning capacity, rarely integrate into host cell chromosomes and are rapidly lost from dividing cells.39 In contrast, lentiviral vectors have a relatively high transgene carrying capacity coupled with an ability to stably transduce both dividing and nondividing cells.18,40 As lentiviral vectors integrate into target cell chromosomes, they represent a powerful tool to genetically modify stem cells, both ex vivo and in vivo. Gene therapy for DMD would benefit from use of a vector able to transduce mature myofibers as well as satellite cells, the primary muscle stem cell which is responsible for muscle regeneration in response to injury.28,29
Kobinger et al. showed Ebola and Mokola pesudotyped lentiviral vectors can transduce mdx skeletal muscle fibers and express mini-dystrophins, partially correcting the dystrophic histopathology when injected into limb muscles.16 Our data extend this analysis by showing that lentiviral vectors carrying a µDys gene can stably transduce both myofibers and satellite cells, leading to amelioration of the dystrophic pathology for at least 2 years. Importantly, although the level of transduction was not sufficient to completely halt ongoing myofiber turnover, dystrophin levels remained stable due to ongoing muscle regeneration supported by satellite cells, a proportion of which carried integrated copies of the µDys vector. Our studies add to growing evidence that lentiviral vectors are able to transduce quiescent satellite cells in vivo as well as cultured myoblasts in vitro.16,17,21,41 In several of these reports, reporter gene–positive cells were observed in muscle sections after intrauterine gene delivery with the anatomical criteria of satellite cells. However, other researchers have reported that cells of hematopoietic origin could settle in the satellite cell niche position.42 Therefore, it was important to know whether transduced satellite-like cells possess the ability to support muscle formation and regeneration both in vitro and in vivo. In our study, muscle mononuclear cells isolated a year after vector administration proliferated in myogenic primary culture conditions, and formed myotubes that expressed the µDys/eGFP gene carried by the lentiviral vector. We also observed that these cells were able to differentiate into µDys/eGFP-positive myofibers in vivo after transplantation into muscles of mdx4cv mice. Thus, stably transduced satellite cells retain integrated copies of lentiviral vectors and can express therapeutic genes for at least 2 years in dystrophic mice. These results suggest that lentiviral vectors could be useful for permanent genetic correction of myogenic stem cells in vivo.
The lentiviral vectors used in this study carried an expression cassette encoding a µDys/eGFP fusion protein gene. This vector afforded a number of advantages over delivering either a standard reporter or a µDys gene. First, the µDys protein has been shown to have a profound ability to rescue the dystrophic phenotype when expressed in transgenic mice or delivered to adult animals using rAAV vectors.5,7,11,13 Also, this fusion protein can be easy to detect and allows one to distinguish transduced cells from revertant fibers that exist in mdx mouse muscles.34,43 Although some reporter genes may be easier to detect following vector administration, they do not provide protection against the dystrophic phenotype, preventing the ability to monitor cell fate in mdx muscles for extended periods of time. Finally, the use of the α-skeletal actin promoter and enhancer (HSA; ref. 33) allowed the transgene to be expressed only in differentiating myocytes, myotubes, and myofibers, preventing gene expression in nonmyogenic cells that might be transduced by the vector. The µDys/eGFP fusion protein showed an ability to correct histopathological abnormalities, in an analogous manner to that obtained following rAAV vector delivered microdystrophins (note that the microdystrophins carried by rAAV vectors lack the carboxy-terminal domain, which is retained in the present constructs5,33 (Figure 1a). A key difference, however, was the significantly reduced degree of spread of the lentiviral vectors compare with rAAV vectors in injected muscles. Intramuscular injection of rAAV vectors can transduce nearly all the myofibers in injected muscles,44 whereas here we saw the transduction between ~20 and 60% of the muscle cross-sectional area. This reduced level of transduction likely accounts for the significant, but incomplete rescue of muscle specific force-generating levels (Figure 3c) and the inability to confer significant protection from eccentric contraction-induced injury (Table 1). Nonetheless, we did observe a marked reduction in the presence of centrally nucleated myofibers in transduced cells as well as an increase in myofiber diameter (Figure 3) and improved overall muscle morphology (Figures 2 and 4). We did not observe a marked decrease in centrally nucleated fibers in regions of the muscle that had lower levels of dystrophin, in contrast to results obtained using rAAV-mediated delivery of a microdystrophin.11 These differences could potentially be explained by a greater clustering of positive fibers in the lentiviral-injected muscles, which can leave larger areas of muscle without dystrophin expression (Figure 4). Indeed, studies in transgenic mice have suggested that mosaic but widely dispersed dystrophin expression can allow positive fibers to provide mechanical protection to adjoining dystrophin-negative fibers.36
Table 1.
Force development (kN/m2) by mdx4cv TA muscles in situ following 1 (LC1) or 2 (LC2) cycles of eccentric-contraction
Application of lentiviral technology to treat muscle disorders must overcome several problems. The biggest concern is the issue of safety, as integrating retroviral vectors have been associated with leukemia in hematopoietic therapies.45 Although we did not detect tumors in any of our treated animals, we followed a relatively small number of mice. Use of the α-skeletal actin promoter could be advantageous in this regard, as this promoter/enhancer is transcriptional active only in postmitotic muscle cells.33,46 A second problem relates to immune reactions against the transgene and vector. Our previous studies involving lentiviral vector delivery to adult mice suggested that vectors were eliminated fairly quickly, likely due to an immune response.21 However, groups that have delivered vectors expressing β-galactosidase in utero observed long-term expression that was not obviously associated with an immune response.20,41 In this study, delivery at postnatal day 2 of a lentiviral vector expressing the µDys/eGFP fusion gene led to expression of the transgene for at least 2 years. Injection of neonatal mice likely elicits tolerance to the vector, and the use of the highly muscle-specific HSA promoter may also facilitate evasion of a host immune response.47 These observations suggest that early diagnosis and gene therapy might be beneficial for treatment of this severe muscular dystrophy. The other major limitation of lentiviral vectors is their relatively poor spread in injected tissues. This limitation derives in large part from the relatively low titer of lentiviral preparations compared with rAAV. In our hands, a typical lentiviral preparation may include up to 1010 transducing units/ml, while it is not difficult to prepare rAAV at concentrations >1013 vector genomes/ml (refs. 7,21). One could consider using a combination of vector systems, with AAV vectors being used for transduction of pre-existing myogenic cells, and lentiviral vectors being used to stably transduce myogenic stem cells (either in vivo or ex vivo) to support muscle regeneration that would inevitably occur during normal exercise or following gradual loss of nonintegrated AAV vector genomes. We conclude that lentiviral-mediated gene transduction of both muscle fibers and satellite cells represents a promising approach to the treatment of muscular dystrophy.
Materials and Methods
Constructing a lentiviral transfer vector bearing HSA-ΔR4-R23/Δ71–78/eGFP (Lv-HSA-µDys/eGFP). A µDys/eGFP expression cassette was generated in several cloning steps. We first replaced TAGGAA (underlined nucleotides are the stop code of dystrophin) with TCTAGA (XbaI site) in pSV40pAΔ71–78 (ref. 5) using the QuikChange-XL Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA) (forward primer: 5′-GG AAACTGACAC AATTCTAGAA GTCTTTTCCAC-3′ reverse primer: 5′-GTGGAAAAGACTTCTAGA ATTGTGTCAGT TTCC-3′). A PCR-amplified XbaI-flanked eGFP-coding sequence without its translational initiation region (forward primer: 5′-GCTCTAGAGGTGAGCA AGGGCGAGGAG-3′ reverse primer: 5′-CCTCTAGAATTCCGGCCGC TTTAC-3′) was then inserted in frame to generate pSV40pAΔ71–78-eGFP. A 2,222-bp HindIII fragment from this plasmid was used to replace the 1,478-bp HindIII fragment in the pCK6-ΔR4-R23/Δ71–78 (ref. 5) to generate pCK6-ΔR4-R23/Δ71–78-eGFP (CK6-µDys/eGFP). A 4,358-bp EagI fragment containing ΔR4-R23/Δ71–78-eGFP (µDys/eGFP) in pCK6ΔR4-R23/Δ71–78-eGFP was inserted into a plasmid carrying the human α-skeletal actin promoter, a hybrid intron between the HSA first intron and the SV40 VP1 intron, and the SV40 polyadenylation site (pHSAvpSV40pA) (ref. 33) at the NotI site to generate pHSA-ΔR4-R23/Δ71–78-eGFP-pA. Finally, a 7,233-bp ClaI–PacI fragment from pHSA-ΔR4-R23/Δ71–78-eGFP-pA was inserted into pRRL-cPPT-CMV-X-PRE-SIN at ClaI and PacI sites to generate the lentiviral transfer vector bearing HSA-ΔR4-R23/Δ71–78-eGFP, which we refer to as Lv-HSA-µDys/eGFP.
Lentiviral transfer vectors and viral preparation. Lentiviral vectors were generated as described previously.21,40 Titers were assayed on HeLa or 3T3 cells with serial dilutions of vector preparations. The titer of these vector stocks were also estimated by measuring viral p24gag antigen using the human immunodeficiency virus-1 p24 Antigen Assay kit (Beckman Coulter, Fullerton, CA) and also compared to the titer of vectors with reporter genes, such as Lv-MSCV-eGFP, as described.21
Mouse strains and vector administration. C57BL/6Ros.Cg-Dmdmdx4cvJ (referred to herein as mdx4cv) and C57BL/6J mice were originally obtained from the Jackson Laboratory (Bar Harbor, MI) then maintained as breeding colonies at the University of Washington. Injection of vectors into muscles was performed as described previously.48 Briefly, 2-day-old mice were put on ice to anesthetize with hypothermia, and then injected with 5 µl of viral preparations with titers from 1.0 × 108 transducing units/ml into each TA muscle.
Immunostaining cultured muscle cells and frozen muscle sections. Cultured muscle cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized with 1% Triton X-100 for 10 minutes before immunostaining. These cells were then incubated with anti-GFP antibody (1:1,000 dilution; Molecular Probes, Eugene, OR) for 1 hour, followed by incubation with either Alexa 488- or Alexa 594-conjugated secondary antibodies (1:1,200) (Molecular Probes) for 1 hour. The 10-µm muscle sections were immunostained as described previously.21 Briefly, air-dried sections were rinsed with phosphate-buffered saline and treated with 1% normal goat serum for blocking before incubation with primary antibody, either a rabbit anti-GFP antibody (1:1,000 dilution; Molecular Probes) or an anti-N terminal dystrophin antibody (1:600) (ref. 49). Secondary antibodies were then applied using either, anti-rabbit (1:1,200) -Alexa488, -Alexa594, or -horseradish peroxidase (Molecular Probes), the latter visualized with diaminobenzidine (Vector Laboratories, Burlingame, CA). For fluorescence, nuclear staining and mounting with 500 ng/ml of 4′,6-diamidino-2-phenylindole, dihydrochloride (Molecular Probes) with VECTASHIELD (Vector Laboratories) was performed. Images were collected on a Nikon E1000 microscope (Nikon, Tokyo, Japan) under identical conditions using a Spot II CCD camera (Diagnostic Instruments, Sterling Heights, MI). Then percentage of centrally nucleated fibers and range of myofiber diameters were analyzed using an image analysis system, ImagePro-Plus (Media Cybernetics, Silver Spring, MD).
Muscle contractility assays. One year postinjection, mice were anesthetized with 2,2,2-tribromoethanol (Sigma, St Louis, MO) to maintain a depth of anesthesia that prevented all responses to tactile stimuli, and the contractile properties of the TA hindlimb muscles were measured in situ as previously described.13 Briefly, the tendons of the TA muscle were surgically exposed to immobilize the knee joint on the test apparatus, firmly secure the distal tendon to a position-force dual-mode servomotor with surgical suture, and to position needle electrodes flanking the peroneal nerve for stimulating the muscle. The muscle was positioned for optimal force production, and stimulated with 0.2-ms pulses at increasing frequencies to establish maximum isometric twitch (Pt) and tetanic (Po) force production. After determination of isometric contractile properties, muscles were subjected to a 4-minute repeated stimulation protocol to assess resistance to contraction-induced fatigue. Muscles were maximally stimulated for 300 ms at 2-second interval for the duration of the fatigue protocol, and once each at 60 seconds and 300 seconds after the completion of the protocol to assess force recovery. To determine the susceptibility of muscles to contraction-induced injury, each muscle was subjected to a stretch of 20% of optimal length while maximally stimulated, which was repeated after an additional 10 seconds of inactivity as described previously.13 At the completion of testing, the muscles were surgically excised, trimmed of any adherent nonmuscle tissue, weighed, and processed for storage and histological examination. For data analysis, we selected muscles with relatively high expression of dystrophin, being defined here as displaying between 10 and 30% of µDys/eGFP-positive myofibers.
Isolation, culturing, and transplantation of muscle mononuclear cells. Mononuclear cells were harvested from mdx4cv TA hindlimb muscles 1 year after injection with Lv-HSA-µDys/eGFP, as well as from an untreated wild-type mouse TA muscle as a control, for culturing and transplantation back into TA muscles of secondary recipient mdx4cv mice. Cryosections of each donor TA muscle were observed for GFP fluorescence, and higher expressing muscles were selected for the culture and transplantation procedures. TA muscles from 1-year-old C57BL/6 and injected mdx4cv mice were excised, minced, and digested in phosphate-buffered saline (pH 7.2; GIBCO, Invitrogen, Grand Island, NY) with final concentrations of 1 mmol/l CaCl2, 0.2% Collagenase II (Worthington Biochemical, Lakewood, NJ), and 1.2 U/ml Dispase (Worthington Biochemical) at 37 °C for 45 minutes, and then filtered through 70- and 40-µm nylon filters (BD Falcon, Franklin Lakes, NJ). Mononuclear cells were cultured on 0.67% gelatin-coated plates in medium containing F10 (GIBCO, Carlsbad, CA) medium with 10 mmol/l CaCl2 (F10C) plus 15% horse serum (GIBCO) and 5 ng/ml recombinant human basic FGF-2 (R&D systems, Minneapolis, MN). At day 7, differentiation was induced by rinsing the cultures and switching them to medium containing F10C with 1.5% horse serum and 6 mg/ml insulin for 48 hours, followed by refeeding cultures with F10C with 15% horse serum and insulin.21,37 At day 14, cells were fixed with 2% paraformaldehyde and 0.5% sucrose, then stained with antibodies against GFP followed with an Alexa488-conjugated secondary antibody.
Freshly isolated muscle mononuclear cells from each injected TA muscle were resuspended independently at a concentration of 2 × 105 cells/30 µl in phosphate-buffered saline, and then transplanted by direct injection into TA muscles of 3-week-old mdx4cv mice under isofluorane anesthesia. The same numbers of cells from age-matched C57BL/6 TA muscles were also injected as a control. Two weeks after transplantation, mice were killed, TA muscles harvested, cryosectioned, and observed via fluorescence microscopy (NIKON E1000; Nikon). These muscle sections were stained with an anti-GFP (Molecular Probes) antibody and visualized with an Alexa488-conjugated secondary anti-rabbit IgG (Molecular Probes).
Statistical analysis. Data were expressed as means ± SEM. Statistical comparisons were performed using one-factor or two-factor analysis of variance followed by Fisher's protected least significant difference test. A P value of <0.05 was considered statistically significant.
Acknowledgments
We thank Luigi Naldini and William R. Osborne for providing lentiviral vector backbones, and Stephen Hauschka for primary culture conditions and advice. These studies were supported by grants from the National Institutes of Health (NS46788, AG015434, and AG033610, to J.S.C.) and the Ministry of Education, Science, and Culture of Japan Grants-in-Aid (18890145, 20591004 to E.K.). S.L. was supported by a Development Grant from the Muscular Dystrophy Association.
REFERENCES
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C., and , Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Emery AE., and , Muntoni F.2003. Duchenne Muscular Dystrophy. Oxford University Press: Oxford [Google Scholar]
- Toussaint M, Chatwin M., and , Soudon P. Mechanical ventilation in Duchenne patients with chronic respiratory insufficiency: clinical implications of 20 years published experience. Chron Respir Dis. 2007;4:167–177. doi: 10.1177/1479972307080697. [DOI] [PubMed] [Google Scholar]
- Cox GA, Cole NM, Matsumura K, Phelps SF, Hauschka SD, Campbell KP, et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993;364:725–729. doi: 10.1038/364725a0. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- DelloRusso C, Scott JM, Hartigan-O'Connor D, Salvatori G, Barjot C, Robinson AS, et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci USA. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano R, Ishizaki M, Maeda Y, Uchida Y, Kimura E., and , Uchino M. Transduction of full-length dystrophin to multiple skeletal muscles improves motor performance and life span in utrophin/dystrophin double knockout mice. Mol Ther. 2008;16:825–831. doi: 10.1038/mt.2008.23. [DOI] [PubMed] [Google Scholar]
- Roberts ML, Wells DJ, Graham IR, Fabb SA, Hill VJ, Duisit G, et al. Stable micro-dystrophin gene transfer using an integrating adeno-retroviral hybrid vector ameliorates the dystrophic pathology in mdx mouse muscle. Hum Mol Genet. 2002;11:1719–1730. doi: 10.1093/hmg/11.15.1719. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Nalbantoglu J, Petrof BJ, Ebihara S, Guibinga GH, Tinsley JM, et al. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum Gene Ther. 1999;10:1299–1310. doi: 10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y, et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther. 2004;10:821–828. doi: 10.1016/j.ymthe.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Takeda S., and , Miyagoe-Suzuki Y. Gene therapy for muscular dystrophies: current status and future prospects. BioDrugs. 2001;15:635–644. doi: 10.2165/00063030-200115100-00001. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JS., and , Rando TA.eds) (2006Duchenne Muscular Dystrophy: Advances in Therapeutics Taylor and Francis: New York [Google Scholar]
- Inagaki K, Lewis SM, Wu X, Ma C, Munroe DJ, Fuess S, et al. DNA palindromes with a modest arm length of greater, similar 20 base pairs are a significant target for recombinant adeno-associated virus vector integration in the liver, muscles, and heart in mice. J Virol. 2007;81:11290–11303. doi: 10.1128/JVI.00963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger GP, Louboutin JP, Barton ER, Sweeney HL., and , Wilson JM. Correction of the dystrophic phenotype by in vivo targeting of muscle progenitor cells. Hum Gene Ther. 2003;14:1441–1449. doi: 10.1089/104303403769211655. [DOI] [PubMed] [Google Scholar]
- MacKenzie TC, Kobinger GP, Louboutin JP, Radu A, Javazon EH, Sena-Esteves M, et al. Transduction of satellite cells after prenatal intramuscular administration of lentiviral vectors. J Gene Med. 2005;7:50–58. doi: 10.1002/jgm.649. [DOI] [PubMed] [Google Scholar]
- Kafri T, Blömer U, Peterson DA, Gage FH., and , Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Seppen J, Barry SC, Harder B., and , Osborne WR. Lentivirus administration to rat muscle provides efficient sustained expression of erythropoietin. Blood. 2001;98:594–596. doi: 10.1182/blood.v98.3.594. [DOI] [PubMed] [Google Scholar]
- MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S, et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther. 2002;6:349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- Li S, Kimura E, Fall BM, Reyes M, Angello JC, Welikson R, et al. Stable transduction of myogenic cells with lentiviral vectors expressing a minidystrophin. Gene Ther. 2005;12:1099–1108. doi: 10.1038/sj.gt.3302505. [DOI] [PubMed] [Google Scholar]
- Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, et al. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci USA. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenneville SP, Chapdelaine P, Skuk D, Paradis M, Goulet M, Rousseau J, et al. Autologous transplantation of muscle precursor cells modified with a lentivirus for muscular dystrophy: human cells and primate models. Mol Ther. 2007;15:431–438. doi: 10.1038/sj.mt.6300047. [DOI] [PubMed] [Google Scholar]
- Ikemoto M, Fukada S, Uezumi A, Masuda S, Miyoshi H, Yamamoto H, et al. Autologous transplantation of SM/C-2.6+ satellite cells transduced with micro-dystrophin CS1 cDNA by lentiviral vector into mdx mice. Mol Ther. 2007;15:2178–2185. doi: 10.1038/sj.mt.6300295. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Kimura E, Han JJ, Li S, Fall B, Ra J, Haraguchi M, et al. Cell-lineage regulated myogenesis for dystrophin replacement: a novel therapeutic approach for treatment of muscular dystrophy. Hum Mol Genet. 2008;17:2507–2517. doi: 10.1093/hmg/ddn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM., and , Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA., and , Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal. J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F., and , Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford GE, Faulkner JA, Crosbie RH, Campbell KP, Froehner SC., and , Chamberlain JS. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol. 2000;150:1399–1410. doi: 10.1083/jcb.150.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kimura E, Ng R, Fall BM, Meuse L, Reyes M, et al. A highly functional mini-dystrophin/GFP fusion gene for cell and gene therapy studies of Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:1610–1622. doi: 10.1093/hmg/ddl082. [DOI] [PubMed] [Google Scholar]
- Phelps SF, Hauser MA, Cole NM, Rafael JA, Hinkle RT, Faulkner JA, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- Rafael JA, Sunada Y, Cole NM, Campbell KP, Faulkner JA., and , Chamberlain JS. Prevention of dystrophic pathology in mdx mice by a truncated dystrophin isoform. Hum Mol Genet. 1994;3:1725–1733. doi: 10.1093/hmg/3.10.1725. [DOI] [PubMed] [Google Scholar]
- Neville C, Rosenthal N, McGrew M, Bogdanova N., and , Hauschka S. Skeletal muscle cultures. Methods Cell Biol. 1997;52:85–116. [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P., and , Chamberlain JS. Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated virus vectors. Mol Ther. 2006;13:241–249. doi: 10.1016/j.ymthe.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Flotte TR. Gene therapy progress and prospects: recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2004;11:805–810. doi: 10.1038/sj.gt.3302233. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Gregory LG, Waddington SN, Holder MV, Mitrophanous KA, Buckley SM, Mosley KL, et al. Highly efficient EIAV-mediated in utero gene transfer and expression in the major muscle groups affected by Duchenne muscular dystrophy. Gene Ther. 2004;11:1117–1125. doi: 10.1038/sj.gt.3302268. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Morgan JE, Watkins SC., and , Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., and , Fischer A. Gene therapy for severe combined immunodeficiency: are we there yet. J Clin Invest. 2007;117:1456–1465. doi: 10.1172/JCI30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KJ., and , Hardeman EC. Quantitative analysis of the human α-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268:719–725. [PubMed] [Google Scholar]
- Hartigan-O'Connor D, Kirk CJ, Crawford R, Mulé JJ., and , Chamberlain JS. Immune evasion by muscle-specific gene expression in dystrophic muscle. Mol Ther. 2001;4:525–533. doi: 10.1006/mthe.2001.0496. [DOI] [PubMed] [Google Scholar]
- Kimura E, Maeda Y, Arima T, Nishida Y, Yamashita S, Hara A, et al. Efficient repetitive gene delivery to skeletal muscle using recombinant adenovirus vector containing the Coxsackievirus and adenovirus receptor cDNA. Gene Ther. 2001;8:20–27. doi: 10.1038/sj.gt.3301359. [DOI] [PubMed] [Google Scholar]
- Rafael JA, Cox GA, Corrado K, Jung D, Campbell KP., and , Chamberlain JS. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J Cell Biol. 1996;134:93–102. doi: 10.1083/jcb.134.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]