Abstract
Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder caused by mutations in the dystrophin gene that result in the absence of functional protein. Antisense-mediated exon-skipping is one of the most promising approaches for the treatment of DMD because of its capacity to correct the reading frame and restore dystrophin expression, which has been demonstrated in vitro and in vivo. In particular, peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs) have recently been shown to induce widespread high levels of dystrophin expression in the mdx mouse model. Here, we report the efficiency of the PPMO-mediated exon-skipping approach in the utrophin/dystrophin double-knockout mouse (dKO) mouse, which is a much more severe and progressive mouse model of DMD. Repeated intraperitoneal (i.p.) injections of a PPMO targeted to exon 23 of dystrophin pre-mRNA in dKO mice induce a near-normal level of dystrophin expression in all muscles examined, except for the cardiac muscle, resulting in a considerable improvement of their muscle function and dystrophic pathology. These findings suggest great potential for PPMOs in systemic treatment of the DMD phenotype.
Introduction
Duchenne muscular dystrophy (DMD) is a fatal muscle-wasting disorder caused by mutations in the dystrophin gene. The majority of mutations disrupt the open reading frame, resulting in the absence of a functional dystrophin protein at the sarcolemma of muscle fibers. The related allelic disorder Becker muscular dystrophy is caused by mutations that create shortened but in-frame transcripts with production of partially functional dystrophin, leading to a milder phenotype.1 Antisense-induced exon-skipping strategies that aim to remove the mutated or additional exon(s) to restore the reading frame have been shown to induce the expression of these “Becker muscular dystrophy-like” shortened forms of dystrophin protein, retaining crucial functions.2,3,4,5 Antisense oligonucleotide–mediated exon-skipping uses different oligonucleotide chemistries to target specific exon(s). The most commonly used 2′-O-methyl phosphorothioate and phosphorodiamidate morpholino oligomers (PMO) have recently been tested in small clinical trials by local intramuscular injections (refs. 6,7). Although the local restoration of dystrophin expression detected in patients confirmed the proof of principle for this therapeutic approach in human subjects, an effective therapy for DMD requires a systemic correction.
Previous work using PMOs has demonstrated a restoration of dystrophin expression in multiple muscle groups following systemic intravenous8 or intraperitoneal9 delivery in mdx mice and more recently in dystrophic dogs,10 but high-dose multi-injection protocols were required. Peptide-conjugated PMO oligomers (PPMOs) have since been shown to induce a uniform, widespread, and high level of dystrophin protein expression, leading to an increase in muscle strength in the mdx mouse.11,12,13,14
The dystrophin-deficient mdx mouse has historically been used as the primary model of DMD, although this mouse does not experience the severe, body-wide dystrophy that considerably shortens lifespan in humans. The mild phenotype of the mdx mouse is partly attributed to compensatory overexpression of the dystrophin-related protein utrophin, as knockout of both dystrophin and utrophin in mice causes progressive muscle wasting, impaired mobility, and premature death.15,16 Therefore, double-knockout (dKO) mice, which present a much more severe and progressive dystrophic phenotype than mdx mice, represent a more appropriate model to test the therapeutic potential of the antisense approach. Here, we tested the hypothesis that the cell-penetrating peptide–conjugated oligomer we previously used in mdx mice11 could ameliorate the pathology associated with severe muscular dystrophy in dKO mice. We demonstrate that repeated intraperitoneal (i.p.) injections of a PPMO induce a widespread restoration of dystrophin expression in all muscles examined, except for the cardiac muscle, resulting in a considerable improvement of the dystrophic phenotype of these mice.
Results
Dystrophin expression in dKO mice after PPMO treatment
To test whether PPMOs could improve the severe dystrophic pathology of dKO mice, initial experiments were conducted with a similar treatment to that previously used in mdx mice.11 The dKO mice were injected weekly into the i.p. cavity with 5 mg/kg/week of PPMO beginning at 10 days of age. This regimen, which induces a variable level of exon 23 skipping and dystrophin expression in the different muscles of mdx mice,11 was not sufficient to rescue the severe pathology of the dKO mice. Treated mice displayed no phenotypic improvement and an equivalent reduced lifespan, as seen in untreated controls (average lifespan of 8.2 weeks, Supplementary Figure S1).
We therefore decided to increase the dosage and injected dKO mice i.p. with 25 mg/kg/week for 6 weeks beginning at 10 days of age. Treated mice were killed 6 weeks after the last injection, at 13 weeks of age. This survival, never seen in untreated dKO mice, was a first indication of treatment efficacy.
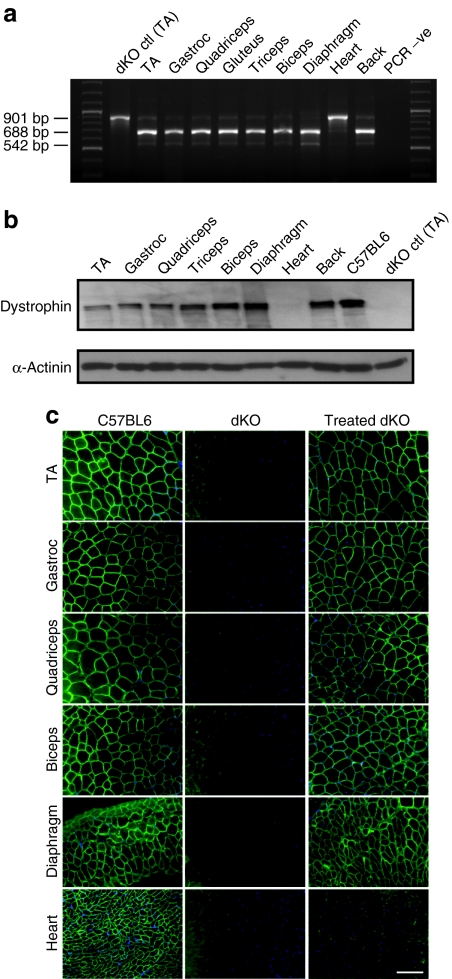
Total RNA extracted from the treated muscles was analyzed by reverse transcriptase-PCR (RT-PCR) (Figure 1a). The full-length transcript is represented by an amplicon of 901 bp, and the in-frame transcript excluding the mutated exon 23 is represented by a 688-bp product. Treatment with PPMO at a dosage of 25 mg/kg/week induced an almost complete exon 23 skipping in all skeletal muscles examined, except in cardiac muscle where only a faint band corresponding to the skipped product could be detected (Figure 1a). Skipping efficiency appeared particularly high in the diaphragm where the full-length transcript was no longer detectable. The shorter 542-bp amplicon corresponds to a skipped transcript lacking exons 22 and 23 and has been previously reported.4,17
Figure 1.
Dystrophin expression in tissues from dKO mice following six once-weekly intraperitoneal injections of PPMO at 25 mg/kg/week. (a) Reverse transcriptase-PCR analysis to detect exon 23 skipping efficiency at the RNA level. The 901-bp product represents the full-length transcript, and the products of 688 and 542 bp represent transcripts that exclude exon 23 and exons 22 and 23, respectively. (b) Western blot to detect dystrophin expression in tissues from PPMO-treated dKO mice, compared with untreated dKO and C57BL6 control mice (top gel). Equal loading of 50-µg protein is shown for each sample with α-actinin expression detected as loading control (bottom gel). (c) Immunostaining of muscle tissue cross-sections to detect dystrophin expression and localization in C57BL6 normal control mice (left panel), untreated dKO mice (middle panel), and PPMO-treated mice (right panel; N = 5). Muscle tissues analyzed were from tibialis anterior (TA), gastrocnemius, quadriceps, biceps, diaphragm, and heart muscles. Bar = 100 µm. dKO, double-knockout; PPMO, peptide-conjugated phosphorodiamidate morpholino oligomer.
Immunofluorescence performed on tissue sections revealed restoration of dystrophin in all muscles analyzed except for the heart (Figure 1c). Widespread expression of dystrophin protein over multiple sections within each muscle group was detected in hind limb (biceps and triceps), forelimb (tibialis anterior, gastroc, and quadriceps), and back muscles. As predicted by RT-PCR results, the level of dystrophin expression was particularly high in diaphragm muscle which was almost undistinguishable from normal C57BL6 diaphragm. Western blotting on samples from treated dKO mice confirmed the above results (Figure 1b). Of particular significance, considering the method of injection, were the distal muscle groups such as biceps and triceps, where levels approaching 80% could be detected. Low levels of dystrophin expression (around 2%) could be detected in the heart when the sensitivity of the western blot was increased by longer exposure (data not shown).
PPMO treatment averts the onset of dystrophic pathology in the dKO mice
In order to evaluate the functional correction in skeletal muscle following the PPMO treatment, we first assessed the restoration of the dystrophin-associated protein complex (DAPC). Dystrophin and the DAPC play a major structural role in muscle by forming a link between the internal cytoskeleton actin and the extracellular matrix, but the DAPC also has important signaling functions via neuronal nitric oxide synthase and other components.18,19 In the absence of functional dystrophin protein, the DAPC fails to localize correctly at the sarcolemma and its function is therefore compromised. Analysis of muscle sections from PPMO-treated dKO mice revealed the expression of the DAPC component proteins including α-sarcoglycan, β-sarcoglycan, β-dystroglycan, and neuronal nitric oxide synthase and their successful relocalization at the sarcolemma (Figure 2).
Figure 2.
Expression of dystrophin restores the dystrophin-associated protein complex (DAPC) to the sarcolemma in the PPMO-treated dKO mice. DAPC components α- and β-sarcoglycan, β-dystroglycan, and nNOS were detected by immunostaining in tissue cross-section of tibialis anterior (TA) muscles from PPMO-treated dKO mice (right panel), compared with C57BL6 normal mice (left panel) and untreated dKO mice (middle panel). Bar = 100 µm. dKO, double-knockout; nNOS, neuronal nitric oxide synthase; PPMO, peptide-conjugated phosphorodiamidate morpholino oligomer.
Histologically, dKO muscles are characterized by large inflammatory infiltrates and a high percentage of centrally nucleated myofibers, which reflects the continuous degeneration–regeneration process. Hematoxylin and eosin staining reveals a significant improvement of the muscle histopathology after PPMO treatment (Figure 3a). Treated muscles displayed no evidence of infiltrating mononuclear cells and a drastic reduction in the percentage of centrally nucleated myofibers (~9%) when compared to age-matched untreated dKO mice (~82%) (Figure 3b). Moreover, the restoration of dystrophin expression in treated muscles induced a significant shift in myofiber size as illustrated in Figure 3c. Although dKO muscles show an increased number of small fibers as a result of the degeneration–regeneration cycles, treated muscles reveal a uniformity of the muscle fibers similar to that observed in the C57BL6 controls, reflecting the functionality of the restored dystrophin.
Figure 3.
PPMO treatment improves muscle histopathology in dKO mice. (a) The upper panel shows immunofluorescent staining for dystrophin (green) and nuclei (blue) of tibialis anterior cross-sections from C57BL6 normal control mice (left), untreated dKO mice (middle) and PPMO-treated mice (right). The lower panel shows hematoxylin and eosin staining of the same muscles. (b) Percentage of myofibers with centronucleation (N ≈ 1,000 myofibers/cohort). (c) Box plots showing variance of the muscle fiber cross-sectional area (N ≈ 1,000/cohort). Boxes represent the middle quartiles from the 25th to the 75th percentiles, and the bar demonstrates the high and low values. ***P < 0.001 when compared to untreated dKO mice. CN, centronucleation; dKO, double-knockout; PPMO, peptide-conjugated phosphorodiamidate morpholino oligomer.
In contrast to mdx mice, dKO mice display a very severe dystrophic phenotype, including abnormal waddling gait, contracted and stiff limbs, and very pronounced kyphosis as a result of the degenerative process (Figure 4a). The reduced musculature considerably affects the mobility of the mice, especially during the last few weeks of life. Supplementary Video S1 illustrates this seriously decreased ambulation in a 9-week-old control dKO mouse. The appearance and behavior of age-matched PPMO-treated dKO mice are strikingly different. Their dystrophic pathology appears greatly improved as the animals demonstrate only a minimal kyphosis and contracted limbs (Figure 4b). Furthermore, their mobility, their food seeking behavior, and the turgor of the tail are markedly improved and appear close to normal as demonstrated in Supplementary Video S2.
Figure 4.
PPMO treatment averts the onset of dystrophic pathology in the dKO mice. (a) Photograph of an untreated dKO mouse at 10 weeks of age, displaying a strong kyphosis and joint contractures compared with (b) a PPMO-treated mouse at 10 weeks of age, looking healthy. (c) Measurement of serum creatine kinase (CK) levels as an index of ongoing muscle membrane instability in PPMO-treated dKO mice (N = 5) compared with untreated dKO mice (N = 9; ***P < 0.005) and C57BL6 normal control mice (N = 6). dKO, double-knockout; PPMO, peptide-conjugated phosphorodiamidate morpholino oligomer.
In addition, treated dKO mice displayed a massive reduction in serum creatine kinase levels when compared with untreated dKO mice (Figure 4c). This significant drop to normal levels indicated a whole-body reduction in muscle degeneration consistent with the widespread restoration of dystrophin expression.
PPMO treatment improves muscle function in dKO mice
Encouraged by the widespread restoration of dystrophin expression and the considerable improvement of the dystrophic phenotype of the treated mice, we investigated in more detail their muscle function and contractile properties.
We first assessed the forelimb muscle strength of the treated and control mice 2 weeks after the last injection using a functional test of grip force strength.20,21 As expected, untreated dKO mice displayed a much reduced grip strength compared to age-matched C57BL6 controls as illustrated in Figure 5a. On the other hand, treated dKO showed a remarkable improvement of their grip strength, no longer different from the normal controls, consistent with the particularly high restoration of dystrophin expression observed in the forelimbs.
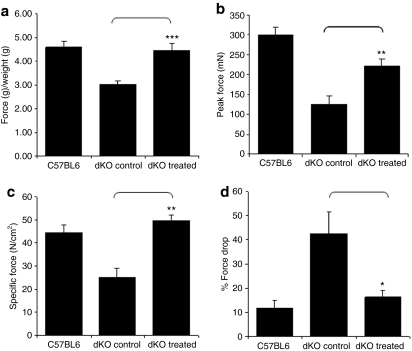
Figure 5.
PPMO treatment improves muscle function in dKO mice. (a) Forelimb muscle function was assessed using a functional grip strength test to determine the physical improvement of PPMO-treated dKO mice compared with untreated dKO mice and C57BL6 normal control mice (N = 6/cohort). ***P < 0.005 compared to untreated dKO mice and no significant difference to C57BL6 control mice. (b) Extensor digitorum longus (EDL) muscles of PPMO-treated dKO mice were analyzed for their maximal force (peak force) producing capacity compared to untreated dKO mice and C57BL6 control mice. (c) Maximal force was also normalized for cross-sectional area to assess specific force. **P < 0.01 compared to untreated dKO mice and no significant difference to C57BL6. (d) The percentage of Force drop is assessed by measuring the force deficit following a series of five eccentric contractions. *P < 0.05 compared to untreated dKO mice and no significant difference to C57BL6 control mice. Error bars are shown as mean ± SEM (N = 5/cohort). dKO, double-knockout; PPMO, peptide-conjugated phosphorodiamidate morpholino oligomer.
We next investigated force production in hindlimb muscles of treated and control mice using the extensor digitorum longus muscle. Both peak force and specific force, after normalization with the cross-sectional area of the muscles, appeared dramatically reduced in untreated dKO mice, as a result of their severe muscle degeneration (Figure 5b,c). PPMO treatment considerably improved dKO muscle function such that specific force was no longer significantly different from that generated by wild-type mice (Figure 5c).
In addition, we tested the ability of the restored dystrophin to protect dKO myofibers from exercise-induced damage, by assessing maintenance of force production following a series of eccentric contractions. The protocol applied resulted in a reduction of initial muscle contractile force to the extent of ~42% in untreated dKO mice compared to only ~12% in C57BL6 mice (Figure 5d). Treated dKO muscles appeared largely protected from contraction-induced injury as the percentage of force drop was reduced to ~16%. Taken together, these results indicate that the widespread restoration of dystrophin expression induced by the PPMO greatly improves muscle function in the severely affected dKO mice.
Discussion
Many therapeutic approaches have been investigated for DMD, including gene or cell therapy, nonsense mutation suppression and homologous gene upregulation (for review, see refs. 22,23). Among these, antisense-mediated exon-skipping has recently gained increasing attention as its ability to restore dystrophin expression has been demonstrated in several studies.5,9,24,25 Clinical trials to evaluate local antisense oligonucleotide exon-skipping in DMD patients have successfully been conducted in the Netherlands6 and in the United Kingdom, using 2′-O-methyl phosphorothioate and PMO, respectively, and systemic trials are currently in progress. Nonetheless, major challenges remain, including achievement of widespread and sustained restoration of dystrophin accompanied with minimal adverse effects. Therefore, the ideal compound will have to show efficacy at low dose, be able to target all of the tissues affected by the lack of dystrophin, including the heart, have long-term effects, and be easily administered. Although previous work investigating antisense oligonucleotide–based exon-skipping failed to achieve some of these objectives, recent studies using PPMO have described very promising findings. Cell-penetrating peptide–conjugated PMO have indeed been shown to induce widespread, uniform, and sustained restoration of dystrophin in most of the skeletal muscles and the cardiac muscle in mdx mice.12,13
In this study, we investigated the therapeutic potential of such peptide-conjugated PMO-mediated exon-skipping in the severely affected dKO mice and demonstrated an impressive prevention of their dystrophic pathology. Treatment with PPMO at a dosage of 25 mg/kg/week for 6 weeks induced almost complete exon 23 skipping in all the muscles examined, except the heart, as revealed by the RT-PCR analysis. This very efficient exon-skipping was associated with a widespread restoration of dystrophin in the same muscles. Levels of exon-skipping and dystrophin expression were particularly high in the diaphragm of the treated dKO mice, which appeared almost indistinguishable from wild-type diaphragm. Of particular significance was the restoration of dystrophin protein—levels approaching 80%—that was detected in distal muscle groups such as triceps and biceps. In contrast, exon-skipping and dystrophin expression were only minimal in the cardiac muscle, which differs from previously reported results. A recent study using the same PPMO oligomer demonstrated a dystrophin restoration of ~20% of normal levels following intravenous injection in mdx mice.12 This difference in heart dystrophin level likely results from the method of administration. In fact, in mdx mice, correction of dystrophin expression in the heart was more effective via intravenous delivery as compared to i.p. or subcutaneous routes12,13,14 (N. Jearawiriyapaisarn, P. Sazani, and R. Kole, unpublished results). In the particular case of dKO mice, being able to treat the animal very early in life was crucial to demonstrate that PMO-induced dystrophin can largely prevent onset of the dystrophic process that normally begins at ~14 days of age.26 Intraperitoneal delivery was the only feasible route of treatment of neonatal animals. However, in older animals and, more importantly, in DMD patients, intravenous infusions of PPMO may lead to more significant dystrophin expression in the heart.
Initial experiments conducted with a dose of 5 mg/kg/week of PPMO as previously used in the mdx mice11 did not lead to any phenotypic improvement of the dKO mice, although some level of dystrophin expression could be detected in mdx controls following the same treatment. This could be explained by low and heterogeneous levels of dystrophin restoration induced with this dose in the different muscles (100% in diaphragm, and 3–8% in distal muscles as described in ref. 11), which would therefore not be sufficient to compensate the severe degeneration of the dKO muscles.
In contrast to dystrophin-deficient mdx mice, whose mild phenotype can be attributable, at least in part, to compensatory overexpression of utrophin at the sarcolemma,27 dKO mice display an earlier onset of dystrophy, more extensive muscle wasting, significantly reduced musculature, contractures of the joints, and a considerably shortened lifespan.15 It is possible that a low-dose treatment such as 5 mg/kg/week should be administered even earlier than 10 days in these mice to prevent the onset of dystrophy, but this could not be tested because of the genotyping steps required, which prevents us from injecting dKO mice before 10 days of age. The dramatically reduced lifespan of the dKO mice, together with the early onset of dystrophy, might represent a very extreme situation where high levels of dystrophin restoration have to be achieved very quickly. Repeated low-dose treatment, which could slowly restore dystrophin expression, might therefore not appear effective in the dKO mouse model when compared to the mdx model. A recent study using naked PMO in mdx mice has demonstrated the significant impact of dosing regimen28 and shown that repeated administration of low doses of PMO could increase the number of dystrophin positive fibers compared with that observed following a single large bolus injection. The lack of functional rescue observed in dKO mice treated with similar repeated low doses of PPMO presumably results from the rapid progression of the disease, possibly suggesting that the dKO model might not be appropriate for evaluating low-dose regimens. However, the comparatively longer time scale of disease progression in DMD patients might allow slow accumulation and restoration of dystrophin through repeated low-dose treatment, which could be clinically applicable.
Increasing the dosage to 25 mg/kg/week in dKO mice allowed a widespread, higher level, and more uniform restoration of dystrophin expression in the different skeletal muscles. As a consequence, we found that DAPC protein components were correctly relocalized and morphological properties of the treated muscles were greatly improved. Restoration of dystrophin expression induced a significant shift in myofiber size and a dramatic reduction in the percentage of centrally nucleated myofibers when compared to age-matched untreated dKO mice. In addition, a massive drop in serum creatine kinase levels was found in treated dKO mice compared with untreated controls, indicating the wide protective effect of restored dystrophin on myofiber integrity.
In order to evaluate in more detail the benefit of the treatment, we performed a series of functional tests starting with assessing the forelimb strength. Treated mice demonstrated a remarkable improvement of grip strength, similar to the wild-type controls, consistent with the particularly high level of dystrophin expression observed in the forelimbs. We next assessed hind limb muscle function and showed significant increase of peak force and drastic reduction in the percentage of force drop following a series of eccentric contractions. These results confirmed the functionality of the restored dystrophin, which protects the myofibers from exercise-induced damage. Remarkably, the specific force generated by treated dKO extensor digitorum longus muscle was no longer different from that recorded in wild-type mice, even though dystrophin restoration in hind limb muscles was estimated between 50 and 60% of normal levels by western blot (for tibialis anterior and gastrocnemius muscles, respectively). However, the quantification of restored protein represents an average of total amount and does not reveal the distribution of the dystrophin within the muscle. Immunostaining data on the other hand reflected a uniform expression of dystrophin, suggesting that each fiber might express the protein but at a lower level. This uniformity could therefore explain the impressive results of specific force recovery following PPMO treatment.
Altogether, these results demonstrate the functionality of the restored dystrophin and represent the molecular, morphological, and functional improvement of the dystrophic pathology of these mice.
Although control dKO mice display a very severe dystrophic phenotype including extensive muscle wasting, contracture of the joints, a very pronounced kyphosis, and a dramatically reduced motility (as illustrated in Supplementary Video S1), treated dKO mice show almost return-to-normalcy for most of the examined parameters. PPMO treatment strikingly prevented kyphosis and contractures and remarkably improved their motility as shown in Supplementary Video S2.
The severe pathology of dKO mice results in a considerably shortened lifespan (80% mortality by 10 weeks of age) and no control dKO mice have survived beyond 12 weeks of age as illustrated in Supplementary Figure S1. Although we did not perform a detailed lifespan analysis, we observed a clear improvement as treated mice looked very healthy when killed at 13 weeks of age (which is older than any control). The appearance and behavior of 13-week-old treated mice were still close to normal as shown at 9 weeks of age in Supplementary Video S2. Although we decided to kill treated mice 6 weeks after the last injection in order to further investigate dystrophin restoration and muscle properties, we also kept few of them for longer term experiments. These mice, reinjected once a month with 25 mg/kg of PPMO in order to maintain a good level of dystrophin expression, are now 26 week old and still look very healthy.
In conclusion, this study demonstrates for the first time the efficiency of the antisense-mediated exon-skipping approach in the utrophin/dystrophin dKO mouse, which is a much more severe and progressive mouse model of DMD. Alternative approaches using adeno-associated viral vectors to deliver mini genes have also been shown to preserve muscle function and extend lifespan in these severely affected dKO mice.29,30,31 These studies demonstrate the efficacy and long-term effect of gene therapy protocols for DMD. However, as the viability of viral vectors in the clinics, especially considering potential immune responses against the viral capsid, still needs to be established, an antisense compound with minimal toxicity would be highly desirable.
Here, we report a remarkable prevention of the dystrophic pathology and improvement of the muscle function of the severely affected dKO mice following repeated i.p. injections of a cell-penetrating peptide–conjugated PMO. These findings indicate that PPMOs exhibit therapeutic, drug-like effects not only in relatively benign mdx mouse model but also in very severe DMD phenotype of dKO mouse, suggesting great potential for these compounds in the systemic treatment of DMD.
Materials and Methods
Morpholino oligomer and animal experiments. The PMO, designated M23D(+7–18), anneals to the last seven nucleotides of mouse dystrophin exon 23 and the first 18 nucleotides of intron 23 and has been described previously.24 The PMO, prepared as a conjugate with the cell-penetrating peptide, was supplied by AVI Biopharma (Corvallis, OR) and is referred to here as PPMO.11
The PPMO was resuspended in sterile purified water at a concentration of 50 mg/ml and stored at 4 °C until required. The PPMO diluted in normal saline was delivered to 10-day-old dKO mice by the i.p. route at a dosage of 25 mg/kg/week for a 6-week treatment. Mice were injected at 10 days of age instead of 1 day of age previously reported for mdx mice11 because of the genotyping steps required for the dKO. Control dKO mice (referred to as untreated dKO mice) were injected with an equivalent volume of normal saline. Control dKO mice show a very severe dystrophic phenotype and were killed at humane end point that varies between 8 and 12 weeks of age depending on the severity of their pathology. No control dKO mice ever survived over the age of 12 weeks in our animal facility. Treated mice were killed at 13 weeks of age (6 weeks after the last injection) by CO2 inhalation and blood was collected from the jugular vein immediately after killing to determine serum creatine kinase levels. Analysis of serum creatine kinase levels was performed by the pathology laboratory (Mary Lyon Centre, Medical Research Council, Harwell, Oxfordshire, UK). Muscles were snap-frozen in liquid nitrogen–cooled isopentane and stored at −80 °C before further analysis. All animal experiments were carried out in the Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK and performed according to the guidelines and protocols approved by the Home Office.
Immunohistochemistry and histology. Sections of 8-µm thickness were cut from at least two-thirds of the muscle length of tibialis anterior, gastrocnemius, quadriceps, gluteus, biceps, triceps, diaphragm, back muscles, and cardiac muscle at 100-µm intervals. The intervening muscle sections were collected for subsequent RT-PCR analysis. Routine hematoxylin and eosin staining was used to examine overall muscle morphology. The cryosections were then examined for dystrophin expression using the mouse monoclonal antibody NCL-DYS2 (Novocastra, Newcastle upon Tyne, UK). DAPC protein detection was also performed using a rabbit polyclonal antibody to neuronal nitric oxide synthase and mouse monoclonal antibodies to β-dystroglycan, α- and β-sarcoglycan according to manufacturer's instructions (Novocastra). Polyclonal antibody was detected by goat-anti-rabbit IgGs Alexa 488 and the monoclonal antibodies were used with an M.O.M. kit (Vector laboratories, Burlingame, CA).
RNA isolation and RT-PCR analysis. Total RNA was isolated from intervening muscle sections collected during cryosection using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Renfrew, UK). Aliquots of 200 ng of total RNA were used for RT-PCR analysis using the Access RT-PCR System (Promega, Southampton, UK) in a 50 µl reaction using the external primers Ex 20Fo (5′-CAGAATTCTGCCAATTGCTGAG-3′) and Ex 26Ro (5′-TTCTTCAGCTTGTGTCATCC-3′). The complementary DNA synthesis was carried out at 45 °C for 45 minutes, directly followed by the primary PCR of 30 cycles of 94 °C (30 seconds), 58 °C (1 minutes), and 72 °C (2 minutes). Two microliters of these reactions were then reamplified in nested PCRs by 22 cycles of 94 °C (30 seconds), 58 °C (1 minutes), and 72 °C (2 minutes) using the internal primers Ex 20Fi (5′-CCCAGTCTACCACCCTATCAGAGC-3′) and Ex 26Ri (5′-CCTGCCTTTAAGGCTTCCTT-3′). PCR products were analyzed on 2% agarose gels.
Western blot analysis. Total protein was extracted from muscle samples with Newcastle buffer (3.8% sodium dodecyl sulfate, 75 mmol/l Tris–HCl pH 6.7, 4 mol/l urea, 10% β-mercaptoethanol, 10% glycerol, 0.001% bromophenol blue) and quantified using the bicinchoninic acid protein assay kit, according to the manufacturer's instructions (Perbio Science, Chester, UK). Samples were denatured at 95 °C for 5 minutes before 50 µg of protein was loaded in a 5% polyacrylamide gel with a 4% stacking gel. Gels were electrophoresed for 4–5 hours at 100 V and blotted to a polyvinylidene fluoride membrane overnight at 40 V. Blots were blocked for 1 hour with 10% nonfat milk in phosphate-buffered saline–Tween buffer. Dystrophin and α-actinin proteins were detected by probing the membrane with 1:100 dilution of NCL-DYS1 primary antibody (monoclonal antibody to dystrophin R8 repeat; Novocastra) and 1:200 dilution of α-actinin primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) respectively. An incubation with a mouse horseradish peroxidase–conjugated secondary antibody (1:2,000) or goat horseradish peroxidase–conjugated secondary antibody (1:160,000) allowed visualization using ECL Analysis System (GE Healthcare, Amersham, UK). Membranes were converted to numerical pictures by scanning and band intensities were analyzed using the ImageJ 1.33a software (http://rsb.info.nih.gov/ij/).
Muscle function analysis. Functional grip strength analysis was performed on treated and control mice at 8 weeks of age using a commercial grip strength monitor (Chatillon, Elancourt, France). Each mouse was held 2 cm from the base of the tail, allowed to grip a bar attached to the apparatus with their fore paws, and pulled gently until they released their grip. The force exerted was recorded from four sequential tests, averaged at 1 minute apart.
Peak force, specific force, and force drop were measured from the extensor digitorum longus muscle dissected from the hind leg of the treated and control mice. During dissection and experiments, muscles were bathed in oxygenated (95% O2–5% CO2) Krebs–Hensley solution composed of (mmol/l): NaCl, 118; NaHCO3, 24.8, KCl, 4.75; KH2PO4, 1.18; MgSO4, 1.18; CaCl2, 2.54; glucose, 10 (ref. 32). Contractile properties were measured using previously described techniques.33 In brief, a silk suture tied onto the exposed tendon at each end of the muscle was used to attach the proximal end of the muscle to a lever arm system connected to a force transducer (model 300B; Aurora Scientific, Aurora, Ontario, Canada) and the distal end to a clamp. The force transducer was in turn attached to a stimulator (model 701B; Aurora Scientific). The equipment was controlled using the signal interface model 604A (Aurora Scientific) and results were recorded by the DMC software (version 4.1.4; Aurora Scientific). The muscle was held vertically and stimulated by an electric field generated by two platinum electrodes placed longitudinally on each side of the muscle. The muscle was subjected to single pulses of 0.2 milliseconds in duration at 30 V while the optimum length (Lo) for development of maximum isometric twitch force was determined. Fiber length (Lf) was determined by multiplying the figure obtained for Lo by the predetermined fiber length to muscle length ratio of 0.45 (ref. 34). A force–frequency curve was generated at 10-Hz intervals from 10 to 100 Hz during a 350-millisecond stimulus. The maximum isometric force (Po) was calculated from the peak of the curve, as were data required to calculate time to half-peak and time to half-relaxation. Eccentric contractions were performed during a 0.75-second protocol. The muscle was stimulated into tetanus at the frequency required to generate the maximum isometric force. After 0.25 seconds, while contracting tetanically, the muscle was stretched at a rate of one fiber length per second for 0.15 seconds, equating to a total stretch of 15% of fiber length. Shortening of the muscle an equal distance either side of Lf was designed to enhance force development.33 The difference in maximum force produced during tetanus between the first (ECC1) and the fifth (ECC5) eccentric contraction was expressed as a percentage of ECC1 to calculate the percentage force drop. Forces were normalized (N/cm2) by dividing the fiber length by the wet weight of the muscle, which was measured at the end of the experiment after tendons had been removed. All data were digitized and analyzed using the DMA software (version 3.2; Aurora Scientific).
Statistical analysis. All results are expressed as mean values ± SEM unless otherwise stated. Differences between treated and control cohorts were determined using an unpaired Student's t-test.
SUPPLEMENTARY MATERIALFigure S1. Life span of control dKO mice.Video S1. Control dKO mouse.Video S2. PPMO-treated dKO mouse.
Supplementary Material
Life span of control dKO mice.
Control dKO mouse.
PPMO-treated dKO mouse.
Acknowledgments
We thank Tertious Hough, Clinical Pathology Laboratory, Mary Lyon Centre, Medical Research Council, Harwell, UK for clinical testing of blood samples. This work was supported by the Medical Research Council, the Association Monegasque contre les myopathies, the Duchenne Parent Project de France, and the Muscular Dystrophy Association of the United States.
REFERENCES
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H., and , Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Sherratt TG, Vulliamy T, Dubowitz V, Sewry CA., and , Strong PN. Exon skipping and translation in patients with frameshift deletions in the dystrophin gene. Am J Hum Genet. 1993;53:1007–1015. [PMC free article] [PubMed] [Google Scholar]
- Dunckley MG, Manoharan M, Villiet P, Eperon IC., and , Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, et al. Local restoration of dystrophin expression with the morpholino oligomer AV1-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD., and , Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Steinhaus JP, et al. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol Ther. 2007;15:1587–1592. doi: 10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P, et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S, et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS., and , Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Honeyman K, McClorey G, Fletcher S., and , Wilton SD. Improved antisense oligonucleotide induced exon skipping in the mdx mouse model of muscular dystrophy. J Gene Med. 2002;4:644–654. doi: 10.1002/jgm.295. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Newey SE, Benson MA, Ponting CP, Davies KE., and , Blake DJ. Alternative splicing of dystrobrevin regulates the stoichiometry of syntrophin binding to the dystrophin protein complex. Curr Biol. 2000;10:1295–1298. doi: 10.1016/s0960-9822(00)00760-0. [DOI] [PubMed] [Google Scholar]
- Oliver PL, Keays DA., and , Davies KE. Behavioural characterisation of the robotic mouse mutant. Behav Brain Res. 2007;181:239–247. doi: 10.1016/j.bbr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- Wells DJ. Therapeutic restoration of dystrophin expression in Duchenne muscular dystrophy. J Muscle Res Cell Motil. 2006;27:387–398. doi: 10.1007/s10974-006-9081-6. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Perkins KJ, Krag TO., and , Khurana TS. Therapeutics for Duchenne muscular dystrophy: current approaches and future directions. J Mol Med. 2004;82:102–115. doi: 10.1007/s00109-003-0484-1. [DOI] [PubMed] [Google Scholar]
- Gebski BL, Mann CJ, Fletcher S., and , Wilton SD. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet. 2003;12:1801–1811. doi: 10.1093/hmg/ddg196. [DOI] [PubMed] [Google Scholar]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Mateddu A, Marchei F, Clerk A., and , Serra G. Muscular weakness in the mdx mouse. J Neurol Sci. 1993;120:71–77. doi: 10.1016/0022-510x(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Hirst RC, McCullagh KJ., and , Davies KE. Utrophin upregulation in Duchenne muscular dystrophy. Acta Myol. 2005;24:209–216. [PubMed] [Google Scholar]
- Malerba A, Thorogood FC, Dickson G., and , Graham IR. Dosing regimen has a significant impact on the efficiency of morpholino oligomer-induced exon skipping in mdx mice. Hum Gene Ther. 2009;20:955–965. doi: 10.1089/hum.2008.157. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P, Allen JM, Finn E., and , Chamberlain JS. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther. 2008;16:1539–1545. doi: 10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, Fu FH., and , Xiao X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin deficient mice. J Orthop Res. 2009;27:421–426. doi: 10.1002/jor.20781. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Woledge RC., and , Curtin NA. Effects of UCP3 genotype, temperature and muscle type on energy turnover of resting mouse skeletal muscle. Pflugers Arch. 2009;457:857–864. doi: 10.1007/s00424-008-0552-z. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT., and , Faulkner JA. Power output of fast and slow skeletal muscles of mdx (dystrophic) and control mice after clenbuterol treatment. Exp Physiol. 2000;85:295–299. [PubMed] [Google Scholar]
- Brooks SV., and , Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol (Lond) 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Life span of control dKO mice.
Control dKO mouse.
PPMO-treated dKO mouse.