Abstract
Drug delivery with microbubbles and ultrasound is gaining more and more attention in the drug delivery field due to its noninvasiveness, local applicability, and proven safety in ultrasonic imaging techniques. In this article, we tried to improve the cytotoxicity of doxorubicin (DOX)-containing liposomes by preparing DOX-liposome-containing microbubbles for drug delivery with therapeutic ultrasound. In this way, the DOX release and uptake can be restricted to ultrasound-treated areas. Compared to DOX-liposomes, DOX-loaded microbubbles killed at least two times more melanoma cells after exposure to ultrasound. After treatment of the melanoma cells with DOX-liposome-loaded microbubbles and ultrasound, DOX was mainly present in the nuclei of the cancer cells, whereas it was mainly detected in the cytoplasm of cells treated with DOX-liposomes. Exposure of cells to DOX-liposome-loaded microbubbles and ultrasound caused an almost instantaneous cellular entry of the DOX. At least two mechanisms were identified that explain the fast uptake of DOX and the superior cell killing of DOX-liposome-loaded microbubbles and ultrasound. First, exposure of DOX-liposome-loaded microbubbles to ultrasound results in the release of free DOX that is more cytotoxic than DOX-liposomes. Second, the cellular entry of the released DOX is facilitated due to sonoporation of the cell membranes. The in vitro results shown in this article indicate that DOX-liposome-loaded microbubbles could be a very interesting tool to obtain an efficient ultrasound-controlled DOX delivery in vivo.
Introduction
Doxorubicin (DOX), also called adriamycin, is one of the most frequently used anticancer drugs. DOX is used for the treatment of different solid and hematopoietic cancers such as breast cancer, osteosarcomas, aggressive lymphomas, and leukemias. Different mechanisms explain its cytotoxic activity.1 They include DNA intercalation, lipid peroxidation, and inhibition of topoisomerase II. The use of free DOX is rather limited because of the severe side effects. Indeed, besides damaging tumors, it also causes cardiotoxicity and nephrotoxicity.1 Additionally, the efficacy of free DOX is also hampered by multidrug resistance, originating from the P-glycoprotein and topoisomerase II resistance.1 Because of these problems associated with free DOX treatment, DOX has been encapsulated inside liposomes. These liposomes contain PEG (polyethylene glycol) chains at their surface to prevent recognition by the reticuloendothelial system (so-called stealth liposomes). This results in the passive accumulation of stealth liposomes in the tumor vasculature due to the enhanced permeability and retention effect.2 In 1995, the liposomal DOX formulations Doxil and Caelyx became FDA-approved for the treatment of AIDS-related Kaposi's sarcoma and ovarian cancer. Although Doxil strongly reduced the cardiotoxicity of DOX in clinical trials, other side effects occurred. Several patients suffered from mucositis and the hand and foot syndrome due to the localization of the liposomes in skin capillaries.1 Therefore, many research groups try to enhance the targeting of DOX to the tumors by attaching ligands or antibodies to DOX-loaded vehicles or by incorporating DOX in stimuli-responsive carriers like pH and temperature-responsive nanocarriers.1,3
In the past, ultrasound has been used as an external trigger to induce drug release from drug-loaded carriers. In these experiments, low frequency (<1 MHz) ultrasound was used. More recently, it has been demonstrated that high-frequency ultrasound (1–10 MHz), when combined with diagnostic microbubbles, can enhance the intracellular delivery and extravasation of drugs.4,5,6,7,8,9 These effects have been attributed to inertial cavitation of the microbubbles. Cavitation is the alternate growing and shrinking of microbubbles under the influence of an ultrasonic field.10 When the ultrasound intensity is high enough, microbubbles can implode due to the inertia of the inrushing fluid (inertial cavitation). As a result, several groups have shown that fluid streams and microjets develop that can transiently perforate the membranes of nearby cells and hence enhance the intracellular uptake of drugs.9,11,12,13,14 This phenomenon is called sonoporation. Additionally, it has been shown that such microjets can also transiently perforate blood vessels and thus induce extravasation of large molecules.15,16
Several papers report on the synergistic effect of DOX and ultrasound.17 However, these papers mainly focused on ultrasound-assisted intracellular delivery of free DOX18,19 or DOX encapsulated in micelles or liposomes.20,21,22,23 The forces associated with the inertial cavitation of the microbubbles may (i) massively release the encapsulated drug from the nanocarriers and (ii) improve the intracellular uptake of DOX due to sonoporation of the cell membranes. However, a major drawback of co-injecting DOX-liposomes and microbubbles is the fact that DOX-liposomes can still extravasate and accumulate in undesired tissues (not exposed to ultrasound), like the skin capillaries.1 This can still result in unwanted side effects. Coupling of the liposomes to the microbubbles could prevent this, as microbubbles attached to the microbubble wall will no longer be able to extravasate. This study aimed to further improve ultrasound-mediated delivery of DOX-liposomes. Therefore, we designed “DOX-loaded microbubbles” through avidin–biotin binding of DOX-containing liposomes to the lipid shell of microbubbles (DOX-liposome-loaded microbubbles) (Figure 1). DOX delivery by such constructs could be attractive, as it would take profit of both the sonoporation effect and targeting potential of ultrasound. Indeed, microbubbles carrying DOX-liposomes at their surface are expected to be too large to extravasate in undesired tissue (i.e., not treated by ultrasound). Only after ultrasound-mediated release of DOX-liposome, extravasation can occur at especially these regions that are treated with ultrasound. This paper shows the killing of tumor cells by DOX-liposome-loaded microbubbles and explains the underlying mechanisms.
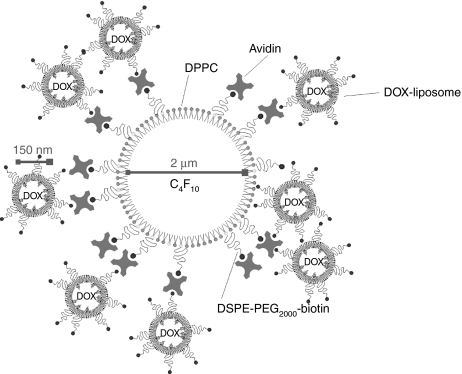
Figure 1.
Schematic presentation of a DOX-liposome-loaded microbubble (not in scale). Biotinylated DOX-containing liposomes were attached to the surface of a biotinylated lipid microbubble with the aid of an avidin molecule. The mean sizes of liposomes and microbubbles are indicated on the picture. C4F10, perfluorobutane; DOX, doxorubicin; DPPC, dipalmitoylphosphatidylcholine; DSPE-PEG2000-biotin, ((1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl(polyethyleneglycol)2000)).
Results
Design and characterization of DOX-liposome-loaded microbubbles
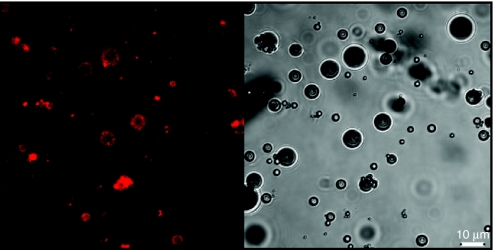
As schematically presented in Figure 1, we aimed to construct lipid microbubbles loaded with DOX-liposomes. Therefore, we first prepared DOX-containing liposomes composed of 55 mol% dipalmitoylphosphatidylcholine, 40 mol% cholesterol, and 5 mol% DSPE-PEG-biotin ((1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl(polyethyleneglycol)2000)). After loading the liposomes with DOX and gel permeation chromatography purification, DOX-liposomes with an average diameter of 147 nm were obtained. We also prepared lipid-shelled microbubbles that contained 15 mol% DSPE-PEG-biotin in their shell. The biotinylated microbubbles were subsequently incubated with an excess of avidin to saturate the biotin molecules at their surface. After removal of the excess of avidin, the microbubbles were incubated with the biotinylated DOX-liposomes to couple the DOX-liposomes on the microbubbles via an avidin–biotin bridge (Figure 1). The binding of the DOX-liposomes, which are fluorescent due to the presence of DOX, on the microbubbles was confirmed via confocal laser scanning microscopy: Figure 2 clearly shows that the surface of the microbubbles becomes surrounded by DOX-containing liposomes. The amount of DOX-liposomes that was bound to the microbubbles was estimated by removing the unbound DOX-liposomes from the microbubbles via centrifugation. We measured that 65% of the DOX-liposomes was attached to the microbubbles. Knowing that we mixed about 1 × 109 microbubbles with 50 µg of DOX encapsulated in liposomes, each microbubble contains about 3.25 × 10−8 µg DOX.
Figure 2.
Confocal laser scanning microscopy image (left) and corresponding transmission image (right) of DOX-liposome-loaded microbubbles. The DOX-liposomes were visualized using the fluorescence of the bound doxorubicin. DOX, doxorubicin.
Efficacy of DOX-liposome-loaded microbubbles after ultrasound treatment
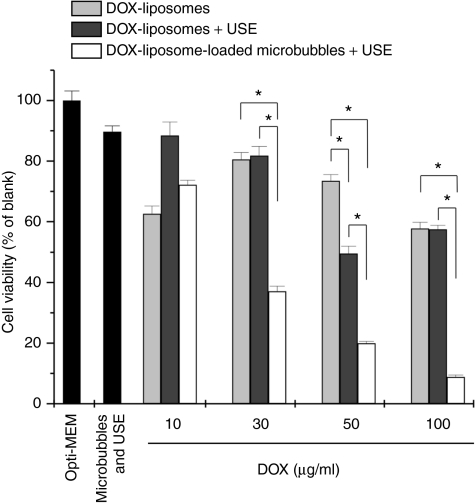
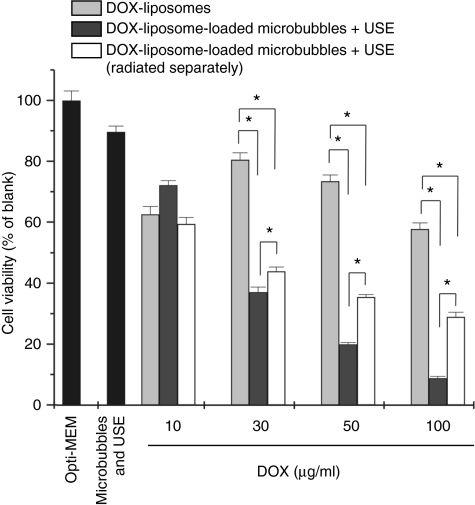
In Figure 3, the killing of cancer cells by DOX-liposome-loaded microbubbles, in the presence of ultrasound, and DOX-liposomes is compared. The cell killing by DOX-liposomes (light gray bars) was rather limited; the highest concentrations (50 and 100 µg/ml) killed about 50% of the cells. In contrast, DOX-liposomes attached onto the microbubbles (white bars) were by far more toxic to the cells after ultrasound application. We also observed a nice correlation between the liposome concentration present on the DOX-liposome-loaded microbubbles and the cell viability, which was less clear in case DOX-liposomes were used.
Figure 3.
Cell viability of the melanoma cells after treatment with DOX-liposomes without (light gray bars) and with ultrasound exposure (USE) (dark gray bars) and DOX-liposome-loaded microbubbles after ultrasound exposure (white bars) as a function of the DOX concentration in the OptiCell. For higher doxorubicin concentrations, more doxorubicin liposomes were used, whereas the microbubble concentration was always 109 microbubbles per OptiCell unit. *P < 0.05. DOX, doxorubicin.
Some groups described a synergistic effect of ultrasound on the killing of cells by DOX-liposomes24 and DOX-micelles,22,25,26 though we did not observe an outspoken improvement of the cell killing by DOX-liposomes when ultrasound was applied (Figure 3: dark gray bars). Most authors use low-frequency ultrasound (20–100 kHz), which is known to favor cavitation at relatively low intensities, even in the absence of microbubbles. In our experiment, we exposed the DOX-liposomes to 1 MHz frequency ultrasound in the absence of microbubbles. Under these ultrasound conditions, it is well known that cavitation is limited and probably too low to release the DOX from the liposomes or to perforate cell membranes. In contrast, DOX-liposome-loaded microbubbles in the presence of ultrasound significantly lowered the overall viability of the melanoma cells. We observed previously that the ultrasound conditions used in this study may detach a (small) part of the cells from the OptiCell membrane. Therefore, we also studied the effect of microbubbles (not loaded with DOX-liposomes) and ultrasound on the viability of the melanoma cells. As Figure 3 shows, this reduced the cell viability to about 10%. Even though if we take this into account, DOX-liposome-loaded microbubbles and ultrasound seemed much more efficient in killing cancer cells than free DOX-liposomes.
Intracellular localization of DOX
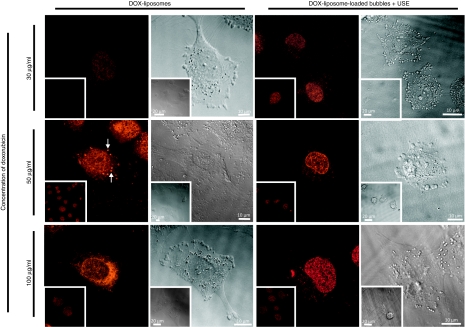
We tried to gain more insight into the intracellular DOX concentrations in the melanoma cells (Figure 4). Different concentrations of DOX-liposomes and DOX-liposome-loaded microbubbles were added to the cells. After 4 hours, the cells were washed and DOX uptake was visualized by confocal laser scanning microscopy. At the lowest DOX concentration (30 µg/ml), we could detect more DOX in cells exposed to DOX-liposome-loaded microbubbles and ultrasound than in cells exposed to DOX-liposomes. This was less obvious at higher DOX concentrations used. The intracellular distribution of DOX seemed to strongly depend on the way the DOX was delivered to the cells. It was almost exclusively localized in the nuclei when the cells were treated with the DOX-liposome-loaded microbubbles and ultrasound, whereas DOX was found in both the cytoplasm and the nucleus of cells treated with DOX-liposomes. Sometimes a punctuated pattern could be seen in the cytoplasm of these cells (indicated by white arrows in Figure 4), which suggests that the DOX locates in endosomes. Two simultaneously occurring phenomena may explain the different intracellular distribution of DOX. First, after exposure of cells to DOX-liposome-loaded microbubbles and ultrasound, free DOX (released from the liposomes destroyed by the ultrasound) probably enters the cells and accumulates in the nucleus because of its high affinity for DNA,27 which is abundantly present in the nucleus. Second, Schlicher et al.28 recently described the existence of exocytosis after exposure of cells to ultrasound and microbubbles to reseal the pores in the cell membranes. The transport of vesicles from the inside to the outside of the cell may limit endocytosis and thus reduce the amount of free DOX or DOX-liposomes that is taken up by endocytosis.
Figure 4.
Confocal laser scanning microscopy images and corresponding transmission images of the uptake of DOX in melanoma cells treated with DOX-liposomes and DOX-liposome-loaded microbubbles after ultrasound exposure. Cells were treated and incubated for 4 hours. Subsequently, the cells were washed and immediately visualized with the confocal microscope. At a given concentration of doxorubicin, the same laser intensities were used to visualize the uptake of DOX delivered by the two approaches. In case of 100 µg/ml DOX, a lower laser intensity was used than in the case of 50 µg/ml and 30 µg/ml. This explains why the 100 µg/ml images are less fluorescent. DOX, doxorubicin; USE, ultrasound exposure.
The higher amount of DOX in the nuclei after exposure of the cells to DOX-liposome-loaded microbubbles and ultrasound suggests the following delivery mechanism. Applying ultrasound destroys the liposomes on the microbubbles releasing free DOX near the cell membranes, which can enter cells more easily than DOX-liposomes. Second, ultrasound may also increase the amount of free DOX and DOX-liposomes that enter the cells via perforations in the membranes. In the following paragraphs, we further investigate the mechanisms that may explain the stronger cell killing by DOX-loaded microbubbles and ultrasound.
Is the stronger cell killing by DOX-liposome-loaded microbubbles due to an ultrasound-mediated release of free DOX from the liposomes?
Several hypotheses have been proposed to explain the synergistic effect of ultrasound on the biological activity of anticancer drugs. The first mechanism postulates that ultrasound treatment of DOX-liposomes results in the release of DOX from the liposomes that subsequently enters the cells via passive diffusion and pinocytosis.29 To verify this hypothesis, a direct effect of the cavitating microbubbles on the cell membrane should be avoided so that only the cytotoxic effect coming from DOX can be taken into account. Therefore, we performed an experiment in which the DOX-liposome-loaded microbubbles were first exposed to ultrasound (using the same settings as in Figure 3) in an “empty” OptiCell (i.e., without melanoma cells). Subsequently, this “medium” (i.e., the debris of radiated DOX-liposome-loaded microbubbles) was transferred to an OptiCell in which melanoma cells were growing. In this experiment, sonoporation of the melanoma cells (by cavitating and imploding microbubbles cavitating) was thus avoided. After 4 hours of incubation, the “medium” was removed and the cell viability was measured 48 hours later. Figure 5 shows that the debris of radiated DOX-liposome-loaded microbubbles (white bars) showed a stronger tumor cell killing than DOX-liposomes (light gray bars). These data indicate that exposure of DOX-liposome-loaded microbubbles to ultrasound most likely results in the release of free DOX, which is known to cause a stronger cell killing than DOX-liposomes. Figure 5 reveals that the killing of melanoma cells by the debris of radiated DOX-liposome-loaded microbubbles was significantly lower than that of DOX-liposome-loaded microbubbles and ultrasound (especially at higher DOX concentrations). It suggests that the cell killing by DOX-liposome-loaded microbubbles and ultrasound is explained by both the release of free DOX and the cavitation of the microbubbles that perforates the cell membranes.
Figure 5.
Cell viability of melanoma cells after treatment with DOX-liposomes (light gray bars), DOX-liposome-loaded microbubbles (dark gray bars), and DOX-liposome-loaded microbubbles that were first radiated with ultrasound. The debris of the radiated DOX-liposome-loaded microbubbles was applied to the cells (white bars). For higher doxorubicin concentrations, more doxorubicin liposomes were used, whereas the microbubble concentration was always 109 microbubbles per OptiCell unit. *P < 0.05. DOX, doxorubicin; USE, ultrasound exposure.
We recently loaded PEGylated plasmid DNA– or small interfering RNA–containing liposomes (lipoplexes) onto the same type of microbubbles as reported in this study.6,7 We could show by dynamic light scattering that exposure of the lipoplex-loaded microbubbles resulted in the release of intact lipoplexes. However, after exposure of the DOX-liposome-loaded microbubbles to ultrasound, we were no longer able to detect DOX-liposomes. This further suggests that indeed a substantial part of the DOX-liposomes becomes destroyed upon applying ultrasound to the DOX-liposome-loaded microbubbles. Probably, this results in the release of free DOX that can enter the cells either via passive diffusion or through the perforations in the cell membranes, as discussed in the next paragraph.
Is the stronger cell killing by DOX-liposome-loaded microbubbles due to an improved cellular uptake of DOX?
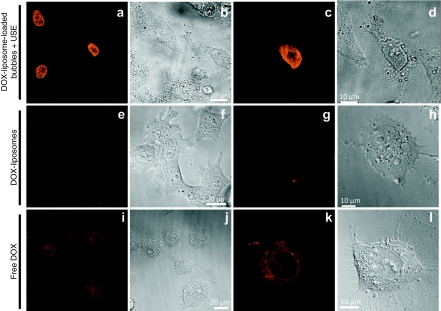
Several groups have studied the perforation of cell membranes by ultrasound.9,28,30,31 Scanning electron microscopy images and uptake of fluorescent molecules after sonoporation have proven that cavitating microbubbles can indeed transiently disrupt cell membranes that allow compounds to enter cells. In our study, pore formation might enhance the intracellular uptake of both free DOX and DOX-liposomes. To further evaluate this hypothesis, we studied the cellular uptake of DOX shortly (i.e., 15–30 minutes) after exposure of melanoma cells to (i) DOX-liposome-loaded microbubbles and ultrasound (Figure 6a–d), (ii) DOX-liposomes (Figure 6e–h), and (iii) free DOX (Figure 6i–l). Almost immediately after exposure of the melanoma cells to DOX-liposome-loaded microbubbles and ultrasound, a substantial part of the cells contained very high levels of DOX in their nuclei (Figure 6a,c). In sharp contrast, melanoma cells exposed to DOX-liposomes hardly contained DOX after 15 minutes (Figure 6e,g). Free DOX is known to be easily taken up by cells through a combined process of passive diffusion and active transport mechanisms.29 Therefore, we also studied the uptake of free DOX (Figure 6i–l). After 15 minutes, cells treated with free DOX contained clearly visible amounts of DOX (Figure 6i,k) in the cytoplasm with only very little fluorescence in the nucleus of the cells. However, the amount of DOX internalized by the cells was still much lower than the DOX content in the cells exposed to DOX-liposome-loaded microbubbles and ultrasound. Note that in the case of DOX liposome-loaded microbubbles, a part of the cells had taken up extreme amounts of DOX 15 minutes after exposure to ultrasound (these were probably the cells that were in contact with cavitating and imploding microbubbles), whereas the others were only weakly fluorescent (Figure 6a). In the case of free DOX, the fluorescence was rather equal in all the cells that suggests that DOX uptake occurred to the same extent in all the cells.
Figure 6.
Confocal laser scanning microscopy images of the cellular uptake of DOX in melanoma cells after 15–30 minutes of incubation time with different DOX formulations. (a,c,e,g,i,k) Confocal laser scanning microscopy images and (b,d,f,h,j,l) corresponding transmission images. a–d show the uptake of DOX after incubation with DOX-liposome-loaded microbubbles. e–h show the uptake of DOX-liposomes and i–l the uptake of free DOX. c,d,g,h,k,i present a close-up of a single cell. DOX, doxorubicin.
Several papers have reported the instant uptake of larger molecules after sonoporation.32,33,34 It has been shown that the microstreams developing around a cavitating microbubble, and especially the shock waves and microjets associated with microbubble cavitation, can result in the formation of transient pores in the cell membrane.35,36 Pore sizes between 100 nm and a few micrometers in size have been reported,28,31 implying that a rather small molecule like DOX should be able to enter the cell through such cell membrane pores.
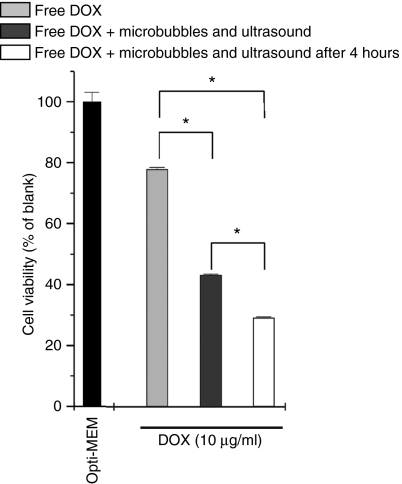
To further evaluate the hypothesis that microbubbles in the presence of ultrasound enhance the cellular uptake of DOX, we compared the killing of cells that had been exposed to free DOX and a mixture of free DOX, microbubbles, and ultrasound. As presented in Figure 7, microbubbles in combination with ultrasound significantly enhanced the cytotoxicity of free DOX (dark gray bars). This supports the hypothesis that sonoporation indeed improves the cellular uptake of free DOX. We also performed a second experiment in which we first exposed the melanoma cells to free DOX; after the 4-hour incubation time, the cells were carefully washed and treated with microbubbles and ultrasound. As can be seen in Figure 7 (white bars), this even resulted in a stronger killing that may be due to the fact that a certain time after internalization of free DOX, melanoma cells become more sensitive to sonoporation. Cells treated with microbubbles and ultrasound are often irregularly shaped.30 This was also observed in our experiments (Figure 6d). Despite their clearly damaged cell membrane, most cells are capable of resealing their membrane wounds,30,37 which was confirmed in our (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) experiment: only 10% of the BLM cells was metabolically inactive after exposure of the cells to microbubbles and ultrasound (Figure 3). It might be possible that due to the DOX-induced cytotoxicity, the cells are less able to generate cell repair mechanisms necessary to repair the cell membrane after sonoporation.
Figure 7.
Cell viability of melanoma cells after treatment with free DOX (light gray bars) and free DOX with microbubbles and ultrasound (dark gray bars and white bars). Cells were simultaneously treated with free DOX, microbubbles, and ultrasound (dark gray bars) or incubated for 4 hours with free DOX, washed and then treated with microbubbles and ultrasound (white bars). For higher DOX concentrations, more DOX-liposomes were used, whereas the microbubble concentration was always 109 microbubbles per OptiCell unit. *P < 0.05. DOX, doxorubicin.
Discussion
We succeeded in coupling DOX-containing liposomes onto the lipid shell of gas-filled microbubbles. DOX-liposome-loaded microbubbles exposed to ultrasound killed much more tumor cells than DOX-liposomes. We showed that this is due to (i) an ultrasound-triggered release of DOX from the DOX-liposomes present on the microbubbles and (ii) an enhanced uptake of the released DOX by the cells. Based on the results in this article, we hypothesize that DOX-liposome-loaded microbubbles in combination with ultrasound may significantly improve the in vivo efficacy of Doxil, being the liposomal DOX formulation that is nowadays used in the clinic. Indeed, a local ultrasound-triggered release of free DOX in the tumor may enhance the cell killing by Doxil, as free DOX is expected to be more efficient than DOX-liposomes. Also, DOX-liposome-loaded microbubbles may show less side effects than Doxil, as in tissues not exposed to ultrasound, the micron-sized DOX-liposome-loaded bubbles (i) will stay intact and thus will not release DOX-liposomes and (ii) will not extravasate into these tissues. Moreover, the cavitation and implosion of the microbubbles in the tumor microvasculature may perforate endothelial cells and enhance the extravasation of the released DOX.15,16 Although a direct contact between the tumor cells and the imploding microbubbles might be restricted by the tumor endothelium, exposure of these endothelial cells to imploding DOX-carrying microbubbles might selectively stimulate the uptake of DOX in these cells and in this way potentiate the destruction of the tumor vasculature.
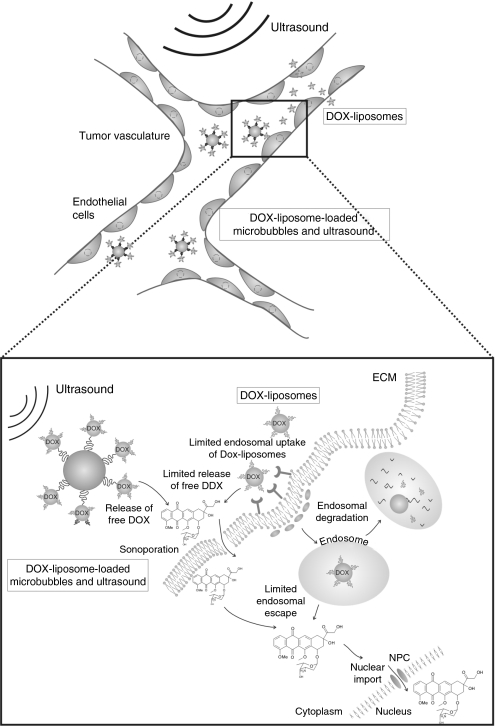
As schematically shown in Figure 8, we hypothesize that the enhanced cellular uptake of DOX in endothelial cells may improve DOX to slow down angiogenesis and to break down the nutrient supply of the tumor cells.38 The in vivo biological response of the DOX-liposome-loaded microbubbles may be further enhanced by attaching ligands to the microbubbles that specifically bind to the tumor endothelium.39,40
Figure 8.
Schematic representation of the proposed working mechanism of DOX-liposome-loaded microbubbles compared to free DOX-liposomes in the tumor vasculature. When the microbubbles are exposed to ultrasound, the liposomes locally release the encapsulated doxorubicin. The cavitating and imploding microbubbles also improve the cell membrane permeability that enhances the amount of doxorubicin that is taken up by, especially, the endothelial cells. DOX, doxorubicin; ECM, extracellular membrane; NPC, nuclear pore complex.
Although several papers describe the use of ultrasound to improve drug release from nanoparticles and to enhance cellular uptake,25,26,41,42,43 only few have been published on the combined use of ultrasound and drug-loaded microbubbles to improve antitumor treatment.44,45 Tartis et al. already developed a paclitaxel-containing ultrasound contrast agent.45 In this study, the paclitaxel was dissolved in triacetin oil, which makes this type of microbubbles unsuited for hydrophilic drugs like DOX. Gao and colleagues were the first to report the design of a DOX-loaded microbubble that was able to release the DOX upon ultrasound treatment.44 The DOX was present in the shell of perfluorocarbon nanoparticles that vaporized and formed DOX-carrying microbubbles upon warming till 37 °C. Although they obtained good results both in vitro and in vivo, we believe that clinical application of the DOX-liposome-carrying microbubbles described in our study may be more straightforward as both DOX-liposomes (Doxil) and lipid microbubbles (e.g., the contrast agent Definity) are already used in clinical settings. Another advantage of the DOX-liposome-loaded microbubbles is their high DOX loading efficiency. We estimated the amount of encapsulated DOX attached to one microbubble to be 3.25 × 10−8 µg. Doxil is given as a single dose of 20 mg/m2. This corresponds to a dose of 40 mg for an adult of about 80 kg. To reach this dose, we should administer about 1.23 × 1012 DOX-liposome-loaded microbubbles. The recommended dose of Definity, which contains 1.2 × 1010 microbubbles/ml, is 10 µl/kg body weight that makes 1010 microbubbles for a person of 80 kg. This is about 100 times lower than the number of DOX-loaded microbubbles we must inject to administer 40 mg of DOX. However, it has been demonstrated that Definity doses that are 1,000 times higher than the recommended dose are well tolerated in primates.46,47 Also, the local release of the DOX in the tumor after exposure of the DOX-liposome-loaded microbubbles to ultrasound will most likely allow us to reduce the dose below 40 mg. The calculations above demonstrate that we may expect clinical effects in humans at a typically used microbubble doses.
Materials and Methods
Preparation and characterization of lipid microbubbles containing DSPE-PEG-biotin. Liposomes containing dipalmitoylphosphatidylcholine and DSPE-PEG-biotin in a 85:15 molar ratio were prepared as previously described.6 Briefly, the chloroform-dissolved lipids were put in a round-bottomed flask and the solvent was removed via evaporation followed by flushing with nitrogen. The obtained lipid film was subsequently hydrated in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (20 mmol/l HEPES, pH 7.4) at a final lipid concentration of 5 mg/ml and incubated overnight at 4 °C to allow the formation of liposomes. The resulting liposomes were first extruded through a polycarbonate membrane (pore size of 0.2 µm) using a mini-extruder (Avanti Polar Lipids, Alabaster, AL). Subsequently, the extruded liposomes were sonicated with a 20 kHz probe (Branson 250 Sonifier; Branson Ultrasonics, Danbury, CT) in the presence of perfluorobutane gas (MW 238 g/mol; F2 Chemicals, Preston, UK). After sonication, the microbubbles were washed (to remove the excess of lipids) with 3 ml fresh HEPES buffer and finally resuspended in 5 ml fresh HEPES buffer. To allow the attachment of biotinylated DOX-liposomes, the biotinylated microbubbles were incubated with 500 µl avidin (10 mg/ml) and incubated for 10 minutes at room temperature. Subsequently, the microbubbles were centrifuged and washed again with 3 ml fresh HEPES buffer. Finally, the microbubbles were resuspended in 5 ml HEPES buffer. The mean size of these microbubbles was around 2 µm and their size distribution ranged between 0.5 and 10 µm.
Preparation and characterization of biotinylated DOX liposomes. Liposomes containing dipalmitoylphosphatidylcholine, cholesterol, and DSPE-PEG-biotin in a 60:40:5 molar ratio were prepared as described above. Liposomes were loaded with DOX following the method previously established by Bolotin et al.48 After removal of the chloroform, the lipid film was hydrated with ammonium sulfate buffer (250 mmol/l). The resulting liposomes were extruded through a polycarbonate membrane (pore size of 0.1 µm) as described above. Subsequently, the liposomes were dialyzed against pure distilled water overnight in a dialysis bag (MWCO 10,000, Spectra/Por Biotech; Spectrum Laboratories, Compton, CA) to remove the ammonium sulfate between the liposomes. Liposomes were loaded with DOX by mixing 1 ml of liposomes with 1 mg of DOX. This mixture was incubated for 4 hours at 65 °C. Afterward, the free DOX was removed by passing the DOX-loaded liposomes over a Sephadex column (Sephadex G-75; Sigma-Aldrich, Bornem, Belgium). Loading efficiency was determined by measuring the absorbance of DOX in the DOX-liposome fraction and the free DOX at 450 nm and was around 90%. The average hydrodynamic diameter of the PEGylated DOX-liposomes was determined by dynamic light scattering (Autosizer 4700; Malvern, Worcestershire, UK).
Attachment of biotinylated DOX-liposomes to avidinylated microbubbles. 50 µl of biotinylated DOX-liposomes was mixed with 1 ml avidinylated microbubbles and incubated at room temperature for 5 minutes. The attachment of DOX-liposomes to the microbubbles was visualized using a Nikon EZC1-si confocal laser scanning microscope (Nikon, Brussels, Belgium) equipped with a ×40 objective. The 491 nm line of this microscope was used to excite the DOX, and the DOX was detected with the 580 nm detector.
Cell culture. BLM cells (melanoma cells)49 were cultured in Dulbecco's modified Eagle's medium (DMEM) with the growth factor F12 and phenol red containing 2 mmol/l glutamine, 10% heat deactivated fetal bovine serum (FBS), 1% penicillin–streptomycin (Gibco, Merelbeke, Belgium), and HEPES buffer (100 mmol/l, pH 7.4).
Cytotoxicity measurements. Cells were grown to 90% confluency in OptiCell units (Biocrystal, Westerville, OH) in a humidified incubator at 37 °C and 5% CO2. Subsequently, cells were washed with 10 ml of phosphate-buffered saline (Gibco) and DOX-liposome-loaded microbubbles, DOX-liposomes or free DOX (all in Opti-MEM; Gibco) were added to the cells. Therefore, we first mixed an appropriate amount of biotinylated DOX-liposomes (containing 10, 30, 50, or 100 µg of DOX) with 1 ml of the avidinylated microbubbles. After 5 minutes of incubation at room temperature, Opti-MEM was added to a final volume of 10 ml. The medium was prepared in a similar way for the experiments without microbubbles except that the microbubbles were substituted by an equal amount of Opti-MEM. The 10 ml medium was completely added to the OptiCell units (surface 50 cm2). Subsequently, the cells were placed in a water bath at 37 °C with an absorbing rubber at the bottom and immediately subjected to ultrasound radiation. The ultrasound radiation was performed by moving in 15 seconds a 22 mm ultrasound probe (Sonitron 2000; Rich-Mar, Inola, OK) over the whole surface of the OptiCell. In all the experiments with ultrasound, we used the following settings: 1 MHz, 50% duty cycle, and an ultrasound intensity of 2 W/cm2. These settings correspond to a total energy delivery of 15 J/cm2 for the whole OptiCell plate, with an ultrasound peak intensity of 0.17 MPa (mechanical index 0.17). Unless otherwise stated, cells were immediately treated with ultrasound after addition of DOX-liposomes, DOX-liposome-loaded microbubbles, or free DOX. Four hours after the addition of the DOX-liposomes, the DOX-liposome-loaded microbubbles, and free DOX, we removed the medium and washed the cells two times with phosphate-buffered saline before adding fresh culture medium. After 48 hours, the cells were incubated with 0.5 mg/ml MTT labeling reagent (Cell Proliferation Kit I; Roche Diagnostics, Vilvoorde, Belgium) for 4 hours. Afterward, the solubilization solution was added and cells were incubated overnight at 37 °C. The next day, the absorbance of each plate was measured at 590 nm (OD590) to determine the amount of formed formazan and at 690 nm as a reference. The cell viability was calculated as follows:
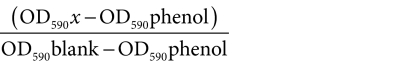 |
Experiments were performed at least three times, and the results shown here are representative of the results obtained in the different cytotoxicity measurements. The error bars in the graphs are originated from different samples that were taken from one OptiCell plate and separately measured at 590 nm and 690 nm.
Cellular uptake of DOX in the BLM cells. Cells were incubated with DOX-liposomes, DOX-liposome-loaded microbubbles, and free DOX according to the protocols described above. The DOX in cells was visualized with a Nikon EZC1-si confocal microscope equipped with a ×60 objective. The 491 nm line of this microscope was used to excite the DOX, and the DOX was detected with the 580 nm detector.
Statistical analysis. All the data in this report are expressed as mean ± SD. For the transfection results, the Student's t-test was used to determine whether data groups differed significantly from each other. A P value lower than 0.05 was considered statistically significant.
Acknowledgments
I.L. is supported by the Fund for Scientific Research–Flanders (Belgium). The financial support of this institution is acknowledged with gratitude. Fien Vandeputte is thanked for practical assistance. The financial support of the European Union (via the FP7 projects Arise and Sonodrugs) is acknowledged.
REFERENCES
- Patil RR, Guhagarkar SA., and , Devarajan PV. Engineered nanocarriers of doxorubicin: a current update. Crit Rev Ther Drug Carrier Syst. 2008;25:1–61. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y., and , Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Andresen TL, Jensen SS., and , Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ferrara KW. Driving delivery vehicles with ultrasound. Adv Drug Deliv Rev. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentacker I, De Smedt SC, Demeester J, Van Marck V, Bracke M., and , Sanders NN. Lipoplex-loaded microbubbles for gene delivery: a Trojan horse controlled by ultrasound. Adv Funct Mater. 2007;17:1910–1916. [Google Scholar]
- Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC., and , Sanders NN. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. J Control Release. 2008;126:265–273. doi: 10.1016/j.jconrel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Lentacker I, Vandenbroucke RE, Lucas B, Demeester J, De Smedt SC., and , Sanders NN. New strategies for nucleic acid delivery to conquer cellular and nuclear membranes. J Control Release. 2008;132:279–288. doi: 10.1016/j.jconrel.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Lentacker I, Wang N, Vandenbroucke RE, Demeester J, De Smedt SC., and , Sanders NN. Ultrasound exposure of lipoplex loaded microbubbles facilitates direct cytoplasmic entry of the lipoplexes. Mol Pharm. 2009;6:457–467. doi: 10.1021/mp800154s. [DOI] [PubMed] [Google Scholar]
- Sboros V. Response of contrast agents to ultrasound. Adv Drug Deliv Rev. 2008;60:1117–1136. doi: 10.1016/j.addr.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Duvshani-Eshet M, Baruch L, Kesselman E, Shimoni E., and , Machluf M. Therapeutic ultrasound-mediated DNA to cell and nucleus: bioeffects revealed by confocal and atomic force microscopy. Gene Ther. 2006;13:163–172. doi: 10.1038/sj.gt.3302642. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA., and , Staples BJ. Ultrasonic drug delivery—a general review. Expert Opin Drug Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Meijering BD, Juffermans LJ, van Wamel A, Henning RH, Zuhorn IS, Emmer M, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- Price RJ, Skyba DM, Kaul S., and , Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- Vancraeynest D, Havaux X, Pouleur AC, Pasquet A, Gerber B, Beauloye C, et al. Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur Heart J. 2006;27:237–245. doi: 10.1093/eurheartj/ehi479. [DOI] [PubMed] [Google Scholar]
- Yu T, Wang Z., and , Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem. 2004;11:95–103. doi: 10.1016/S1350-4177(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Siu T, Jackson J, Burt H., and , Chiao M.2007Drug uptake enhancement using sonodynamic effects at 4 MHz—a potential application for micro-ultrasonic-transducers IEEE Trans Biomed Eng 546 Pt 2): 1153–1156. [DOI] [PubMed] [Google Scholar]
- Yu T, Huang X, Hu K, Bai J., and , Wang Z. Treatment of transplanted adriamycin-resistant ovarian cancers in mice by combination of adriamycin and ultrasound exposure. Ultrason Sonochem. 2004;11:287–291. doi: 10.1016/j.ultsonch.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Fain HD., and , Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Husseini GA, Rapoport NY, Christensen DA, Pruitt JD., and , Pitt WG. Kinetics of ultrasonic release of doxorubicin from Pluronic P105 micelles. Colloids Surf B Biointerfaces. 2002;24:253–264. [Google Scholar]
- Husseini GA., and , Pitt WG. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1137–1152. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport NY, Christensen DA, Fain HD, Barrows L., and , Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- Frenkel V, Etherington A, Greene M, Quijano J, Xie J, Hunter F, et al. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006;13:469–479. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Husseini GA., and , Pitt WG. The use of ultrasound and micelles in cancer treatment. J Nanosci Nanotechnol. 2008;8:2205–2215. doi: 10.1166/jnn.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int J Pharm. 2004;277:155–162. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V., and , Prausnitz MR. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med Biol. 2006;32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Dai X, Yue Z, Eccleston ME, Swartling J, Slater NK., and , Kaminski CF. Fluorescence intensity and lifetime imaging of free and micellar-encapsulated doxorubicin in living cells. Nanomedicine. 2008;4:49–56. doi: 10.1016/j.nano.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Coppage ML, Vaidya S., and , Miller MW. Transient poration and cell surface receptor removal from human lymphocytes in vitro by 1 MHz ultrasound. Ultrasound Med Biol. 1999;25:999–1008. doi: 10.1016/s0301-5629(99)00039-3. [DOI] [PubMed] [Google Scholar]
- Prentice P, Cuschieri A, Dholakia K, Prausnitz M., and , Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nat Phys. 2005;1:107–110. [Google Scholar]
- Karshafian R, Samac S, Banerjee M, Bevan PD, Burns PN. Ultrasound-induced uptake of different size markers in mammalian cells. Abstracts IEEE Ultrasonics Symp. 2005. pp. 1–4.pp. 13–16.
- Khanna S, Hudson B, Pepper CJ, Amso NN., and , Coakley WT. Fluorescein isothiocynate-dextran uptake by chinese hamster ovary cells in a 1.5 MHz ultrasonic standing wave in the presence of contrast agent. Ultrasound Med Biol. 2006;32:289–295. doi: 10.1016/j.ultrasmedbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kodama T, Doukas AG., and , Hamblin MR. Shock wave-mediated molecular delivery into cells. Biochim Biophys Acta. 2002;1542:186–194. doi: 10.1016/s0167-4889(01)00177-x. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Uchida T, Ogawa K, Yamashita N., and , Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- Wu J., and , Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Ross JP, Cai X, Chiu JF, Yang J., and , Wu J. Optical and atomic force microscopic studies on sonoporation. J Acoust Soc Am. 2002;111:1161–1164. doi: 10.1121/1.1448340. [DOI] [PubMed] [Google Scholar]
- Molema G, Meijer DK., and , de Leij LF. Tumor vasculature targeted therapies: getting the players organized. Biochem Pharmacol. 1998;55:1939–1945. doi: 10.1016/s0006-2952(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Eliaz RE., and , Szoka FC. Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–2601. [PubMed] [Google Scholar]
- Sawant RM, Cohen MB, Torchilin VP., and , Rokhlin OW. Prostate cancer-specific monoclonal antibody 5D4 significantly enhances the cytotoxicity of doxorubicin-loaded liposomes against target cells in vitro. J Drug Target. 2008;16:601–604. doi: 10.1080/10611860802228954. [DOI] [PubMed] [Google Scholar]
- Myhr G., and , Moan J. Synergistic and tumour selective effects of chemotherapy and ultrasound treatment. Cancer Lett. 2006;232:206–213. doi: 10.1016/j.canlet.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Rosenthal I, Sostaric JZ., and , Riesz P. Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, et al. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23:4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kennedy AM, Christensen DA., and , Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48:260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartis MS, McCallan J, Lum AF, LaBell R, Stieger SM, Matsunaga TO, et al. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol. 2006;32:1771–1780. doi: 10.1016/j.ultrasmedbio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Karshafian R, Bevan PD, Williams R, Samac S., and , Burns PN. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol. 2009;35:847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Unger EC, Porter T, Culp W, Labell R, Matsunaga T., and , Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Bolotin EM, Cohen R, Bar L, Emanuel N, Ninio S, Lasic DD, et al. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J Liposome Res. 1994;4:455–479. [Google Scholar]
- Quax PH, van Muijen GN, Weening-Verhoeff EJ, Lund LR, Danø K, Ruiter DJ, et al. Metastatic behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its type-1 inhibitor, and urokinase-mediated matrix degradation. J Cell Biol. 1991;115:191–199. doi: 10.1083/jcb.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]