Abstract
Treatment of functional bowel disorders of irritable bowel-type (IBS) in children remains a difficult task because of a lack of drugs with low adverse event profile. We here report the results of a treatment study in 203 children (66 boys and 137 girls) age 4 to 18 years (mean: 10.5±4.5 years) with typical IBS symptoms with abdominal pain and either predominant diarrhea (n=50), constipation (n=56), alternating stool frequency (n=28) or unspecific pain (n=69). The average duration of symptoms prior to therapy was 175 days. Most (95%) patients up to age 11 were treated with a daily dose of 10 drops of Symbioflor 2 (SF2) (SymbioPharm, Herborn) (cells and autolysate of 1.5–4.5x107 CFU of bacteria of Escherichia coli type), in the elder children 77% received this dosage, while the remaining received a higher dose up to 30 drops/day. Treatment lasted 43 days on average. Results: All patients tolerated the treatment well and without adverse events. The key IBS symptoms (abdominal pain, stool frequency) as well as the other symptoms (bloating, mucous and blood in stool, need for straining at stools, urge to defecate) improved significantly during treatment. Global assessment of therapy by parents and doctors was altogether positive. In summary these data confirm efficacy and tolerability of this probiotic compound in children and adolescents and supplement published data of probiotic IBS therapy in adults.
Abstract
Die Behandlung des kindlichen Reizdarmsyndroms (RDS) ist schwierig, da bislang wenige nebenwirkungsarme Medikamente vorliegen. Wir berichten hier die Ergebnisse einer offenen Anwendungsbeobachtung bei 203 Kindern (66 Jungen und 137 Mädchen) im Alter von 4 bis 18 Jahren (mittleres Alter: 10,5±4,5 Jahre), die die typischen Symptome eines RDS vom Typ „Schmerzen und Diarrhöe” (n=50), „Schmerzen und Obstipation” (n=56), „Schmerzen und alternierende Stuhlfrequenz” (n=28) und „unspezifische Schmerzen” (n=69) gemäß Rom-III-Kriterien hatten. Die durchschnittliche Dauer der Beschwerden bis zum Therapiebeginn waren 175 Tage. Die Patienten bis 11 Jahre wurden in der Regel (95%) mit 10 Tropfen Symbioflor-2 (SF2) (SymbioPharm, Herborn) (Zellen und Autolysat von 1,5–4,5x107 Bakterien vom Typ Escherichia coli) behandelt, bei den älteren Kindern erhielten 77% diese Dosierung, die übrigen eine höhere Dosierung bin zu 30 Tropfen/Tag. Die Behandlung dauerte im Mittel 43 Tage. Ergebnisse: Alle Patienten vertrugen die Behandlung ohne Nebenwirkungen. Die Kernsymptome des RDS (Bauchschmerzen, Stuhlfrequenz) wie auch die weiteren Symptome (Meteorismus, Stuhlbeimengungen von Schleim, Notwendigkeit starken Pressens, imperativer Stuhldrang) besserten sich alle signifikant im Verlauf der Behandlung. Die globale Bewertung der Therapie durch Eltern und Ärzte war positiv. Insgesamt bestätigen die in der vorliegenden Anwendungsbeobachtung dokumentierte Wirksamkeit und Verträglichkeit die bisherigen Erfahrungen mit SF2 bei Kindern und Jugendlichen und die publizierten Daten der Behandlung von Erwachsenen mit RDS.
Introduction
The irritable bowel syndrome (IBS) is a functional bowel disorder and characterized by a number of symptoms including abdominal pain or discomfort and disturbed bowel habits. Other typical IBS symptoms are diffuse abdominal pain, bloating, excessive passing of gas, irregular bowel movements with improvement of symptoms after defecation, and/or the feeling of incomplete evacuation. Morphological abnormalities are missing that sufficiently could explain the symptoms, as are diagnostic criteria and proven pathophysiological concepts; hence a therapeutic concept based on such findings is lacking as well.
The diagnosis is usually based of the recording of the typical symptom pattern, the absence of alarm symptoms, and the exclusion of a number of differential diagnoses. In the present observational study, the recently published diagnostic criteria of the Rome III consensus for IBS have been applied [1], [2], (Table 1 (Tab. 1)). This classification was meanwhile also applied to childhood functional bowel disorders [3] and has replaced the older terminology that used “recurrent abdominal pain” as diagnostic label [4].
Table 1. Rome III criteria for irritable bowel syndrome (IBS) in children and adults.
IBS is a frequent disease in the general population. The worldwide prevalence ranged between 10 and 20% in the adult and adolescent population, and women are more often affected than men. The disease has substantial consequences on quality-of-life of patients and induces high direct and indirect medical costs [5].
For pediatric patients, very few data are available. The recently published children and adolescent health survey (KIGGS) collected – among others – representative data on pain in children age 3–17 years in Germany [6]. In children between 3 and 10 years, “belly pain” was the most frequent pain location, while children age 11 to 17 most frequently reported headaches, followed by abdominal and back pains [7].
Non-medicinal general management strategies including exercises, stress reduction, and relaxation techniques may serve as supplementary options in addition to drug therapy of key symptoms, e.g. regulation of stool consistency, against bloating, spasmolytic therapy and modulation of gastrointestinal motility. Only a few new drug developments have found their way into clinical routine in the last years [8], but most have never been tested in children.
Since a number of years, prebiotic and probiotic therapies have seen a renaissance [9] in clinical use and are most often used in functional gastrointestinal disorders [10]. Their low adverse event profile [11] also supports their use in childhood functional bowel disorders but respective studies are rather scarce [12] – they are mostly used with dermatological and allergic diseases [13].
Symbioflor® 2 (SF2) (SymbioPharm GmbH, Herborn, Germany) contains both living as well as non-living Escherichia coli bacteria (cells and autolysate of 1.5–4.5x107 bacteria) and is – based on current knowledge – effective in the therapy of functional bowel disorders [14]. We here report the open label use of SF2 in 203 children age 4 to 18 with IBS symptoms according to Rome-III criteria.
Patients and methods
Type of study
This is an observational study (Anwendungsbeobachtung, AWB) according to §67-6 of the respective law (Arzneimittelgesetz, AMG) in Germany. The study was conducted according to the “Recommendations for planning and conductance of observational studies” (Empfehlungen zur Planung und Durchführung von Anwendungsbeobachtungen) of the German drug approval authorities (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) as of November 12th, 1998, in accordance with the European guideline E11 of the Committee for Proprietary Medicinal Products (CMPM) of the European Agency for the Evaluation of Medicinal Products (EMEA) (CPMP/ICH/2711/99: Clinical investigation of medicinal products in the pediatric population, December, 2000) and in agreement with the guideline CPMP/EWP/462/95 (Note for guidance on clinical investigation of medicinal products in children, March 17th, 1997).
According to the respective rules, the study did not interfere with medical routine in treatment of childhood abdominal pain but left the management up to the doctor's decision; this included also the dosage and the duration of treatment, and eventually needed supplementary therapy. Doctors were asked to document each patient's data (age, gender, height. weight) and treatment with SF2 during the course of the study. Retrospective inclusion of patients was not permitted. Patients that fulfilled inclusion and exclusion criteria (Table 1 (Tab. 1)) but were not treated by SF2 were also documented.
The current observational study did not legally require informed consent of patients and their parents, respectively in addition to what is required with every medical intervention, since patients received a therapy based on the doctors sole decision, and no additional risk was involved, data storage and statistical evaluation is allowed in routine praxis as well. Only anonymous data were used for evaluation that did not interfere with legal requirements regarding handling of personalized data and the medical confidentiality rules of the doctor.
According to §67-6 of the AMG, the conductance of the study was registered with the legal representative of private practitioners in Germany (Kassenärztliche Bundesvereinigung), the German drug approval authorities (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) and the association of health insurances (Spitzenverbände der Krankenkassen). The study was conducted with an approved medical product. According to AMG, this requires prescription of the drug by a doctor and the usage of regular commercially available products, in addition to individual indication and patient selection.
Doctors and patients
This observational study was conducted between October 8th, 2007 (first inclusion) and August 12th, 2008 (last final examination) in 14 general practitioner and pediatric private practices with altogether 203 children in two age ranges, between 4 and 11 years and between 12 and 18 years.
General course of study, duration, dosage
Children age 4 to 18 with IBS that would receive SF2 anyway were to include into the study. It was required to observe the children until significant improvement of symptoms had occurred but for a maximum of 3 months.
SF2 had to be used according to the doctors prescription. The doctor was also asked to document the duration of therapy and the dosage prescribed. The regular dosage in children – as indicated on the patient information sheet – is 1x10 drops/day diluted in water and taken midday, but in adolescents the dosage can be increased up to the adult dosage (30 drops/day).
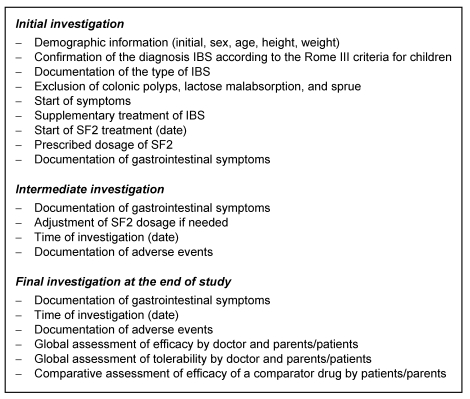
Because of the non-interfering character of the observational study, neither the type nor the timing of subsequent doctor visits were ruled, and their number and intervals was left up to the individual decision of the treating doctor. However, to be able to estimate the treatment success, it was advised to conduct and intermediate office consultation about 2 weeks after the initial visit, and a final examination after 3 months. The intermediate consultation could also be used to eventually adjust the drug dosage. The investigations to perform at the single visits are listed in Table 2 (Tab. 2).
Table 2. Clinical investigations during the observational study.
Symptom documentation
At any office visit, the presence and severity of the following IBS symptoms were registered: number of stools (per day or per week), stool consistency (hard/lumpy, formed, soft/mushy, liquid, alternating), mucus with stool (yes/no), abdominal pain (yes/no), bloating (yes/no), passing of gas (yes/no), passage of stools (yes/no).
Doctors were also asked to overall estimate the treatment success between “very good” and “poor”, and parents and adolescent patients were asked to compare SF2 treatment with previous therapies used and to evaluate SF between “much better” and “much worse”. Doctors and parents/patients were also asked to evaluate the overall tolerability and any adverse event between “very good” and “unacceptable”.
Statistical evaluation
For the analysis, all documented evaluation sheets were used, and all patients were included (intent-to-treat analysis, ITT). Documented variables are presented with their descriptive values (mean, standard deviation), or as distribution tables in case of qualitative variables. Inference statistics must be regarded as exploratory and non-confirmatory.
Results
Demographics and diagnoses
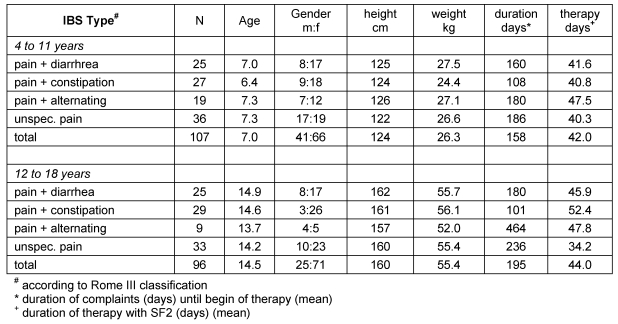
A total of 203 children and adolescents in two age groups (4 to 11 years and 12 to 18 years) participated in the study and were distributed unequally among the four clinical subtypes of IBS: pain and diarrhea (n=50), pain and constipation (n=56), pain and alternating bowel habits (n=28) and unspecified pain (n=68). All children suffered from IBS according to the Rome III classification (Table 3 (Tab. 3)).
Table 3. Sociographic and clinical information.
Table 3 (Tab. 3) shows the key demographic variables gender, age, height and weight of all patients grouped by age and clinical IBS type. There were no differences noted between the four IBS subtypes with respect to gender, age, and height and weight (age-adjusted).
An IBS diagnosis according to the Rome III classification requires at least 2 months of symptom persistence; accordingly, the doctors participating in this study started treating IBS in the patients after an average 80 days. However, substantial differences occurred depending on the type of IBS symptoms: in general, diarrhea-predominant and constipation-predominant IBS were treated relatively early (median less than 100 days) while IBS alternating and unspecified IBS were started being treated after more than 100 days (Table 3 (Tab. 3)).
Dosage of SF2, other medication
In 77.4% of cases, patients received the standard children dosage of 1x10 drops per days of SF2, and change of dosing was rare (5% of cases). Only 6 patients received other medication in addition to SF2, and usually for a few days only: 4 x lactulose, 1 x Gastrosil®, 1 x Iberogast®.
Duration of treatment
Mean treatment duration with SF2 ranged between 40 and 50 days in both age groups and all types of IBS (Table 3 (Tab. 3)), with the exception of “unspecified IBS”: these patients were treated an average of 34.2 days. Differences between age groups and IBS types were not significant (p=0.1919).
Efficacy of therapy
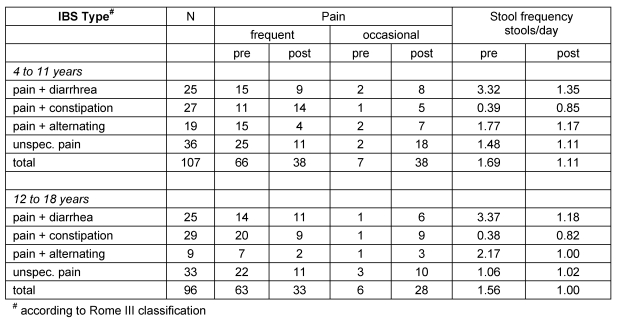
Table 4 (Tab. 4) summarizes the efficacy of SFG2 treatment for the core IBS symptoms abdominal pain and stool frequency (see above) between initial and final examination, broken down by age groups and IBS types. All changes are significant (p<.001).
Table 4. Efficacy of SF2 treatment for key IBS symptoms.
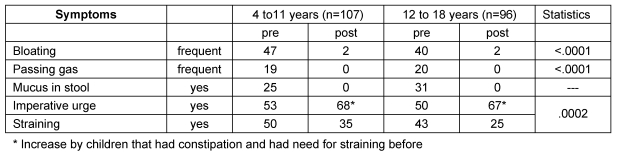
Also, other IBS associated symptoms such as bloating, passage of gas, mucus in stools and altered stool passage (urge, need for straining) were significantly improved with SF2 (p<.001) (Table 5 (Tab. 5)).
Table 5. Efficacy of SF2 treatment for other IBS symptoms (absolute numbers).
Global assessment of efficacy
Table 5 (Tab. 5) also summarizes the global assessment of treatment efficacy by the doctor. In the majority of cases (81.8%) the therapy was judged as “very good” or “good”. This is valid for all age groups and all clinical IBS types, with the exception of the IBS alternators age 12 to 18, where doctors evaluated only 55.5% of therapies as “good” or better. However this refers only to a very small number of patients (n=9).
Comparison with previously used medication
Only about 40% patients/parents made respective evaluations thus had experience with other IBS therapies. SF2 was judged as better in 77% of cases, and in only a very few instances, SF2 was regarded as “rather worse” or “much worse” than the comparator drug, with no differences between IBS subtypes and age groups. Previously given drugs were besides diet (1 times), herbal preparations (Iberogast®, 15x), yeast preparations (Parenterol®, 11x) and chemically defined laxatives (lactulose, 10x).
Tolerability, adverse events
Except for two out of 203 cases, patients and parents rated the overall tolerability of SF2 as good or better (98.6%). This is in nearly total agreement with the doctors rating of tolerability: except for two cases 98.5% of treatments were judged as good or better by the treating physician. No adverse events were noted.
Discussion
The irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that is characterized by a typical symptom constellation, i.e. abdominal pain or complaints associated with disturbed defecation. The diagnosis is based of confirmation of this typical symptom pattern, the missing of alarm symptoms (such as fever, weight loss, and blood with stools), and the exclusion of relevant differential diagnoses.
This observational study used the recently published Rome III diagnostic criteria that have been adjusted to childhood functional bowel disorders [3]. It was investigated whether efficacy and tolerability of a probiotic preparation (SF2) can be found under routine conditions in private practice with children and whether this extends previous studies with the same or similar compounds in adults.
Both physicians a well as parents and (adolescent) patients found the efficacy of the probiotic as good or better in the majority of cases (82% of physicians and 83% of patients) irrespective of the age group and the type of IBS. SF2 was regarded as better than previously used medication (acting on motility or as laxatives) for IBS in all but a few cases.
Therefore, this observational study supplements the increasing number of previously conducted randomized double-blinded and placebo controlled treatment trials in IBS [10] using probiotic preparations that seem to be equivalent or even superior to newly developed chemical compounds for IBS treatment in recent years [14], [15], [16], [17]. This is also confirmed by respective meta-analyses [18], [19], [20], [21].
However, most of these studies have been conducted in adults with IBS. In comparison, only a few studies are available on probiotic treatment of childhood IBS [12], [22], [23].
Henker et al. [12] treated 113 children with acute diarrhea with an E. coli probiotic (Mutaflor®) in a placebo-controlled study and confirmed significant efficacy with respect to response rate, therapy goals and duration to achieve these goals. Bausserman et al. [22] treated 64 children with a lactobacillus GG preparation in comparison to placebo for 6 weeks and found that LGG improved bloating symptoms but not abdominal pain. Gawronska et al. [23] treated 103 children with a similar compound and found moderate therapy efficacy for IBS, but none for pure abdominal pain and functional upper gastrointestinal symptoms. A recent summary [24] of the respective published literature in children concluded that probiotics are not effective in constipation, and a Cochrane meta-analysis summarized that the data base for treatment of childhood IBS is altogether rather insufficient for a final conclusion [25].
Beyond lactobacillus preparations that are confirmed to be effective in adult treatment of IBS [20], [21], other bacterial strains have less frequently been used, and so far only one study has investigated the effects of an inactive E. coli bacterial preparation [26], and another study successfully used a formula of non-living E. coli and Enterococcus feacalis bacteria [14] for therapy of IBS, in addition to the above discussed E. coli treatment of acute childhood diarrhea [12].
The relative homogeneous efficacy of different bacterial strains in the rather heterogeneous gastrointestinal disorder IBS in children and adults [10] calls for an answer to the questions of the underlying mechanism of action of prebiotics and probiotics [26]: It appears unlikely that this will be based on a temporary change of the commensal colonic bacterial flora by new bacterial strains, as was often assumed previously, but instead relies on the stimulation of the innate intestinal immune system through novel bacteria that initiate long-lasting changes in intestinal function. This will have to be studied in the future.
The limitations of the current study are obvious: as this is an observational study, it did not include a control group with either no treatment, or with a placebo treatment. Hence, the data are not controlled for spontaneous variation of symptoms and for a placebo response. Both are, however, known and effective factors contributing to the clinical appearance of symptoms in IBS [27], [28]. Therefore, the efficacy data have to be interpreted with care. A comparison to “treatment as usual” (TAU) in childhood IBS may have allowed to overcome some of the study limitations without randomization into treatment arms as is would have allowed the doctor's decision to be based on clinical criteria only. Finally, while the study applied current diagnostic criteria for childhood IBS, it did not adopt the current standards for efficacy assessment in clinical trials based on “subjective global assessment” (SGA) in IBS, i.e. at least 50% improvement in SGA [29] and therefore may overrate the degree of clinical improvement. Final proof of the efficacy of probiotic treatment in childhood irritable bowel syndromes still requires a double-blinded, randomized and placebo controlled trial.
Notes
Conflicts of interest
The first author (UM) has no conflict of interest, the second author (PE) has a consulting contract with the sponsor (SymbioPharm), the third author (EZ) has performed the statistical evaluation of the study as contract work for the sponsor.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. Available from: http://dx.doi.org/10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–1537. doi: 10.1053/j.gastro.2005.08.063. Available from: http://dx.doi.org/10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130(5):1519–1526. doi: 10.1053/j.gastro.2005.11.065. Available from: http://dx.doi.org/10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958;33(168):165–170. doi: 10.1136/adc.33.168.165. Available from: http://dx.doi.org/10.1136/adc.33.168.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth-Isigkeit A, Thyen U, Stöven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):e152–e162. doi: 10.1542/peds.2004-0682. Available from: http://dx.doi.org/10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 6.Ellert U, Neuhauser H, Roth-Isigkeit A. Schmerzen bei Kindern und Jugendlichen in Deutschland: Prävalenz und Inanspruchnahme medizinischer Leistungen Ergebnisse des Kinder-und Jugend-gesundheitssurveys (KiGGS) [Pain in children and adolescents in Germany: the prevalence and usage of medical services. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)]. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2007;50(5-6):711–717. doi: 10.1007/s00103-007-0232-8. (Ger). Available from: http://dx.doi.org/10.1007/s00103-007-0232-8. [DOI] [PubMed] [Google Scholar]
- 7.Schwille IJ, Giel KE, Ellert U, Zipfel S, Enck P. A community-based survey of abdominal pain prevalence, characteristics, and health care use among children. Clin Gastroenterol Hepatol. 2009;7(10):1062–1068. doi: 10.1016/j.cgh.2009.07.002. Available from: http://dx.doi.org/10.1016/j.cgh.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Hammerle CW, Surawicz CM. Updates on treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14(17):2639–2649. doi: 10.3748/wjg.14.2639. Available from: http://dx.doi.org/10.3748/wjg.14.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramia G. Metchnikoff and the centenary of probiotics: an update of their use in gastroenteric pathology during the age of development. Minerva Pediatr. 2008;60(6):1417–1435. [PubMed] [Google Scholar]
- 10.Krammer H, Neumer F, Enck P. Beeinflussung des Reizdarmsyndroms und der Obstipation durch Pro- und Präbiotika. In: Bischoff SC, editor. Probiotika, Präbiotika, Synbiotika. Stuttgart: Thieme Verlag; 2009. pp. 232–242. [Google Scholar]
- 11.Vlieger AM, Robroch A, van Buuren S, Kiers J, Rijkers G, Benninga MA, te Biesebeke R. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial. Br J Nutr. 2009;102(6):869–875. doi: 10.1017/S0007114509289069. Available from: http://dx.doi.org/10.1017/S0007114509289069. [DOI] [PubMed] [Google Scholar]
- 12.Henker J, Blokhin BM, Bolbot YK, Maydannik VG. Akute Diarrhö bei Säuglingen und Kleinkindern. Erfolgreiche adjuvante Therapie mit dem probiotikum Mutaflor. Pädiat Prax. 2007/2008;71:605–610. [Google Scholar]
- 13.Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475. doi: 10.1002/14651858.CD006475.pub2. Available from: http://dx.doi.org/10.1002/14651858.CD006475.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47(2):209–214. doi: 10.1055/s-2008-1027702. Available from: http://dx.doi.org/10.1055/s-2008-1027702. [DOI] [PubMed] [Google Scholar]
- 15.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. Available from: http://dx.doi.org/10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 16.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind,controlled trial. Aliment Pharmacol Ther. 2007;26(3):475–486. doi: 10.1111/j.1365-2036.2007.03362.x. Available from: http://dx.doi.org/10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173-010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009 doi: 10.1111/j.1365-2036.2008.03853.x. In press. [DOI] [PubMed] [Google Scholar]
- 18.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51(12):1775–1780. doi: 10.1007/s10350-008-9335-z. Available from: http://dx.doi.org/10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 19.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14(17):2650–2661. doi: 10.3748/wjg.14.2650. Available from: http://dx.doi.org/10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. Available from: http://dx.doi.org/10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller D, Rijkers GT, Bengmark S, Enck P, Lenoir-Wijnkoop I, Antoine JM. Guidance for substantiating the evidence of the probiotics beneficial effects. Probiotics in chronic intestinal inflammatory and functional disorders. J Nutrition. doi: 10.3945/jn.109.113746. In press. [DOI] [PubMed] [Google Scholar]
- 22.Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147(2):197–201. doi: 10.1016/j.jpeds.2005.05.015. Available from: http://dx.doi.org/10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Gawronska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177–184. doi: 10.1111/j.1365-2036.2006.03175.x. Available from: http://dx.doi.org/10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 24.Vandenplas Y, Benninga M. Probiotics and functional gastrointestinal disorders in children. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 2):S107–S109. doi: 10.1097/MPG.0b013e3181a1603a. Available from: http://dx.doi.org/10.1097/MPG.0b013e3181a1603a. [DOI] [PubMed] [Google Scholar]
- 25.Huertas-Ceballos AA, Logan S, Bennett C, Macarthur C. Dietary interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev. 2009;(1):CD003019. doi: 10.1002/14651858.CD003019.pub3. Available from: http://dx.doi.org/10.1002/14651858.CD003019.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Enck P, Zimmermann K, Menke G, Müller-Lissner S, Martens U, Klosterhalfen S. A mixture of E.coli (DSM17252) and Enterococcus faecalis (DSM16440) for treatment of the irritable bowel syndrome - a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20:1103–1109. doi: 10.1111/j.1365-2982.2008.01156.x. Available from: http://dx.doi.org/10.1111/j.1365-2982.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 27.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34(2):189–204. doi: 10.1016/j.gtc.2005.02.008. Available from: http://dx.doi.org/10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Enck P, Klosterhalfen S. The placebo response in functional bowel disorders: perspectives and putative mechanisms. Neurogastroenterol Motil. 2005;17(3):325–331. doi: 10.1111/j.1365-2982.2005.00676.x. Available from: http://dx.doi.org/10.1111/j.1365-2982.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 29.Design of Treatment Trials Committee, Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1538–1551. doi: 10.1053/j.gastro.2005.11.058. Available from: http://dx.doi.org/10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]