Abstract
Integrating lentiviral vectors based on the human immunodeficiency virus type-1 (HIV-1) can transduce quiescent cells, which in lung account for almost 95% of the epithelial cell population. Pseudotyping lentiviral vectors with the envelope glycoprotein from the Ebola Zaire virus, the lymphocytic choriomeningitis virus (LCMV), the Mokola virus, and the vesicular stomatitis virus (VSV-G) resulted in transduction of mouse alveolar epithelium, but gene expression in the lung of C57BL/6 and BALB/c mice waned within 90 days of vector injection. Intratracheal delivery of the four pseudotyped lentiviral vectors resulted in transgene-specific T-cell activation in both mouse strains, albeit lower than that achieved by intramuscular injection of the vectors. We performed an adoptive transfer of luciferase-specific T cells, isolated from spleen or lung of donor mice injected with VSV-G-pseudotyped lentivirus vector expressing luciferase into the muscle or lung, respectively, into recipient recombination-activating gene (RAG)–deficient mice transduced in lung with adenovirus expressing firefly luciferase (ffluc2). Gene expression declined within 7 days of adoptive transfer approaching background levels by day 36. Taken together, our results suggest that the loss of transduced cells in lung is due to VSV-G.HIV vector–mediated activation of transgene-specific T cells rather than as result of normal turnover of airway cells.
Introduction
Lentiviral vectors based on the human immunodeficiency virus type-1 (HIV-1) can efficiently transduce dividing and quiescent cells.1 The large packaging capacity of lentiviral vectors in addition to their ability to stably integrate into the host's genome has rendered these vectors effective gene transfer tools.1 Targeting specific cell types is achieved by pseudotyping lentiviral vectors with surface glycoproteins of enveloped viral vectors that have inherent tropism for a specific tissue. HIV-1-based lentiviral vectors are most commonly pseudotyped with the surface glycoprotein of the vesicular stomatitis virus (VSV-G). Pseudotyping with VSV-G allows the production of high-titer vector preparations.1 Furthermore, the broad tropism of VSV-G results in the efficient transduction of various tissue targets. In addition to VSV-G, surface glycoproteins from other enveloped viruses such as Ebola Zaire,2,3 lymphocytic choriomeningitis (LCMV),4 Mokola,5 gibbon ape leukemia,6 respiratory syncytial,2 and severe acute respiratory syndrome virus7 have been used to generate pseudotyped HIV-1-based lentiviral vectors. In addition to HIV-1-based lentiviral vectors, nonprimate lentiviral vectors (i.e., feline immunodeficiency virus) are also being developed,8,9 which have the added advantage of improved safety and minimal risk of generation of wild-type virus by homologous recombination.
Lentiviral vectors are being developed for lung genetic diseases, such as cystic fibrosis, as they can accommodate large genes, transduce quiescent cells, and depending on the pseudotype, can target specific cell populations in airway. To improve the transduction of airway epithelium in vivo, envelope glycoproteins derived from viruses tropic for airway epithelium, such as Ebola,2,3 GP64,8,9 and severe acute respiratory syndrome,7 have been generated. Although their transduction efficiency in in vitro model systems of airways2,7 and the mouse nasal airway epithelium has been encouraging, efficient transduction of lung epithelium by pseudotyped lentiviral vectors remains challenging. Most lung-directed gene transfer studies utilized VSV-G-pseudotyped lentiviral vectors to transduce adult mouse lung or fetal mouse,10 fetal lamb,11 and fetal macaque lung.12 Transduction of mouse nasal airway by VSV-G-pseudotyped lentiviral vectors requires preconditioning or damage of the airway for effective transduction.13,14 In lung VSV-G-pseudotyped HIV vector can transduce the alveolar epithelium and conducting airway epithelium in the absence of tight-junction disruption agents.10,11,12,15
In this study, we evaluated the transduction efficiency of lentiviral vectors pseudotyped with the surface glycoproteins of the Ebola Zaire virus variant NTDL6, LCMV, Mokola, and VSV-G in the lung of C57BL/6 and BALB/c mice. As lentiviral vectors have been shown to activate CD8+ and CD4+ T-cell responses and are being developed as vaccines,16,17,18 we also characterized the potential of these vectors to activate transgene and/or vector-specific T cells following lung delivery, which we hypothesized could compromise the potential of this approach in vivo.
Results
Intratracheal delivery of pseudotyped lentiviral vectors activates GFP-specific T cells
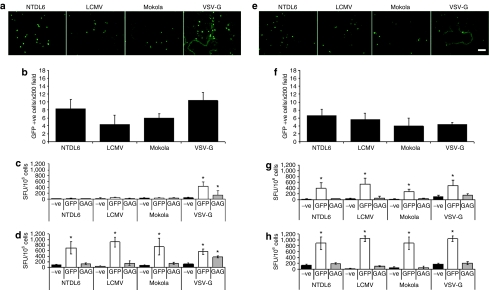
Pseudotyped lentiviral vectors expressing green fluorescence protein (GFP) were delivered intratracheally (i.t.) to C57BL/6 and BALB/c mouse lung in an attempt to determine whether these vectors were capable of activating transgene-and/or vector-specific T cells. Vector-treated mice were killed 21 days later (peak of transgene expression in mouse lung; data not shown). The lungs were fixed for analysis of GFP expression and the spleens harvested for lymphocyte isolation and subsequent analysis for GFP- and/or gag-specific T-cell responses using the interferon-γ (IFNγ) enzyme-linked immunosorbent spot (ELISpot) assay. For C57BL/6 mice, GFP expression was evidenced only in the alveolar epithelium for all vectors at varying degrees of efficiency (Figure 1a,b). VSV-G- and NTDL6-pseudotyped lentiviral vectors were the most efficient vectors followed by the Mokola and LCMV-pseudotypes (Figure 1a,b). Analysis of the splenocytes for GFP- and gag-specific T cells demonstrated significant GFP- and gag-specific T-cell activation only in mice treated with the VSV-G-pseudotyped lentivirus vector (Figure 1c; P < 0.05, analysis of variance, Student–Newman–Keuls test, n = 4). The transduction efficiency of the lentiviral vectors in BALB/c mouse lung was quite different when compared to C57BL/6 mouse lung. In this instance, the NTDL6-pseudotyped lentivirus vector was the most efficient at transducing the alveolar epithelium followed by the LCMV- and Mokola-pseudotyped vectors (Figure 1e,f). The least efficient vector in BALB/c mouse lung was the VSV-G-pseudotyped vector (Figure 1e,f). Most interesting, however, was the pattern of GFP-specific T-cell activation. In BALB/c mouse lung, all four pseudotyped lentiviral vectors activated high frequencies of GFP-specific T cells (Figure 1g). No significant activation of gag-specific T cells was observed for any of the pseudotyped lentiviral vectors.
Figure 1.
GFP gene expression in lung of C57BL/6 and BALB/c mice and subsequent activation of GFP-specific T cells. (a) Representative images of vector-transduced mouse lung from C57BL/6 mice harvested at day 21 and (b) Quantitation of GFP-expressing cells. NTDL6- and VSV-G-pseudotyped vectors were the most efficient vectors at transducing the alveolar epithelium, followed by the Mokola- and LCMV-pseudotyped vectors. (c) Following intratracheal (i.t.) injection only the VSV-G-pseudotyped vector activated GFP-specific and vector-specific T cells. (d) Following intramuscular (i.m.) injection of C57BL/6 mice all four vectors activated significant frequencies of GFP-specific T cells. Interestingly, gag-specific T cells were only observed in C57BL/6 mice injected with the VSV-G-pseudotyped lentivirus vector. (e) Representative images of vector-transduced mouse lung from BALB/c mice harvested at day 21 and (f) Quantitation of GFP-expressing cells. The NTDL6-pseudotyped vector was the most efficient at transducing the alveolar epithelium followed by the LCMV-, Mokola-, and VSV-G-pseudotyped lentiviral vectors. (g) In BALB/c mice, when the pseudotyped lentiviral vectors were injected i.t. there was significant activation of GFP-specific T cells. (h) Following i.m. injection of the four vectors in BALB/c mice a significant GFP-specific T-cell activation was observed for all vectors. T-cell activation was quantified and presented as SFU/106 cells. Results presented are the average of n = 4 ± SD (error bar)*P < 0.05, analysis of variance, Student–Newman–Keuls test. Bar = 100 µm. SFU, spot forming units.
Intramuscular injection of pseudotyped lentiviral vectors activates GFP-specific T cells
To gauge the relevance of the transgene-specific T cells, activated in response to the lung delivery of the pseudotyped lentiviral vectors, we injected the four vectors in the muscle of C57BL/6 and BALB/c mice. It is well documented that direct muscle injection of vectors elicits high frequencies of transgene- and in some instances vector-specific T cells. Lentiviral vectors were pseudotyped with NTDL6, LCMV, Mokola, or VSV-G and injected into the right hindlimb of C57BL/6 or BALB/c mice. A kinetics time course experiment of T-cell activation was performed in C57BL/6 and BALB/c mice, with day 17 chosen to represent the peak of T-cell activation following intramuscular (i.m.) injection of 1–4 × 108 transducing units (TU) (data not shown). C57BL/6 and BALB/c mice (n = 4) were necropsied at day 17 after lentivirus vector injection. The spleens were harvested for lymphocyte isolation and subsequent analysis by IFNγ ELISpot for GFP- and gag (p17/p24)-specific T cells. For both C57BL/6 and BALB/c mice, all four pseudotyped lentiviral vectors activated high frequencies of GFP-specific T cells (Figure 1d,h, respectively). There was also a nonstatistical significant trend of a higher GFP-specific T-cell activation in BALB/c mice. Intriguingly, we observed significant activation of gag-specific T cells only in C57BL/6 mice injected with the VSV-G-pseudotyped lentivirus vector (Figure 1d).
VSV-G mediated GFP transfer in mouse airway is transient
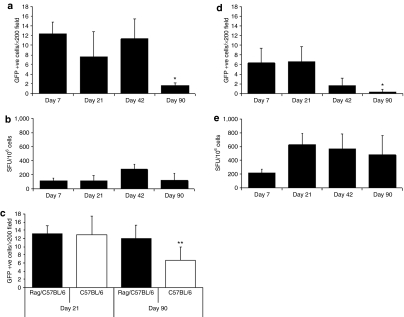
VSV-G-pseudotyped lentivirus vector delivery in mouse lung activates transgene-specific T cells (Figure 1c,g). We next evaluated the impact of these transgene-specific T cells on the long-term gene expression mediated by the delivery of the VSV-G-pseudotyped lentivirus vector to mouse lung. Vector was injected i.t. into C57BL/6 and BALB/c mouse lung and gene transfer efficiency as well as transgene-specific T-cell activation were monitored longitudinally up to day 90 after the single vector dose instillation. Specifically, vector-treated mice were killed at days 7, 21 (peak of transgene expression), 42, and 90 (proposed turnover time of airway epithelium).
In terms of GFP expression, VSV-G-pseudotyped lentivirus vector–mediated gene expression in alveolar epithelium was higher in C57BL/6 than BALB/c mouse lung at days 7, 42, and 90 (P < 0.05, Student's t-test) (Figure 2a,d, respectively). Although gene expression was stable in C57BL/6 mouse lung through to day 42, there was a significant decrease (P < 0.05, analysis of variance, Student–Newman–Keuls test) in the number of GFP-expressing cells by day 90. Interestingly, GFP-specific T cells were present as early as day 7, increased by day 42, and were still present at day 90 after vector injection (Figure 2b). For BALB/c mice, the number of GFP-positive alveolar epithelial cells was low but remained stable to day 21 (Figure 2d). By day 42, however, there was a significant decrease in the number of GFP-expressing cells and by the end of the experiment (day 90) only rare GFP-expressing cells were observed (Figure 2d). When compared to C57BL/6 mice, we observed similar GFP-specific T-cell activation at day 7. However, unlike in C57BL/6 mice, in BALB/c mice GFP-specific T-cell responses peaked at day 21 and remained at high levels through day 90 (the end of the experiment) (Figure 2e). Taken together, the GFP expression and transgene-specific T-cell activation data suggest that GFP-specific T cells destroyed GFP-expressing cells in C57BL/6 and BALB/c mouse lung around 6 weeks after vector injection, evidenced as a significant elevation of GFP-specific T cells and a significant decrease of GFP-expressing cells. Indeed, in BALB/c mice, we found a clear correlation between the high frequencies of GFP-specific T cells (Figure 2e) and the diminution of GFP expression in lung (Figure 2d).
Figure 2.
GFP expression in mouse lung and activation of GFP-specific T cells. (a) VSV-G-pseudotyped lentivirus vector was injected i.t. to C57BL/6 mouse lung at day 0. At day 7 GFP-mediated gene expression was observed in the cells of the alveolar epithelium the numbers of which remained stable to day 42. There was a significant decrease in GFP-expressing cells at day 90. (b) GFP-specific T-cell activation was observed at day 7 and remained stable through day 90, with the exception of day 42 in which T-cell activation increased, although not significantly. (c) Vector was delivered to RAG-deficient/C57BL/6 and C57BL/6 mouse lung and GFP expression assessed at days 21 and 90. GFP expression in RAG-deficient/C57BL/6 mice remained stable while for C57BL/6 mice a significant decrease in GFP-expressing cells was observed at day 90. (d) At day 7 after i.t. injection of vector to BALB/c mouse lung only low numbers of GFP-expressing cells were observed in the alveolar epithelium which significantly decreased at day 90. (e) GFP-specific T-cell activation in BALB/c mouse lung was observed at day 7 that increased at day 21 and remained stable through to day 90. T-cell activation was quantified and presented as SFU/106 cells. Results presented are the average of n = 4 ± SD (error bar)* P < 0.05, analysis of variance, Student–Newman–Keuls test, n = 4. **P = 0.02, Student's t-test, n = 5. GFP, green fluorescence protein; SFU, spot forming units.
Longevity of lentivirus vector–mediated GFP expression in the absence of a T-cell response
To directly assess whether transgene- and/or vector-specific T cells were activated in response to lentivirus vector delivery in lung, we assessed long-term gene expression in C57BL/6 mice and in recombination-activating gene (RAG)-deficient mice, which are devoid of major conventional populations of B cells and T cells.19 VSV-G-pseudotyped lentivirus vector was injected i.t. into C57BL/6 and RAG-deficient/C57BL/6 mouse lung with GFP expression being monitored at day 21 (peak of gene expression) and day 90 (proposed turnover time of airway epithelium). In both mouse models, the vector transduced only alveolar epithelium and at day 21 no difference in GFP expression was observed between C57BL/6 and RAG-deficient/C57BL/6 mice (Figure 2c). However, by day 90 GFP expression in the airway epithelium of C57BL/6 mice was significantly reduced (P = 0.02, Student's t-test) when compared to the GFP expression in the airway epithelium of RAG-deficient/C57BL/6 mice, which remained at similar levels to those observed at day 21 (Figure 2c).
Adoptive transfer of transgene-specific T cells activated in response to VSV-G-mediated lentiviral gene transfer ablates transgene-expressing cells
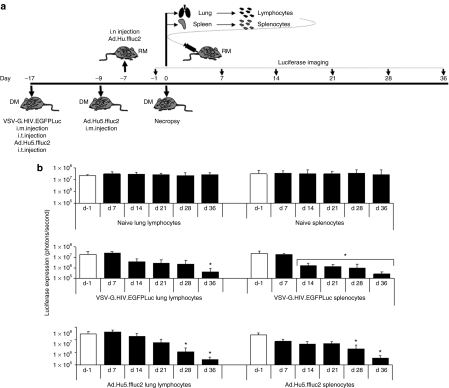
To decipher whether the activated transgene-specific T cells could destroy transgene-expressing cells in mouse lung, we performed a series of adoptive transfer studies. For these studies, we used the luciferase transgene as its use allowed the longitudinal assessment of transgene expression through the use of luminescence. Donor mice consisted of groups of C57BL/6 mice injected in lung or muscle with either VSV-G-pseudotyped lentivirus vector expressing luciferase (Luc) (VSV-G.HIV.EGFPLuc) or Ad.Hu5 vector expressing firefly luciferase (Ad.Hu5.ffluc2) (Figure 3a). As the peak of transgene-specific T-cell activation was contingent upon the vector and route of instillation, groups of donor mice were injected at various time points before the adoptive transfer to allow for the transfer of T cells at the peak of T-cell activation for each group. At day −17, groups of C57BL/6 mice were injected i.m. with VSV-G.HIV.EGFPLuc vector, i.t. with VSV-G.HIV.EGFPLuc, and i.t. with Ad.Hu5.ffluc2 vector. We also included a group of C57BL/6 mice that received an i.t. injection of Ad.Hu5.LacZ to exclude vector-specific killing for those recipient mice that received Ad.Hu5.ffluc2 in lung. At day −9, a group of mice was injected i.m. with Ad.Hu5.ffluc2 vector. The recipients of the donor splenocytes were RAG-deficient/C57BL/6 mice that had been injected i.n. intranasally with Ad.Hu5.ffluc2 vector at day −7.
Figure 3.
Impact on ffluc2 expression following adoptive transfer of activated T cells. (a) Schematic diagram of the experimental design. In brief, donor mice consisted of C57BL/6 mice that were injected i.t. or i.m. with either VSVG.HIV.EGFPLuc or Ad.Hu5.ffluc2 vector. Recipient mice were RAG-deficient/C57BL/6 mice that were injected i.n. with Ad.Hu5.ffluc2 vector. At the peak of T-cell activation, lung-lymphocytes and splenocytes were isolated and transferred intravenously (i.v.) to the recipient mice. As controls, RAG-deficient/C57BL/6 mice were i.v. injected with either lung-lymphocytes or splenocytes isolated from naive C57BL/6 mice. Expression of ffluc2 was monitored through to day 36 after adoptive transfer (b) Quantitation (photons/second) of ffluc2 expression before and up to 36 days after adoptive transfer (*P < 0.05, analysis of variance, Student–Newman–Keuls test). DM, donor mice; i.m., intramuscularly; i.n., intranasally; i.t., intratracheally; RM, recipient mice.
At the time of adoptive transfer (day 0) lymphocytes were isolated from donor mice (lung-lymphocytes from mice administered vector i.t. and splenocytes from mice administered vector i.m.) and intravenously injected into recipient mice in a 1:1 ratio. Specifically, RAG-deficient/C57BL/6 mice were injected intravenously with (i) naive lung-lymphocytes; (ii) naive splenocytes; (iii) Ad.Hu5.ffluc2 activated lung-lymphocytes; (iv) Ad.Hu5.ffluc2 activated splenocytes; (v) VSV-G.HIV.EGFPLuc activated lung-lymphocytes; (vi) VSV-G.HIV.EGFPLuc activated splenocytes; and (vii) Ad.Hu5.LacZ activated lung-lymphocytes. The lung-lymphocytes and splenocytes were subjected to an IFNγ ELISpot to evaluate the level of luciferase-specific T-cell activation. As expected, no luciferase-specific T cells were observed in the naive lung-lymphocytes and naive splenocytes or in the lung lymphocytes isolated from the C57BL/6 mice injected i.t. with the Ad.Hu5.LacZ vector. In lung-lymphocytes isolated from lung of mice injected i.t. with VSV-G.HIV.EGFPLuc or Ad.Hu5.ffluc2 vector, luciferase-specific CD8 T-cell responses were in the order of 600–780 spot forming units/106 cells. For lymphocytes isolated from spleen of mice injected i.m. with VSV-G.HIV.EGFPLuc or Ad.Hu5.ffluc2 vector, luciferase-specific T cells were ~1,200 spot forming units/106 cells.
For the groups of RAG-deficient/C57BL/6 mice that received naive lung-lymphocytes or naive splenocytes from C57BL/6 mice, there was no diminution of ffluc2 expression following adoptive transfer (Figure 3b). Interestingly, for those groups of RAG-deficient/C57BL/6 mice that received (i) naive lung-lymphocytes, (ii) VSV-G.HIV.EGFPLuc activated lung-lymphocytes, and (iii) Ad.Hu5.ffluc2 activated lung-lymphocytes there was an unexplained transient nonsignificant increase of ffluc2 gene expression at day 7, which subsided by day 14 (Figure 3b). For the RAG-deficient/C57BL/6 mouse groups that were injected intravenously with either lung-lymphocytes or splenocytes isolated from mice injected with VSV-G-pseudotyped lentivirus vector or Ad.Hu5.ffluc2 vector, ffluc2 expression waned significantly (P < 0.05, Student's t-test) within 14 and 28 days, respectively, and by day 36 neared the detection sensitivity of the Xenogen imaging system (Caliper Life Sciences, Hopkinton, MA) (Figure 3b). This effect was achieved from lymphocytes of mice immunized with vector administered either i.t. (lung-derived lymphocytes) or i.m. (splenocytes).
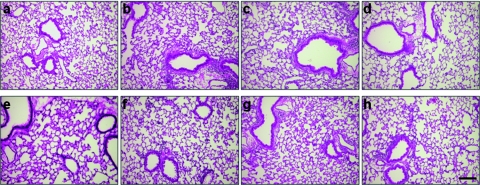
The impressive diminution of ffluc2 expression warranted the evaluation of the lung tissue of the recipient RAG-deficient/C57BL/6 mice at day 36 (the end of the experiment) for signs of inflammation. We observed no evidence of inflammation in the lung sections of any of the treated RAG-deficient/C57BL/6 mice (Figure 4) when compared to the control groups that consisted of RAG-deficient/C57BL/6 mice injected with naive lung-lymphocytes or naive splenocytes (Figure 4a,e, respectively).
Figure 4.
Pathology of recipient mouse lung tissue after adoptive transfer of naive or vector-activated lung-lymphocytes or splenocytes. Lungs were harvested and evaluated for signs of cellular infiltration at day 36 after adoptive transfer of (a) naive lung-lymphocytes; (b) VSV-G.HIV.EGFPLuc activated lung-lymphocytes; (c) Ad.Hu5.ffluc2 activated lung-lymphocytes; (d) no adoptive transfer; (e) naive splenocytes; (f) VSV-G.HIV.EGFPLuc activated splenocytes; (g) Ad.Hu5.ffluc2 activated splenocytes; (h) Ad.Hu5.LacZ activated lung-lymphocytes. Representative tissue sections counterstained with hematoxylin and eosin are shown. Bar = 100 µm.
Discussion
Lentiviral vectors are being developed for the efficient delivery of large therapeutic genes to the diseased airway epithelium. Interestingly, most airway-directed lentiviral vector gene transfer studies have been performed in the mouse nasal airway8,13,14 as this tissue models key features of the human conducting airway such as tight-junction formation and airway cell composition,20,21 and combined with its small surface area and ease of delivery allows for the assessment of gene transfer efficiency and preferential tropism. Few studies have delivered lentiviral vectors directly to the lung airways in vivo.10,11,12
Sinn et al. demonstrated that GP64-pseudotyped lentiviral vectors (based on the feline immunodeficiency virus) expressing firefly luciferase can effectively transduce the BALB/c mouse nasal airway epithelium resulting in yearlong transgene expression.8,9 However, in another study Kremer et al. showed that GP64-pseudotyped lentivirus vector–mediated gene transfer to C57BL/6 mouse nose was low and waned within 6 months of vector inoculation.22 As it has been proposed that the turnover of the mouse airway epithelium is in the order of 3 months (ref. 23) the diminution of LacZ-expressing cells in the Kremer study could be explained as normal turnover of positively transduced terminally differentiated airway cells. In light, however, of the recent report that the mouse airway epithelium turnover is in the order of >12 months (ref. 24) a plausible explanation for the loss of gene expression with time in the Kremer study22 is the activation of cytolytic T cells.
We hypothesized that lentivirus vector–mediated transduction of resident lung antigen–presenting cells could activate transgene- and/or vector-specific T cells that could negatively impact on effective gene transfer to mouse lung epithelium. When four different pseudotyped lentiviral vectors were injected i.t. into BALB/c mice, we observed high levels of transgene-specific T cells, the frequencies of which were similar to those observed following i.m. injection of these vectors. In C57BL/6 mice, only one of the four vectors, the VSV-G-pseudotyped lentivirus vector, activated both transgene- and vector-specific T cells following i.t. delivery. This difference in T-cell activation between BALB/c and C57BL/6 mice did not appear to depend on the vector performance in lung as the NTDL6-, LCMV-, and Mokola-pseudotyped vectors transduced the alveolar epithelium of both mouse models equally well. Interestingly, in the longitudinal gene expression studies, the kinetics of GFP-specific T-cell activation between the two mouse models was different. The peak of GFP-specific T-cell activation for C57BL/6 mice was found to be around day 42 whereas for BALB/c mice it appeared to be around day 21. When we correlated the frequency of GFP-specific T-cell activation with the corresponding number of GFP-expressing cells in the airway of each vector-treated animal, we observed a pattern of diminution of GFP expression soon after the peak of GFP-specific T-cell activation. As the VSV-G-pseudotyped lentivirus vector was equally efficient at activating GFP-specific T cells in both mouse strains and given the availability of RAG-deficient/C57BL/6 mice, we studied the cytolytic properties of C57BL/6 lung-lymphocytes and also splenocytes activated in response to lung or muscle delivery of either VSV-G-pseudotyped lentivirus vector or adenovirus vector. We confirmed the previous findings that adoptive transfer of adenovirus-activated splenocytes in RAG-deficient/C57BL/6 mice can destroy transgene-expressing cells.25,26,27 We also found that lymphocytes isolated from lung of C57BL/6 mice injected i.t. with adenovirus can destroy transgene-expressing cells. Most importantly, we demonstrate that VSV-G-pseudotyped lentiviral vectors also have the potential to elicit cytolytic transgene-specific T cells when delivered to the lung of C57BL/6 mice.
It has become increasingly apparent that the therapeutic benefit of viral-mediated gene therapy may be hindered by the activation of destructive T cells activated in response to the therapeutic gene product and/or the gene transfer vector.28 Indeed, it is long known that adenovirus vector delivery to lung activates cytolytic transgene-specific T cells29 that have hampered the development of adenovirus as a gene therapy vector. Our findings suggest that similar issues are associated with the application of pseudotyped lentiviral vectors in lung, which may hinder the development of these vectors for gene therapy of pulmonary genetic diseases, such as cystic fibrosis. In this study, we used the GFP and luciferase reporter genes to study transgene-specific T-cell activation following lentivirus-mediated gene transfer to mouse lung. Both these transgenes have been demonstrated to elicit transgene-specific T cells in C57BL/6 and BALB/c mice.30 Nonimmunogenic transgenes, including rhesus α-fetoprotein31 and the rhesus β-chain of chorionic gonadotropin,32 which express “self”-antigen have demonstrated lack of transgene-specific immune response activation in large-animal gene transfer studies.31 However, for gene therapy of genetic diseases caused by specific gene mutation(s), it is highly probable that expression of the “normal” therapeutic gene product will be recognized by the host as a “non-self”-antigen thus activating transgene-specific T cells with potentially adverse effects. Indeed, using computational analysis, we predicted the likelihood of a CFTR (cystic fibrosis transmembrane conductance regulator)-specific T-cell activation following expression of the therapeutic CFTR gene in cystic fibrosis subjects with the most common mutation, a deletion of phenylalanine at residue 508 (ΔF508) (ref. 33). We proposed that expression of the therapeutic CFTR in cystic fibrosis subjects with the ΔF508 CFTR mutation could be subject to antigen crosspresentation or activation of quiescent CFTR-specific T cells. For the development of lentiviral vectors for gene therapy of pulmonary diseases, we propose the evaluation of the impact of transgene-specific T cells in lentiviral-based preclinical animal studies.
Materials and Methods
DNA constructs and vector production. The helper packaging construct pCMVΔR8.2 encoding for the HIV helper function, the transfer vectors pHR.CMV.GFP encoding for the GFP and pCH.CMV.EGFPLuc (A. Ibrahimi, G.V. Velde, V. Reumers, J. Toelen, I. Thiry, C. Vandeputte et al., manuscript submitted) encoding enhanced GFP and luciferase (luc) a kind gift from Dr Zeger Debyser (Katholieke Universiteit Leuven, Belgium), and plasmids pcDNA.NTDL6, pcDNA.LCMV, pcDNA.Mokola, or pcDNA.VSV-G encoding, respectively, for the NTDL6, LCMV, Mokola, VSV-G viral envelope were used to generate pseudotyped lentivirus vector as previously described.3,34 Pseudotyped lentivirus vector was produced by triple transfection of 293T cells using the CaPO4 precipitation method.2 Vector was resuspended in complete Dulbecco's modified Eagle's medium and stored at −80 °C. TU/ml was determined for each vector stock by counting GFP-positive cells by limiting dilution on 293T cells. All preparations of lentivirus vector underwent quality control for presence of replication-competent lentivirus by monitoring p24 antigen expression using the p24 ELISA (Alliance HIV-1 p24 ELISA; PerkinElmer, Waltham, MA) as previously shown.2 Lentivirus vector stocks with titers ranging from 0.1 to 6 × 109 TU/ml were used. E1/E3-deleted replication-deficient Ad.Hu5 vectors expressing either β-galactosidase (LacZ) or ffluc2 (average titer of 5 × 1012 particles/ml) were created as previously described.30,35 All experiments involving the production and in vivo evaluation of the pseudotyped lentiviral or adenoviral vectors were performed under biosafety level 2 containment, as approved by the Institutional Biosafety Committee of the University of Pennsylvania.
Mice. For experiments C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and used at 6–8 weeks of age. Mice were housed under specific pathogen-free conditions at the University of Pennsylvania's Translational Research Laboratories. All animal procedure protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. For muscle injections: groups of 4–5 mice were anaesthetized with ketamine/xylazine and injected i.m. in the right hindlimb with 0.5–3 × 108 TU (two sites with 25 µl) with pseudotyped lentivirus vector. Mice were killed after 17 days and the spleens harvested. For lung i.t. injections: the trachea was exposed through a midline incision, and 0.5 to 3 × 108 TU of virus in a final volume of 50 µl of phosphate-buffered saline (PBS), was instilled into the trachea with a 27 gauge needle. Upon completion of the instillation, the needle was removed and pressure gently applied over the tracheal puncture site. The skin and fascia were closed in one layer with interrupted sutures (4-0 Vicryl). Vector (0.5 to 3 × 108 TU) was also delivered intranasally as previously described.36 At necropsy, the lungs were fix-inflated with 10% neutral buffered formalin at room temperature (RT) in the dark for 24 hours. The lungs were washed twice in PBS and then frozen with optimal cutting temperature compound. Cryosections (10 µm) were prepared and mounted with Vectashield containing 4′,6-diamidino-2-phenylindole. Transduction efficiency was estimated by examining 10 high-power (×200) fields from cryosections with a 200-µm interval. For intravenous injections, the solution up to 300 µl was injected via the tail vein. All experiments were performed in quadruplicate on at least two separate occasions.
Adoptive transfer of splenocytes and lung-lymphocytes. C57BL/6 mice were injected i.t. or i.m. with either VSV-G-pseudotyped lentivirus vector expressing luciferase (Luc) or adenovirus vector serotype 5 (Ad.Hu5) expressing firefly luciferase (ffluc2) or β-galactosidase (LacZ). The mice were killed at the peak of transgene-specific T-cell activation in either spleen or lung, which was day 17 for VSV-G-pseudotyped lentivirus vector delivered i.m. or i.t., Ad.Hu5 vector delivered i.t.; and day 8 for the Ad.Hu5 vector delivered i.m. The splenocytes and lung-lymphocytes were then harvested and a 300 µl dose, containing either 107 splenocytes or 2.5 × 106 lung-derived lymphocytes, injected intravenously via the tail vein (one donor mouse = one recipient mouse) into recipient RAG-deficient mice on C57BL/6 background (C.129S7(B6)-Rag1tm1Mom/J; Jackson Laboratory) which had been injected intranasally with Ad.Hu5 vector expressing ffluc2 7 days before. Expression of ffluc2 in the recipient RAG-deficient/C57BL/6 mice was monitored the day before and up to 36 days after the adoptive transfer.
Cell isolation.
Splenocytes: Spleens were harvested from the treated mice and transferred into 1× Liebowitz's-15 (L-15; Cellgro, Mediatech, Herndon, VA) at RT. The tissues were homogenized and filtered through a 70-µm cell strainer. Cells were centrifuged for 5 minutes at 1,600 r.p.m. at 24 °C and the cell pellet resuspended in fresh L-15 media and centrifuged for 5 minutes at 1,600 r.p.m. at 24 °C. The cell pellet was resuspended in complete media (Dulbecco's modified Eagle's medium, 10% heat-inactivated fetal bovine serum, 1% penicillin–streptomycin, 10 mmol/l Hepes, 0.1 mmol/l nonessential amino acids and 10−6 M 2-mercaptoethanol) and the cells were overlaid onto a Ficoll-Paque gradient layer and centrifuged for 20 minutes at 2,000 r.p.m. at 24 °C. The resulting highly pure population of spleen-derived lymphocytes was harvested from the interface, washed in PBS and centrifuged for 5 minutes at 1,600 r.p.m. at 24 °C. The splenocytes were resuspended in complete media.
Lung-lymphocytes: Lungs were inflated through the trachea with 1 ml solution of 300 U/ml collagenase type-1 (Worthington Biochemical, Lakewood, NJ) and 50 U/ml Dnase-1 (Sigma-Aldrich, St Louis, MO) in RPMI (Invitrogen, Carlsbad, CA). The trachea was removed and lungs incubated on a shaker at 37 °C for 1 hour. The tissue remnants were homogenized through a 70-µm filter (BD Bioscience, Franklin Lakes, NJ) and the cell suspension collected and centrifuged for 5 minutes at 1,600 r.p.m. at 24 °C. The cell pellet was then resuspended in fresh L-15 media and centrifuged for 5 minutes at 1,600 r.p.m. at 24 °C. The pellet was resuspended in 40% percoll solution (GE Healthcare, Piscataway, NJ) and underlayed with 70% percoll solution to create a gradient. This gradient was then centrifuged for 20 minutes at 1,800 r.p.m. at 24 °C. The resulting cell interphase, containing the purified lung-lymphocytes, was carefully collected in a new sterile tube and the cells washed twice with PBS. Following centrifugation for 5 minutes at 1,600 r.p.m. the cells were resuspended in complete media.
IFNγ ELISpot assay. The assay was performed using the ELISpot Mouse Set (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. Briefly, a 96-well ELISpot plate was precoated with 5.0 µg/ml of anti-mouse IFNγ capture antibody overnight at 4 °C. Wells were then blocked with complete culture medium for a minimum of 2 hours at RT. Splenocytes were added to wells at a density of 5 × 105 cells/well and stimulated with the GFP and the p17, p24 (gag)-specific peptide libraries or the H2-Kd luciferase GFQSMYTFV epitope.37 Cells were incubated at 37 °C, 5% CO2 for 20 hours. As positive control phorbol myristate acetate (0.05 µg/ml); ionomycin (1 µg/ml) (ref. 38) was used. As a negative control cells were incubated in the absence of peptide. Following overnight incubation, wells were vigorously washed with water, followed by PBS/0.05% Tween-20 and subsequently incubated with 2.0 µg/ml of biotinylated anti-mouse IFNγ detection antibody for 2 hours at RT. Following three PBS/0.05% Tween-20 washes, the wells were incubated with 5 µg/ml of streptavidin–horseradish peroxidase antibody for 1 hour at RT. Wells were washed with PBS/0.05% Tween-20, followed by PBS and developed using the 3-amino-9-ethylcarbazole substrate set (BD Pharmingen). Color development was monitored and stopped 5 minutes later by washing well with distilled water. After drying overnight at RT, spots were counted using an ELISpot reader.
Imaging. Mice were injected intraperitonealy with 200 µl of 15 mg/ml D-luciferin (Caliper Life Sciences) (i.e., 10 µl luciferin per 1 g body weight). Five minutes later mice were anaesthetized with ketamine/xylazine and imaged within 10 minutes of anesthesia using the IVISR Xenogen imaging system. Quantitation of signal (photons per second) was calculated using the Living Image 3.0 Software (Caliper Life Sciences).
Statistical analysis. Statistical analysis of the data presented was performed using the SigmaStat 3.1 program (SPSS, Chicago, IL). Statistical significance was set at P = 0.05 and statistical power at 0.80. Results are presented as a mean ± SD. Student's t-test was used for two-group comparisons and analysis of variance Student–Newman–Keuls test for multiple-group comparisons.
Acknowledgments
We thank Deirdre McMenamin and Regina Munden for invaluable assistance with animal studies; Julie Johnston and Arbans Sandhu from Penn Vector for supplying the pseudotyped lentiviral vectors. This work was supported by grants from the CFF RDP center grant R881, the CFP01 (HL051746), and GSK. C.L.B. is supported by a CFF predoctoral scholarship. J.M.W. is an inventor on patents licensed to Targeted Genetics, GlaxoSmithKline, and ReGenX. In addition, he holds stock in, receives a grant from, and consults for ReGenX.
REFERENCES
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Kobinger GP, Weiner DJ, Yu QC., and , Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Medina MF, Kobinger GP, Rux J, Gasmi M, Looney DJ, Bates P, et al. Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung. Mol Ther. 2003;8:777–789. doi: 10.1016/j.ymthe.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Beyer WR, Westphal M, Ostertag W., and , von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol. 2002;76:1488–1495. doi: 10.1128/JVI.76.3.1488-1495.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Schwartz JP, Tanaka K, Brady RO., and , Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol. 1998;72:8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz J, Buchholz CJ, Engelstädter M, Uckert W, Bloemer U, Schmitt I, et al. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology. 2000;273:16–20. doi: 10.1006/viro.2000.0394. [DOI] [PubMed] [Google Scholar]
- Kobinger GP, Limberis MP, Somanathan S, Schumer G, Bell P., and , Wilson JM. Human immunodeficiency viral vector pseudotyped with the spike envelope of severe acute respiratory syndrome coronavirus transduces human airway epithelial cells and dendritic cells. Hum Gene Ther. 2007;18:413–422. doi: 10.1089/hum.2006.194. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Burnight ER, Hickey MA, Blissard GW., and , McCray PB. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol. 2005;79:12818–12827. doi: 10.1128/JVI.79.20.12818-12827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Goreham-Voss JD, Arias AC, Hickey MA, Maury W, Chikkanna-Gowda CP, et al. Enhanced gene expression conferred by stepwise modification of a nonprimate lentiviral vector. Hum Gene Ther. 2007;18:1244–1252. doi: 10.1089/hum.2006.127. [DOI] [PubMed] [Google Scholar]
- Buckley SM, Howe SJ, Sheard V, Ward NJ, Coutelle C, Thrasher AJ, et al. Lentiviral transduction of the murine lung provides efficient pseudotype and developmental stage-dependent cell-specific transgene expression. Gene Ther. 2008;15:1167–1175. doi: 10.1038/gt.2008.74. [DOI] [PubMed] [Google Scholar]
- Yu ZY, McKay K, van Asperen P, Zheng M, Fleming J, Ginn SL, et al. Lentivirus vector-mediated gene transfer to the developing bronchiolar airway epithelium in the fetal lamb. J Gene Med. 2007;9:429–439. doi: 10.1002/jgm.1039. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Lee CI, Ekert JE, McDonald R, Kohn DB, Plopper CG, et al. Lentiviral vector gene transfer into fetal rhesus monkeys (Macaca mulatta): lung-targeting approaches. Mol Ther. 2001;4:614–621. doi: 10.1006/mthe.2001.0497. [DOI] [PubMed] [Google Scholar]
- Limberis M, Anson DS, Fuller M., and , Parsons DW. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther. 2002;13:1961–1970. doi: 10.1089/10430340260355365. [DOI] [PubMed] [Google Scholar]
- Johnson LG, Olsen JC, Naldini L., and , Boucher RC. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M., and , Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V., and , Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- Garcia Casado J, Janda J, Wei J, Chapatte L, Colombetti S, Alves P, et al. Lentivector immunization induces tumor antigen-specific B and T cell responses in vivo. Eur J Immunol. 2008;38:1867–1876. doi: 10.1002/eji.200737923. [DOI] [PubMed] [Google Scholar]
- Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C, et al. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J Virol. 2008;82:86–95. doi: 10.1128/JVI.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S., and , Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Grubb BR., and , Boucher RC.1999Pathophysiology of gene-targeted mouse models for cystic fibrosis Physiol Rev 79(1 Suppl): S193–S214. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Hopkins PJ, Bourne AJ, Boucher RC., and , Martin AJ. Airway gene transfer in mouse nasal-airways: importance of identification of epithelial type for assessment of gene transfer. Gene Ther. 2000;7:1810–1815. doi: 10.1038/sj.gt.3301317. [DOI] [PubMed] [Google Scholar]
- Kremer KL, Dunning KR, Parsons DW., and , Anson DS. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J Gene Med. 2007;9:362–368. doi: 10.1002/jgm.1025. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR., and , Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Rawlins EL., and , Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Moscioni AD, Qian Y, Qian R, Chen Y., and , Wilson JM. Fas-Fas ligand interactions play a major role in effector functions of cytotoxic T lymphocytes after adenovirus vector-mediated gene transfer. Hum Gene Ther. 1999;10:259–269. doi: 10.1089/10430349950019048. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ertl HC., and , Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC., and , Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., and , Yang Y. Innate immune recognition of viruses and viral vectors. Hum Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Su Q., and , Wilson JM. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ., and , Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal WK, Rose E, Zhou H, Langston C, Rice K, Carey D, et al. Multiple advantages of alpha-fetoprotein as a marker for in vivo gene transfer. Mol Ther. 2000;2:640–648. doi: 10.1006/mthe.2000.0198. [DOI] [PubMed] [Google Scholar]
- Zoltick PW., and , Wilson JM. A quantitative nonimmunogenic transgene product for evaluating vectors in nonhuman primates. Mol Ther. 2000;2:657–659. doi: 10.1006/mthe.2000.0204. [DOI] [PubMed] [Google Scholar]
- Figueredo J, Limberis MP., and , Wilson JM. Prediction of cellular immune responses against CFTR in patients with cystic fibrosis after gene therapy. Am J Respir Cell Mol Biol. 2007;36:529–533. doi: 10.1165/rcmb.2006-0313CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Kobinger GP, Passini MA, Wilson JM., and , Wolfe JH.2002Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins Mol Ther 5(5 Pt 1): 528–537. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Figueredo J, Calcedo R., and , Wilson JM. Activation of CFTR-specific T Cells in cystic fibrosis mice following gene transfer. Mol Ther. 2007;15:1694–1700. doi: 10.1038/sj.mt.6300210. [DOI] [PubMed] [Google Scholar]
- Limberis MP., and , Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Bell CL., and , Wilson JM. Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 2009;16:441–447. doi: 10.1038/gt.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. Immunodeficiency of aging: restorative effects of phorbol ester combined with calcium ionophore. J Immunol. 1986;137:805–808. [PubMed] [Google Scholar]