Abstract
Background and purpose:
In adults, neurogenesis persists in the hippocampus and the subventricular zone (SVZ), and this is important for learning and memory. Inhibitors of COX-2 suppress ischaemia-induced neurogenesis in the hippocampus. Here, we have determined the effects of COX-2 inhibitors on neurogenesis throughout the normal adult mouse brain.
Experimental approach:
Young adult mice were treated with COX-2 inhibitors, and the proliferation of neural progenitor cells was measured in the SVZ and hippocampus. In addition, the local uptake of lentiviral vectors in the rostral migratory stream enabled the formation of new neurons in the olfactory bulb (OB) to be assessed.
Key results:
The COX-2 inhibitor meloxicam suppressed progenitor cell proliferation in the SVZ and hippocampus. A significant decrease in the appearance of new neurons in the OB was also observed. Similar effects on progenitor proliferation in the SVZ were seen with nimesulide. The absence of COX-2 expression in the proliferating progenitors in vivo, and the lack of effect of the COX-2 inhibitors on the growth rate of a cultured progenitor cell line, suggest that the effect is indirect. The specific expression of COX-2 in resting microglia that closely associate with the proliferating progenitor cells provides for a possible site of action.
Conclusions and implications:
Treatment with a COX-2 inhibitor results in a substantial inhibition of adult neurogenesis. Studies on human tissues are warranted in order to determine if this effect extends to humans, and whether inhibition of neurogenesis should be considered as an adverse effect of these drugs.
Keywords: meloxicam, nimesulide, adult neurogenesis, microglia, COX-2, hippocampus, subventricular zone, neural stem cells
Introduction
Until recently, it was believed that the adult mammalian brain did not have the ability to generate new functional neurons (Scharfman and Hen, 2007). This notion has now been dispelled with the observations that stem cell niches exist within the subventricular zone (SVZ) of the lateral ventricles, and in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus (Zhao et al., 2008). Neural stem cells within that lateral wall of the SVZ generate transient amplifying cells that generally differentiate into neuroblasts that migrate through the rostral migratory stream (RMS) and become granule neurons and periglomerular neurons in the olfactory bulb (OB) (Zhao et al., 2008). However, in some circumstances, these cells can be diverted from their normal path and migrate to sites of injury (Arvidsson et al., 2002; Parent et al., 2002). Neurons born in the adult SGZ migrate into the granule cell layer of the DG and become dentate granule cells (Zhao et al., 2008). Adult neurogenesis is a highly adaptive process that declines dramatically with age (Kempermann, 2006), with both increases and decreases being associated with various disease states (Scharfman and Hen, 2007).
Recent studies have established that neural progenitor cell (NPC) proliferation and differentiation are dependent on the cellular composition and molecular characteristics of the niche in which they reside (van et al., 2002; Alvarez-Buylla and Lim, 2004). Within the niche, a wide range of molecular cues have been implicated in neurogenesis including neurotransmitters (Santarelli et al., 2003; Jiang et al., 2005), growth factors (Kuhn et al., 1997; Doetsch et al., 2002; Jin et al., 2003; Ghashghaei et al., 2007; Zhao et al., 2007) and cytokines (Ekdahl et al., 2003; Butovsky et al., 2006).
Neurogenesis is under the control of both autocrine and paracrine signalling pathways. Previous studies have identified mechanisms for non-neural cell contribution to the regulation of neurogenesis (Shen et al., 2004; Wurmser et al., 2004). For example, resident brain microglia influence neurogenesis based on their ability to transform into an activated state during injury. In this context, Battista et al. (2006) studied the levels of microglia activation in adrenalectomized rats, and correlated stages of activation with an increase in progenitor cell number in the hippocampus. They further characterized the profile of cytokine expression, and found a positive correlation between microglia activation, expression of TGF-β and neurogenesis (Battista et al., 2006). Similarly, activation of microglia contributed to the maintenance of neurogenesis and spatial learning abilities in adult mice, possibly via the release of insulin growth factor-1 and brain-derived neurotrophic factor (Ziv et al., 2006). Microglia-conditioned medium can promote neurogenesis in cell cultures derived from the SVZ (Walton et al., 2006). Activated microglia can also direct the migration of NPCs and influence their differentiation via a variety of pro-inflammatory mediators (Aarum et al., 2003).
Some commonly prescribed drugs have robust effects on neurogenesis. These include antidepressants, mood stabilizers, cognitive enhancers, anesthetics, steroids and statins (Scharfman and Hen, 2007). In many cases, the positive effects on neurogenesis might play a direct role in the therapeutic value of the drug. However, this is a difficult link to establish. On the other hand, inhibition of adult neurogenesis might be considered as an adverse effect of the drug, yet there might be some circumstances in which this could be beneficial.
The prostaglandin-synthesizing enzyme, COX-2, has largely been studied in the context of inflammation and pain, with selective inhibitors developed primarily as non-steroidal anti-inflammatory drugs (NSAIDs). Low levels of COX-2 expression might limit the adverse effects of these drugs. However, this enzyme is expressed at high levels in discrete populations of neurons within cortical and limbic regions (Yamagata et al., 1993). Recent studies have found COX-2 to be a multifunctional neural modulator affecting synaptic plasticity (Kaufmann et al., 1996; Bazan, 2001) and glutamate-mediated cytotoxicity (Nogawa et al., 1997). Additionally, COX-2 is important in cell proliferation and has been implicated in growth and progression of a variety of tumour types (Shono et al., 2001; Thun et al., 2002). COX-2 inhibitors can also suppress the increases in neurogenesis in the hippocampus that follows acute global ischaemia (Kumihashi et al., 2001) and status epilepticus (Jung et al., 2006). However, the effects of COX-2 inhibitors on basal neurogenesis, particularly in the SVZ, have not been fully explored. In this study, we investigate the effects of meloxicam and nimesulide, two clinically licensed COX-2 inhibitors (Davis and Brogden, 1994; Turck et al., 1996) on neurogenesis in the normal adult mouse brain, in particular the SVZ region. Our results show that COX-2 inhibitors can substantially (>90%) inhibit neurogenesis in the brain of adult mice by an indirect mechanism of action, possibly involving COX-2 function in microglia.
Methods
Animals
All procedures were performed in accordance with UK Home Office regulations (Animals Scientific Procedures Act 1986). In general, studies were carried out on young adult female C57BL/6 mice (Harlan UK Ltd, 6 weeks). At the end of each experiment, the animals were deeply anaesthetized with pentobarbitone (80 mg·kg−1 i.p.; Sigma, St Louis, MO, USA), and transcardially perfused with 10 mL saline followed by 50 mL paraformaldehyde (PFA) (4% in 0.1 M phosphate buffer). Brains were removed and post-fixed in 4% PFA at 4°C for 2 h.
Treatment regimen
Meloxicam was purchased from Sigma, while nimesulide from Tocris (Ellisville, MO, USA). All drugs were dissolved in 80% dimethylsulphoxide. Meloxicam was administered at 10 or 20 mg·kg−1 (i.p.), once per day for 5, 7 or 10 days (see text for details). Nimesulide was administered daily at 10 mg·kg−1 (i.p.) for 5 days. 5-Bromo-2′-deoxyuridine (BrdU; Sigma) was administered three times a day for 2 consecutive days at 50 mg·kg−1 (i.p.) at 2 h intervals. In parallel studies, we have found that a number of drugs dissolved in the same vehicle and delivered by the same route have no effect on cell proliferation in the SVZ (see Goncalves et al., 2008). We conclude that neither the vehicle nor the injection protocol affects cell proliferation in the SVZ.
Lentiviral vector injections
We used the vector pRRL.SIN.PPT.CMV.GFPpre in which the CMV promoter drives expression of enhanced green fluorescent protein (GFP) as previously described (Goncalves et al., 2008). For the labelling of dividing progenitors and their progeny, 1 mL of virus stock (2–5 × 108 TU·mL−1) was injected at a rate of 0.2 µL·min−1 using a micro-infusion pump into the start of the RMS (AP +0.75 mm from bregma; ML +1.2 mm; DV −1.7 mm) as previously described (Goncalves et al., 2008). The animals were prepared for histology 2 weeks after virus injection.
Immunohistochemistry
For Ki-67 counts in the SVZ, blocks of brain tissue containing the SVZ were dissected, post-fixed in 4% PFA (2 h at 4°C) and transferred to 20% sucrose (overnight at 4°C) then blocked in OCT embedding compound (BDH, Essex, UK) and snap-frozen in liquid nitrogen. Tissues were kept at −80°C until ready for further processing. Coronal sections (20 mm) were cut in the cryostat. For quantitative analysis in the hippocampus and at the RMS/OB border, 5 mm paraffin wax sagittal sections, stained for BrdU and/or Ki-67 (see below), were used. For GFP staining, the brains were embedded in 10% gelatin (Sigma) and 40 mm sagittal sections cut on a vibratome. Immunohistochemistry for cryostat and gelatin-embedded sections was performed by washing in Tris-buffered saline (TBS), 3 × 10 min. After they were blocked with 1% BSA for 15 min, the sections were incubated overnight at 4°C with the primary antibody. The following day, the sections were washed with TBS (3 × 10 min) before incubating with secondary antibody for 2 h (Alexa Fluor 488 or Alexa Fluor 594, 1:1000, Invitrogen, Paisley, UK) and Hoechst 33342 (Sigma Aldrich, Dorset, UK, 1:10 000). Following a further three washes with TBS, the sections were mounted onto glass slides with Mowiol. Primary antibodies and dilutions used were Ki-67 (SP6, Laboratory Vision Neomarkers, Thermo Fisher Scientific, Runcom, Cheshire, UK, 1:100) and anti-GFP (Abcam, Cambridge, UK, 1:1000). Immunohistochemistry for paraffin sections was performed as follows: Sections were first dewaxed in xylene, then heated in citric acid (10 mM, pH 6), until boiling, then washed under running tap water for 5 min. Sections were then blocked with 1% BSA for 15 min, followed by overnight incubation at 4°C with the primary antibody, before incubation with the corresponding fluorescent secondary antibody (Alexa Fluor 488 or Alexa Fluor 594, 1:1000, Invitrogen) and/or Hoechst 33342 (Sigma, 1:10 000). Primary antibodies used on paraffin wax sections were Ki-67 (SP6, Laboratory Vision Neomarkers, 1:100), COX-2 (Abcam, 1:400) and COX-2 (Santa Cruz, Insight Biotechnology Ltd, Wembley, UK, 1:250). For BrdU staining, sections were treated for 10 min at 60°C with 1 M HCl in TBS to denature DNA. Tissue was then washed in running tap water before blocking and subsequent incubation with anti-BrdU antibody (Dako, Dako UK Limited, Cambridgeshire, UK, 1:100), at 4°C overnight. For double staining with BrdU and COX-2, sections were first stained for COX-2 as described above, then fixed for 30 min in 4% PFA, after which the BrdU staining protocol was followed as above.
Cell counting
In general, Ki-67- and BrdU-positive cells were counted as previously described (Goncalves et al., 2008). In brief, for Ki-67 quantification in the SVZ, 20 µm sections were cut coronally throughout the lateral ventricle from the level of the optic tract entry into the brain in the rostral direction. For each animal, at least four sections spaced 300 µm apart taken in the caudal–rostral direction were randomly selected to undergo immunohistochemistry. Sections of the same areas sampled throughout the ventricle were selected for all animals to allow appropriate comparison of results. Although cell proliferation was generally restricted to the lateral wall, we counted all the proliferating cells within a four-cell deep layer around the ventricle. However, for the key experiment, we also counted Ki-67-positive cells within a defined area of the lateral wall (300 µM in length and 50 µM in depth) at the juncture between the lateral wall and the beginning of the RMS. For BrdU quantification, 6 µm sagittal sections were cut from 0.4 mm laterally from the midline, and every fifth section taken up for immunohistochemistry. At least four sections per animal were analysed, all from the same bregma levels between animals. Cells were counted (without knowledge of treatments) under high power (×40) using an Apotome Zeiss microscope (Carl Zeiss, Hertfordshire, UK). The average number of labelled cells per section was determined for each animal, and results are expressed as the mean per animal group.
GFP labelling and number of mature GFP-labelled neurones in the OB
Sagittal sections (40 µm) of the OB from vehicle and drug-treated animals were stained for GFP to identify cells expressing viral vectors as previously described (Goncalves et al., 2008). To ensure anatomical consistency, the RMS was used as a reference point, and only sections showing the entry level of the RMS into the OB were studied. The whole OB was examined at ×20 magnification using an Apotome Zeiss microscope. Total number of mature and immature GFP-labelled neurones, identified by morphological analysis, within the OB per section, was counted by an experimenter unaware of the treatments.
Cell culture and cell proliferation assay
The Cor-1 neural stem cell line and methods for culture have been described in detail elsewhere (Conti et al., 2005). Briefly, cells were expanded as adherent cultures on gelatin-coated flasks (Iwaki, Jencons, Leicestershire, UK) in Euromed-N media (Euroclone, Milan, Italy) supplemented with N2 (in-house preparation) and 10 ng·mL−1 each of epithelial growth factor (EGF) and fibroblast growth factor-2 (FGF-2) (PeproTech EC Limited, London, UK). For cell proliferation assays, 104 cells were seeded in 100 µL full growth media into individual wells of a 96-well microtitre place. Drugs were added after approximately 24 h culture, and the plates were transferred to an IncuCyte live cell imaging station (for details, see http://www.essen-instruments.com/index.php). High-definition phase images were captured from two fields of each well every 2 h. Cell proliferation rates were indexed by measuring the degree of cell confluence as a percentage of the field area. Control experiments showed a linear relationship between cell number and confluence over the initial phase of growth, and that this held until 80% confluence.
Statistical analysis
Student's two-sided t-test was used for all statistical analysis. P < 0.05 was taken to show a significant difference between means.
Results
Meloxicam and nimesulide inhibit cell proliferation in the SVZ
The effects of the COX-2 inhibitors on basal neurogenesis in the SVZ of young adult (6-week-old) mice were assessed after treatment with meloxicam (20 mg·kg−1, i.p.) or nimesulide (10 mg·kg−1) for 5 days. Cell proliferation rates in the SVZ were then determined using Ki-67 as the proliferation marker (Rose et al., 1994). Remarkably, treatment with meloxicam was associated with a ∼90% reduction in the number of Ki-67-positive cells counted around the whole ventricular wall in the SVZ (Figure 1). Most of the proliferating cells were in the lateral wall, with greatest density close to the junction with the RMS. A similar substantial reduction in cell proliferation (down to 6.6 ± 0.88 from 27+/ to 1.7, mean ± SEM, n = 4, P ≤ 0.0004) was seen when cells were counted in a defined area of the lateral wall (see Methods for details). A substantial reduction of cell proliferation was also seen following treatment with another COX-2 inhibitor, nimesulide (Figure 1). The SVZ-derived neuroblasts continue to proliferate as they migrate along the RMS before entering the OB (Luskin, 1993; Bonfanti and Theodosis, 1994; Menezes et al., 1995). Whereas the meloxicam treatment (in this instance 10 mg·kg−1 for 7 days) again substantially suppressed the number of proliferating cells in the SVZ (data not shown), it had no effect on the number of proliferating neuroblasts at the RMS/OB boundary (control group 153 ± 5.0, treated group 145 ± 5.2 both values mean ± SEM, n = 4 animals). These data suggest that in contrast to cells within the SVZ, migrating neuroblasts do not require COX-2 activity for proliferation.
Figure 1.
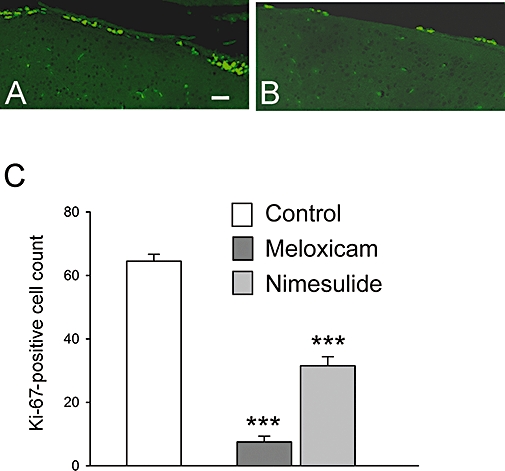
Effect of meloxicam and nimesulide on cell proliferation in the SVZ. Six-week-old mice were treated with meloxicam (20 mg·kg−1) or nimesulide (10 mg·kg−1) for 5 days as described in Methods. Sagittal sections were cut throughout the SVZ and stained for Ki-67. Examples of Ki-67-positive cells in the lateral wall of the SVZ in control and treated animals are shown in A and B respectively. Quantification of Ki-67-positive cells is shown in C. The results are expressed as the mean determined from groups of four animals. Bars show SEM. Scale bar is 75 µm.
Meloxicam inhibits cell proliferation in the hippocampus
The above data might point to regional differences in the sensitivity of proliferating cells to meloxicam treatment. Based on this, it was of interest to determine if cell proliferation in the DG in the hippocampus was also sensitive to meloxicam treatment. To this end, 6-week-old mice treated with vehicle or meloxicam (10 mg·kg−1, i.p., for 7 days) were additionally pulsed with BrdU (50 mg·kg−1, i.p., for 2 days) to label proliferating cells. Quantification of BrdU-stained cells in the DG showed that treatment with meloxicam caused a significant decrease in cell proliferation (∼40%) (Figure 2A–C). Therefore, it would appear that COX-2 inhibitors can inhibit NPC proliferation within two stem cell niches, but have little if any effect on proliferation of neuroblasts that migrate from the niche.
Figure 2.
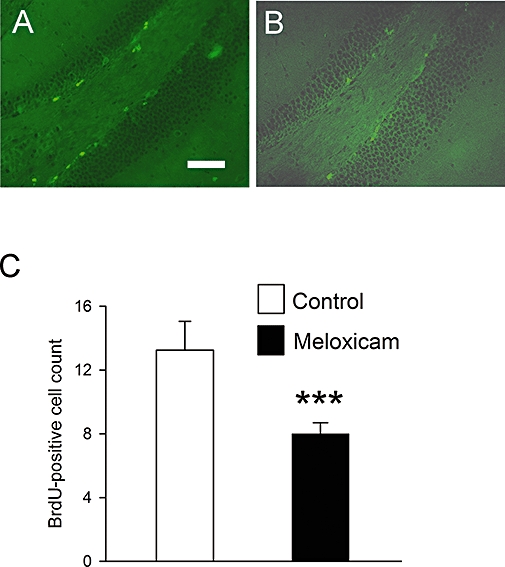
Effect of meloxicam on cell proliferation in the DG. Six-week-old mice were treated with meloxicam (20 mg·kg−1) for 10 days. On the final 2 days, BrdU (50 mg·kg−1) was injected to label proliferating cells. Sagittal sections were cut throughout the DG and stained for BrdU to assess proliferation. Examples of BrdU-positive cells in the DG of the hippocampus in control (A) and treated (B) animals are shown. Quantification of BrdU-positive cell numbers is shown in C. Values are the means from groups of four animals. Bars are SEM. Scale bar is 150 µm.
Meloxicam inhibits the appearance of new neurons in the OB
The majority of cells generated in the SVZ are destined to migrate and populate the OB with new neurons, a process that takes around 2 weeks (Bernier and Parent, 1998). The local uptake of lentiviral vectors at injection sites close to the SVZ allows for the fate mapping of neuroblasts and their progeny in the OB (Mizrahi, 2007; Goncalves et al., 2008). In order to determine to what extent meloxicam might reduce the number of new neurons that appear in the OB, we injected a GFP-expressing lentiviral vector into the RMS in close proximity to the lateral ventricle 1 day after the cessation of drug treatment (meloxicam, 20 mg·kg−1, i.p., daily for 10 days). It has previously been established by others (Mizrahi, 2007), and confirmed by us (unpublished data) that all such GFP-expressing cells migrating in the RMS and arriving in the OB express PSA-NCAM and/or NeuN confirming their identity as neuroblasts. The animals were killed 2 weeks later, and sections of the OB cut at the level of entry of the RMS and stained for viral vector encoded GFP. Visual inspection indicated a marked reduction in the number of undifferentiated and differentiated neurons within the OB in the meloxicam-treated group (Figure 3A, B). Cell counting confirmed the very clear reduction (∼94%) in neuronal numbers between the drug-treated and control groups, and showed this to be statistically significant (Figure 3C).
Figure 3.
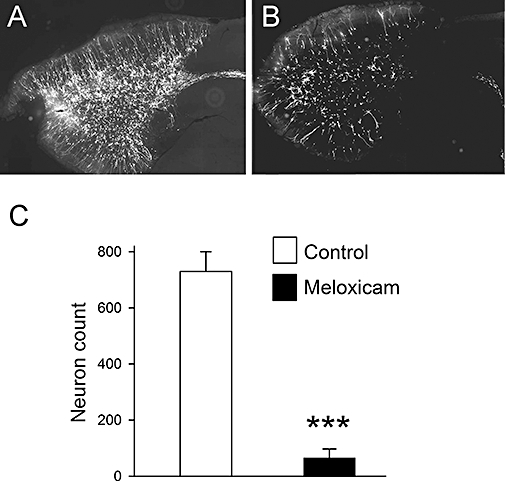
Effect of meloxicam on the appearance of new neurons in the OB. Six-week-old mice were treated with meloxicam (20 mg·kg−1) for 10 days. Then, 24 h after cessation of drug treatment, viral vectors encoding GFP were injected directly into the exit point of the SVZ adjacent to the origin of the RMS to label migratory neuroblasts. Two weeks later, animals were killed, and 40 µm sagittal sections obtained from the OB. The micrographs show GFP labelling at the level of entry of the RMS into the OB for a control animal (A) and a treated one (B). (C) Neuron counts in the OB, with each value being the mean determined from at least four animals. Bars show SEM.
Meloxicam and nimesulide do not inhibit proliferation of cultured progenitors
In order to determine if COX-2 inhibitors have direct effects on proliferating progenitor cells, we tested the effects of the two drugs on the recently characterized Cor-1 neural stem cell line (Conti et al., 2005; Goncalves et al., 2008). When grown on a gelatin-coated substrate in the presence of EGF and FGF, these cells can be expanded essentially indefinitely without loss of their ability to differentiate into neurons, astrocytes or oligodendrocytes (Pollard et al., 2006). Using high-definition phase contrast live cell imaging, the growth of the cells was monitored over a 48 h period of exposure to meloxicam or nimesulide (at up to 5 µM). The results from a typical and representative experiment are shown in Figure 4A. Neither drug affected the growth rates of the cells. In addition, there were no detectable changes in cell morphology or viability at any time point (Figure 4B,C). These data suggest that the effects of the COX-2 inhibitors on progenitor proliferation in vivo might be indirect.
Figure 4.
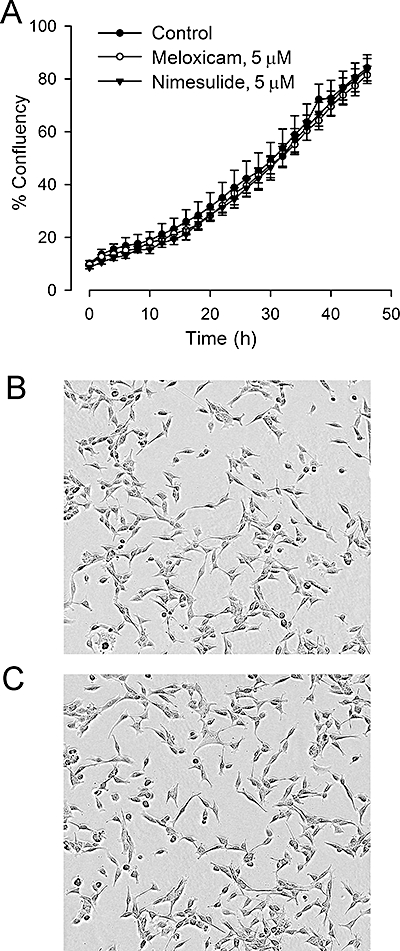
Effects of meloxicam and nimesulide on proliferation of cultured progenitor cells. Meloxicam and nimesulide were added to cultures of the Cor-1 progenitor cell line that had been established for 24 h in individual wells of a 96-well microtitre plate (see Methods for details). Growth curves were monitored by live cell imaging over the next 48 h. Results in (A) show a representative example of an experiment, with each value representing the mean ± SEM obtained from six replicate wells. Results in (B) and (C) show representative micrographs of control and meloxicam-treated cells obtained approximately 24 h after addition of the drug.
COX-2 expression in the adult SVZ and DG
Failure to detect a direct effect of the COX-2 inhibitors on progenitor cell proliferation in vitro prompted us to examine the expression pattern of COX-2 within the neural stem cell niches. We were unable to detect COX-2 expression in any Ki-67-positive cells in either the SVZ or hippocampus (shown for the SVZ in Figure 5A,B). However, COX-2 was highly expressed in a distinct population of cells that were not only intimately associated with Ki-67-positive cells in the lateral wall of the ventricle (Figure 5C), but could also be found scattered throughout the SVZ. The morphology of the COX-2-positive cells largely resembled resting microglia, characterized by spindle-shaped cell bodies and numerous long, thin, branched processes (Kadowaki et al., 2007). Double labelling with an established microglial marker (Iba1) confirmed that the COX-2-positive cells were microglia.
Figure 5.
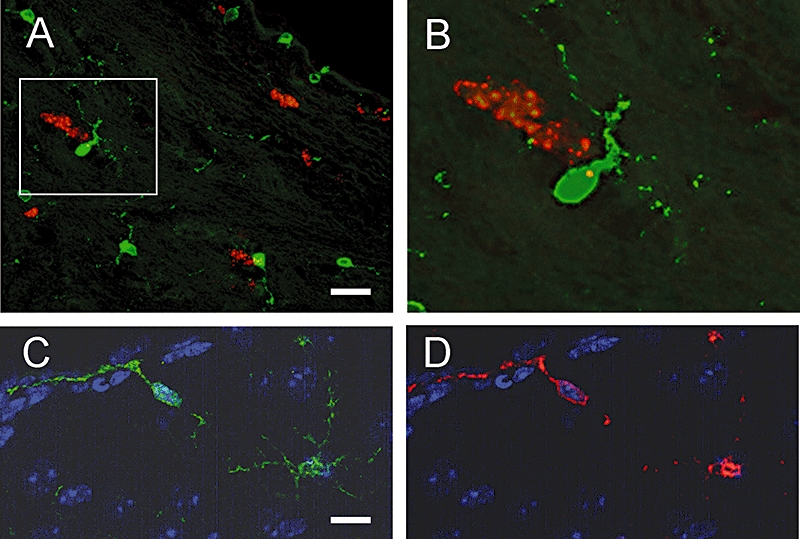
Expression of COX-2 in the SVZ of the adult mouse brain. (A) and (B) Representative micrographs from coronal sections of the SVZ double stained for COX-2 (green) and Ki-67 (red). Box in (A) highlights a microglial cell. A higher magnification image of the cell is shown in (B). COX-2 was not seen in Ki-67-positive cells, but was found to be expressed in cells that closely associate with the proliferating cells. (C) and (D) Similar sections stained for COX-2 (green) and Iba1 (red), demonstrating that COX-2 is present in microglia around the ventricle. Scale bar in A is 40 µm, and 20 µm for (C) and (D).
Discussion and conclusions
In the present study, we have investigated the effects of COX-2 inhibitors on adult neurogenesis, with a particular focus on the SVZ. Under normal circumstances, neurogenesis in this region continues throughout adulthood, but declines dramatically with age (Kempermann, 2006). The main purpose of SVZ neurogenesis is to supply the OB with new neurons, and this is correlated with memorization of odours in animals (Rochefort et al., 2002). In humans, there is considerable interest in the contribution of neurogenesis to the pathology of neurodegenerative diseases. Interestingly, odour discrimination appears to be an early symptom in both Parkinson's disease (Berendse et al., 2001) and Alzheimer's disease (Doty et al., 1987; Koss et al., 1987; Serby et al., 1991; Mesholam et al., 1998). In both disease states, reduced SVZ neurogenesis has been correlated with the severity of the disease (Mesholam et al., 1998). We also examined cell proliferation in the hippocampus where neurogenesis has also been described in humans (Eriksson et al., 1998).
Our results have shown dramatic reductions (of ∼90%) in cell proliferation in the SVZ following as little as 5 days of treatment with meloxicam. Similar, albeit less pronounced, reductions are seen following treatments with nimesulide. As might be expected, the dramatic effects of meloxicam treatment on cell proliferation within the SVZ were reflected in a very substantial (∼95%) decline in the number of new neurons that appear in the OB 2 weeks after cessation of drug treatment. The drug treatments had no clear effects on the tissue integrity and/or morphology of the SVZ and OB. These differences in neurogenesis were detected using two different methodologies (proliferation in SVZ and appearance of new neurons in the OB). This unequivocally demonstrates that COX-2 inhibitors can reduce the number of new neurons that appear in the brains of young adult animals. Within the SVZ, slowly proliferating stem cells give rise to a population of rapidly proliferating transient amplifying cells that differentiate into migratory neuroblasts (Doetsch et al., 1997). Interestingly, we found that meloxicam did not affect the proliferation of neuroblasts at the distal end of the RMS. This implies that the effects are restricted to proliferation within the SVZ, suggesting that COX-2 function is required for the proliferation of stem cell and/or transient amplifying cell populations. The effects of meloxicam on each specific NPC type were not assessed, but rather on the whole population of NPCs taken together. Further studies are required to determine if there are distinct effects on the various subtypes and/or regional effects within the SVZ. The effects of meloxicam are not restricted to the SVZ as similar effects on cell proliferation can be seen in the DG of the hippocampus. Other studies also support a role for COX-2 in neurogenesis in the adult hippocampus. For example, COX-2 knockout mice have been shown to have impaired neurogenesis in the hippocampus, and treatment with COX-2 inhibitors suppresses the increased progenitor cell proliferation induced by ischaemia (Sasaki et al., 2003).
In principle, the COX-2 inhibitors could block neurogenesis by acting directly on the proliferating cells, or indirectly via effects on other cell types. In order to determine if COX-2 activity is required in a cell autonomous manner, we tested the effects of the two COX-2 inhibitors on the recently characterized Cor-1 neural stem cell line (Conti et al., 2005). When grown on a gelatin-coated substrate in the presence of EGF and FGF, these cells can be expanded essentially indefinitely without loss of their ability to differentiate into neurons, astrocytes or oligodendrocytes (Pollard et al., 2006). Previously, we have shown that drugs that inhibit neurogenesis in the SVZ have their effects mirrored on cultured Cor-1 cells (Goncalves et al., 2008). Surprisingly, we did not find any effect of meloxicam or nimesulide on the growth rates of Cor-1 cells, suggesting that the effects of the drugs seen in vivo might not reflect a direct effect on these proliferating cells. In support of this, we have not been able to detect COX-2 expression in the rapidly proliferating cells in the SVZ or hippocampus. However, we can readily detect expression in resting microglia, cells that we have shown to be intimately associated with proliferating progenitors. This was apparent both in the SVZ and DG. Thus, one possibility is that meloxicam and nimesulide are inhibiting a COX-2-related function in resting microglia.
Microglia have been proposed as important players in neurogenesis both in the absence and presence of tissue damage. In pathological conditions, microglial cells become activated after changes in the milieu by altering their morphology and the pattern of secreted factors such as immune cytokines (Perry et al., 2003). The unique profile of inflammatory factors, which depends on the mode of activation and/or the severity of inflammation, can affect neurogenesis in different ways. For example, microglial activation by endotoxins generated during uncontrolled inflammation creates an environment that is detrimental to neurogenesis (Butovsky et al., 2006). On the contrary, mild acute inflammation induces an adaptive immunity response mediated by IL-4 and IFN-γ that stimulates neurogenesis (Butovsky et al., 2006). Indeed, the release of TGF-β by activated microglia increases neurogenesis in the adult DG (Battista et al., 2006). Our results suggest that resting microglia might have a major impact on basal rates of neurogenesis, with other evidence in the literature also supporting this hypothesis (Aarum et al., 2003; Morgan et al., 2004; Walton et al., 2006).
The fact that COX-2 is expressed in resting microglia in the human brain (Tomimoto et al., 2000) and that COX-2 inhibitors reduce adult neurogenesis in mice, is of some concern given that COX-2 inhibitors are used clinically. The rationale behind the pharmacological use of selective COX-2 inhibitors is that they cause fewer severe side effects in the gastrointestinal tract than traditional NSAIDs (Hinz et al., 2007). However, COX-2 is not only involved in pathological processes, such as acute and chronic inflammatory states, but it is also involved in many adaptive processes including regulation of blood pressure, kidney function, ulcer and wound healing. Hence, its suppressive properties may cause adverse cardiovascular and kidney effects (Hinz et al., 2007). As a result, the prescription of COX-2 inhibitors is limited for the relief of signs and symptoms of osteoarthritis and rheumatoid arthritis in patients at high risk of developing serious gastrointestinal disorders (Lipsky, 2001). Further studies are warranted in order to determine if the results of this study extend to humans treated with COX-2 inhibitors. If inhibition of adult neurogenesis is found in humans, it might be wise to further restrict the prescription of COX-2 inhibitors, especially in children and adolescents.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council.
Glossary
Abbreviations:
- BDNF
brain-derived neurotrophic factor
- BrdU
bromodeoxyuridine
- DG
dentate gyrus
- EGF
epithelial growth factor
- FGF-2
fibroblast growth factor-2
- GFP
green fluorescent protein
- IFN-γ
interferon gamma
- IGF-1
insulin growth factor-1
- IL-4
interleukin-4
- NPC
neural progenitor cell
- NSAIDs
non-steroid anti-inflammatory drugs
- OB
olfactory bulb
- PG
prostaglandin
- PSA-NCAM polysialic
neural cell adhesion molecule
- RMS
rostral migratory stream
- SVZ
subventricular zone
- TGF-β
transforming growth factor-beta
Conflict of interest
No one associated with this paper has any conflict of interest relating to the publication.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bazan NG. COX-2 as a multifunctional neuronal modulator. Nat Med. 2001;7:414–415. doi: 10.1038/86477. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Parent A. Bcl-2 protein as a marker of neuronal immaturity in postnatal primate brain. J Neurosci. 1998;18:2486–2497. doi: 10.1523/JNEUROSCI.18-07-02486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience. 1994;62:291–305. doi: 10.1016/0306-4522(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. Plos Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R, Brogden RN. Nimesulide. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1994;48:431–454. doi: 10.2165/00003495-199448030-00009. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Weimer JM, Schmid RS, Yokota Y, McCarthy KD, Popko B, et al. Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev. 2007;21:3258–3271. doi: 10.1101/gad.1580407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Hinz B, Renner B, Brune K. Drug insight: cyclo-oxygenase-2 inhibitors – a critical appraisal. Nat Clin Pract Rheumatol. 2007;3:552–560. doi: 10.1038/ncprheum0619. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van CJ, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23:237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Nakadate K, Sakakibara S, Hirata K, Ueda S. Expression of Iba1 protein in microglial cells of zitter mutant rat. Neurosci Lett. 2007;411:26–31. doi: 10.1016/j.neulet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. They are not too excited: the possible role of adult-born neurons in epilepsy. Neuron. 2006;52:935–937. doi: 10.1016/j.neuron.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Koss E, Weiffenbach JM, Haxby JV, Friedland RP. Olfactory detection and recognition in Alzheimer's disease. Lancet. 1987;1:622. doi: 10.1016/s0140-6736(87)90256-x. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumihashi K, Uchida K, Miyazaki H, Kobayashi J, Tsushima T, Machida T. Acetylsalicylic acid reduces ischemia-induced proliferation of dentate cells in gerbils. Neuroreport. 2001;12:915–917. doi: 10.1097/00001756-200104170-00010. [DOI] [PubMed] [Google Scholar]
- Lipsky PE. Introduction. The role of COX-2-specific inhibitors in clinical practice. Am J Med. 2001;110(Suppl 3A):1S–2S. doi: 10.1016/s0002-9343(00)00676-8. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Smith CM, Nelson KC, Luskin MB. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol Cell Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Mizrahi A. Dendritic development and plasticity of adult-born neurons in the mouse olfactory bulb. Nat Neurosci. 2007;10:444–452. doi: 10.1038/nn1875. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90:89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16(Suppl 1):i112–i120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DS, Maddox PH, Brown DC. Which proliferation markers for routine immunohistology? A comparison of five antibodies. J Clin Pathol. 1994;47:1010–1014. doi: 10.1136/jcp.47.11.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitagawa K, Sugiura S, Omura-Matsuoka E, Tanaka S, Yagita Y, et al. Implication of cyclooxygenase-2 on enhanced proliferation of neural progenitor cells in the adult mouse hippocampus after ischemia. J Neurosci Res. 2003;72:461–471. doi: 10.1002/jnr.10595. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–4381. [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Wakita H, Lin JX, Budka H. Cyclooxygenase-2 is induced in microglia during chronic cerebral ischemia in humans. Acta Neuropathol. 2000;99:26–30. doi: 10.1007/pl00007402. [DOI] [PubMed] [Google Scholar]
- Turck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol. 1996;35(Suppl 1):13–16. doi: 10.1093/rheumatology/35.suppl_1.13. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004;304:1253–1255. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]


