Abstract
Limited packaging capacity hinders adeno-associated virus (AAV) gene therapy. A recent study seems to have provided a solution to this problem. Allocca et al. reported that AAV-5 could package an 8.9 kb vector genome. Here we tested whether this approach can be used to deliver a large genome for Duchenne muscular dystrophy (DMD) gene therapy. We first evaluated AAV-5 packaging of an 8.2 kb genome. This vector carries two independent reporter gene cassettes, one for alkaline phosphatase (AP) and another for LacZ. Viral yield was log-fold lower than that of a regular AAV-5. Nevertheless, both AP and LacZ genes were detected in purified virus. Injection to dystrophic muscle resulted in both AP and LacZ expression. On electron microscopy, virion structure appeared normal. Surprisingly, we did not find the full-length single-stranded viral genome by alkaline gel electrophoresis. Neither did we see the full-length double-stranded replication forms in adenovirus coinfected cells. We suspect that AP and LacZ expression may have come from partially packaged 5′ or 3′-half of the genome. Additional studies revealed failure of AAV-5 to package and express an 8.7 kb minidystrophin gene cassette. In summary, our results do not support the extraordinary packaging capacity of AAV-5.
Introduction
Adeno-associated virus (AAV) has become one of the most favorite gene delivery vehicles over the last two decades.1,2,3,4 Recent clinical success further raises the hope of treating inherited diseases with AAV gene therapy.5,6,7 Although a highly efficient and quite safe viral vector,8 AAV has a major limitation. It is generally believed that the maximal packaging capacity of an AAV vector is 5 kb. This presents a great hurdle for therapeutic expression cassettes that exceed this limit.
To overcome this obstacle, a number of single and dual vector approaches have been explored with various levels of success.9,10 Dual vector approaches often require complicated molecular engineering and transgene reconstitution may highly depend on the target gene sequence.11,12,13 On the other hand, several groups have reported packaging of a ~6 kb genome in a single AAV virion.14,15,16 Though encouraging, a 6 kb packaging capacity remains insufficient for many therapeutic genes such as a 7 kb minidystrophin gene for Duchenne muscular dystrophy (DMD) gene therapy.17
Recently, Allocca et al. screened a series of AAV serotypes for their tolerance to large viral genome.18 Surprisingly, they noticed a transgene and purification method independent packaging of an up to 8.9 kb genome by AAV serotype 5 (AAV-5).18 Importantly, they achieved structural and functional amelioration of recessive Stargardt's disease in a mouse model by delivering an 8.9 kb adenosine triphsophate–binding cassette transport family gene, ABCA4, expression cassette to the retina.18 Encouraged by this observation, here we tested whether AAV-5 can be used to deliver a large genome to dystrophin-deficient mdx mice, a murine DMD model. We examined two vector genomes including (i) an 8.2 kb genome carrying two intact reporter gene expression cassettes for alkaline phosphatase (AP, at the 5′ end) and β-galactosidase (LacZ, at the 3′ end), and (ii) an 8.7 kb genome containing the 7 kb ΔH2-R15 minidystrophin gene. The viruses derived from these two genomes were referred to as AV.AP.LacZ and AV.ΔH2-R15.
In contrast to the results reported by Allocca et al.,18 we failed to detect incorporation of the full length of these oversized genomes in AAV-5 virus. Immunostaining with N-terminal and C-terminal specific antibodies also failed to reveal minidystrophin expression in AV.ΔH2-R15 infected mdx muscle. Interestingly, we did observe coexpression of AP and LacZ in AV.AP.LacZ infected mdx muscle. However, this expression appeared to have derived from a partial packaging of either the 5′ or the 3′ end of the genome. In summary, our results do not support serotype-specific packaging of an oversized genome by AAV-5.
Results
Characterization of AAV-5 AV.AP.LacZ virus
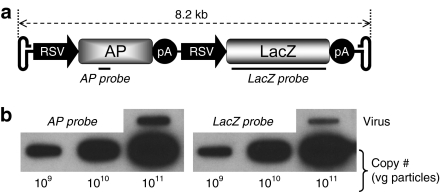
To examine AAV-5 packaging of a large genome, we constructed an 8.2 kb template by flanking two independent reporter gene cassettes with AAV-2 inverted terminal repeats (ITRs) (Figure 1). Each expression cassette contains its own transcriptional regulatory elements including a Rous sarcoma virus (RSV) promoter and a SV40 pA signal. The 5′-end cassette expresses the AP gene and the 3′-end one expresses the LacZ gene (Figure 1).
Figure 1.
AAV-5 packaging of an 8.2 kb viral genome. (a) Schematic outline of the putative full-length viral genome. The 5′-half genome contains an AP expression cassette and the 3′-half genome contains a LacZ expression cassette. RSV, Rous sarcoma virus promoter; pA, SV40 polyadenylation signal; AP, alkaline phosphatase gene; LacZ, β-galactosidase gene. The locations of the AP and LacZ probes are marked. (b), A representative slot blot of the purified AV.AP.LacZ virus. vg, viral genome.
After confirming expression, the template plasmid was used for AAV-5 production.19 After four rounds of isopycnic ultracentrifugation in cesium chloride gradient, we obtained a viral stock with a physical titer of ~7 × 108 viral genome (vg) particles/µl. Both AP and LacZ sequences were detected by slot blot (Figure 1b). To compare the packaging efficiency with that of an ideal sized genome, we generated AAV-5 AV.EGFP virus using the same protocol. AV.EGFP has a 4.7 kb genome and it expresses the enhanced green fluorescence protein (EGFP).20 Consistent with Allocca et al.,18 the yield of AV.EGFP was approximately tenfold higher than that of AV.AP.LacZ (data not shown).
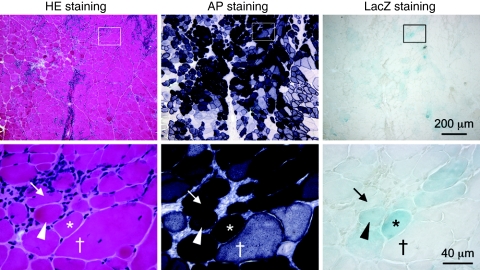
Next, we delivered AV.AP.LacZ virus to the tibialis anterior muscle of adult mdx mice. One month later, we examined AP and LacZ expression by histochemical staining (Figure 2). Robust AP expression was observed whereas LacZ staining appeared weak. Importantly, the relative levels of AP expression closely mirrored that of LacZ expression in the corresponding cells in serial sections (Figure 2).
Figure 2.
Characterization of AV.AP.LacZ infection in dystrophic muscle. Representative photomicrographs of HE, AP, and LacZ stained muscle sections. Transgene expression was detected at 1 month after AV.AP.LacZ infection (N = 4 muscles). Bottom panels are high magnificent photomicrographs of the boxed areas in the respective low power images. Asterisk, a myofiber with high level AP expression also showed high LacZ expression; Cross, a myofiber with relatively weak AP expression also showed minimal LacZ expression; Arrow, a myofiber with robust AP but poor LacZ expression; Arrowhead, a myofiber with relatively high level LacZ but weak AP expression.
Wild-type AAV-5 virion is a 25 nm icosahedral particle.21 To determine whether a large genome has alternated virion structure, we compared AV.AP.LacZ and AV.EGFP by transmission electron microscopy (Figure 3a). In both cases, more than 98% viral particles were uniform fully packaged virions with an average size of approximately 25 nm. No gross change was detected in AV.AP.LacZ stock (Figure 3a).
Figure 3.
Characterization of AV.AP.LacZ viral particle and the vector genome. (a) Representative electron microscopic images of AV.AP.LacZ and AV.EGFP viral particles. AV.EGFP carries a 4.7 kb vector genome. (b) A representative alkaline gel Southern analysis of the AV.AP.LacZ viral genome. (c) A representative Southern blot examination of the replication forms of AV.AP.LacZ virus. The probes used are marked for each blot. Results in each panel were obtained from at least three independent experiments.
To determine whether the full 8.2 kb genome was incorporated in viral particle, we examined purified virus by alkaline gel Southern blot (Figure 3b). Surprisingly, we did not see the 8.2 kb full-length band. Instead, AP and LacZ probes yielded distinctive sets of bands, all smaller than 5 kb. The AP probe revealed two prominent bands migrated at ~3.6 kb and ~3.9 kb. There also appeared a faint smear around 5 kb. Interestingly, the LacZ probe revealed a ~5 kb band and some barely visible low molecular weight smear (Figure 3b). It is currently not clear why the AP and LacZ probes yielded the viral genome of different lengths. Nevertheless, our result supports a model of segmental rather than full-length genome packaging.
To confirm this unexpected finding, we examined replication forms of the AV.AP.LacZ virus. Hela cells were coinfected with AV.AP.LacZ and adenovirus, Ad.dl802.22 Low molecular weight Hirt DNA was extracted 24 hours later. Southern blot was performed using either the AP or LacZ probe (Figure 3c).22 Consistent with our alkaline gel result, we failed to detect the 8.2 kb replication monomer. Using the AP probe, we observed a series of bands migrated at ~3.6 kb, ~7.2 Kb and ~11 Kb. The size of these bands appears to correlate with the replication form monomer, dimer and trimer of a ~3.6 kb viral genome (Figure 3c). A similar sets of replication form bands corresponding to a 5 kb viral genome were revealed by the LacZ probe (Figure 3c).
Characterization of AAV-5 AV.ΔH2-R15 virus
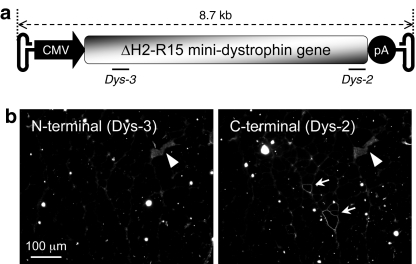
Our ultimate goal is to deliver a large-size genome for DMD gene therapy. We recently reported a highly functional 7 kb ΔH2-R15 human minidystrophin gene. Here we tested whether functional AAV-5 vectors can be generated to express this minigene. Together with the promoter, pA signal and the flanking viral ITRs, the estimated vector genome size is 8.7 kb (Figure 4a). This should fit within the 8.9 kb packaging capacity suggested by Allocca et al.18
Figure 4.
AV.ΔH2-R15 fails to express the minidystrophin gene in mdx muscle. (a) Schematic outline of the putative full-length AV.ΔH2-R15 genome. The locations of the Dys-3 and Dys-2 antibodies are marked. (b) Representative photomicrographs of serial muscle sections stained with a human dystrophin hinge-1 specific Dys-3 antibody or a dystrophin C-terminal domain specific antibody (Dys-2). Arrow, rare occurring revertant fibers detected by the Dys-3 antibody. Arrowhead, infiltration of immunoglobulin in necrotic myofiber.
AV.ΔH2-R15 viral stock was generated the same way as described above. Electron microscopy revealed normal sized particles at ~25 nm (data not shown). Slot blot with 5′ and 3′-end minidystrophin gene probes suggested that the purified viruses contained sequences from both ends (data not shown). A total of 8 × 109 vg particles of AV.ΔH2-R15 were delivered to each tibialis anterior muscle in four adult mdx mice. Immunofluorescence staining was performed at 50 days postinfection. To allow for full-length minidystrophin detection, we used two independent antibodies. Dys-3 specifically reacts with the N-terminal end of human dystrophin whereas Dys-2 recognizes dystrophin C-terminal domain from any species. We were only able to find rare occurring revertant fibers with the Dys-2 antibody (Figure 4). Additional alkaline gel and adenovirus coinfection Southern blot experiments confirmed the absence of full-length genome packaging (data not shown).
Discussion
From many standpoints, nano-sized AAV appears to be an ideal gene delivery vehicle.1,2,3,4 However, a broad application of AAV gene therapy has been hindered by the small viral packaging capacity. A recent report challenged AAV packaging dogma.18 Allocca et al. examined AAV-1, 2, 3, 4, 5, 7, 8, and 9. Surprisingly they found that AAV-5 could tolerate a genome as large as 8.9 kb.18 It was recently shown that the assembly and the overall mechanical stiffness of the parvovirus are influenced by the interaction between the viral genome and capsid.23 Because AAV-5 is phylogenetically the most divergent AAV serotype,24 it seems plausible that AAV-5 capsid could be more adaptable to a larger genome.
To capitalize this intriguing finding for DMD gene therapy, we examined AAV-5 packaging and expression from two large viral genomes. We first tested an 8.2 kb genome containing both AP and LacZ expression cassettes. Consistent with Allocca et al.,18 we detected both AP and LacZ sequences in purified virus (Figure 1). Further, we observed AP and LacZ coexpression in mdx muscle (Figure 2). Despite the encouraging initial results, we were surprised that there was no apparent difference between AAV-5 virions carrying a larger or normal sized genome (Figure 3a). Additional molecular characterization suggests that the purified viral stock actually represents a mixture of two partially packaged viruses. One contains the AP expression cassette and the other the LacZ expression cassette (Figures 3b,c and 5a). The observed coexpression may likely reflect efficient coinfection of the same cell by two simultaneously delivered viruses.25
Figure 5.
AAV-5 packaging of normal and large sized viral genomes. (a) Schematic presentation of AV.AP.LacZ (left panel) and AV.ΔH2-R15 (right panel) packaging. Middle panel depicts AAV-5 packaging of a wild-type (wt) sized genome. AV.AP.LacZ and AV.ΔH2-R15 are consisted of particles carrying either the 5′-half or the 3′-half of the vector genome. In AV.AP.LacZ, independent packaging of each expression cassette by individual viral particle resulted in AP and LacZ coexpression in the same muscle cell. In AV.ΔH2-R15, failure to express minidystrophin is most likely due to an incomplete packaging of the full-length cassette in a single virion. Also included are a representative histochemical and dystrophin immunostaining images from AV.AP.LacZ or AV.ΔH2-R15 infected mdx muscle. The immunostaining image from normal muscle reveals sarcolemmal dystrophin expression. N, the N-terminal end of minidystrophin; C, the C-terminal end of minidystrophin. (b) Transgene expression from AAV-5 carrying different size viral genomes. For virus with a normal sized genome, both the 5′ and the 3′-end inverted terminal repeats (ITRs) are encapsidated in mature viral particle. For these with an oversized genome, we hypothesize that there is only one ITR in each mature virion. For a large but highly recombinogenic target gene, we speculate that transgene expression may have derived from homologous recombination between the overlapping regions of partially packaged viral genomes.
To further confirm this contradictory observation, we evaluated AAV-5 packaging of an 8.7 kb genome containing a single expression cassette for a 7 kb minidystrophin gene (Figure 4). On slot blot, electron microscopy, and alkaline gel and adenovirus coinfection Southern blots, we observed results similar to these of AV.AP.LacZ. The only difference was that we did not see minidystrophin expression whereas both AP and LacZ expression were detected in AV.AP.LacZ infected muscle (Figures 2 and 4).
Our results suggest that AAV-5 cannot package a genome ≥8.2 kb. Similar to AAV-2,26,27,28 infectious single ITR AAV-5 particle is generated when viral genome exceeds the maximal packaging capacity (Figure 5). However, the production of such single ITR virion is substantially less efficient.26,27 We suspect that a similar mechanism may also explain (at least partially) the findings from Allocca et al.18 We speculate that AAV-5 virus generated by Allocca et al. may contain partially packaged viral genomes. A potential sequence overlap between viruses carrying the 5′-end and 3′-end genome may reconstitute expression by homologous recombination (Figure 5b).29,30 Because overlapping-based reconstitution depends on the target gene sequence,12 the lack of minidystrophin expression may likely reflect a poor recombination efficiency of the ΔH2-R15 minigene (Figure 4).
An interesting aspect of our model is whether the 5′- and the 3′-ITRs are equally efficient in generating single ITR viral particles. Although histochemical staining revealed much intense AP expression (Figure 2), we believe that this may not necessarily suggest a preferential packaging of the AP expression cassette. It is well established that the sensitivity of the AP staining method is much higher than that of the LacZ staining method.12,25,31 Future studies are needed to completely resolve this issue.
Together with the results from Dong et al.32 and Wu et al.33 we believe that AAV-5 may not encapsidate a viral genome ≥8.2 kb. The maximal carrying capacity of a single AAV particle remains limited.
Materials and Methods
Construction of the cis proviral plasmids. pcis.AP.LacZ (also called YL203) was generated by inserting an RSV-LacZ expression cassette downstream of the AP expression cassette in a previously reported AAV packaging plasmid, pcis.RSV.AP.34 The RSV-LacZ expression cassette was obtained from an already described plasmid, pcis.RSV.LacZ.35 pcis.CMV.ΔH2-R15 (also called YL198) was generated by PCR-mediated multistep cloning. Briefly, the C-terminal domain of the dystrophin gene was PCR amplified using pDysEHpA (a gift from Jeffery Chamberlain, University of Washington, Seattle, WA) as the template and the following pair of primers, forward primer 5′-tcgagactttgccaaggtac (DL1070); reverse primer 5′-gcgcGCATGCaagccatgaatttcatatggatatcacg (DL1071, the underlined capital nucleotides represent the Sph I site introduced during cloning. This site is located downstream the 3′ end of the minidystrophin gene). The PCR product was digested with BstB I and Sph I and swapped into pcis.CMV.ΔR4/ΔC to obtain an intermediate plasmid, pYL197.36 The Nsi I/BsB I fragment of pYL197 was then replaced by a 5,574 bp fragment from pHSA.ΔH2-R15 (also called pYL66).17 The resulting plasmid was named pYL198. The region obtained by PCR and the regions containing cloning junctions were confirmed by DNA sequencing.
AAV-5 production. AAV-5 stock was generated according to a previously published protocol.19 Experimental virus was purified from crude viral lysate through four rounds of cesium chloride banding.
Animal studies. Animal experiments were performed in accordance with the NIH and institutional guidelines of the University of Missouri. Male mdx mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Recombinant AAV-5 virus was delivered to the tibialis anterior muscle according to a previously described protocol (6 × 109 vg particles/muscle for AV.AP.LacZ and 8 × 109 vg particles/muscle for AV.ΔH2-R15).20 LacZ and AP expression was analyzed by histochemical staining as described before.12,19 Minidystrophin expression was determined by immunofluorescence staining using a human dystrophin hinge-1 specific antibody (Dys-3; Novocastra, Newcastle, UK) and a dystrophin C-terminal domain specific antibody (Dys-2, Novocastra) according to our published protocol.37
Electron microscopy. Purified AAV-5 virus was placed on a 200 mesh carbon-coated copper grid for five minutes. After four to five rounds of gentle washing in ultra pure water, virus was stained with 1% uranyl acetate for 5 minutes. Viral particles were visualized using a JEOL JEM-1400 transmission electron microscope.
Alkaline gel Southern blot. Viral DNA was extracted from purified virus as reported before.38 After electrophoresis in a 1% alkaline agarose gel, Southern blot was performed using either an AP or a LacZ probe. The AP probe consists of a 359 bp Sac I fragment from pcis.RSV.AP. The LacZ probe consists of a 3,474 bp Not I fragment from pcis.RSV.LacZ.
Examination of replication forms of AAV genome by Southern blot. Hela cells were coinfected with AAV-5 and adenovirus dl802 as described before.22 Low molecular weight Hirt DNA was extracted 24 hours later and resolved in a 1% agarose gel.22 Replication form viral genome was examined by AP and LacZ probes as described in alkaline gel Southern.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR-49419, DD) and the Muscular Dystrophy Association (DD). The authors also thank Robert J McDonald Jr., MD, for the generous support to Duchenne muscular dystrophy research in Duan lab.
REFERENCES
- Flotte TR., and , Berns KI. Adeno-associated virus: a ubiquitous commensal of mammals. Hum Gene Ther. 2005;16:401–407. doi: 10.1089/hum.2005.16.401. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Mol Ther. 2004;10:981–989. doi: 10.1016/j.ymthe.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Srivastava A. Adeno-associated virus-mediated gene transfer. J Cell Biochem. 2008;105:17–24. doi: 10.1002/jcb.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, et al. Investigation of the cause of death in a gene-therapy trial. N Engl J Med. 2009;361:161–169. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]
- Duan D, Yan Z., and , Engelhardt JF.2006Expanding the capacity of AAV vectorsIn: Bloom, ME, SF Cotmore, RM Linden, CR Parrish and JR Kerr (eds). Parvoviruses Hodder Arnold; Distributed in the U.S.A. by Oxford University Press: London, New York. pp. 525–532 [Google Scholar]
- Ghosh A., and , Duan D. Expending adeno-associated viral vector capacity: a tale of two vectors. Biotechnol Genet Eng Rev. 2007;24:165–177. doi: 10.1080/02648725.2007.10648098. [DOI] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yue Y., and , Duan D. Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J Gene Med. 2006;8:298–305. doi: 10.1002/jgm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Lai Y., and , Duan D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther. 2008;16:124–130. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- Grieger JC., and , Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KH Jr, Gorbatyuk OS, Harrison JK, Opie SR, Zolotukhin S., and , Muzyczka N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J Virol. 2004;78:6595–6609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Chen L, Wang J, Huack B, Sarkar R, Zhou S, et al. Complete correction of hemophilia A with adeno-associated viral vectors containing a full-size expression cassette. Hum Gene Ther. 2008;19:648–654. doi: 10.1089/hum.2007.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yan Z, Yue Y, Ding W., and , Engelhardt JF. Enhancement of muscle gene delivery with pseudotyped adeno-associated virus type 5 correlates with myoblast differentiation. J Virol. 2001;75:7662–7671. doi: 10.1128/JVI.75.16.7662-7671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Agbandje-McKenna M, Bowman VD, Moninger TO, Olson NH, Seiler M, et al. Structure of adeno-associated virus serotype 5. J Virol. 2004;78:3361–3371. doi: 10.1128/JVI.78.7.3361-3371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Sharma P, Dudus L, Zhang Y, Sanlioglu S, Yan Z, et al. Formation of adeno-associated virus circular genomes is differentially regulated by adenovirus E4 ORF6 and E2a gene expression. J Virol. 1999;73:161–169. doi: 10.1128/jvi.73.1.161-169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco C, Castellanos M, de Pablo PJ., and , Mateu MG. Manipulation of the mechanical properties of a virus by protein engineering. Proc Natl Acad Sci USA. 2008;105:4150–4155. doi: 10.1073/pnas.0708017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Yue Y, Lai Y, Ye C, Qiu J, Pintel DJ, et al. Trans-splicing adeno-associated viral vector-mediated gene therapy is limited by the accumulation of spliced mRNA but not by dual vector coinfection efficiency. Hum Gene Ther. 2004;15:896–905. doi: 10.1089/hum.2004.15.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS., and , Srivastava A. A novel terminal resolution-like site in the adeno-associated virus type 2 genome. J Virol. 1997;71:1140–1146. doi: 10.1128/jvi.71.2.1140-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Ponnazhagan S., and , Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J Virol. 1996;70:1668–1677. doi: 10.1128/jvi.70.3.1668-1677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Qing K, Ponnazhagan S., and , Srivastava A. Adeno-associated virus type 2 DNA replication in vivo: mutation analyses of the D sequence in viral inverted terminal repeats. J Virol. 1997;71:3077–3082. doi: 10.1128/jvi.71.4.3077-3082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and , Miller AD. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol. 2002;20:697–701. doi: 10.1038/nbt0702-697. [DOI] [PubMed] [Google Scholar]
- Duan D, Yue Y., and , Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- Bell P, Limberis M, Gao G, Wu D, Bove MS, Sanmiguel JC, et al. An optimized protocol for detection of E. coli beta-galactosidase in lung tissue following gene transfer. Histochem Cell Biol. 2005;124:77–85. doi: 10.1007/s00418-005-0793-2. [DOI] [PubMed] [Google Scholar]
- Dong B, Nakai H, Xiao W.Characterization of genome integrity for oversized recombinant AAV vector Mol Therin the press). [DOI] [PMC free article] [PubMed]
- Wu Z, Yang H., and , Colosi P. Effect of genome size on AAV vector packaging. Mol Ther (in the press) [DOI] [PMC free article] [PubMed]
- Duan D, Yue Y, Yan Z, Yang J., and , Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., and , Dongsheng D. Development of multiple cloning site cis-vectors for recombinant adeno-associated virus production. BioTechniques. 2002;33:672, 674, 676–672, 674, 678. doi: 10.2144/02333dd03. [DOI] [PubMed] [Google Scholar]
- Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS., and , Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS., and , Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE., and , Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]