Research over the past 20 years has focused on the development of adeno-associated virus (AAV) DNA delivery vectors for the treatment of several hereditary human diseases. Among viral vectors for gene therapy approaches, AAV has several advantages: (i) the majority of transgenic DNA persists as episomes rather than relying on host chromosome integration, (ii) AAV is considered nonpathogenic, and (iii) natural AAV serotypes transduce most nondividing and dividing cell types.1 Furthermore, recent advancements in the understanding of functional capsid regions, as well as random capsid sequence selection techniques, demonstrate the ability to engineer AAV vectors for enhanced transduction of specific tissues as well as an enhanced ability to evade the immune system.2,3,4
However, despite these desirable attributes, AAV vectors suffer from a major limitation for the treatment of disorders requiring large-gene transfer: the ~26-nm-diameter virus capsid packages a single-strand DNA payload of ~5 kb. However, this packaging dogma was recently challenged by a report of the unique ability of AAV serotype 5 (AAV5) capsids to package genomes of ≈9 kb, which mediated successful transduction in vitro and in vivo.5 Although these data were convincing, the large packaging capacity reported for AAV5 conflicted with the sizes observed with wild-type AAV genomes and modeling predictions for particles within a 25-nm size range.6 The answer to this gene transfer conundrum is now being described by three independent groups who report in this issue of Molecular Therapy studies that resolve the discrepancy between the physical size–imposed limitations of the AAV capsid with the successful biological outcome of transducing large genes.7,8,9 The three studies all report physical packaging of AAV genomes of normal size (~5 kb) but also demonstrate the ability of these normal-sized vectors to produce large functional transgene products following transduction.7,8,9 The solution to this puzzle of “little vector, big gene transduction” is thus the fragmented genome reassembly of AAV payloads.
With respect to AAV vectors, the field now has a working model to help explain the phenomena of AAV-mediated transduction of large genes. With respect to AAV biology, these studies point to the amazing plasticity built into AAV viral biology that ensures that physical laws of packaging are followed while maintaining the functional gene expression required for viral survival. To fully appreciate the issue resolved here, one must recount the pieces of the puzzle of AAV biology that have been generated over the past 10 years.
For example, the first report that AAV could mediate transfer of large DNAs (>4.7 kb) appeared in 1997 and made use of an in vitro packaging system.10 Zhou and Muzyczka demonstrated that AAV particles produced in vitro were able to deliver DNA of ~9 kb. Further scrutiny determined that these particles were incompletely assembled but nevertheless protected the DNA and thereby produced the false impression of virus-mediated gene transfer. Around the same time, two independent research groups (Dong et al.8 and Hermonat et al.11), using traditional AAV replication packaging studies with larger viral genomes, reported that the optimal packaging size for AAV2 was between 4.1 and 4.9 kb. These studies were followed by a report that characterized packaging limits for multiple AAV serotypes (types 1–5) and demonstrated no serotype bias for packaging, but it noted that particles with larger genomes were more sensitive to proteosome-mediated degradation.12 Against this backdrop, Allocca et al. presented data showing that the AAV5 capsid could package a DNase-resistant genome of 8.9 kb in a sequence-independent manner.5 These authors also reported that transduction by these large gene vectors resulted in the synthesis of the transgene product (the 8.9-kb Abca4 gene), which ameliorated the disease phenotype of Abca4-deficient mice.5 Spurred by the exciting implications of these results, Lai et al.,9 Dong et al.,8 and Wu et al.7 independently sought to recapitulate the reported AAV5 packaging capacity and the production of large transgene products.
The data presented by these three groups in this issue of Molecular Therapy challenge the conclusions of the previous study by Allocca et al.5 while nevertheless reporting very similar findings.5,7,8,9 Using different DNA sequences of various sizes, multiple AAV serotypes, and different vector purification techniques, each of these groups reports partial packaging of large DNA as analyzed by Southern blotting.7,8,9 Dong et al. described a vector packaging approach that utilized a plasmid origin of replication and ampicillin-resistance marker embedded in the vector DNA to facilitate recovery of circularized post-transduced AAV genomes.8 Few genomes larger than 5 kb were recovered after infection regardless of the initial vector size, but this was not the case in mice at later times. Recombinant AAV genomes of larger than 5 kb were isolated 21 days after vector administration, implying that partially packaged sequences may have complemented each other to restore a full-expression cassette. Using LacZ and alkaline phosphatase reporter transgene, Lai et al.9 established similar observations. In these instances, no packaged genomes exceeded 5.2 kb, and the recovered viral titers were at least 10-fold less than the titers of particles with wild-type-sized genomes, consistent with earlier studies examining AAV packaging limitations.
These studies were extended further by Wu et al.,7 who used strand-specific probes of the structure of packaged genomes. They established that packaging initiates primarily from the 3′ end until the capsid reaches capacity (5 kb) and that vectors exceeding this size were heterogeneous in length and truncated at the 5′ end. The remainder of unpackaged DNA is thought to be lost (following the “head-full” mechanism).7,13 This concept is also consistent with the proposed mechanism of unidirectional genome packaging into the AAV capsid, which initiates from the 3′ terminus.14 Ironically, the packaging capacity results reported in this issue—that AAV5 capsids cannot harbor DNA that is much larger than 5 kb—contrasts with the reported biological observations: all three groups demonstrate that the large transgene product was synthesized after transduction.7,8,9 This unexpected result of larger gene transduction, given the partial packaging observed in this issue, is consistent with the report by Allocca et al.,5 thereby presenting the gene transfer conundrum of how a “little vector” could physically deliver “big genes” that are functional. The answer is “intracellular reassembly of partial gene fragments.”
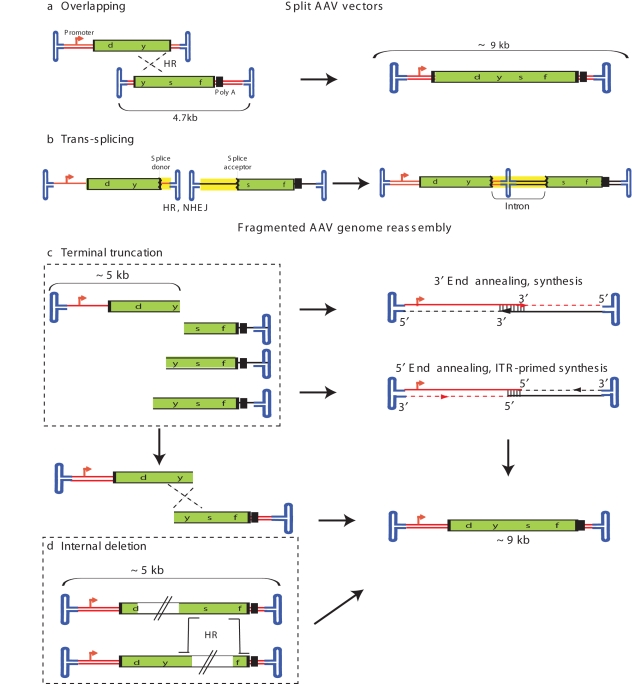
Although this sounds novel, the ability to recombine AAV vector fragments into a functional gene is not new to AAV vector technology and was suggested by all three studies. In fact, nonhomologous end joining and homologous recombination are integral to the natural life cycle of AAV and to the long-term persistence of transgene DNA delivered by AAV vectors.1,15,16 Following infection, the single-strand genome is processed into double-strand circular monomers and concatemers, processes thought to be stimulated by the inverted terminal repeats (ITRs). This tendency has been exploited to reassemble transgenes exceeding the capsid packaging limitation following a cotransduction strategy of split-gene fragments on distinct, yet intact (nontruncated), AAV genomes. In particular, overlapping and trans-splicing AAV vectors (collectively termed split vectors herein) result in the reconstruction of DNA sequences larger than the individual vector packaging capacity via genome concatemerization.15,16,17,18 The primary difference between these technologies (described in early 2000) and the large-transgene packaging results reported in this issue is that AAV split vectors package wild-type-sized genomes having ITRs flanking the single-strand genome (Figure 1). In contrast, the partially packaged genomes are postulated to have one truncated end without an ITR, although this was not directly determined. Specifically, Wu et al. proposed two potential pathways of large-gene reconstruction from the partially packaged AAV vectors: (i) recombination at overlapping homologous genome sequences and (ii) direct annealing of opposite-polarity partial large-transgene fragments (the 5′ and 3′ fragments) followed by single-strand synthesis in both directions primed by the free 3′ ends (Figure 1).7
Figure 1.
Intracellular processing of AAV genomes. (a) Overlapping and (b) trans-splicing AAV split vectors. Fragmented AAV genome reassembly following (c) terminal truncation and (d) internal truncation. HR, homologous recombination; ITR, inverted terminal repeat; NHEJ, nonhomologous end joining.
Another possibility that remains to be tested is that the viral genomes contain sequence deletions yet maintain, and are packaged with, both ITRs. In fact, Lai et al. demonstrate that partially packaged vectors are capable of replication following infection, which would require either two packaged ITRs or ITR correction at the truncated end of the genome, possibly after double-strand monomer/concatamer formation.8 If the partially packaged genomes are missing an ITR, the mechanism of large-gene reassembly from partial genomes is probably different, because the ITRs are thought to stimulate intermolecular recombination and nonhomologous end joining.19,20 This point remains to be definitively tested.
Nevertheless, these three studies (i) provide more detailed understanding of the packaging capacity of AAVs (e.g., it is unlikely that AAV capsids efficiently package genomes significantly larger than the wild-type size), (ii) identify formerly unknown ways in which AAV vectors overcome the size limitation following large-gene delivery (e.g., fragmented genome reassembly), and (iii) stress the importance of understanding of the fundamental biology of AAVs for vector engineering. Although all the points described in these studies are positive and noteworthy, and answer the intriguing question of how big genes can effectively be delivered while still obeying the packaging constraints of AAVs, we must remember that the transition of partial AAV genome vectors toward clinical applications poses further challenges. For example, the efficiency to reconstruct the desired product from genome fragments is controlled by host cell recombination machinery that will be different for certain disorders (e.g., cystic fibrosis vs. cancer) and, more concerning, may vary from patient to patient. Then there are the never-ending technical issues when trying to “scale up” large-gene vectors for human experimentation, as a result of the inherently lower titers. Finally, vectors produced in this manner are packaged with unknown heterogeneous DNA sequences (depending on the site of genome truncation or deletion), which create additional hurdles for approval by the US Food and Drug Administration.
Despite these limitations, these three reports provide a uniform understanding pointing to a likely mechanism for successful performance of AAV “little vector, big gene transduction” experiments and highlight how these new reagents can continue to be an invaluable tool for the understanding and development of more efficient AAV vectors that can package desirable larger genomes for human therapy.
REFERENCES
- McCarty DM, Young SM., Jr, and , Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS.et al. (2008Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles Mol Ther 161252–1260. [DOI] [PubMed] [Google Scholar]
- Maheshri N, Koerber JT, Kaspar BK., and , Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D.et al. (2008Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice J Clin Invest 1181955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsuaga J, Tan RK, Vazquez M, Sumners DW, Harvey SC. Investigation of viral DNA packaging using molecular mechanics model. Biophys Chem. 2002. pp. 101–102.pp. 475–484. [DOI] [PubMed]
- Wu Z, Yang H., and , Colosi P. Effect of genome size on AAV vector production. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nakai H., and , Xiao W. Characterization of genome integrity for oversized recombinant AAV. Mol Ther. 2010;18:87–92. doi: 10.1038/mt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Yue Y., and , Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome ≥8.2 kb. Mol Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., and , Muzyczka N. In vitro packaging of adeno-associated virus DNA. J Virol. 1998;72:3241–3247. doi: 10.1128/jvi.72.4.3241-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat PL, Quirk JG, Bishop BM, Han L. The packaging capacity of adeno-associated virus (AAV) and the potential for wild-type-plus AAV gene therapy vectors. FEBS Lett. 1997;407:78–84. doi: 10.1016/s0014-5793(97)00311-6. [DOI] [PubMed] [Google Scholar]
- Grieger JC., and , Samulski RJ. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Wang D, Van Ginkel FW, Pascual DW., and , Frizzell RA. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- King JA, Dubielzig R, Grimm D., and , Kleinschmidt JA. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 2001;20:3282–3291. doi: 10.1093/emboj/20.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi VW, McCarty DM., and , Samulski RJ. Host cell DNA repair pathways in adeno-associated viral genome processing. J Virol. 2006;80:10346–10356. doi: 10.1128/JVI.00841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BR., and , Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yue Y., and , Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and , Miller AD. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol. 2002;20:697–701. doi: 10.1038/nbt0702-697. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Ma C, Storm TA, Kay MA., and , Nakai H. The role of DNA-PKcs and artemis in opening viral DNA hairpin termini in various tissues in mice. J Virol. 2007;81:11304–11321. doi: 10.1128/JVI.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Storici F, Li C, Choi VW., and , Samulski RJ. AAV recombineering with single strand oligonucleotides. PLoS ONE. 2009;4:e7705. doi: 10.1371/journal.pone.0007705. [DOI] [PMC free article] [PubMed] [Google Scholar]