Abstract
Background and purpose:
Mucopolysaccharidoses (MPS) are lysosomal storage disorders resulting from a deficit of specific lysosomal enzymes catalysing glycosaminoglycan (GAG) degradation. The typical pathology involves most of the organ systems, including the brain, in its severe forms. The soy isoflavone genistein has recently attracted considerable attention as it can reduce GAG synthesis in vitro. Furthermore, genistein is able to cross the blood–brain barrier in the rat. The present study was undertaken to assess the ability of genistein to reduce urinary and tissue GAG levels in vivo.
Experimental approach:
We used mice with genetic deletion of iduronate-2-sulphatase (one of the GAG catabolizing enzymes) which provide a model of MPS type II. Two doses of genistein, 5 or 25 mg·kg−1·day−1, were given, in the diet for 10 or 20 weeks. Urinary and tissue GAG content was evaluated by biochemical and histochemical procedures.
Key results:
Urinary GAG levels were reduced after 10 weeks' treatment with genistein at either 5 or 25 mg·kg−1·day−1. In tissue samples from liver, spleen, kidney and heart, a reduction in GAG content was observed with both dosages, after 10 weeks' treatment. Decreased GAG deposits in brain were observed after genistein treatment in some animals.
Conclusions and implications:
There was decreased GAG storage in the MPSII mouse model following genistein administration. Our results would support the use of this plant-derived isoflavone in a combined therapeutic protocol for treatment of MPS.
Keywords: mucopolysaccharidosis, Hunter syndrome, MPSII mouse model, genistein, glycosaminoglycan
Introduction
Mucopolysaccharidoses (MPS) are lysosomal storage disorders due to the deficit of the enzymes involved in the catabolism of the glycosaminoglycans (GAG) dermatan, heparan, keratan and chondroitin sulphates. Patients with MPS cannot degrade one or more of these mucopolysaccharides, which accumulate inside lysosomes of many tissues, causing a progressive multi-systemic deterioration.
Clinical manifestations can vary in different MPS, as well as in distinct patients within the same disorder, and include hepato-splenomegaly, multiple dysostosis, reduced growth, recurrent infections and a chronic degenerative progression of the disease (Neufeld and Muenzer, 2001). A major CNS involvement is characteristic of the severe forms of MPSI, MPSII and MPSVII, and all forms of MPSIII. Therapies available for MPS are mainly symptomatic and, for some MPS, haemopoietic stem cells transplantation and enzyme replacement therapy (ERT) are available. Due to its limited efficacy and the associated risks of morbidity and mortality, haemopoietic stem cells transplantation is today restricted to MPSI. ERT, although improving the patient's quality of life, is expensive and limited to correction of peripheral symptoms, as no results have been obtained in subjects with a severe CNS involvement, the enzyme being unable to cross the blood–brain barrier (BBB) (Urayama et al., 2007).
A more recent therapeutic approach, such as the substrate reduction therapy (SRT) cannot be applied in its original form to MPS as, for these disorders, the competitor monomer should be a carbohydrate that would be potentially implicated in other metabolic pathways. Therefore, it would be more appropriate in this situation to develop an alternative form of SRT based on a reduction of GAG synthesis (Piotrowska et al., 2006).
Genistein, a soy isoflavone, has recently been put forward as a potential new treatment for MPS (Piotrowska et al., 2006; Piotrowska et al., 2008; Jakobkiewicz-Banecka et al., 2009). Genistein belongs to a group of plant-derived compounds (phytotherapeutics) used to treat disorders such as osteoporosis (Wang et al., 2006), cardiovascular disease (Vera et al., 2007) and menopause (D'Anna et al., 2009). Further therapeutic potential has been shown for cancer (Gu et al., 2005) and for other chronic diseases (Fanti et al., 2006).
In the context of MPS, genistein has been described to decrease the expression of genes encoding for one or more enzymes involved in GAG synthesis (Nikitovic et al., 2003), by inhibiting the tyrosine specific protein kinase activity of the epithelial growth factor (EGF) receptor (EGFR or ErbB1, ENSG00000146648) (Akiyama et al., 1987; Kim et al., 1998; nomenclature follows Alexander et al., 2008).
A protocol for the reduction of GAG synthesis in vitro, mediated by genistein, the gene expression-targeted isoflavone therapy (GET IT), has been described (Piotrowska et al., 2006; Jakobkiewicz-Banecka et al., 2009) and the same authors recently reported a pilot clinical evaluation of MPSIII patients, receiving treatment with genistein (Piotrowska et al., 2008). In addition, genistein crosses the BBB in the rat (Tsai, 2005) and in the mouse (Liu et al., 2008). As there are no preclinical in vivo studies so far to assess the efficacy of genistein on GAG reduction (GET IT protocol) in both systemic and neurological compartments, we undertook such an evaluation in the mouse model for the X-linked, inherited MPSII disease (Hunter syndrome). The effects of genistein in two different dose regimens, 5 or 25 mg·kg−1·day−1 administered for 10 or 20 weeks, were analysed.
The lower dosage, 5 mg·kg−1·day−1, equals the human daily intake used in the published pilot study (Piotrowska et al., 2008) in patients with MPSIII. The higher dose (25 mg·kg−1·day−1) was included as mice have a higher metabolic rate than humans. Our results showed that genistein decreased storage of GAG in several peripheral tissues with some signs of a reduction in brain GAG levels.
Methods
Mice
All animal care and experimental procedures were conducted according to the current national and international animal ethical guidelines. C57BL6 wild-type mice were purchased from Harlan, Italy. C57BL6 iduronate-2-sulphatase (IDS) knockout (IDS-ko) mouse, which provided the model for MPSII (Garcia et al., 2007), was kindly provided by J Muenzer (University of North Carolina, NC, USA) and expanded in our animal house. These IDS-ko mice have been previously characterized and show significantly higher urinary levels of GAG and tissue GAG storage, with respect to age-matched healthy control mice (Friso et al., 2005; Cardone et al., 2006; Garcia et al., 2007). In the present experiments, knockout and wild-type mice were housed in light and temperature controlled conditions, with food and water provided ad libitum. Experiments were performed in hemizygous affected and wild-type male mice. Treated mice, 4 to 5 months old at the beginning of the study, were fed with pellets modified (Mucedola, Milano, Italy) by adding Soyfem (Biofarm, Poznań, Poland) in order to administer the two different dosages of genistein, 5 and 25 mg·kg−1·day−1. Soyfem is a proprietary product and one tablet contains 100 mg of soy seed extract, including 26 mg of isoflavones converted to genistein. This was mixed with the food at a rate corresponding to 37.7 mg isoflavones converted to genistein per kilogram of pellet for the lower dose and to 188.5 mg isoflavones converted to genistein per kilogram of pellet, for the higher dose. The doses were calculated assuming an average body weight of 30 g per mouse and a daily consumption of modified pellet of about 4 g per mouse.
The IDS-ko animals were divided into three dose groups of 10 mice each: one group was treated with genistein, 5 mg·kg−1·day−1, the second group with 25 mg·kg−1·day−1, and the last one, the control group, was fed with unmodified pellet. Moreover, a small group of three wild-type mice were fed with genistein at 25 mg·kg−1·day−1 for up to 20 weeks only to evaluate possible oestrogenic effects of the treatment on their weight. All mice were in individual cages during the treatment period, and animals and food pellets were weighed weekly. During treatment, urine samples were collected using metabolic cages. At both time-points, five IDS-ko mice per dose and five untreated IDS-ko mice were killed. In each genistein-treated mice group, urinary GAG content was evaluated in the whole group of 10 mice before treatment and after 10 weeks' treatment, in the five remaining animals at 20 weeks' follow-up. In addition, three age-paired wild-type untreated mice were killed at each time-point as controls for tissue analysis. Biochemical and histochemical GAG analyses were performed on liver, kidney, spleen, heart and brain, tissues importantly involved in the pathology of this disorder in humans. Study design is shown schematically in Figure 1.
Figure 1.
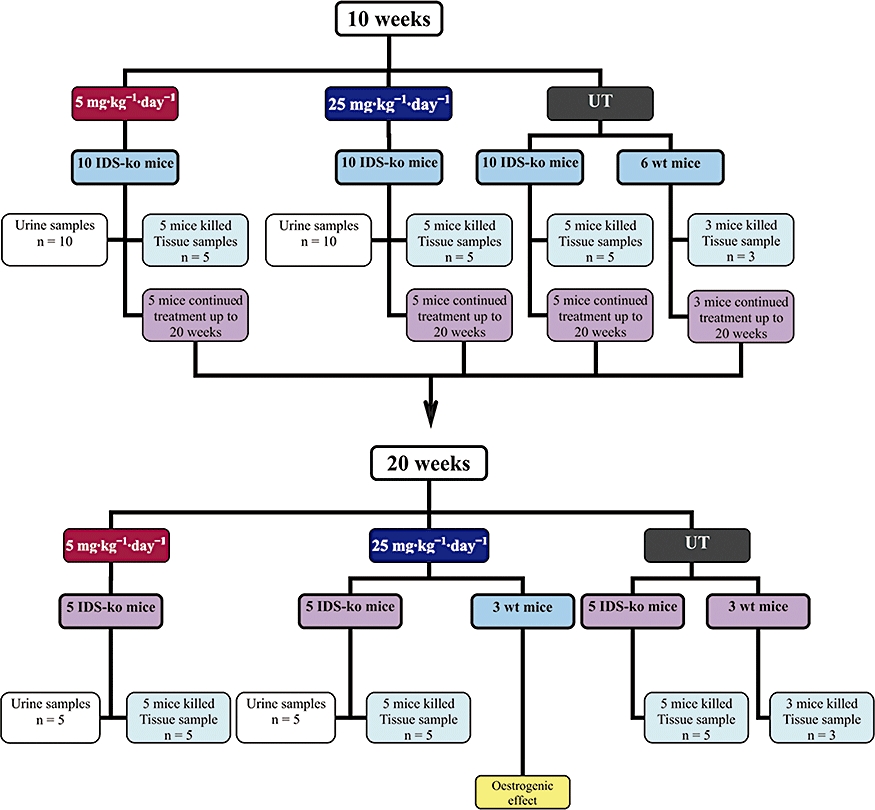
Study design. IDS, iduronate-2-sulphatase; IDS-ko, IDS knockout; UT, untreated mice; wt, wild-type.
Urinary GAG content
Urinary GAG content (calculated as µg GAG per mg creatinine) was determined using the protocol described by de Jong et al. (1992) with modifications. GAG content was determined in 24 h urine samples, diluted 1:16, 1:32, 1:64 and mixed with 8 M 1,9-dimethylmethylene blue chloride (Sigma-Aldrich, Milano, Italy), 0.05 M Na-formate (Sigma-Aldrich) and 0.18 M Tris-HCl (Merck, Milano, Italy) to pH 8.8. A standard curve was prepared using chondroitin sulphate type C (Sigma-Aldrich). Absorbance was measured at 520 nm in a multi-well reader Victor2 1420 (PerkinElmer, Milano, Italy). Urinary creatinine was measured by mixing diluted 1:5 and 1:10 urine with picric acid (Sigma-Aldrich) diluted 1:5 and 7.5 g·L−1 sodium hydroxide. Absorbance was measured at 535 nm and compared with creatinine standard solutions (Sigma-Aldrich).
Tissue GAG content
Tissue GAG content was measured by using Bjornsson's protocol (Bjornsson, 1993), with modifications previously described (Friso et al., 2008). Briefly, tissues were lyophilized and then homogenized in 0.9% NaCl + 0.2% Triton X-100. The assay was performed by diluting 50 µL of blank (water), calibrators (chondroitin sulphate C) or samples with an equal volume of 8 M guanidine HCl, and then 0.3% H2SO4/0.75% Triton X-100. Samples were incubated o/n at 4°C in the presence of Alcian Blue solution. Following centrifugation, pellets were washed with 40% DMSO/0.05% MgCl2 and resuspended in 4 M guanidine HCl/33% isopropanol/0.25% Triton X-100. All reagents were purchased from Sigma-Aldrich. Absorbance was measured at 620 nm. GAG content was calculated as µg·mg−1 protein, and protein content was determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Milano, Italy).
Histochemical analysis
Mice were killed and tissues rapidly dissected and fixed for 48 h in Bouin's solution. Thereafter, tissues were extensively washed in 70% ethyl alcohol, dehydrated and paraffin embedded. Sections (7 µm) were cut, de-paraffined and stained with 1% Alcian Blue (Sigma-Aldrich) solution pH 1.0; 0.1% Nuclear Fast Red (Sigma-Aldrich) was used as counterstain. All sections for the same organ obtained from wild-type, untreated IDS-ko and treated IDS-ko mice were stained at the same time. Sections were assessed without knowledge of the treatments.
Statistical analyses
Results reported in Figure 2 are single animal values, each representing the average of four separate assays performed in duplicate. In the same figure, mean values for each group of samples are also shown. In Figures 3 and 5, data are expressed as mean ± SD.
Figure 2.
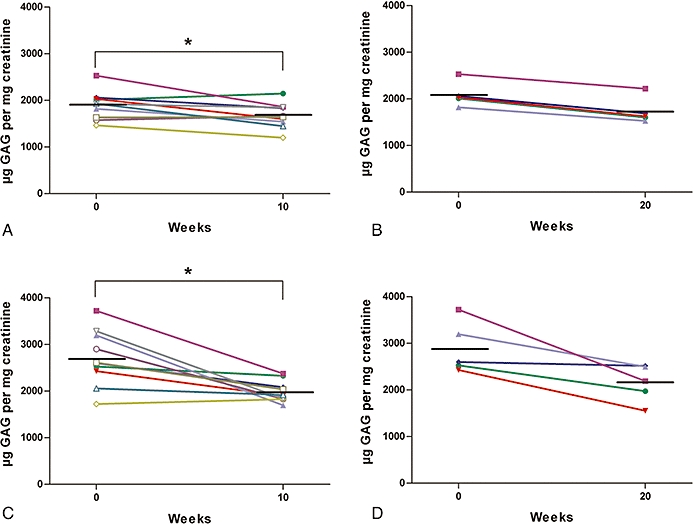
Urinary glycosaminoglycan (GAG) levels in genistein-treated iduronate-2-sulphatase knockout (IDS-ko) mice. (A) 5 mg·kg−1·day−1, 10 weeks' treatment, n = 10; (B) 5 mg·kg−1·day−1, 20 weeks' treatment, n = 5; (C) 25 mg·kg−1·day−1, 10 weeks' treatment, n = 10; (D) 25 mg·kg−1·day−1, 20 weeks' treatment, n = 5. Graphs represent single animal data measured at baseline level (0) and at the end of the follow-up (10 or 20 weeks). Each value represents the average of four separate assays, performed in duplicate, on three different dilutions. Short black horizontal lines show the mean value at each time-point. Urinary GAG levels were significantly decreased in mice treated with either 5 or 25 mg·kg−1·day−1 for 10 weeks (P < 0.05; P < 0.005, respectively, indicated as*; Wilcoxon's signed rank), compared with baseline levels.
Figure 3.
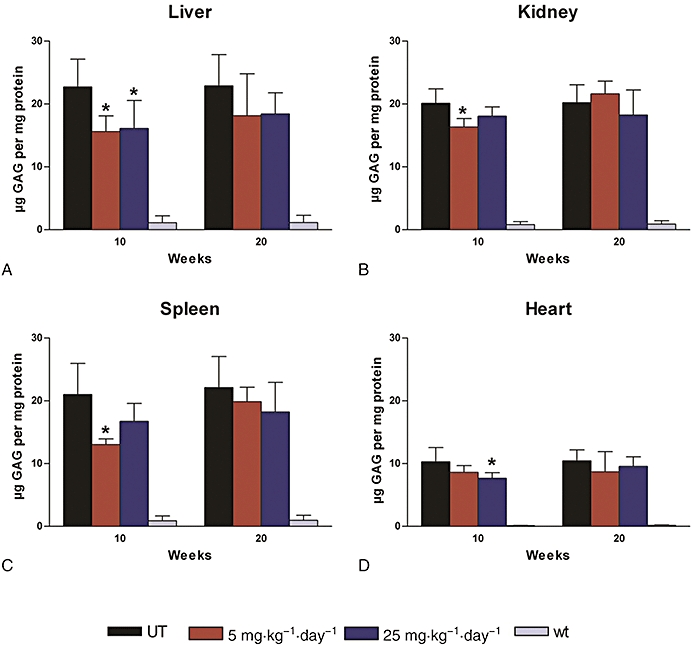
Tissue levels of glycosaminoglycan (GAG) measured biochemically in genistein-treated iduronate-2-sulphatase knockout (IDS-ko) mice. n = 5 for untreated (UT) IDS-ko mice, 5 mg·kg−1·day−1 treated IDS-ko mice, 25 mg·kg−1·day−1 treated IDS-ko mice; n = 3 for untreated wild-type (wt) mice. Values, presented as mean ± SD, resulted significantly decreased, compared with untreated IDS-ko animals (UT), in liver (P < 0.01), kidney (P < 0.01) and spleen (P < 0.005) of IDS-ko mice treated with 5 mg·kg−1·day−1 for 10 weeks, in liver (P < 0.05) and heart (P < 0.05) of IDS-ko mice treated with 25 mg·kg−1·day−1 for 10 weeks. *Statistically significant decrease compared with UT.
Figure 5.
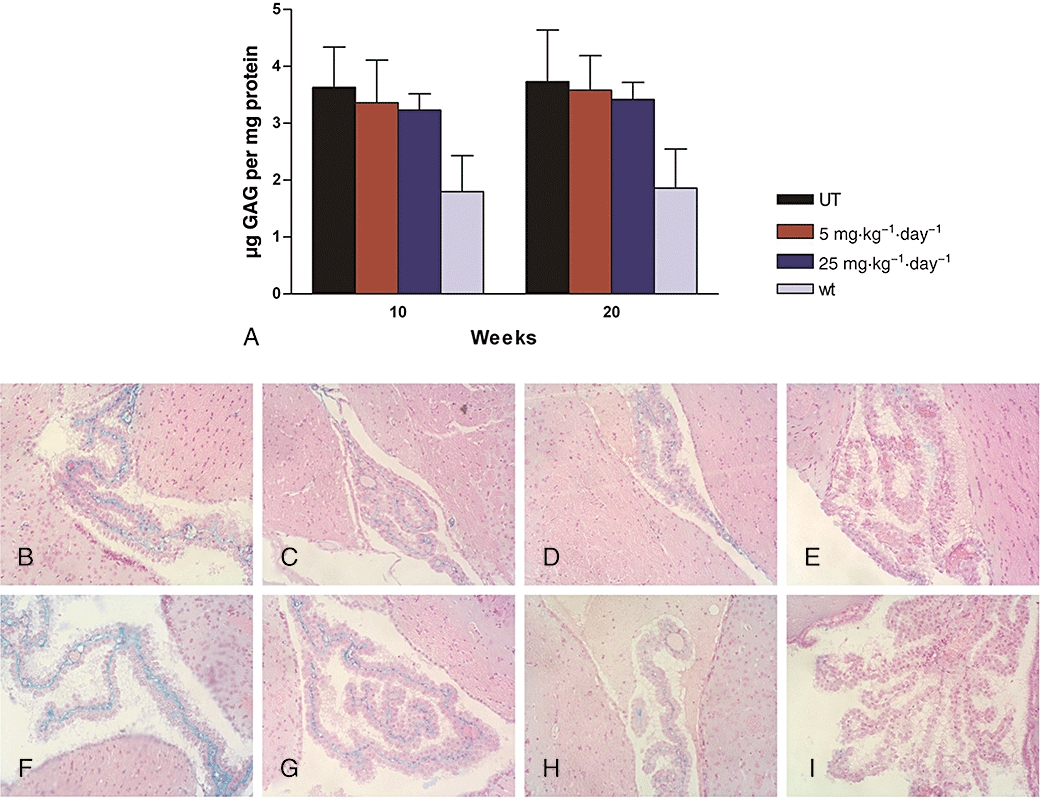
Analysis of glycosaminoglycan (GAG) in brains of genistein-treated iduronate-2-sulphatase knockout (IDS-ko) mice. (A) biochemical analysis: 5 and 25 mg·kg−1·day−1 at 10 and 20 weeks' treatment; n = 5 for untreated (UT) IDS-ko mice, 5 mg·kg−1·day−1 treated IDS-ko mice, 25 mg·kg−1·day−1 treated IDS-ko mice; n = 3 for untreated wild-type (wt) mice, values represent mean ± SD; (B–I) Histochemical analysis performed after 10 and 20 weeks' treatment. Representative sections of 10 (B–E) and 20 (F–I) weeks' treatment include samples from untreated IDS-ko mice (B,F), 5 mg·kg−1·day−1 genistein-treated IDS-ko mice (C,G), 25 mg·kg−1·day−1 genistein-treated IDS-ko mice (D,H) and wild-type mice (E,I). Arrows indicate GAG deposits. Sections (7 µm) were stained with 1% Alcian Blue, counterstained with 0.1% Nuclear Fast Red. Original magnification: ×200.
Statistical evaluation was performed by using Wilcoxon signed rank test for Figure 2 and Student's t-test for the data in Figures 3 and 5. Differences were considered statistically significant when P < 0.05.
Results
Animal weights and genistein intake
No weight difference was observed at any time in the different groups analysed (data not shown). Measurement of food intake confirmed that the animals were receiving the correct genistein dosages (data not shown).
Effects of genistein on urinary GAG
Figure 2 shows urinary GAG content in each animal before treatment (0) and at the end of the treatment (10 or 20 weeks).
After 10 weeks' treatment, there was for six out of 10 mice fed genistein at 5 mg·kg−1·day−1, a decrease of urinary GAG, of up to 26% (Figure 2A). A more consistent reduction, that is, in all 10 animals, of up to 20%, was observed at 20 weeks (Figure 2B). With the higher dose of genistein (25 mg·kg−1·day−1), following 10 weeks of treatment, urinary GAG content was lower in nine out of 10 IDS-ko mice, reaching 37–47% decrease in four animals (Figure 2C). After 20 weeks (Figure 2D), all animals showed a reduced GAG content, up to 36% and 41% in two animals.
On average, the decrease with respect to the starting level was 11% and 24% for mice fed 5 and 25 mg·kg−1·day−1 following 10 weeks of treatment respectively (P < 0.05 and P < 0.005). At 20 weeks, the average decrease in urinary GAG was 17% and 25% for the animals treated with 5 and 25 mg·kg−1·day−1, respectively, and these changes did not reach statistical significance.
Biochemical analyses of GAG deposits in visceral organs after treatment
Data obtained from the biochemical evaluation of tissue GAG levels in mice treated with genistein in their diet for 10 or 20 weeks are summarized in Figure 3.
Overall there was no dose-related effect and the magnitude of the reductions varied between tissues and between animals. There was a decrease of 48% in the GAG content of liver in one animal treated with the lower dose and of 55% with the higher dose. Reductions of 27% in the kidney of one animal following administration of 5 mg·kg−1·day−1, of about 40% in the spleen of three animals and of 39% in one mouse for the two doses respectively, a decrease of 32% in the heart of one animal fed the lowest dose and of 37% in one mouse treated with the higher dose were also found.
After 20 weeks' treatment, a similarly wide variation in results was observed for either dose. In the liver, with the lower dose three animals reached a decrease of 44%, 37% and 27%, while two animals treated with the higher dose showed 37% and 22% reductions in GAG content. No improvement was detected in the kidney (Figure 3B) of mice fed 5 mg·kg−1·day−1 for 20 weeks, and no significant decrease was observed in the same tissue following administration of 25 mg·kg−1·day−1. In the spleen one animal fed 25 mg·kg−1·day−1 showed a 50% decrease; in the heart, one animal fed 5 mg·kg−1·day−1 showed a 54% reduction.
Statistical analysis of the group means showed that the effects of genistein at either high or low dose were not significant in any tissue after 20 weeks' treatment, relative to those in the untreated IDS-ko mice. However, after 10 weeks' treatment, there were significant reductions in all sampled tissues (Figure 3), for one or both dose regimens.
Histochemical analysis of GAG deposits in visceral organs after treatment
The results of histochemical analysis of liver, kidney, spleen and heart, performed after 10 weeks of treatment is shown in Figure 4. Animals treated with the two different doses of genistein were compared with age-matched untreated IDS-ko and wild-type mice.
Figure 4.
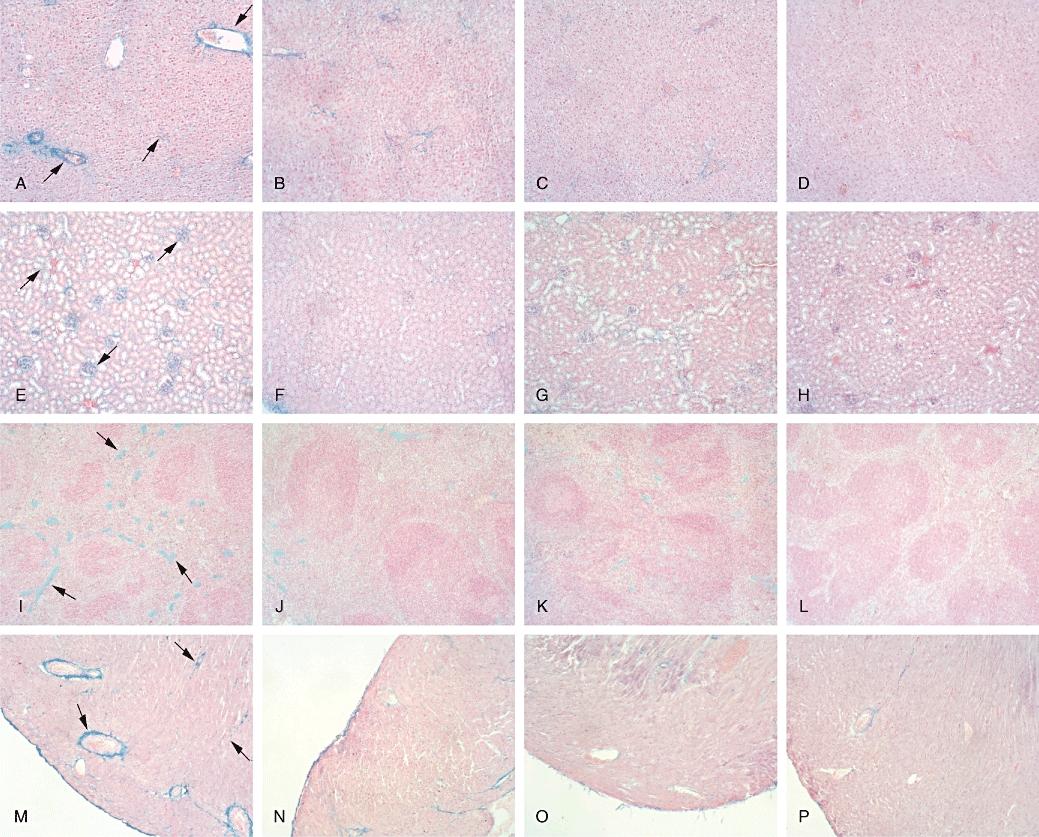
Histochemical analysis of glycosaminoglycan (GAG) in liver, kidney, spleen and heart performed after 10 weeks' treatment. Representative sections of liver (A–D), kidney (E–H), spleen (I–L) and heart (M–P) include samples from untreated iduronate-2-sulphatase knockout (IDS-ko) mice (A, E, I and M), 5 mg·kg−1·day−1 genistein-treated IDS-ko mice (B, F, J and N), 25 mg·kg−1·day−1 genistein-treated IDS-ko mice (C, G, K and O) and untreated wild-type mice (D, H, L and P). Arrows indicate GAG deposits. Sections (7 µm) were stained with 1% Alcian Blue, counterstained with 0.1% Nuclear Fast Red. Original magnification: ×100.
In the liver of untreated IDS-ko animals, accumulation of undegraded GAG was routinely observed around the vessels and in the extracellular matrix of hepatocytes (Figure 4A). Some hepatic lobules showed a vacuolated structure. In the same tissue from healthy controls, a light blue staining was observed (Figure 4D). In the genistein-treated animals (Figures 4B,C), histopathological signs were remarkably improved. Improvements of tissue architecture could be observed both along the hepatic sinusoids and in the parenchyma where the vacuolated structure was less evident.
In the kidney from IDS-ko animals, blue staining was associated with glomerular structures and parenchyma (Figure 4E). An important reduction of GAG storage was observed in the treated animals (Figure 4F,G) with respect to the untreated mice, both in the glomerular and in the parenchymal cells, although some deposits still persisted.
In the spleen of the untreated mouse model, GAG accumulation was mainly associated with connective tissue between the lymphoid follicles and to the vessels (Figure 4I). No storage was observed in the healthy control mice (Figure 4L). Treatment with the two doses of genistein led to a considerable reduction of GAG in the 10 weeks-treated IDS-ko animals (Figure 4J,K), although some storage was still visible in the parenchyma.
Heart sections are shown in Figure 4M–P. In the untreated IDS-ko mice, GAG deposits were laid along the muscle fibres and the vascular endothelium (Figure 4M). In the healthy animals (Figure 4P), a very light blue staining was visible in some areas. Sections obtained after treatment with genistein (5 and 25 mg·kg−1·day−1) showed a considerable reduction of storage in the endothelium and in the extracellular matrix (Figure 4N,O).
Histochemical evaluation performed in the 20 weeks-treated IDS-ko mice showed similar results (data not shown).
Biochemical and histochemical brain analysis
The neurological involvement of the severe form of Hunter syndrome mainly comprises mental retardation and anatomical changes leading to hydrocephalus (Neufeld and Muenzer, 2001). Neuropathological defects were described for the MPSII mouse model as cellular vacuolization in different brain regions (Cardone et al., 2006) and as neuronal necrosis in the brainstem and spinal cord (Garcia et al., 2007). As previously described for the mouse model of MPSIII B (Fu et al., 2007), the animal model for MPSII showed lower accumulation of GAG in brain, compared with other tissues when biochemical analysis was performed. A twofold increase in GAG levels compared with wild-type mice was previously described by Garcia et al. (2007) and confirmed by our current results (Figure 5). However, this increase was variable throughout the groups, and there was no statistically significant difference between the IDS-ko and the wild-type groups of mice. Treatment of the IDS-ko mice with genistein for 10 or 20 weeks at either dose did not induce significant changes in the levels of GAG in brain (Figure 5A), using biochemical analysis. At 10 weeks we observed with both doses, a slight GAG reduction in all the animals except two. On average, in the 5 and 25 mg·kg−1·day−1 dose groups, GAG level was reduced by 6% and 10% respectively compared with basal level. Following 20 weeks' treatment a slight change was detected on the mean GAG content in the brain of mice fed 5 mg·kg−1·day−1, and in two animals we measured a reduction of 10% and 20% respectively. Following administration of 25 mg·kg−1·day−1 for 20 weeks we detected an overall 5% decrease, with two animals showing a GAG reduction of 7% and 18%.
Histochemical analysis of brain GAG content is presented in Figures 5B–I, which report for each genistein dosage and each time, stained sections from one animal of each group. As the major area of GAG accumulation in brain is within the choroid plexus in the ventricular regions (Cardone et al., 2006), we show sections of this area only. Figure 5B and F show the untreated IDS-ko mice while Figure 5E and I show the wild-type healthy controls. In the IDS-ko mice treated with genistein, a similar reduction of GAG staining was visible at both time-points and doses, clearly involving the endothelial cells of the plexus (Figure 5C,D and G,H for 10 and 20 weeks' treatment respectively). These sections were from treated animals that had shown, biochemically, a reduction of GAG levels in whole brain extracts.
Discussion
At present the available therapeutic options for MPS are mainly based on the administration of recombinant enzymes (ERT). However, ERT is extremely expensive, approximately 400 000 euros·year−1 for a 30 kg patient. Furthermore, the neurological involvement, which is a component of most forms of MPS, is unlikely to benefit from ERT. In fact, although positive results have been reported following ERT in neonates (Urayama et al., 2004; 2008;) or using doses far higher than those conventionally used in clinical treatment (Vogler et al., 2005; Tomatsu et al., 2007), with the current dosages and formulation, diffusion of the proteins involved in ERT across the BBB is almost non-existent (Boado et al., 2008). All these problems deserve a solution and would suggest the use of modified enzymes or of very different molecules.
During the last few years, there has been much interest in the use of the phytoestrogen, genistein, as a possible treatment for MPS. For many more years, we have known that GAG synthesis requires growth factors such as EGF and follicle stimulating hormone (Pisano and Greene, 1987; Tirone et al., 1997). By binding to its receptor, EGF induces the activation of its receptor protein kinase activity, which in turn modulates expression of several genes including some involved in GAG synthesis. Such activity of the EGF receptor is inhibited by genistein (Akiyama et al., 1987; Kim et al., 1998; Nikitovic et al., 2003) which, thus, might inhibit GAG synthesis. Genistein could therefore be considered as a new form of SRT, based on inhibition of GAG synthesis and thus be applied in therapeutic protocols to MPS. A similar approach was previously evaluated (Roberts et al., 2006; Roberts et al., 2007) by using rhodamine B in the mouse model for MPSIIIA, leading to a decrease of GAG content in liver and brain. However, rhodamine B may cause mucous membrane and skin irritation in humans, and its long-term adverse effects are unknown (Dire and Wilkinson, 1987). As for genistein, possible problems related to its long-term administration to children could relate to its phytoestrogenic activity, potentially influencing puberty and fertility (Piotrowska et al., 2006). However, studies of genistein toxicity in vivo showed no adverse effects in dogs up to 500 mg·kg−1·day−1 (McClain et al., 2005). Administration in humans of doses of genistein up to 600 mg·day−1 (8 mg·kg−1·day−1) for about 3 months resulted in minor side effects related to its oestrogenic activity (Fischer et al., 2004), while cytogenetic analysis in peripheral blood lymphocytes, obtained from subjects under treatment for prostate cancer, showed no increase in DNA strand breaks (Myltic et al., 2003). Genistein is much less expensive than ERT, and it can be taken orally. In addition, this molecule can cross the BBB in rats and mice (Tsai, 2005; Liu et al., 2008) and GAG reduction in vitro mediated by genistein was recently described (Piotrowska et al., 2006; Jakobkiewicz-Banecka et al., 2009).
Therefore, genistein could help in solving the present limitations of ERT, as well as providing hope for those forms of MPS for which ERT is not yet available. Because of these potential benefits to MPS patients and the community in general, genistein merits a full evaluation in terms of efficacy. Although a pilot clinical trial on MPSIII patients was recently published (Piotrowska et al., 2008), here we present the first preclinical study aimed at a complete analysis of the efficacy of genistein treatment for MPS.
For this, we used the mouse model for Hunter syndrome, the IDS-ko mouse (Garcia et al., 2007), treated with 5 and 25 mg·kg−1·day−1 of genistein in the diet for 10 or 20 weeks. Because of the earlier toxicity evaluations (McClain et al., 2005; McClain et al., 2006), no adverse effects were expected following in vivo administration of such doses. As genistein is a phytoestrogen, we monitored weekly the weight gain of the animals but we did not observe any differences between genistein-fed mice and matched controls.
As for the measurements of GAG, a considerable inter-individual variability was recorded in our experiments and so our results on urinary GAG have been presented as single data analysis. Although urinary levels of GAG were never increased by genistein treatment, the overall reduction of urinary GAG was significant only after 10 weeks' treatment with either dose (5 or 25 mg·kg−1·day−1). However a general decrease of urinary GAG levels was also observed after 20 weeks' treatment, which although not significant, suggested that GAG synthesis was decreased by the treatment. A significant reduction of urinary heparan sulphate levels was recently reported in the pilot study performed in MPSIII patients (Piotrowska et al., 2008).
In the peripheral tissues, biochemical and histochemical analyses showed an overall small decrease after 10 weeks, which was lost after 20 weeks, of genistein-supplemented diet. Different effects on GAG deposits were observed in the peripheral tissues analysed. A decrease was quite consistently measured in liver, spleen and heart, while efficacy of the treatment was less pronounced in kidney. This may be due to the fact that a decrease of GAG content may be more easily detected in tissues, like liver and spleen, which show a high GAG content in the untreated, IDS-ko animals. The same organs are also primarily affected in the human disorder. Also, expression of EGF receptors in the liver is very high compared with other cell types (Berasain et al., 2009), and this may favour genistein efficacy in hepatic tissue.
Beneficial effects of genistein in the CNS of IDS–ko mice were less easily demonstrable as the BBB hinders access to the brain tissue. Although the BBB is more permeable to genistein than to enzyme proteins, previous experiments performed in rats showed that only limited amounts of genistein can reach this compartment (Tsai, 2005). Besides, in the MPSII mouse model we have used here, GAG content of the CNS is low and not widely or uniformly distributed over the brain. Therefore, evaluation of GAG content may be performed more appropriately by histochemical analysis of specific brain sections, particularly those containing the choroid plexus in the ventricular regions, than by biochemical assays of extracts of whole brain. This is probably why we did not find decreased GAG levels in whole brain using biochemical analysis, but were able to show, by histochemistry, a reduction of GAG deposits around the choroid plexus at both doses.
In general, our data on the effects of genistein on pre-existing GAG deposits could be explained by the physiological turnover of GAG in tissues, which was not replenished by new GAG synthesis. The loss of effect of the treatment observed at 20 weeks' versus 10 weeks' exposure might reflect interactions of genistein with EGF receptors. Uninterrupted administration of genistein might cause a progressive saturation of EGF receptors on the cell surface, thus rendering them unavailable for other physiological ligands, which in turn might cause an up-regulation of EGF receptor production. Penar et al. (1997) showed that increasing dosages of genistein caused a proportional increase of EGF receptors, due to a strong decrease of tyrosine kinase activity. Thus the prolonged exposure to genistein might have induced an increased number of EGF receptors, which would overcome the inhibition. Such increased production of EGF receptors could be considered a cytoprotective mechanism, maintaining the cell's ability to respond to the physiological ligands. In this scenario, GAG synthases might also be reactivated, and the inhibitory effect of genistein might be overcome by their restoration. Therefore, we would suggest that interrupted dosing with genistein rather than continuous treatment might provide greater clinical benefit.
In conclusion, this in vivo preclinical evaluation demonstrated for the first time that genistein administration can reduce GAG content of urine and of several peripheral tissues in the animal model for Hunter syndrome. The histochemical evidence for a reduction in GAG levels in and around the choroid plexus would suggest that genistein can indeed cross the BBB in this model and exert beneficial effects in the brain. As the higher dose of 25 mg·kg−1·day−1 of genistein did not show an increased effect compared with 5 mg·kg−1·day−1, in peripheral tissues, probably due to the saturation of the EGF receptors, we would suggest using the lower dose in patients. Moreover, at low doses, possible side effects, related to the oestrogenic activity of the molecule, as well as potential cytotoxic effects due to its interference with signal transduction processes, depending on the tyrosine kinase activity of EGF receptors, should be limited (Piotrowska et al., 2006). Also, mutagenic activities of the molecule, which have been described in in vitro, could not be reproduced in vivo following administration of genistein equal to 8 mg·kg−1·day−1 for about 3 months (Myltic et al., 2003).
Genistein might be used in a combined therapy for those forms of MPS for which ERT is already available, where it might be valuable to target difficult to treat tissues such as brain and heart. In MPSIII, which presents with greater neurological involvement and thus is less likely to benefit from ERT, we believe that genistein could be used to improve patient compliance by integrating present symptomatic therapies. Finally, our results should encourage the synthesis of new molecules, based on genistein and with greater potency, as inhibitors of GAG synthesis and better able to cross the BBB.
Acknowledgments
The authors wish to thank the Italian MPS Association and the University of Padova (Ricerca Scientifica fondi quota ex-60%, year 2008 (grant no. 60A07-2137/08) and 2009 (grant no. 60A07-9239/09) for partly funding the study. We also wish to acknowledge Professor Grzegorz Węgrzyn (University of Gdansk, Poland) for scientific advices and Dr Francesca D'Avanzo for technical assistance and helpful reading of the manuscript.
Glossary
Abbreviations:
- BBB
blood–brain barrier
- EGF
epithelial growth factor
- EGFR
epithelial growth factor receptor
- ERT
enzyme replacement therapy
- GAG
glycosaminoglycan(s)
- IDS
iduronate-2-sulphatase
- IDS-ko
IDS knockout
- MPS
mucopolysaccharidosi(e)s
- SRT
substrate reduction therapy
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd Edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol Bioeng. 2008;99:475–484. doi: 10.1002/bit.21602. [DOI] [PubMed] [Google Scholar]
- Berasain C, Perugorria MJ, Latasa MU, Castillo J, Goñi S, Santamaria M, et al. The epidermal growth factor receptor: a link between inflammation and liver cancer. Exp Biol Med. 2009;234:713–725. doi: 10.3181/0901-MR-12. [DOI] [PubMed] [Google Scholar]
- Bjornsson S. Simultaneous preparation and quantitation of proteoglycans by precipitation with alcian blue. Anal Biochem. 1993;210:282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- Cardone M, Polito VA, Pepe S, Mann L, D'Azzo A, Auricchio A, et al. Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum Mol Genet. 2006;15:1225–1236. doi: 10.1093/hmg/ddl038. [DOI] [PubMed] [Google Scholar]
- D'Anna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, Corrado F, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double-blind, placebo-controlled study. Menopause. 2009;16:320–328. doi: 10.1097/gme.0b013e318186d7e2. [DOI] [PubMed] [Google Scholar]
- Dire DJ, Wilkinson JA. Acute exposure to rhodamine B. J Toxicol Clin Toxicol. 1987;25:603–607. doi: 10.3109/15563658708992660. [DOI] [PubMed] [Google Scholar]
- Fanti P, Asmis R, Stephenson TJ, Sawaya BP, Franke AA. Positive effect of dietary soy in ESRD patients with systemic inflammation – correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]
- Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, Valentine JL, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–170. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- Friso A, Tomanin R, Alba S, Gasparotto N, Puicher EP, Fusco M, et al. Reduction of GAG storage in MPS II mouse model following implantation of encapsulated recombinant myoblasts. J Gene Med. 2005;7:1482–1491. doi: 10.1002/jgm.790. [DOI] [PubMed] [Google Scholar]
- Friso A, Tomanin R, Zanetti A, Mennuni C, Calvaruso F, La Monica N, et al. Gene therapy of Hunter syndrome: evaluation of the efficiency of muscle electro gene transfer for the production and release of recombinant iduronate-2-sulfatase (IDS) Biochim Biophys Acta. 2008;1782:574–580. doi: 10.1016/j.bbadis.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Fu H, Kang L, Jennings JS, Moy SS, Perez A, DiRosario J, et al. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14:1065–1077. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- Garcia AR, Pan J, Lamsa JC, Muenzer J. The characterization of a murine model of mucopolysaccharidosis II (Hunter syndrome) J Inherit Metab Dis. 2007;30:924–934. doi: 10.1007/s10545-007-0641-8. [DOI] [PubMed] [Google Scholar]
- Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512–6517. doi: 10.3748/wjg.v11.i41.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobkiewicz-Banecka J, Piotrowska E, Narajczyk M, Baranska S, Wegrzyn G. Genistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathway. J Biomed Sci. 2009;16:26. doi: 10.1186/1423-0127-16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JG, Wevers RA, Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am J Clin Nutr. 1998;68:1418S–1425S. doi: 10.1093/ajcn/68.6.1418S. [DOI] [PubMed] [Google Scholar]
- Liu LX, Chen WF, Xie JX, Wong MS. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson's disease. Neurosci Res. 2008;60:156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- McClain RM, Wolz E, Davidovich A, Pfannkuch F, Bausch J. Subchronic and chronic safety studies with genistein in dogs. Food Chem Toxicol. 2005;43:1461–1482. doi: 10.1016/j.fct.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McClain RM, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Myltic W, Craciunescu CN, Fischer L, Jeffcoat RA, Koch MA, Lopaczynski W, et al. Lack of significant toxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am J Clin Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs R, Kinzler KW, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill Professional Publishing; 2001. pp. 3421–3452. [Google Scholar]
- Nikitovic D, Tsatsakis AM, Karamanos NK, Tzanakakis GN. The effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by two osteosarcoma cell lines depends on tyrosine kinase and the estrogen receptor density. Anticancer Res. 2003;23:459–464. [PubMed] [Google Scholar]
- Penar PL, Khoshyomn S, Bhushan A, Tritton TR. Inhibition of epidermal growth factor receptor-associated tyrosine kinase blocks glioblastoma invasion of the brain. Neurosurgery. 1997;40:141–151. doi: 10.1097/00006123-199701000-00032. [DOI] [PubMed] [Google Scholar]
- Piotrowska E, Jakobkiewicz-Banecka J, Baranska S, Tylki-Szymanska A, Czartoryska B, Wegrzyn A, et al. Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses. Eur J Hum Genet. 2006;14:846–852. doi: 10.1038/sj.ejhg.5201623. [DOI] [PubMed] [Google Scholar]
- Piotrowska E, Jakóbkiewicz-Banecka J, Tylki-Szymanska A, Liberek A, Maryniak A, Malinowska M, et al. Genistin-rich soy isoflavone extract in substrate reduction therapy for Sanfilippo syndrome: An open-label, pilot study in 10 pediatric patients. Curr Ther Res Clin Exp. 2008;69:166–179. doi: 10.1016/j.curtheres.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano MM, Greene RM. Epidermal growth factor potentiates the induction of ornithine decarboxylase activity by prostaglandins in embryonic palate mesenchymal cells: effects on cell proliferation and glycosaminoglycan synthesis. Dev Biol. 1987;122:419–431. doi: 10.1016/0012-1606(87)90306-x. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Thomas BJ, Wilkinson AS, Fletcher JM, Byers S. Inhibition of glycosaminoglycan synthesis using rhodamine B in a mouse model of mucopolysaccharidosis type IIIA. Pediatr Res. 2006;60:309–314. doi: 10.1203/01.pdr.0000233037.00707.da. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Rees MH, Klebe S, Fletcher JM, Byers S. Improvement in behaviour after substrate deprivation therapy with rhodamine B in a mouse model of MPS IIIA. Mol Genet Metab. 2007;92:115–121. doi: 10.1016/j.ymgme.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Tirone E, D'Alessandris C, Hascall VC, Siracusa G, Salustri A. Hyaluronan synthesis by mouse cumulus cells is regulated by interactions between follicle-stimulating hormone (or epidermal growth factor) and a soluble oocyte factor (or transforming growth factor beta1) J Biol Chem. 1997;272:4787–4794. doi: 10.1074/jbc.272.8.4787. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Ohashi A, Gutierres MA, Oikawa H, Oguma T, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2007;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- Tsai TH. Concurrent measurement of unbound genistein in the blood, brain and bile of anesthetized rats using microdialysis and its pharmacokinetic application. J Chromatogr A. 2005;1073:317–322. doi: 10.1016/j.chroma.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Banks WA, Sly WS. Epinephrine enhances lysosomal enzyme delivery across the blood brain barrier by up-regulation of the mannose 6-phosphate receptor. Proc Natl Acad Sci USA. 2007;104:12873–12878. doi: 10.1073/pnas.0705611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS, Banks WA. Mannose 6-phosphate receptor-mediated transport of sulfamidase across the blood-brain barrier in the newborn mouse. Mol Ther. 2008;16:1261–1266. doi: 10.1038/mt.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera R, Sanchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, et al. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond) 2007;112:183–191. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZL, Sun JY, Wang DN, Xie YH, Wang SW, Zhao WM. Pharmacological studies of the large-scaled purified genistein from Huaijiao (Sophora japonica-Leguminosae) on anti-osteoporosis. Phytomedicine. 2006;13:718–723. doi: 10.1016/j.phymed.2005.09.005. [DOI] [PubMed] [Google Scholar]


