Abstract
Diets rich in fruits and vegetables reduce blood pressure (BP) and the risk of adverse cardiovascular events. However, the mechanisms of this effect have not been elucidated. Certain vegetables possess a high nitrate content and we hypothesized that this might represent a source of vasoprotective nitric oxide via bioactivation. In healthy volunteers, approximately 3h following ingestion of a dietary nitrate load (beetroot juice 500ml) BP was substantially reduced (Δmax - 10.4/8 mmHg); an effect that correlated with peak increases in plasma nitrite concentration. The dietary nitrate load also prevented endothelial dysfunction induced by an acute ischemic insult in the human forearm and significantly attenuated ex vivo platelet aggregation in response to collagen and ADP. Interruption of the enterosalivary conversion of nitrate to nitrite (facilitated by bacterial anaerobes situated on the surface of the tongue), prevented the rise in plasma nitrite, blocked the decrease in BP and abolished the inhibitory effects on platelet aggregation, confirming that these vasoprotective effects were due to the activity of nitrite converted from the ingested nitrate. These findings suggest that dietary nitrate underlies the beneficial effects of a vegetable-rich diet and highlights the potential of a ‘natural’, low cost approach for the treatment of cardiovascular disease.
Keywords: diet, nitric oxide, blood pressure, hypertension, ischemia/reperfusion, platelets, endothelium
Introduction
Perhaps the largest public health initiative in the western world has focused on improvement of diet, particularly in those with a high risk of cardiovascular disease. Trials have shown that diets rich in fruits and vegetables reduce blood pressure (BP; Dietary Approaches to Stop Hypertension; DASH, Vegetarian Diet and BP)1;2 and adverse cardiovascular events3-7. These protective effects have previously been attributed to the high antioxidant vitamin content, yet large clinical trials have failed to provide evidence in support of this thesis8;9. The greatest protection against coronary heart disease afforded by a change in diet is that associated with the consumption of green leafy vegetables (e.g. spinach, lettuce)6. Such vegetables, also including beetroot, commonly have a high inorganic nitrate (NO3−) content10;11. In humans, following absorption through the stomach wall, ~25% of consumed nitrate enters the enterosalivary circulation where it is reduced to nitrite (NO2−) by bacterial nitrate reductases from facultative anaerobes on the dorsal surface of the tongue12-14. This nitrite is swallowed and in the acidic environment of the stomach is reduced to nitric oxide (NO) or re-enters the circulation as nitrite. Indeed, it has been hypothesized that dietary nitrate represents an intravascular source of the pleiotropic, vasoprotective molecule nitric oxide (NO), that supplements conventional NO generation by NO synthases (NOS)15.
Endothelium-derived NO is a potent dilator, governs systemic BP and retards atherogenesis (NO inhibits inflammatory cell recruitment and platelet aggregation)16. Consequently, numerous cardiovascular pathologies (including prehypertension17, hypertension18, atherosclerosis19 and stroke20) are associated with endothelial dysfunction and diminished NO bioactivity. Recently, studies have demonstrated that nitrite confers marked protection against ischemia/reperfusion (I/R) injury in the myocardial, hepatic, renal, pulmonary and cerebral vasculature21;22. This cytoprotective effect has been attributed to reduction of nitrite to NO during ischemia or hypoxemia (conditions that inactivate the enzyme, NO synthase, responsible for endothelial NO synthesis), facilitated by xanthine oxidoreductase (XOR)23, deoxyhaemoglobin21, deoxymyoglobin24 or via chemical acidification. Thus, in an environment where conventional NO synthesis is impaired, nitrite provides an alternative source of (vaso)protective NO. Furthermore it has been proposed that nitrite plays an important physiological role. Indeed, nitrite causes dose-dependent vasodilatation in the brachial artery of normal volunteers, indicating that it may have an important role in maintaining normal cardiovascular homeostasis in addition to its cytoprotective role25.
Studies in humans demonstrate a progressive rise over time in plasma nitrate26 and nitrite14 concentrations following oral administration of sodium or potassium nitrate. We investigated the possibility that a similar increase in these anions can be achieved by consuming dietary nitrate through the consumption of beetroot juice and that this will acutely lower arterial BP, supplement endothelial function (measured by flow-mediated dilatation, FMD) during ischemia and inhibit platelet aggregation as a result of bioconversion to NO.
Methods
Volunteers
The studies were granted full ethics approval by the Local Research Ethics Committee and all subjects gave informed consent (On-line Methods Supplement for inclusion criteria please see http://hyper.ahajournals.org). The study was separated into three phases with distinct recruitment for each phase.
BP study
An open-label cross-over design was utilised with 14 healthy subjects randomized to drink 500 ml of either beetroot juice (Planet Organic, London) or water within 30 min. BP was measured every 15 min for 1h pre- and 3h post-beetroot juice ingestion, then hourly to 6h, with a final reading at 24h. BPs were taken according to a standard protocol (Online Methods Supplement please see http://hyper.ahajournals.org), using an automated BP measuring machine (Omron 705CP (Japan) with the subject seated; three BP measurements were taken at each time point and the mean of the 2nd and 3rd reading was used. Blood samples (5 ml each) were collected into citrate tubes for plasma nitrate and nitrite measurement at baseline and every 30 min for 2h, then hourly up to 6h, with a further measurement at 24 h. Blood samples were centrifuged immediately at 2,200g for 10 min at 4°C. The plasma was collected and stored at −80°C until measurement of nitrite and nitrate concentration. The second part of the study was conducted after a minimum of 7 days.
Interruption of enterosalivary circulation study
The effects of spitting out all saliva during, and for 3h following, beetroot juice ingestion (500 ml) on BP and simultaneous changes in plasma nitrate and nitrite concentration were investigated in a further cross-over study in 6 healthy volunteers, with normal swallowing of saliva as control. In this study blood was collected in lithium heparin tubes, for measurement of plasma potassium. For assessment of effects on platelet aggregation, blood was collected at baseline and at 2.5h into 3.8% trisodium citrate (9:1 v/v), pH 7.4, using a 19-gauge butterfly needle. Platelet-rich-plasma (PRP) was prepared and used for assessment of aggregation in response to collagen or adenosine di-phosphate (ADP) using an adaptation of the Born method27 (Online Methods Supplement please see http://hyper.ahajournals.org).
FMD study
In the second phase of this study endothelial function was assessed in 10 healthy subjects by measuring brachial artery diameter in the non-dominant arm in response to the endothelium-dependent reactive hyperaemia response before and after an ischemic insult as previously described28;29(Online Methods Supplement please see http://hyper.ahajournals.org). In this open-label cross-over study, healthy subjects were randomized to 500 ml of beetroot juice 2h prior to the I/R sequence, or no treatment and returned for the second arm of the study after a minimum of 7 days.
Chemiluminescence
Samples were analysed for nitrite and nitrate using chemiluminescence as described previously30 (Online Methods Supplement please see http://hyper.ahajournals.org).
Data and statistical analysis
We analysed data using the Graph Pad Prism™ Software. All data are expressed as mean ± SEM unless otherwise stated. Data were compared by repeated-measures ANOVA with Dunnett’s post test for comparison with baseline and Bonferroni’s post test for comparison with the control group. In all cases, P<0.05 was considered statistically significant.
Results
There were no significant differences in the general characteristics of the individuals recruited for the separate phases of the study (Table S1, Online Supplement please see http://hyper.ahajournals.org). Beetroot juice was generally well tolerated by the subjects. Beeturia (red urine) and red stools were common, expected effects. The mean concentration of nitrate in the beetroot juice was 45.0±2.6 mMol/L (2.79g/L BP study), and 34.0±0.1 mMol/L (2.11 g/L, spitting study), whereas nitrite was below the limits of detection (<50 nMol/L).
A dietary nitrate load raises circulating nitrite and nitrate levels
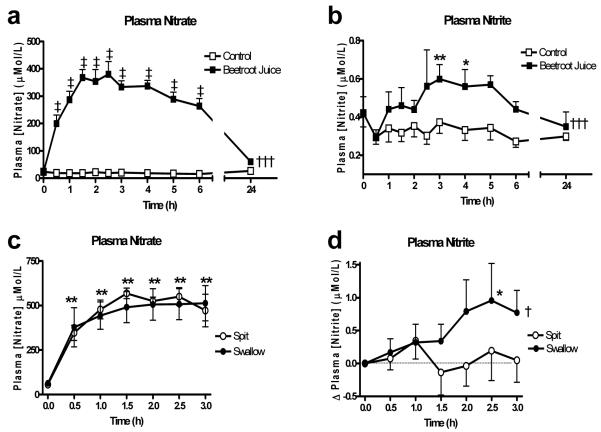
No changes in plasma nitrate or nitrite concentration were found following ingestion of water. In contrast following consumption of juice, there was a rapid rise (~16-fold) in nitrate concentration appearing after the first 30 min, peaking at 1.5h and remaining at this level up to 6h following ingestion (P<0.001 compared to control). Nitrate levels showed a trend to remain elevated at 24h following beetroot juice compared to water (P=0.05). Plasma nitrite also increased significantly (2-fold) following beetroot juice ingestion. An effect that reached a peak at 3h and remaining at this raised level up until 5 h after juice ingestion. Levels had returned to near baseline by 24h (Figure 1). Plasma K+ concentration increased rapidly following beetroot juice ingestion peaking by 1h but had returned to baseline levels by 3h (Online Supplement Figure S1, please see http://hyper.ahajournals.org).
Figure 1.
The effect of beetroot juice on the plasma concentrations of (a) nitrate and (b) nitrate and the effects of spitting versus swallowing of saliva on plasma concentrations of (c) nitrate, (d) nitrite. Data expressed as mean±SEM. Significance shown as: ANOVA of curve of †††P<0.001 beetroot juice vs. control, †P<0.05 spitting vs. swallowing followed by ‡P<0.001 Bonferroni post test of beetroot juice v control, *P<0.05, **P<0.01 Dunnett’s post test compared to baseline.
Dietary nitrate lowers BP
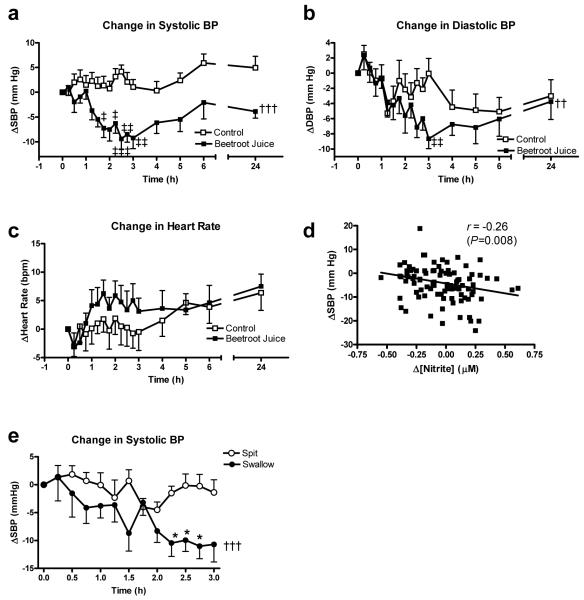
There were no differences in BP between the two groups during the hour prior to ingestion of beetroot juice or water. However, BP began to decrease from 1h following ingestion of juice compared to the water control (Figure 2). The peak difference in systolic BP was achieved at 2.5h following ingestion with a drop of 10.4±3.0 mmHg (P<0.01), whilst the peak differences in diastolic BP and MAP were seen at 3h post ingestion, with changes of 8.1±2.1 mmHg and 8.0±2.1 mmHg respectively (both P<0.01, Figure 2). At 24h, systolic BP was 4.4 mmHg lower with beetroot juice than water, although not statistically significantly different (P=0.058). However, systolic BP was significantly reduced by ~6 mmHg at 24h after beetroot juice ingestion compared to −1 h (106.2±2.8 and 112.4±3.4 mmHg respectively, P<0.01) (Figure 2). There were no differences in diastolic BP at 24h. The mean heart rate was not significantly altered over the 1-6h period following beetroot juice or water ingestion (70.2±0.3 and 69.0±0.5 bpm respectively, Figure 2). The changes in BP were related to the plasma nitrite concentration as demonstrated by a significant inverse correlation between the change in plasma nitrite concentration and the change in systolic BP from baseline (Pearson’s r=−0.26, P=0.008, Figure 2). However, there was no significant correlation between change in plasma nitrate concentration and change in systolic BP (Pearson’s r=−0.17, P>0.05).
Figure 2.
The effect of beetroot juice on the change from baseline in (a) systolic BP (, (b) diastolic BP, (c) heart rate, (d) correlation of change in BP with change in plasma nitrite concentration and (e) effect of spitting versus swallowing of saliva on changes in systolic BP following beetroot juice. Data expressed as mean ± SEM. Significance shown as: ††P<0.01, †††P<0.001 ANOVA of curve of beetroot juice vs. control, ‡P<0.05, ‡‡P<0.01, ‡‡‡P<0.001 Bonferroni post test of beetroot juice vs. control; †P<0.001 compared to control pre-I/R, ‡P<0.05 compared to post-I/R with beetroot juice.
The functional effects of an acute dietary nitrate load are due to nitrite
Interrupting the enterosalivary circulation by spitting out all saliva blocked the rise in plasma nitrite concentration at 2.5h (Figure 1) (P<0.05) but had no significant effect on plasma nitrate or plasma K+ concentration (Online Supplement Figure S1,please see http://hyper.ahajournals.org). Spitting also blocked the reduction in SBP (P<0.05 compared to swallowing; Figure 2). Platelet aggregation to ADP and collagen were inhibited 2.5h after beetroot ingestion (both P<0.001), however no inhibition of platelet aggregation was seen with spitting (see Online Supplement, Figure S2,please see http://hyper.ahajournals.org).
Dietary nitrate protects against endothelial dysfunction
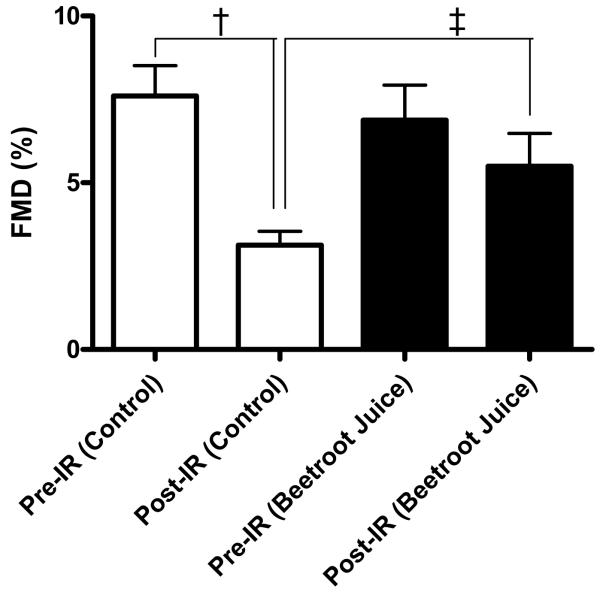
I/R of the forearm significantly reduced the FMD response in control subjects resulting in an approximate ~60 % suppression of the FMD response (P<0.001, Figure 3). In contrast, whilst beetroot juice did not alter pre-ischemic brachial artery dilatation, it protected against I/R-induced depression of this response as evidenced by complete prevention of ischemia-induced endothelial dysfunction (P<0.05, Figure 3).
Figure 3.
Flow mediated dilatation (FMD) in ischemia-reperfusion (I/R) in control and the effect of beetroot juice. Data expressed as mean±SEM. Significance shown as: †P<0.001 compared to control pre-I/R, ‡P<0.05 compared to post-I/R with beetroot juice.
Discussion
Determining how vegetables confer protection against cardiovascular disease, and exploiting these to therapeutic advantage, is likely to have considerable health and economic implications. Whilst the protective effects of vegetable-rich diets have previously been attributed to the antioxidant vitamin content, recent large scale clinical trials have failed to provide evidence in support of this hypothesis8;9. In this study we have shown that ingestion of dietary nitrate (beetroot juice) results in increased plasma nitrite concentration via bioconversion in vivo. This bioactive nitrite substantially decreases BP, inhibits platelet aggregation and prevents endothelial dysfunction in healthy volunteers. These findings suggest that dietary nitrate likely plays a major role in mediating the beneficial effects of a vegetable-rich diet.
Following ingestion of beetroot juice plasma nitrate concentration increased rapidly (within 30 min), peaking at 1.5h. In contrast, the appearance of nitrite in the circulation was considerably slower, peaking at 2.5-3h. The vast majority of absorbed inorganic nitrate is ultimately excreted in the urine, but up to 25% of plasma nitrate is also excreted in the saliva31;32. The exact mechanism for this concentrating effect is unknown, but the consequence is the provision of substrate for the nitrate reductases expressed by bacteria that colonize the dorsal surface of the tongue, resulting in the reduction of nitrate to nitrite. This nitrite is swallowed and in the acidic environment of the stomach is then reduced to NO or re-enters the circulation as nitrite (Figure 4)33. In the present study the beetroot juice consumed contained substantial amounts of nitrate, but undetectable quantities of nitrite, supporting the thesis that the delayed appearance of nitrite is likely due to in vivo processing and that this enterosalivary circuit likely underlies the time-lag in the appearance of nitrite in the plasma observed following ingestion of beetroot juice. That this is the pathway employed to elevate circulating nitrite concentration following a nitrate load is supported by the finding in the 2nd volunteer study where interruption of this circuit, by avoidance of swallowing of saliva14 for 3h subsequent to beetroot ingestion, blocked the rise in plasma nitrite but not nitrate concentration.
Figure 4.
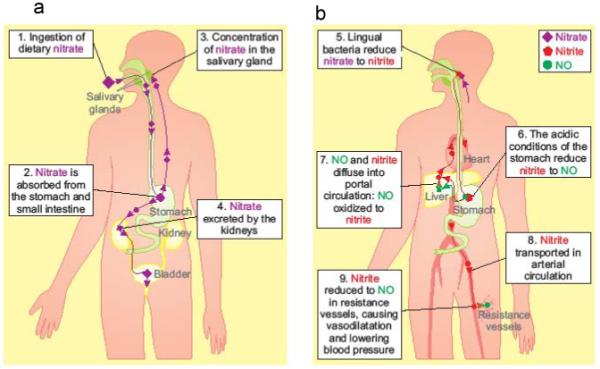
The fate of dietary nitrate, derived from consuming beetroot juice. Systemically absorbed nitrate is concentrated 10-fold in the salivary glands (Left hand panel) and undergoes an enterosalivary circulation where it is reduced to nitrite by bacterial nitrate reductases on the dorsal surface of the tongue, and swallowed into the stomach providing a source of systemically available nitrite/NO. Right hand panel- nitrite is transported in the arterial circulation to resistance vessels, where lower O2 tension favors the reduction of nitrite to NO, causing vasodilatation, with consequent lowering of BP.
Beetroot juice ingestion lowered BP in healthy volunteers. There was a lag period of approximately 1-2h, following ingestion, with a peak drop in BP occurring after 3-4 h. This time course of reduction in BP correlated with the appearance and peak levels of nitrite in the circulation; an effect that was absent in individuals within whom the enterosalivary circuit was disrupted by avoidance of swallowing. These observations, together with the fact that plasma nitrite, and not nitrate, concentration correlated with the decreases in BP implicates nitrite as the likely functional mediator of the beetroot juice-induced effects on BP.
Exactly how nitrite mediates this decrease in BP is uncertain however, recent evidence demonstrates that nitrite is a potent vasodilator in the human forearm25, and it is likely that such vasodilator activity underlies the BP effects evidenced here. This activity of nitrite has been attributed to its chemical reduction to the potent vasodilator NO. Until recently, it was assumed that the only route for NO synthesis in vivo was via NOS activity and that nitrite and nitrate were inert biological end-products of NO metabolism. However, Benjamin et al., and Lundberg et al., both independently demonstrated, in 1994, that nitrite derived from dietary nitrate was a substrate for NOS-independent production of NO in the acidic conditions of the human stomach12;13. That this might be a mechanism that operates in the cardiovascular system has attracted considerable attention over the past 5 years. In 1995 Zweier and co-workers demonstrated that during ischemic conditions in the heart sufficient acidiosis develops permitting NO generation from endogenously stored nitrite34. More recently this potential for nitrite-derived NO production has been clearly demonstrated in both in vitro and in vivo animal models of I/R injury in various organs23;35. Whilst this conversion is, in part, brought about by chemical acidification as in the stomach, there is an additional component that has been attributed to the reductant activity of at least, but principally, two distinct proteins; XOR and deoxyhemoglobin21. In addition to occurring under ischemic conditions there is mounting evidence to support the thesis that nitrite reduction also occurs in physiological conditions within the blood vessel, resulting in alterations in vasoactivity and BP in both animal models and normal volunteers25;36. It is likely that nitrate-derived nitrite generation, in the present study, provides an intravascular store of NO that results in arterial dilatation within the microcirculation to produce a decrease in peripheral resistance and hence a reduction in BP (Figure 4).
Support for the thesis that nitrate was the component of the beetroot juice responsible for the effects seen comes from a recent study where supplementation of dietary nitrate by administration of sodium nitrate (0.1 mmol/kg/day) to healthy volunteers over three days reduced diastolic (but not systolic) BP by 3.7 mmHg compared to sodium chloride37. In addition the loss of functionality of the beetroot juice in terms of BP by spitting of saliva further implicates dietary nitrate. It has, however, been proposed that the high K+ content of fruit and vegetables could account for the BP lowering effects of such a diet38. In the present study K+ concentration was measured in 6 different samples of beetroot juice and found to be 92.88 ± 0.68 mMol/L. Ingestion of the juice resulted in a rapid rise in plasma K+ concentration, however, levels had returned to baseline by 2.5 h and this rise was not significantly altered by avoidance of swallowing suggesting that the effects of beetroot juice on BP were independent of K+ levels.
The in vivo half life of nitrite (~1.5h) in the present studies is much longer than the ex-vivo half life of <2 min39, suggesting that nitrite is continuously produced from nitrate (which has a long half life of ~8h) via the enterosalivary circulation. Nitrite is readily distributed throughout the body40;41. More recently it has been demonstrated in rats that the absorption of nitrite across the abdominal cavity is rapid, and that it is then widely and rapidly distributed, reaching near steady-state concentrations in all tissues assessed within about 5 min42. It is possible that this uptake of nitrite results in the provision of stores of nitrite that are then slowly released back into the circulation over time and such a mechanism may underlie the sustained effects of nitrite on BP reduction in the present study.
Nitrite also confers marked protection against I/R and hypoxic injury, an effect that has been demonstrated in the myocardial, hepatic, renal, pulmonary and cerebral vasculature21;22. This activity of nitrite has been attributed to its reduction to NO during ischemia facilitated predominantly by XOR or deoxyhaemoglobin21. In keeping with NO bioactivity, we also show that the beneficial effects of a dietary nitrate load are not limited to BP, but also include a reversal of the endothelial dysfunction associated with I/R injury in the brachial artery and inhibition of ex vivo platelet aggregation responses to the aggregating stimuli ADP and collagen. These cytoprotective effects of beetroot juice consumption likely relate to the elevation of systemic nitrite (which is converted to protective NO particularly during ischemia), since the responses were measured at a time-point associated with the peak in plasma nitrite concentration and interruption of the enterosalivary circuit for nitrate reduction abolished these effects. In addition, that dietary nitrate is protective against I/R injury is supported by the very recent findings of Bryan and colleagues43 in mice elegantly demonstrating the reduction of myocardial infarct damage following increases in dietary nitrite and nitrate intake. Many pre-clinical studies support the concept that NO is protective in I/R44 and NO is well known to inhibit platelet aggregation45;46 however, there has been limited translation of protective effects of NO in human studies (e.g. the ISIS-4 trial using the organic nitrate, isosorbide mononitrate47). This may be due to timing of treatment or the difference between types of NO donor; specifically it is important to note that significant free radical production is associated with organic nitrate activity and it is possible that this may be an important mechanism underlying the failure to demonstrate protection.
In 2002 the WHO reported that ~11 % of all disease burden was a direct consequence of the deleterious effects of chronic hypertension. The economic burden is therefore immense and cost-effective interventions would be of considerable value. Studies in patients and normal volunteers show that hypertension is associated with impaired endogenous NO synthesis and we propose that provision of an alternative NOS-independent source of NO in the form of dietary nitrate, such as beetroot juice, would restore NO levels and improve hypertension. Current treatment guidelines target individuals with established hypertension and vascular disease, pathologies of considerable mechanistic complexity that are suitably matched with an ever increasingly complex treatment regime. However, recent calls suggest that treatment should occur before disease has evolved in individuals with ‘pre-hypertension’48, or even in normotensives. Such a preventative strategy in relatively ‘well’ individuals would require a minimally interventional approach, based on, and optimised through an understanding of physiological processes. Our data support the thesis that dietary nitrate is likely to have been a major contributor to the BP lowering effects of the fruit- and vegetable-rich diets in previous studies49, and demonstrate that nitrate is likely to underlie the cardioprotective effect of vegetables. In addition, it is also of interest to consider that the BP response to a high fruit and vegetable diet was considerably greater in hypertensives compared to normotensives in the ‘DASH’ study1, and therefore it is possible that the BP effect of dietary nitrate, evidenced in our study of normotensives, will be heightened in hypertensives. Therefore, we advocate consumption of a diet high in nitrate (i.e. a ‘natural’ strategy) to treat (pre-) hypertension and to protect individuals at risk of adverse vascular events.
Perspectives
In summary, an acute dietary nitrate load causes a marked reduction in BP in normotensives, reduces platelet activation and protects against experimentally-induced endothelial I/R injury; effects that correlate with a rise in circulating levels of nitrite derived from dietary nitrate. We hypothesise that this mechanism likely accounts for much of the cardioprotective effects of vegetables and suggests an important role of high dietary nitrate in delaying the development and treatment of prehypertension and hypertension.
Supplementary Material
Acknowledgements
We thank the subjects for participating in this study and Prof Tim Warner for his guidance with the platelet studies.
Sources of funding: AJH is supported by a Wellcome Trust Senior Research Fellowship.
Footnotes
Disclosures: None
Reference List
- 1.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, DASH Collaborative Research Group A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood-pressure-lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet. 1983;1:5–10. doi: 10.1016/s0140-6736(83)91557-x. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 4.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 5.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 6.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 7.Willett WC. Diet and health: what should we eat? Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- 8.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 9.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 10.Ysart G, Miller P, Barrett G, Farrington D, Lawrance P, Harrison N. Dietary exposures to nitrate in the UK. Food Addit Contam. 1999;16:521–532. doi: 10.1080/026520399283669. [DOI] [PubMed] [Google Scholar]
- 11.Petersen A, Stoltze S. Nitrate and nitrite in vegetables on the Danish market: content and intake. Food Addit Contam. 1999;16:291–299. doi: 10.1080/026520399283957. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 17.Plavnik FL, Ajzen SA, Christofalo DM, Barbosa CS, Kohlmann O., Jr. Endothelial function in normotensive and high-normal hypertensive subjects. J Hum Hypertens. 2007;21:467–472. doi: 10.1038/sj.jhh.1002164. [DOI] [PubMed] [Google Scholar]
- 18.Giansante C, Fiotti N. Insights into human hypertension: the role of endothelial dysfunction. J Hum Hypertens. 2006;20:725–726. doi: 10.1038/sj.jhh.1001951. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosentino F, Volpe M. Hypertension, stroke, and endothelium. Curr Hypertens Rep. 2005;7:68–71. doi: 10.1007/s11906-005-0057-5. [DOI] [PubMed] [Google Scholar]
- 21.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr., Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 22.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-Derived Nitric Oxide Protects the Rat Kidney against Ischemia/Reperfusion Injury In Vivo: Role for Xanthine Oxidoreductase. J Am Soc Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 23.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 25.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 26.McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211–214. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bednar B, Condra C, Gould RJ, Connolly TM. Platelet aggregation monitored in a 96 well microplate reader is useful for evaluation of platelet agonists and antagonists. Thromb Res. 1995;77:453–463. doi: 10.1016/0049-3848(95)93881-y. [DOI] [PubMed] [Google Scholar]
- 28.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 29.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannenbaum SR, Weisman M, Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 32.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 34.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 35.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias-Junior CA, Gladwin MT, Tanus-Santos JE. Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Radic Biol Med. 2006;41:1764–1770. doi: 10.1016/j.freeradbiomed.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 38.Adrogue HJ, Madias NE. Sodium and Potassium in the Pathogenesis of Hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 40.Parks NJ, Krohn KJ, Mathis CA, Chasko JH, Geiger KR, Gregor ME, Peek NF. Nitrogen-13-labeled nitrite and nitrate: distribution and metabolism after intratracheal administration. Science. 1981;212:58–60. doi: 10.1126/science.7209517. [DOI] [PubMed] [Google Scholar]
- 41.Thayer JR, Chasko JH, Swartz LA, Parks NJ. Gut reactions of radioactive nitrite after intratracheal administration in mice. Science. 1982;217:151–153. doi: 10.1126/science.6211766. [DOI] [PubMed] [Google Scholar]
- 42.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 43.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 45.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loscalzo J. Nitric Oxide Insufficiency, Platelet Activation, and Arterial Thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 47.ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. The Lancet. 1995;345:669–682. [PubMed] [Google Scholar]
- 48.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr., Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 49.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.