Abstract
In this article we consider the molecular basis of sensing and signalling by the extracellular calcium-sensing receptor. We consider the nature of its ligands and sensing modalities, the identities of its major protein domains and their roles in sensing, signalling and trafficking as well as the significance of receptor homo- and hetero-dimerization. Finally, we consider the current, incomplete, state of knowledge regarding the requirements for ligand-specific signalling.
This article is part of a themed section on Molecular Pharmacology of GPCR. To view the editorial for this themed section visit http://dx.doi.org/10.1111/j.1476-5381.2010.00695.x
Keywords: calcium-sensing receptor, class C GPCR, Venus Fly Trap domain, cysteine-rich domain, dimerization, heptahelical domain
Introduction
Class C GPCRs
The extracellular calcium sensing receptor (CaR) belongs to class C of the G-protein-coupled receptor superfamily. Class C G-protein-coupled receptor (GPCRs) also include metabotropic glutamate receptors (mGlu-s) of which eight isoforms are recognized, GABA(B) receptors, T1R taste receptors (T1R1-3), the GPRC6A cationic amino acid receptor and various orphans including putative pheromone receptors (Pin et al., 2004; Bräuner-Osborne et al., 2007). These proteins are characterized by large N-terminal extracellular regions composed of 400–500 residue bilobed Venus Fly Trap (VFT) domains for nutrient binding tethered to GPCR heptahelical (HH) signalling domains. In most, but not all cases – the GABA(B) receptor is a notable exception (Hu et al., 2000) – 60–70 residue Cysteine-rich domains act as necessary signal transmission units interposed between the VFT and HH domains. Class C receptors also exhibit large cytoplasmic C-terminal domains which act as scaffolds for the assembly of intracellular signalling units and for the binding of proteins that direct trafficking to specific compartments (Huang and Miller, 2007).
The calcium-sensing receptor: physiological roles
The calcium-sensing receptor (CaR) is widely, almost ubiquitously, expressed (Brown and MacLeod, 2001). In addition to being expressed in endocrine glands such as the parathyroid and various tubular segments of the kidney, it is also expressed in the gastrointestinal tract, mesenchymal tissues including cartilage and bone, and even the brain in which it is expressed in neurons, glial cells and their precursors, apparently acting to modulate synaptic transmission and/or facilitate development (Hofer and Brown, 2003; Yano et al., 2004). High level expression in the subfornical organ, which has a recognized role in ionic strength sensing (Rogers et al., 1997), indicates that CaR-mediated inputs may also modulate whole body salt and water homeostasis.
Expression cloning of the CaR (Brown et al., 1993) revealed the molecular basis of extracellular Ca2+ (Ca2+o) – dependent feedback control of parathyroid hormone (PTH) secretion and other key aspects of whole body calcium homeostasis (reviews: Brown et al., 1995; 1998;). Parathyroid chief cells synthesize and secrete PTH, which acts on the renal proximal tubule to suppress inorganic phosphate reabsorption and stimulate the synthesis of calcitriol as well as the thick ascending limb and distal tubule to promote Ca2+ reabsorption (review: Houillier et al., 2003). These effects restrain renal calcium losses and boost intestinal calcium absorption, thereby elevating the serum Ca2+ concentration. The attendant fall in the serum inorganic phosphate concentration limits the risk of calcium-phosphate precipitation.
As Ca2+o rises towards the lower limit of its normal range (around 1.0–1.1 mM) CaRs in the parathyroid trip a switch that turns off continued PTH release to close the feedback loop (review: Brown and MacLeod, 2001). As Ca2+o rises still further, CaRs in the renal tubules further adjust the Ca2+o and inorganic phosphate levels by directly suppressing Ca2+ reabsorption in the cortical thick ascending limb (reviews: Ba and Friedman, 2004; Gamba and Friedman, 2009) and antagonizing the effect of PTH to restore inorganic phosphate reabsorption in the proximal tubule (Ba et al., 2003). Downregulation of the CaR and inorganic phosphate transporter Na+/Pi-2 in the proximal tubule in response to a chronically elevated phosphate diet provides protection from hyperphosphatemia (Riccardi et al., 2000).
Calcium-sensing receptor agonists, modulators and regulatory modalities
In addition to what is generally considered to be its primary physiological agonist Ca2+, the CaR is sensitive to various other biochemical species and modalities such as ionic strength and pH (Quinn et al., 1998; 2004;), and even temperature (Breitwieser et al., 2004) – pointing to roles beyond, but perhaps complementary, to its well-recognized role in the regulation of calcium metabolism.
Other activators that largely mimic the effect of Ca2+, and are therefore considered to be agonists, include the inorganic divalent cation Mg2+, which is a less potent activator than Ca2+, various other divalent and tervalent inorganic cations including Gd3+, the polyamines spermine and spermidine, antibiotics of the aminoglycoside class and cationic peptides including polyArg and amyloid peptides (reviews: Brown and MacLeod, 2001; Ward and Riccardi, 2002). The molecular basis for this extraordinary promiscuity is, thus far, unexplained.
L-amino acids including, in particular, aromatics, short aliphatics and small polar amino acids are physiological modulators of CaR function (Conigrave et al., 2000a,b;). These amino acid activators markedly sensitize the receptor to Ca2+o–induced activation of intracellular Ca2+ mobilization but have lesser impacts on Ca2+o-stimulated phosphatidylinositol-specific phospholipase-C (PI-PLC) (Rey et al., 2005) and ERK1/2 (Lee et al., 2007) activities. Pharmacologically significant modulators include calcimimetics of the phenylalkylamine (Shoback et al., 2003; Nemeth et al., 2004) and other classes (Dauban et al., 2000; Kessler et al., 2004a), which are positive allosteric modulators (Hebert, 2006; Nemeth, 2006), and calcilytics, which are negative allosteric modulators that stabilize the receptor in one or more inactive conformations (Nemeth et al., 2001; Kessler et al., 2004b; 2006; Nemeth, 2004; Balan et al., 2009).
Variations in pH and ionic strength modulate the receptor's extracellular Ca2+ sensitivity. Ca2+o sensitivity is enhanced by increased pH and reduced by decreased pH (Quinn et al., 2004) suggesting that the receptor may have pH-sensing properties in compartments in which dynamic variations in pH arise including, for example, the medullary collecting tubule, in which the receptor is expressed on the apical membrane (Sands et al., 1997) and the stomach in which the CaR is expressed on the basolateral membranes of gastric mucosal cells (Cheng et al., 1999; Rutten et al., 1999). In addition, Ca2+o sensitivity is enhanced by decreased ionic strength (e.g. arising from reduced serum Na+ concentration) and reduced by increased ionic strength (Quinn et al., 1998) providing a possible explanation for the high level of CaR expression in the subfornical organ (Rogers et al., 1997).
Calcium-sensing receptors: signalling mechanisms
In response to the binding of activators, the CaR's HH transmembrane domains couple to various heterotrimeric G-proteins, including Gq/11, Gi/o and G12/13 to initiate intracellular signalling pathways upstream of PI-PLC, MAP kinases including Erk1/2, p38 and JNK, PI-3 kinase, monomeric G-proteins such as Rho that regulate interactions with the cytoskeleton, and inhibition of adenylyl cyclase (reviews: Brown and MacLeod, 2001; Hofer and Brown, 2003; Ward, 2004; Brennan and Conigrave, 2009). This functional plasticity requires the assembly of complex signalling networks involving interactions between docked heterotrimeric G-proteins and the receptor's C-terminal domain. As a consequence of ligand diversity and functional plasticity, the CaR is particularly susceptible to the impact of mutations throughout its extracellular and intracellular domains (reviews: Brown, 1999; Thakker, 2004) and it may be possible in future to tailor pharmacotherapy to restore receptor function in individuals with CaR mutations that significantly impair receptor function (Hu and Spiegel, 2007).
Problems impeding a complete molecular pharmacological description
How do extracellular calcium-sensing receptors achieve their nutrient and multi-modal sensing tasks? How do they provide high fidelity signalling, differentiating between signals and integrating information from diverse inputs to operate effectively in diverse tissue contexts to control cell fate and function? The solution of these complex issues requires careful analysis on a tissue-by-tissue basis, taking account of the physiologically relevant nutrients and sensing modalities for the compartment and tissue, and taking advantage of modern molecular techniques. In the following sections we review what is currently known of the CaR's ligand binding, signalling and trafficking functions focusing on the structures and functions of its key protein domains.
Major protein domains
Venus FlyTrap domain: major site of nutrient sensing
The CaR's N-terminal Venus FlyTrap (VFT) domain extends from residues 20 to 536. Like the corresponding N-terminus of metabotropic glutamate receptors, the CaR's N-terminus is homologous to nutrient-binding bacterial periplasmic binding proteins (O'Hara et al., 1993; Brauner-Osborne et al., 1999) and, based on the solved crystal structures of homologous mGlus including mGlu-1 (Kunishima et al., 2000) as well as mGlu-3 and mGlu-7 (Muto et al., 2007), takes the form of a bilobed structure in which ligand binding sites are located within the bilobed cleft and at the interprotomeric interface of homodimers (Figure 1). By analogy with the mGlu-1 VFT domain (Kunishima et al., 2000; Tsuchiya et al., 2002), the CaR's dimeric VFT domains are likely to adopt four major conformations: open-open, open-closed, closed-open and closed-closed, in which the closed forms favour receptor activation and are stabilized upon ligand binding.
Figure 1.
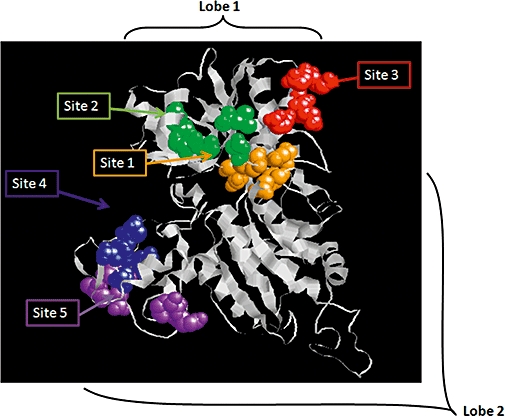
Molecular model of a CaR protomeric VFT domain. A model of a single subunit based on the mGlu-1 crystal structure 1EWK (Kunishima et al., 2000). Putative Ca2+o binding sites (1–5) were identified by aromatized terbium luminescence analysis of globular sub-domains (Huang et al., 2009). Site ‘1’ also corresponds to the conserved L-amino acid-binding site of class C GPCRs raising the possibility that Ca2+ and amino acid binding are closely associated. CaR, calcium-sensing receptor; mGlu, metabotropic glutamate receptor; GPCR, G-protein-coupled receptor; VFT domain, Venus Fly Trap domain.
In addition to its role in nutrient sensing, the VFT domain is an important site of dimerization (Ray et al., 1999) and appears to be the primary location of Ca2+ ion binding (Huang et al., 2007b; 2009;) although additional cation binding site(s) have been identified in the HH transmembrane domains (Ray and Northup, 2002). The nature of the CaR VFT domain's interaction with the immediately contiguous Cysteine-rich domain is currently uncertain (see below) but turning moments induced by closure of the VFT domains are believed to control the conformation of the HH domains and thus the probability of G-protein docking and activation.
The VFT domain exhibits tight conservation of residues required for binding the α-amino and α-carboxylate groups of L-amino acid ligands in metabotropic glutamate receptors and other amino acid binding class C GPCRs (Table 1) and, indeed, on the basis of analyses of chimeric receptors (Mun et al., 2004) and mutations (Zhang et al., 2002b; Mun et al., 2005) is the site of broad-spectrum L-amino binding in the CaR.
Table 1.
Aligned α-carboxylate and α-amino VFT domain binding residues from a multiple sequence alignment of class C GPCRs
| rmGlu-1 | S165 | T188 | D208 | Y236 | D318 |
| rmGlu-3 | S151 | T174 | D194 | Y222 | D301 |
| hCaR | S147 | S170 | D190 | Y218 | E297 |
| mT1R1 | T150 | S173 | D193 | Y221 | E302 |
| mT1R3 | S147 | S170 | D190 | Y218 | E301 |
| mGPRC6A | S149 | T172 | D192 | Y220 | D303 |
Known α-amino and α-carboxylate binding residues from the crystal structures of the VFT domains of rat mGlu-1 (Kunishima et al., 2000) and rat mGlu-3 (Muto et al., 2007) are presented together with aligned residues for various L-amino acid binding members of GPCR class C including the human isoform of the CaR and mouse isoforms of T1R2, T1R3 and GPRC6A. The table has been modified (Conigrave and Hampson, 2006). The Protein Database accession numbers used in the analysis were as follows: NP_058707.1 (rmGlu-1), NP_001099182.1 (rmGlu-3), NP_000379.2 (hCaR), NP_114073.1 (mT1R1), NP_114078.1 (mT1R3), NP_694711.1 (mGPRC6A).
CaR, calcium-sensing receptor; GPCR, G-protein-coupled receptor; GPRC6A, G-protein-coupled receptor family C (class C) member 6A; mGlu, metabotropic glutamate receptor; VFT domain, Venus Fly Trap domain.
Cysteine-rich domain: signal transmission
The CaR's VFT domain connects with its HH domain via a 62 residue Cys-rich domain (Figure 2; Hu et al., 2000) and a 14 residue linker (Ray et al., 2007). Although the otherwise conserved Cys-rich domain is absent in GABA(B) receptors (Hu et al., 2000), molecular analyses indicate that the CR domain plays a critical role in transmitting nutrient-derived signals from the VFT domain to the HH domains in other class C GPCRs. Thus, deletion of the entire CR domain eliminated high Ca2+o concentration-induced activation of PI-PLC with no significant effect on surface expression (Hu et al., 2000) and mutational analyses indicate that all nine Cys residues are required for normal receptor function (Fan et al., 1998; Ray et al., 1999; Hu et al., 2000).
Figure 2.
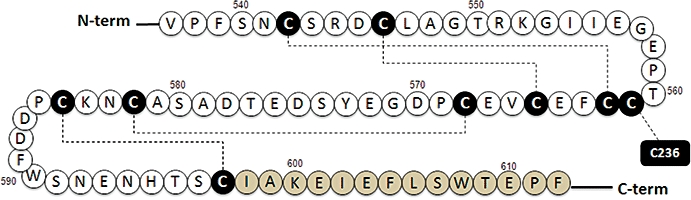
Schematic representation of the CaR's Cysteine rich domain. The Cys-rich (CR) domain has nine conserved cysteine residues (black circles), all of which are predicted to participate in di-sulfide bonds (broken lines). In total there are four predicted intra-domain di-sulfides and one disulfide between CaR residues 561 in the CR domain and 236 in lobe 2 of the VFT domain. A 14 amino acid linker (grey circles) supports signal transmission from the VFT domain to the heptahelical domain. CaR, calcium-sensing receptor; VFT domain, Venus Fly Trap domain.
Although no structures are available for any of the CaR's major domains, the recent solution of a crystal structure for the entire extracellular domain of the rat Group II metabotropic glutamate receptor, mGlu-3 (Muto et al., 2007), provides new insights into the structural relationships between the CR domains and contiguous VFT and HH domains. In particular, the structure defines roles for the entire complement of nine conserved Cys residues in the CR domains. In the case of four pairs of CR domain Cysteines (mGlu-3 residues C509 and C528; C513 and C531; C534 and C546; and C549 and C562) a network of intra-domain disulfides stabilizes a rigid rod-like structure composed of several anti-parallel sheets to provide the VFT domain with caliper-like control over the HH domain (Muto et al., 2007). In the case of the remaining mGlu-3 CR domain Cys residue, C527 (which aligns to CaR residue 561), an interdomain disulfide forms with C240 (aligning to CaR residue 236) in lobe-2 of the VFT domain. This disulfide appears to adjust the angle at which turning moments induced in the VFT domains are transmitted to the HH domains.
An inter-domain disulfide between the homologous Cys residues in mGlu-2 was predicted previously (Rondard et al., 2006) but, despite conservation of both Cys residues, was excluded for the CaR based on analyses of proteolytic fragments released from an engineered tobacco etch virus cleavage site between the VFT and CR domains (Hu et al., 2001). The discrepancy between the crystal structure-based findings of Muto et al. for mGlu-3 (Muto et al., 2007) and mutational analysis of Rondard et al. for mGlu-2 (Rondard et al., 2006), on the one hand, and Hu et al. for the CaR (Hu et al., 2001) on the other, is currently unexplained. It suggests, however, that interdomain di-sulfide bridge formation in the CaR may be relatively unstable (review: Hu and Spiegel, 2007) and perhaps sensitive to ligand binding.
The HH domain: interactions with G-proteins
Members of the GPCR superfamily exhibit a characteristic HH domain consisting of seven helices connected by alternating intracellular loops (iL-s) and extracellular loops (eL-s) (Pierce et al., 2002; Karnik et al., 2003; Rosenbaum et al., 2009). The N-terminus of the HH module is continuous with the N-terminal extracellular domain of the receptor and the C-terminus of the module extends into the cytoplasm as the C-terminal domain. The HH domain is considered critical for the docking and activation of hetero-trimeric G-proteins. Crystal structures of class A GPCRs including bovine rhodopsin (Palczewski et al., 2000) and more recently β2-adrenergic (Cherezov et al., 2007; Rasmussen et al., 2007) and β1-adrenergic (Warne et al., 2008) receptors, have greatly facilitated molecular modelling efforts aimed at understanding the mechanism(s) of GPCR activation. According to the current view of class A receptor action, ligand binding in a pocket formed by a cylindrical arrangement of HH helices releases inhibitory constraints on the docking and binding of G-proteins on the interior surface of the receptor (Kobilka and Schertler, 2008).
Although models based on the solved class A GPCR structures have been applied with some success to class C GPCRs including the CaR (Miedlich et al., 2004; Petrel et al., 2004), the nature of the interfaces between oligomeric HH domains, together with the selectivities and stoichiometries of receptor: G-protein binding are undefined. Nevertheless, significant progress has been made on the activation mechanism of the homologous mGlu-5 receptors, which exhibit subunit interchange between dimeric HH domains (Brock et al., 2007). In addition, mutational analysis has demonstrated that CaR-dependent activation of PI-PLC, which is Gq/11-dependent, is dependent on residues in both intracellular loops 2 and 3 (Figure 3; Chang et al., 2000).
Figure 3.
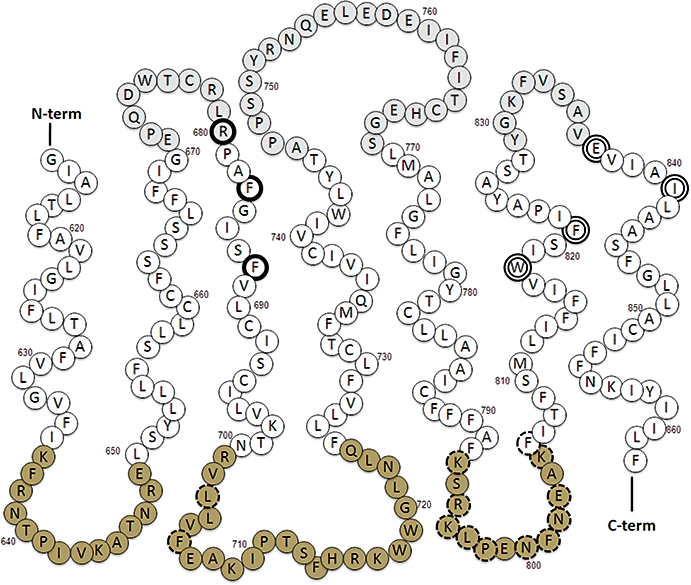
Schematic representation of the CaR's heptahelical domain. The seven transmembrane helices are shown together with the alternating intracellular loops (residues in dark grey) and extracellular loops (residues in light grey). Residues that interact with both calcimimetics and calcilytics are enclosed in double-lined circles. Residues that interact with calcilytics alone are enclosed in single, bold circles. Residues in iL-2 and iL-3 that support Gq/11-dependent activation of PI-PLC are highlighted with broken-lines. CaR, calcium-sensing receptor; PI-PLC, phosphatidylinositol-specific phospholipase-C.
Unlike, the mGlus which tend to be specialized for either the activation of Gq/11, in the case of Group I receptors, or Gi/o in the case of the Group II and Group III receptors, the CaR is unusually pleiotropic, coupling to both Gq/11 and Gi/o, as well as G12/13 (reviews: Hofer and Brown, 2003; Ward, 2004; Brennan and Conigrave, 2009). It may even couple to Gs under some circumstances (see below). Although the signalling potential of these G-protein partners is clear, the broader physiological significance of these interactions is, at present, largely unknown. A notable exception is the role of Gq/11 in CaR-mediated control of PTH secretion as parathyroid-specific ablation of both Gq and G11 in mice induced severe neonatal primary hyperparathyroidism (Wettschureck et al., 2007).
Gq and G11 appear to interact with the CaR via residues in intracellular loop-2 (iL-2) and intracellular loop-3 (iL-3) as alanine scanning mutagenesis in both iL-2 and iL-3 impaired PI-PLC activation (Chang et al., 2000). In the case of iL-2 (residues 700 to 727 in the bovine isoform), L704 and F707 were critical for high Ca2+o-mediated coupling to PI-PLC. In the case of iL-3 (residues 794–807), two closely associated patches were identified between residues R796 and P799 and between residues N801 and F807 (Chang et al., 2000). The naturally occurring FHH-related mutation of the human CaR, R795W (R796 in the bovine isoform), which exhibits dominant negative activity, is located nearby.
The analysis described above for CaR coupling to Gq/11 has not yet been extended to other heterotrimeric G-proteins to which the CaR couples such as Gi/o or G12/13. In addition, the CaR's G-protein preference switches from Gi/o in normal mammary epithelial cells to Gs in two breast cancer cell lines, thus reversing the polarity of its control over cAMP synthesis (Mamillapalli et al., 2008) with potential significance for cancer cell growth and/or metastasis. The mechanism that underlies this effect is currently unknown.
C-terminal domain: Dynamic regulation of signalling and expression
The human CaR's C-terminal domain is composed of residues 863–1078 (Figure 4; Garrett et al., 1995) and plays key roles in signalling, expression, trafficking, cooperativity and desensitization (reviews: Bai, 2004; Ward, 2004; Breitwieser, 2006; Hu and Spiegel, 2007). Based on analyses of truncation mutants and alanine scanning, the immediate membrane proximal loop (residues 863–874) promotes receptor expression and is required for PI-PLC activation (Ray et al., 1997). In addition, several residues between 874 and 888, although not required for receptor expression, are necessary for PI-PLC activation. Residues beyond 887 although not required for PI-PLC activation (Ray et al., 1997) are implicated in alternative downstream signalling pathways. In addition, residues 868–886 appear to contribute to cooperativity and protect the receptor from desensitization (Gama and Breitwieser, 1998); see Figure 4.
Figure 4.

Schematic representation of the CaR's intracellular C-terminal domain. The C-terminal domain supports receptor expression and activation of PI-PLC (residues 863–874; double circles), activation of PI-PLC alone (highlighted in grey), as well as cooperativity and resistance to desensitization (residues 868–886 indicated by broken line). Residues 960–990 (labelled in black) provide a high-affinity binding site for filamin-A. The major PK-C phosphorylation site at T-888 is labelled ‘▾’. CaR, calcium-sensing receptor; PI-PLC, phosphatidylinositol-specific phospholipase-C.
Various potential PK-C phosphorylation sites have been identified in the intracellular loops and C-terminal domain. Of these, T888 in the proximal C-terminal domain is considered to be the primary site and phosphorylation of this residue modulates receptor function, acting to uncouple PI-PLC and thus reduce sensitivity to elevated Ca2+o (Bai et al., 1998; Jiang et al., 2002). Interestingly, recent work employing an antibody that binds selectively to a CaR-tail phospho-peptide centred on T888 demonstrates that the activated receptor exhibits repetitive T888 phosphorylation and dephosphorylation (Davies et al., 2007). Although the full significance of this result is uncertain, it suggests a mechanism by which activator-induced low frequency oscillations in Ca2+i concentration might arise (Davies et al., 2007). In addition, T888 phosphorylation appears to promote G-protein receptor kinase-2 mediated desensitization via sequestration of alpha-q subunits and/or beta-arrestin binding to the HH domain (Pi et al., 2005; Lorenz et al., 2007). The tendency for T888 to undergo repetitive phosphorylation and dephosphorylation upon receptor activation may also contribute to the CaR's well-recognized resistance to desensitization (Brown and MacLeod, 2001).
In addition to its role in PI-PLC signalling, the C-terminal domain interacts either directly or indirectly with intracellular proteins that modulate or mediate receptor trafficking, subcellular localization and downstream signalling pathways (Huang and Miller, 2007). Interactions with inwardly rectifying K+ channels, Kir4.1 and Kir4.2, for example, provide a mechanism by which alterations in Ca2+o modulate renal salt and water transport (Huang et al., 2007a). Binding to filamin-A, on the other hand, establishes a link to the actin cytoskeleton to direct receptors to specific subcellular compartments for the creation of signalling scaffolds (Awata et al., 2001; Hjalm et al., 2001; Zhang and Breitwieser, 2005). Filamin may mediate, for example, the CaR's interactions with caveolin, thereby targeting the receptor to plasma membrane caveolae (Kifor et al., 1998). In addition, the association between the CaR and filamin is required for coupling between the receptor and ERK 1/2 (Awata et al., 2001; Hjalm et al., 2001) and may permit G12/13 control of small G-proteins including Rho, upstream of PI-4 kinase and phospholipase D (Pi et al., 2002; Rey et al., 2005). Two filamin-A binding sites have been reported: a high affinity site located in the approximate region 960–990 (Awata et al., 2001; Hjalm et al., 2001; Zhang and Breitwieser, 2005) and a lower affinity, membrane proximal binding site that appears to contribute to Ca2+o-induced ERK1/2 activation (Zhang and Breitwieser, 2005).
Other CaR binding partners including the E3 ubiquitin ligase, dorfin (Huang et al., 2006) and AMSH (Herrera-Vigenor et al., 2006; Reyes-Ibarra et al., 2007) promote intracellular trafficking to proteasomes for protein degradation. Surprisingly, recent studies employing co-immunoprecipitation and RNAi techniques indicate that trafficking of the CaR to the plasma membrane in COS-7 and HEK293 cells requires receptor activity modifying proteins (RAMPs) including RAMP-1 and RAMP-3 (Bouschet et al., 2005; Bouschet et al., 2008). These findings were unexpected as RAMPs have been considered specific partners of Class B GPCRs such as the calcitonin gene-related peptide receptor for which they modulate receptor expression, ligand selection and signalling properties (Hay et al., 2006; Sexton et al., 2006).
Roles of dimers in tissue-specific receptor function
Homodimers
Whether expressed heterologously in HEK293 cells or constitutively in cells with a primary role in extracellular Ca2+-sensing, the CaR functions primarily as homodimers and both covalent and non-covalent interactions support dimerization at the interface between neighbouring VFT domains (reviews: Bai, 2004; Hu and Spiegel, 2007). Two asymmetric intermolecular disulfide bonds form between residues C129 and C131 of dimeric receptors (Ray et al., 1999). In addition, non-covalent interactions involving L112 and L156 promote dimer stability (Jiang et al., 2004). Dimerization is independent of agonist or modulator binding, and appears to arise at the time of insertion of the newly translated subunits into the endoplasmic reticulum (ER) membrane (Pidasheva et al., 2006).
Dimerization promotes trafficking to the plasma membrane, perhaps by the mutual masking of peptides that act as ER retention signals (Chang et al., 2007). If this is correct, CaR homodimers might operate in a manner analogous to GABA(B) heterodimers in which GABA(B2) residues R714-P820 mask an ER retention signal located in the GABA(B1) C-terminal domain (Pagano et al., 2001). Dimerization also appears to be required for normal receptor function. Firstly, co-expression of receptor mutants disabled in either their VFT domains or HH domains restored extracellular Ca2+-stimulated PI-PLC (Bai et al., 1999). Secondly, the CaR homolog, mGlu-5 exchanges loops of its HH domains upon receptor activation (Brock et al., 2007) suggesting that the dimeric VFT-CR domain apparatus induces a functionally important interchange between associated HH domains.
Heterodimerization with other class C GPCRs: a mechanism for tissue-specific nutrient and multi-modal sensing?
In addition to forming functional homodimers, the CaR appears to form physiologically important heterodimers with other class C GPCRs dependent on their patterns of expression. Consistent with this idea, the CaR and mGlu-1α were co-immunoprecipitated from bovine brain (Gama et al., 2001). Furthermore, the CaR formed disulfide-linked dimers with mGlu-1α or mGlu-5 when co-expressed with these receptors in HEK293 cells, acquiring glutamate-induced receptor internalization and exhibiting enhanced cell surface expression in the presence of the mGlu-1/5 binding partner Homer 1c (Gama et al., 2001).
The CaR also forms heterodimers with co-expressed GABA(B) receptor subunits as revealed by co-immunoprecipitation analyses of growth plate chondrocytes (Cheng et al., 2007) as well as whole brain and hippocampal neurons (Chang et al., 2007). Interestingly, CaR expression was differentially modulated by co-expressed GABA(B1) or GABA(B2) receptors in HEK293 cells (Chang et al., 2007). Whereas GABA(B2) markedly enhanced CaR surface expression, possibly by shielding a putative ER retention signal in the CaR tail, GABA(B1) suppressed CaR total and surface expression and promoted internalization (Chang et al., 2007). On the other hand, the CaR promoted the expression of both GABA(B) subunits suggesting that it may play a role as a chaperone for the expression of some class C GPCRs. Taken together, the results suggest that the CaR preferentially forms heterodimers with other class C GPCRs but the mechanisms by which specific heterodimers are favoured are currently undefined.
A domain-based survey of CaR-ligand interactions
As noted above, the CaR mediates multi-modal, multi-metabolic sensing not only of nutrients including Ca2+ and L-amino acids but also ionic strength, pH and even temperature (Conigrave et al., 2000a; Breitwieser et al., 2004). Although the molecular requirements for the sensing of Ca2+o and amino acids as well as calcimimetics and calcilytics have been the subject of considerable scrutiny, the mechanisms that underlie the sensing of other modalities are largely unknown. Thus, the following discussion concentrates on Ca2+ and other multivalent cations, L-amino acids and calcimimetics and calcilytics.
Ca2+ and other multivalent cations
Although the CaR binds Ca2+ with apparently low affinity, it exhibits pronounced positive cooperativity in its control of proximal signalling pathways including PI-PLC and intracellular Ca2+ mobilization as well as its inhibitory control of PTH secretion (review: Brown and MacLeod, 2001). In particular, Hill coefficients for the Ca2+o-dependent activation of proximal signalling events are typically around 3–5 and approach 8–12 for the control of PTH secretion, indicating the existence of multiple interacting binding sites in receptor dimers or even oligomeric arrays. As a consequence, the concentration-response relationships are very steep at the points of inflection; for PTH secretion from normal human parathyroid cells this occurs at a Ca2+o concentration of around 1.1 mM (Conigrave et al., 2004; Mun et al., 2009).
The location(s) of the Ca2+ binding sites have been controversial. Chimeric receptors composed of the VFT domain of the CaR fused to the CR domain, HH domain and intracellular C-terminal domain of mGlu-1 exhibit normal or near-normal Ca2+o sensitivity in assays of PI-PLC activity (Hauache et al., 2000) and intracellular Ca2+ mobilization (Mun et al., 2004), consistent with the idea that the CaR's Ca2+ binding sites are located in the VFT domain. The interpretation of these experiments is compromised, however, by reports that mGlu-1 and some other mGlu-s are also Ca2+o-sensitive (Kubo et al., 1998; Tabata et al., 2002); for a review see: Tabata and Kano, 2004. In addition, studies of CaRs in which the entire extracellular domain has been replaced by the signal peptide of bovine rhodopsin (so-called ‘headless’ receptors), demonstrate that Ca2+o sensing can arise from the HH domain in the complete absence of the VFT domain (Ray and Northup, 2002; Mun et al., 2004) and indicate that there is at least one Ca2+ binding site outside the VFT domain. Alanine scanning mutagenesis in the junction between transmembrane helices VI and VII, thus involving the third extracellular loop, identified a peptide (residues 819–837) required for Ca2+o-induced receptor activation but its significance for cation binding is unknown (Hu et al., 2005).
Mutational analysis has also been employed in attempts to locate Ca2+o binding sites but is of limited value unless impaired receptor function and impaired cation binding can be causally associated. Based on molecular modelling, Silve et al. (Silve et al., 2005) reported the presence of a Ca2+ binding site in the region of a conserved glutamate residue E297 in the VFT domain. They noted that E297K is an inactivating mutation associated with FHH whereas E297D is an activating mutation associated with ADH. The activating effect of the conservative mutation E297D was postulated to arise from the creation of an enlarged divalent cation binding pocket (Silve et al., 2005).
More recent analysis using aromatized terbium luminescence to probe for Ca2+o binding sites, together with molecular modelling and mutational analysis, suggests the existence of several functionally significant Ca2+o binding sites in the VFT domain (Huang et al., 2007b; Huang et al., 2009). In particular, the independent expression of three globular subdomains corresponding to the hinge region of the VFT domain and distinct lobe 1 and lobe 2 proteins permitted analyses of Ca2+o binding and associated conformational changes (Huang et al., 2009). Ca2+ binding ‘site 1’ in subdomain-1 was defined by residues S147, S170, D190, Y218 and E297, which are all highly conserved in class C GPCRs (Table 1) and known to contribute to the core amino acid binding site in mGlu-1 (Kunishima et al., 2000; Tsuchiya et al., 2002) as well as mGlu-3 and mGlu-7 (Muto et al., 2007). Based on its apparent Ca2+-binding affinity (Kd around 0.5 mM) it seems likely that this site is normally occupied at physiologically relevant Ca2+o concentrations and, therefore, may not contribute directly to the changes in receptor structure that arise from physiologically relevant changes in Ca2+o concentration (between around 1.1–1.3 mM). Instead ‘site 1’ may stabilize a receptor configuration necessary for Ca2+ binding to coupled lower affinity sites or a closely associated L-amino acid binding site.
The interpretation of the physiological significance of any of a large number of low affinity Ca2+-binding sites is complicated by the demonstration that various multivalent cations including the inorganic polyvalent cations, Gd3+ and Mg2+ (review: Brown and MacLeod, 2001), as well as polyamines such as spermine (Quinn et al., 1997), aminoglycoside antibiotics (Ward et al., 2002; Gibbons et al., 2008) and cationic peptides including PolyArg (Brown et al., 1991) and amyloid peptides (Ye et al., 1997) are all CaR activators. This extraordinary promiscuity is not clearly explained by currently reported binding analyses and the negatively charged surface(s) that mediate these effects are undefined.
L-Amino acids
L-Amino acids act as positive allosteric modulators promoting Ca2+o-induced intracellular Ca2+ mobilization in CaR-expressing HEK293 and CHOK1 cells (Conigrave et al., 2000a,b;) as well as normal human parathyroid cells (Conigrave et al., 2004). In single cells, L-amino acids induce low frequency oscillations in the presence of submaximal Ca2+o concentrations (Young and Rozengurt, 2002) that arise from the activation of a signalling pathway coordinated by filamin binding to the C-terminal domain (Rey et al., 2005). In addition, L-amino acids suppress PTH secretion (Conigrave et al., 2004), an effect that is impaired in primary hyperparathyroidism (Mun et al., 2009). The apparent requirement for a threshold extracellular Ca2+ level (typically around 0.5–1.0 mM in CaR-expressing HEK293 cells and parathyroid cells) indicates that the amino acid and Ca2+ binding sites interact. Furthermore, the L-amino acid and phenylalkylamine binding sites also exhibit positive cooperativity (Zhang et al., 2002a).
Analysis of CaR/mGlu-1 chimeric receptors and a headless CaR construct expressed in HEK293 cells localized the L-amino acid binding site to the VFT domain (Mun et al., 2004) indicating that the L-glutamate binding site in mGlu-1 is conserved in the CaR as a broad-spectrum L-amino acid binding site (Conigrave and Hampson, 2006). Consistent with this idea, the double mutant T145A/S170T markedly impaired L-Phe sensitivity but had little or no effect on Ca2+o-sensing in CaR-expressing HEK293 cells (Mun et al., 2005). As noted above, this site may be closely associated with a moderately high affinity Ca2+ binding site located in the hinge region (Huang et al., 2009) providing a potential explanation for the positive interactions between Ca2+ and L-amino acids (Conigrave et al., 2007).
Calcimimetics and calcilytics
Calcimimetics are organic compounds that act as agonists or positive allosteric modulators, mimicking the effects of elevated Ca2+o on downstream signalling pathways. Calcilytics, on the other hand, are CaR inhibitors. In general, unlike amino acids, these compounds bind in the HH domains. The first classes of calcimimetics and calcilytics to be identified were phenylalkylamines including the calcimimetics NPS R467 and R568 (Nemeth et al., 1998; Nemeth, 2006) and the calcilytics NPS 2143 and NPS 89636 (Nemeth et al., 2001); for a review see: Nemeth (2002). NPS R467, R568 and their orally active analog cinacalcet are all stereoselective, positive allosteric modulators.
Unlike L-amino acids, which exhibit selectivity for specific signalling pathways including activation of low frequency intracellular Ca2+ oscillations (Rey et al., 2005) and modestly enhance the Ca2+o sensitivity of ERK 1/2 activation (Lee et al., 2007), phenylalkylamine calcimimetics exhibit broad specificity for CaR-linked signalling pathways (review: Brennan and Conigrave, 2009). Consistent with these effects cinacalcet is a potent activator of the CaR in vivo and has been successfully introduced for the medical therapy of secondary hyperparathyroidism in the context of chronic renal failure (Nagano, 2006; Wüthrich et al., 2007). Its place in the treatment of primary hyperparathyroidism is less certain due to the effectiveness of modern surgical techniques (Bilezikian et al., 2009; Khan et al., 2009) but it is effective in long-term biochemical control for 1 year or more (Shoback et al., 2003; Peacock et al., 2005). Calcilytics, on the other hand, are being evaluated in the treatment of osteoporosis (Nemeth, 2004) although a reported inhibitory effect on osteoblast differentiation and function may limit their utility in this disorder (Dvorak et al., 2004).
Based on studies with chimeric and ‘headless’ receptors, as well as molecular modelling and mutational analyses, calcimimetics and calcilytics bind in the HH domain (Hauache et al., 2000; Ray and Northup, 2002; Miedlich et al., 2004; Mun et al., 2004; Petrel et al., 2004) acting either positively or negatively to modulate the transmission of molecular signals to the G-protein docking site. Consistent with the idea that the binding sites of positive and negative allosteric modulators at least partially overlap, mutations of residues in transmembrane helix VI (Y818, F821) and VII (E837, I841) impaired the effects of the calcimimetics R-568 and calindol as well as calcilytics including NPS 2143 and Calhex 231 (Miedlich et al., 2004; Petrel et al., 2004). Mutations of residues in helix III (R680, F684, F688), however, selectively impaired the effects of calcilytics (Miedlich et al., 2004; Petrel et al., 2004); see Figure 3. Of the key HH domain residues identified in these studies, E837 between eL-3 and helix VII appears to be of particular interest as mutations of this residue can switch the efficacy of allosteric modulators between positive and negative (Miedlich et al., 2004; Hu et al., 2006).
Conclusions and future directions
Class C GPCRs exhibit dazzling promiscuity for ligands together with pluripotent activation of signalling pathways. The CaR's ability to control Gq/11, Gi/o and G12/13 appears to be an extreme example. Despite this craziness it works! In addition, molecular analysis is starting to locate ligand binding sites and unravel the mechanisms of receptor activation including the internal transmission of signals, coordination of G-protein docking and formation of signalling scaffolds. Despite the impressive progress to date, the recognition that different ligands/signals induce distinct receptor conformations linked to the selective recruitment of signalling pathways indicates that important tasks in molecular analysis still lie ahead.
Acknowledgments
The authors wish to thank the Australian Research Council for an Australian Postgraduate Award (M.A.K.) and the National Health and Medical Research Council of Australia for project grant support (A.D.C.). The authors also wish to thank A/Prof Charles Collyer for his assistance with the preparation of Figure 1.
Glossary
Abbreviations:
- ADH
Autosomal Dominant Hypocalcemia
- AMSH
associated molecule with the SH3 domain of STAM
- CaR
calcium-sensing receptor
- ERK
extracellular regulated kinase
- FHH
familial hypocalciuric hypercalcemia
- GPCR
G-protein-coupled receptor
- GPRC6A
G-protein-coupled receptor family C (class C) member 6A
- mGlu
metabotropic glutamate receptor
- VFT domain
Venus Fly Trap domain
References
- Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem. 2001;276:34871–34879. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium. 2004;35:229–237. doi: 10.1016/j.ceca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol. 2003;285:F1233–F1243. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- Bai M. Structure-function relationship of the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:197–207. doi: 10.1016/j.ceca.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Kifor O, Quinn SJ, Brown EM. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc Natl Acad Sci USA. 1999;96:2834–2839. doi: 10.1073/pnas.96.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca2+o-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem. 1998;273:21267–21275. doi: 10.1074/jbc.273.33.21267. [DOI] [PubMed] [Google Scholar]
- Balan G, Bauman J, Bhattacharya S, Castrodad M, Healy D, Herr M, et al. The discovery of novel calcium sensing receptor negative allosteric modulators. Bioorg Med Chem Lett. 2009;19:3328–3332. doi: 10.1016/j.bmcl.2009.04.044. [DOI] [PubMed] [Google Scholar]
- Bilezikian J, Khan AA, Potts JT. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Regulation of calcium-sensing-receptor trafficking and cell-surface expression by GPCRs and RAMPs. Trends Pharmacol Sci. 2008;29:633–639. doi: 10.1016/j.tips.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner-Osborne H, Jensen AA, Sheppard PO, O'Hara P, Krogsgaard-Larsen P. The agonist-binding domain of the calcium-sensing receptor is located at the amino-terminal domain. J Biol Chem. 1999;274:18382–18386. doi: 10.1074/jbc.274.26.18382. [DOI] [PubMed] [Google Scholar]
- Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. Calcium sensing receptors and calcium oscillations: calcium as a first messenger. Curr Top Dev Biol. 2006;73:85–114. doi: 10.1016/S0070-2153(05)73003-9. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, Miedlich SU, Zhang M. Calcium sensing receptors as integrators of multiple metabolic signals. Cell Calcium. 2004;35:209–216. doi: 10.1016/j.ceca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Brennan SH, Conigrave AD. Regulation of cellular signal transduction pathways by the extracellular calcium-sensing receptor. Curr Pharmaceut Biotechnol. 2009;10:270–281. doi: 10.2174/138920109787847484. [DOI] [PubMed] [Google Scholar]
- Brock C, Oueslati N, Soler S, Boudier L, Rondard P, Pin JP. Activation of a dimeric metabotropic glutamate receptor by intersubunit rearrangement. J Biol Chem. 2007;282:33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]
- Brown EM. Physiology and pathophysiology of the extracellular calcium-sensing receptor. Am J Med. 1999;106:238–253. [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular Calcium Sensing and Extracellular Calcium Signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Brown EM, Katz C, Butters R, Kifor O. Polyarginine, polylysine, and protamine mimic the effects of high extracellular calcium concentrations on dispersed bovine parathyroid cells. J Bone Miner Res. 1991;6:1217–1225. doi: 10.1002/jbmr.5650061112. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Brown EM, Pollak M, Seidman CE, Seidman JG, Chou YH, Riccardi D, et al. Calcium-ion-sensing cell-surface receptors. N Engl J Med. 1995;333:234–240. doi: 10.1056/NEJM199507273330407. [DOI] [PubMed] [Google Scholar]
- Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing receptor: its role in health and disease. Annu Rev Med. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- Chang W, Chen TH, Pratt S, Shoback D. Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J Biol Chem. 2000;275:19955–19963. doi: 10.1074/jbc.M909613199. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, et al. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, et al. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology. 1999;116:118–126. doi: 10.1016/s0016-5085(99)70235-0. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Tu C, Rodriguez L, Chen TH, Dvorak MM, Margeta M, et al. Type B gamma-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992. doi: 10.1210/en.2007-0653. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave A, Mun H, Brennan SC. Physiological significance of L-amino acid sensing by extracellular Ca(2+)-sensing receptors. Biochem Soc Trans. 2007;35:1195–1198. doi: 10.1042/BST0351195. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Hampson DR. Broad-spectrum amino acid sensing by class 3 G-protein coupled receptors. Trends Endocrinol Metab. 2006;17:398–407. doi: 10.1016/j.tem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. Cooperative multi-modal sensing and therapeutic implications of the extracellular Ca2+-sensing receptor. Trends Pharm Sci. 2000a;21:401–407. doi: 10.1016/s0165-6147(00)01546-7. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA. 2000b;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave AD, Mun H-C, Delbridge L, Quinn SJ, Wilkinson M, Brown EM. L-amino acids regulate parathyroid hormone secretion. J Biol Chem. 2004;279:38151–38159. doi: 10.1074/jbc.M406373200. [DOI] [PubMed] [Google Scholar]
- Dauban P, Ferry S, Faure H, Ruat M, Dodd RH. N1-Arylsulfonyl-N2-(1-aryl)ethyl-3-phenylpropane-1,2-diamines as novel calcimimetics acting on the calcium sensing receptor. Bioorg Med Chem Lett. 2000;10:2001–2004. doi: 10.1016/s0960-894x(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Davies SL, Ozawa A, McCormick WD, Dvorak MM, Ward DT. Protein kinase C-mediated phosphorylation of the calcium-sensing receptor is stimulated by receptor activation and attenuated by calyculin-sensitive phosphatase activity. J Biol Chem. 2007;282:15048–15056. doi: 10.1074/jbc.M607469200. [DOI] [PubMed] [Google Scholar]
- Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GF, Ray K, Zhao XM, Goldsmith PK, Spiegel AM. Mutational analysis of the cysteines in the extracellular domain of the human Ca2+ receptor: effects on cell surface expression, dimerization and signal transduction. FEBS Lett. 1998;436:353–356. doi: 10.1016/s0014-5793(98)01165-x. [DOI] [PubMed] [Google Scholar]
- Gama L, Breitwieser GE. A carboxyl-terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem. 1998;273:29712–29718. doi: 10.1074/jbc.273.45.29712. [DOI] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- Gamba G, Friedman PA. Thick ascending limb: the Na+: K+: 2Cl- co-transporter, NKCC2, and the calcium-sensing receptor. Pflugers Arch. 2009;458:61–76. doi: 10.1007/s00424-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JE, Capuano IV, Hammerland LJ, Hung BCP, Brown EM, Hebert SC, et al. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- Gibbons CE, Maldonado-Pérez D, Shah AN, Riccardi D, Ward DT. Calcium-sensing receptor antagonism or lithium treatment ameliorates aminoglycoside-induced cell death in renal epithelial cells. Biochim Biophys Acta. 2008;1782:188–195. doi: 10.1016/j.bbadis.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hauache OM, Hu J, Ray K, Xie R, Jacobson KA, Spiegel AM. Effects of a calcimimetic compound and naturally activating mutations on the human Ca2+ receptor and on Ca2+ receptor/metabotropic glutamate chimeric receptors. Endocrinology. 2000;141:4156–4163. doi: 10.1210/endo.141.11.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hebert SC. Therapeutic use of calcimimetics. Annu Rev Med. 2006;57:349–364. doi: 10.1146/annurev.med.57.121304.131328. [DOI] [PubMed] [Google Scholar]
- Herrera-Vigenor F, Hernández-García R, Valadez-Sánchez M, Vázquez-Prado J, Reyes-Cruz G. AMSH regulates calcium-sensing receptor signaling through direct interactions. Biochem Biophys Res Commun. 2006;347:924–930. doi: 10.1016/j.bbrc.2006.06.169. [DOI] [PubMed] [Google Scholar]
- Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nature Reviews Molec Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- Houillier P, Nicolet-Barousse L, Maruani G, Paillard M. What keeps serum calcium levels stable? Joint Bone Spine. 2003;70:407–413. doi: 10.1016/s1297-319x(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Hu J, Hauache O, Spiegel AM. Human Ca2+ receptor cysteine-rich domain. Analysis of function of mutant and chimeric receptors. J Biol Chem. 2000;275:16382–16389. doi: 10.1074/jbc.M000277200. [DOI] [PubMed] [Google Scholar]
- Hu J, Reyes-Cruz G, Goldsmith PK, Spiegel AM. The Venus's-flytrap and cysteine-rich domains of the human Ca2+ receptor are not linked by disulfide bonds. J Biol Chem. 2001;276:6901–6904. doi: 10.1074/jbc.C000865200. [DOI] [PubMed] [Google Scholar]
- Hu J, McLarnon SJ, Mora S, Jiang J, Thomas C, Jacobson KA, et al. A region critical in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ J Biol Chem. 2005;280:5113–5120. doi: 10.1074/jbc.M413403200. [DOI] [PubMed] [Google Scholar]
- Hu J, Jiang J, Costanzi S, Thomas C, Yang W, Feyen J, et al. A missense mutation in the seven-transmembrane domain of the human Ca2+ receptor converts a negative allosteric modulator into a positive allosteric modulator. J Biol Chem. 2006;281:21558–21565. doi: 10.1074/jbc.M603682200. [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM. Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med. 2007;11:908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Niwa J, Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem. 2006;281:11610–11617. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- Huang C, Miller RT. The calcium-sensing receptor and its interacting proteins. J Cell Mol Med. 2007;11:923–934. doi: 10.1111/j.1582-4934.2007.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, et al. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol. 2007a;292:F1073–F1081. doi: 10.1152/ajprenal.00269.2006. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Yang W, Butters R, Lee H-W, Li S, et al. Identification and dissection of Ca(2+)-binding sites in the extracellular domain of Ca(2+)-sensing receptor. J Biol Chem. 2007b;282:19000–19010. doi: 10.1074/jbc.M701096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochem. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Minet E, Zhang Z, Silver PA, Bai M. Modulation of interprotomer relationships is important for activation of dimeric calcium-sensing receptor. J Biol Chem. 2004;279:14147–14156. doi: 10.1074/jbc.M307422200. [DOI] [PubMed] [Google Scholar]
- Jiang YF, Zhang Z, Kifor O, Lane CR, Quinn SJ, Bai M. Protein kinase C (PKC) phosphorylation of the Ca2+o-sensing receptor (CaR) modulates functional interaction of G proteins with the CaR cytoplasmic tail. J Biol Chem. 2002;277:50543–50549. doi: 10.1074/jbc.M205798200. [DOI] [PubMed] [Google Scholar]
- Karnik SS, Gogonea C, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol Metab. 2003;14:431–437. doi: 10.1016/j.tem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Ruat M, Dauban P, Dodd R. N(2)-benzyl-N(1)-(1-(1-naphthyl)ethyl)-3-phenylpropane-1,2-diamines and conformationally restrained indole analogues: development of calindol as a new calcimimetic acting at the calcium sensing receptor. Bioorg Med Chem Lett. 2004a;14:3345–3349. doi: 10.1016/j.bmcl.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Kessler A, Faure H, Roussanne M, Ferry S, Ruat M, Dauban P, et al. N(1)-Arylsulfonyl-N(2)-(1-(1-naphthyl)ethyl)-1,2-diaminocyclohexanes: a new class of calcilytic agents acting at the calcium-sensing receptor. Chembiochem. 2004b;5:1131–1136. doi: 10.1002/cbic.200400049. [DOI] [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Rognan D, Césario M, Ruat M, et al. N1-Benzoyl-N2-[1-(1-naphthyl)ethyl]-trans-1,2-diaminocyclohexanes: development of 4-chlorophenylcarboxamide (calhex 231) as a new calcium sensing receptor ligand demonstrating potent calcilytic activity. J Med Chem. 2006;49:5119–5128. doi: 10.1021/jm051233+. [DOI] [PubMed] [Google Scholar]
- Khan A, Grey A, Shoback D. Medical management of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:373–381. doi: 10.1210/jc.2008-1762. [DOI] [PubMed] [Google Scholar]
- Kifor O, Diaz R, Butters R, Kifor I, Brown EM. The calcium-sensing receptor is localized in caveolin-rich plasma membrane domains of bovine parathyroid cells. J Biol Chem. 1998;273:21708–21713. doi: 10.1074/jbc.273.34.21708. [DOI] [PubMed] [Google Scholar]
- Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Lee H, Mun H-C, Lewis NC, Crouch MF, Culverston EL, Mason RS, et al. Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation. Biochem J. 2007;404:141–149. doi: 10.1042/BJ20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Frenzel R, Paschke R, Breitwieser GE, Miedlich SU. Functional desensitization of the extracellular calcium-sensing receptor is regulated via distinct mechanisms: role of G protein-coupled receptor kinases, protein kinase C and beta-arrestins. Endocrinology. 2007;148:2398–2404. doi: 10.1210/en.2006-1035. [DOI] [PubMed] [Google Scholar]
- Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlich SU, Gama L, Seuwen K, Wolf RM, Breitwieser GE. Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J Biol Chem. 2004;279:7254–7263. doi: 10.1074/jbc.M307191200. [DOI] [PubMed] [Google Scholar]
- Mun H, Culverston E, Franks A, Collyer C, Clifton-Bligh R, Conigrave A. A double mutation in the extracellular Ca2+-sensing receptor's Venus fly trap domain that selectively disables L-amino acid sensing. J Biol Chem. 2005;280:29067–29072. doi: 10.1074/jbc.M500002200. [DOI] [PubMed] [Google Scholar]
- Mun H-C, Brennan SC, Delbridge L, Wilkinson M, Brown EM, Conigrave AD. Adenomatous human parathyroid cells exhibit impaired sensitivity to L-amino acids. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2714. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun HC, Franks AH, Culverston EL, Krapcho K, Nemeth EF, Conigrave AD. The Venus Fly Trap domain of the extracellular Ca2+-sensing receptor is required for L-amino acid sensing. J Biol Chem. 2004;279:51739–51744. doi: 10.1074/jbc.M406164/200. [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano N. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacol Ther. 2006;109:339–365. doi: 10.1016/j.pharmthera.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. The search for calcium receptor antagonists (calcilytics) J Mol Endocrinol. 2002;29:15–21. doi: 10.1677/jme.0.0290015. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Nemeth EF. Misconceptions about calcimimetics. Ann N Y Acad Sci. 2006;1068:471–476. doi: 10.1196/annals.1346.044. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Heaton W, Miller M, Fox J, Balandrin M, Van Wagenen B, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, van Wagenen BC, Delmar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth EF, Delmar EG, Heaton WL, Miller MA, Lambert LD, Conklin RL, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Therap. 2001;299:323–331. [PubMed] [Google Scholar]
- O'Hara PJ, Sheppard PO, Thogersen H, Venezia D, Haldeman BA, McGrane V, et al. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:135–141. doi: 10.1210/jc.2004-0842. [DOI] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Dauban P, Dodd R, Rognan D, Ruat M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem. 2004;279:18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, et al. Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol. 2005;19:1078–1087. doi: 10.1210/me.2004-0450. [DOI] [PubMed] [Google Scholar]
- Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucleotide exchange factor. Endocrinology. 2002;143:3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CaSR mutants retained intracellularly. Hum Mol Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nature Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Kniazeff J, Goudet C, Bessis A, Liu S, Galvez J, et al. The activation mechanism of class-C G-protein coupled receptors. Biol Cell. 2004;96:335–342. doi: 10.1016/j.biolcel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Bai M, Brown EM. pH sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Kifor O, Trivedi S, Diaz R, Vassilev P, Brown EM. Sodium and ionic strength sensing by the calcium receptor. J Biol Chem. 1998;273:19579–19586. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, et al. The Ca2+-sensing receptor: a target for polyamines. American J Physiology. 1997;273(4):C1315–C1323. doi: 10.1152/ajpcell.1997.273.4.C1315. Pt 1. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Ray K, Northup J. Evidence for distinct cation and calcimimetic compound (NPS 568) recognition domains in the transmembrane regions of the human Ca2+ receptor. J Biol Chem. 2002;277:18908–18913. doi: 10.1074/jbc.M202113200. [DOI] [PubMed] [Google Scholar]
- Ray K, Fan GF, Goldsmith PK, Spiegel AM. The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J Biol Chem. 1997;272:31355–31361. doi: 10.1074/jbc.272.50.31355. [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization. Implications for function of monomeric Ca(2+) receptor. J Biol Chem. 1999;274:27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- Ray K, Adipietro KA, Chen C, Northup JK. Elucidation of the role of peptide linker in calcium-sensing receptor activation process. J Biol Chem. 2007;282:5310–5317. doi: 10.1074/jbc.M609610200. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- Reyes-Ibarra AP, García-Regalado A, Ramírez-Range I, Esparza-Silva AL, Valadez-Sánchez M, Vázquez-Prado J, et al. Calcium-sensing receptor endocytosis links extracellular calcium signaling to parathyroid hormone-related peptide secretion via a Rab11a-dependent and AMSH-sensitive mechanism. Mol Endocrinol. 2007;21:1394–1407. doi: 10.1210/me.2006-0523. [DOI] [PubMed] [Google Scholar]
- Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, et al. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflugers Arch. 2000;441:379–387. doi: 10.1007/s004240000436. [DOI] [PubMed] [Google Scholar]
- Rogers KV, Dunn CK, Hebert SC, Brown EM. Localization of calcium receptor mRNA in the adult rat central nervous system by in situ hybridization. Brain Res. 1997;744:47–56. doi: 10.1016/s0006-8993(96)01070-0. [DOI] [PubMed] [Google Scholar]
- Rondard P, Liu J, Huang S, Malhaire F, Vol C, Pinault A, et al. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J Biol Chem. 2006;281:24653–24661. doi: 10.1074/jbc.M602277200. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten MJ, Bacon KD, Marlink KL, Stoney M, Meichsner CL, Lee FP, et al. Identification of a functional Ca2+-sensing receptor in normal human gastric mucous epithelial cells. Am J Physiol. 1999;277(3):G662–670. doi: 10.1152/ajpgi.1999.277.3.G662. Pt 1. [DOI] [PubMed] [Google Scholar]
- Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, et al. Complexing receptor pharmacology: modulation of family B G protein-coupled receptor function by RAMPs. Ann N Y Acad Sci. 2006;1070:90–104. doi: 10.1196/annals.1317.076. [DOI] [PubMed] [Google Scholar]
- Shoback DM, Bilezikian JP, Turner SA, McCary LC, Guo MD, Peacock M. The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003;88:5644–5649. doi: 10.1210/jc.2002-021597. [DOI] [PubMed] [Google Scholar]
- Silve C, Petrel C, Leroy C, Bruel H, Mallet E, Rognan D, et al. Delineating a Ca2+ binding pocket within the venus flytrap module of the human calcium-sensing receptor. J Biol Chem. 2005;280:37917–37923. doi: 10.1074/jbc.M506263200. [DOI] [PubMed] [Google Scholar]
- Tabata T, Aiba A, Kano M. Extracellular calcium controls the dynamic range of neuronal metabotropic glutamate receptor responses. Mol Cell Neurosci. 2002;20:56–68. doi: 10.1006/mcne.2002.1118. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kano M. Calcium dependence of native metabotropic glutamate receptor signaling in central neurons. Mol Neurobiol. 2004;29:261–270. doi: 10.1385/MN:29:3:261. [DOI] [PubMed] [Google Scholar]
- Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:275–282. doi: 10.1016/j.ceca.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc Natl Acad Sci USA. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Ward DT, Riccardi D. Renal physiology of the extracellular calcium-sensing receptor. Pflugers Arch. 2002;445:169–176. doi: 10.1007/s00424-002-0914-x. [DOI] [PubMed] [Google Scholar]
- Ward DT, McLarnon SJ, Riccardi D. Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J Am Soc Nephrol. 2002;13:1481–1489. doi: 10.1097/01.asn.0000015623.73739.b8. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, et al. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+-sensing receptor. Mol Endocrinol. 2007;21:274–280. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- Wüthrich R, Martin D, Bilezikian J. The role of calcimimetics in the treatment of hyperparathyroidism. Eur J Clin Invest. 2007;37:915–922. doi: 10.1111/j.1365-2362.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- Yano S, Brown EM, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35:257–264. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ye C, Ho-Pao CL, Kanazirska M, Quinn SJ, Rogers K, Seidman CE, et al. Amyloid-beta proteins activate Ca2+-permeable channels through calcium-sensing receptors. J Neurosci Res. 1997;47:547–554. doi: 10.1002/(sici)1097-4547(19970301)47:5<547::aid-jnr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Am J Physiol Cell Physiol. 2002;282:C1414–C1422. doi: 10.1152/ajpcell.00432.2001. [DOI] [PubMed] [Google Scholar]
- Zhang M, Breitwieser GE. High affinity interaction with filamin A protects against calcium-sensing receptor degradation. J Biol Chem. 2005;280:11140–11146. doi: 10.1074/jbc.M412242200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jiang Y, Quinn SJ, Krapcho K, Nemeth EF, Bai M. L-Phenylalanine and NPS R-467 synergistically potentiate the function of the extracellular calcium-sensing receptor through distinct sites. J Biol Chem. 2002a;277:33736–33741. doi: 10.1074/jbc.M200978200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Qiu W, Quinn SJ, Conigrave AD, Brown EM, Bai M. Three adjacent serines in the extracellular domains of the CaR are required for L-amino acid-mediated potentiation of receptor function. J Biol Chem. 2002b;277:33727–33735. doi: 10.1074/jbc.M200976200. [DOI] [PubMed] [Google Scholar]


