Abstract
Intratumoral injections of a replication-incompetent adenovirus (Ad) expressing melanoma differentiation–associated gene-7/interleukin-24 (Ad.mda-7), a secreted cytokine displaying cancer-selective, apoptosis-inducing properties, profoundly inhibits prostate cancer (PC) growth in immune-incompetent animals. In contrast, Ad.mda-7 is ineffective in PCs overexpressing antiapoptotic proteins such as Bcl-2 or Bcl-xL. However, intratumoral injections of a conditionally replication-competent Ad (CRCA) in which expression of the adenoviral E1A gene is driven by the cancer-specific promoter of progression-elevated gene-3 (PEG-3) and which simultaneously expresses mda-7/interleukin (IL)-24 in the E3 region of the Ad (Ad.PEG-E1A-mda-7), a cancer terminator virus (CTV), is highly active in these cells. A major challenge for gene therapy is systemic delivery of nucleic acids directly into an affected tissue. Ultrasound (US) contrast agents (microbubbles—MBs) are viable candidates for gene delivery/therapy. Here, we show that MB/Ad.mda-7 complexes targeted to DU-145 cells using US dramatically reduced tumor burden in xenografted nude mice. Additionally, US-guided MB/CTV delivery completely eradicated not only targeted DU-145/Bcl-xL-therapy-resistant tumors, but also nontargeted distant tumors (established in the opposite flank), thereby implementing a cure. These findings highlight potential therapeutic applications of this novel image-guided gene therapy technology for advanced PC patients with metastatic disease.
Introduction
Prostate cancer (PC) is the most common cancer and the second leading cause of cancer-related deaths in men in the United States.1 At present, no effective therapy is available for metastatic PC.2 Advanced PC is refractory to conventional anticancer treatments because of frequent overexpression of antiapoptotic proteins Bcl-2 and/or Bcl-xL.3,4 The melanoma differentiation–associated gene-7/interleukin-24 (mda-7/IL-24) is a secreted cytokine having broad-spectrum, cancer-selective, apoptosis-inducing properties that profoundly inhibits PC cell growth.5 Adenovirus (Ad)-mediated delivery of mda-7/IL-24 (Ad.mda-7) has shown dramatic antitumor effects in animal models and in clinical trials.6,7,8,9 However, forced overexpression of Bcl-2 or Bcl-xL renders PC cells resistant to Ad.mda-7 (ref. 4). In contrast, a conditionally replication-competent Ad (CRCA) (a cancer terminator virus—CTV), which expresses mda-7/IL-24 (Ad.PEG-E1A-mda-7) can abrogate acquired resistance of PC cells mediated through Bcl-2 and/or Bcl-xL overexpression causing growth arrest and apoptosis and selectively replicating in PC xenografted cells in athymic nude mice. Moreover, the CTV completely eradicates not only primary tumors but also distant tumors following repeated intratumoral injections into the primary tumor site.10,11
A major challenge for effective gene therapy is the ability to specifically deliver nucleic acids and potentially toxic gene products directly into diseased tissue. Progress in gene therapy has been hampered by concerns over the safety and practicality of viral vectors, particularly for intravenous delivery, and the inefficiency of currently available nonviral transfection techniques.12 Viruses are appealing delivery vectors because of their ability to efficiently transfer genes with sustained and robust expression. Recombinant Ads are one of the most common gene transfer vectors utilized in human clinical trials, but systemic administration of this virus is thwarted by host innate and adaptive antiviral immune responses, which can limit and/or preclude repetitive treatment regiments.13
The quest for novel, safe, and more efficient systemic gene delivery systems has recently highlighted ultrasound (US) contrast agents (microbubbles—MB) as a potential candidate for enhancing delivery of molecules to target tissue.14,15,16,17 Currently used US contrast agents (MBs) contain high-molecular weight gases with less solubility and diffusivity, which improves MB persistence and allows passage through the microcirculation. MBs can be injected in peripheral veins, because the more robust bubbles can recirculate through the systemic circulation numerous times, surviving for several minutes within the bloodstream.17,18 The ideal MB diameter most likely is between 2.5 and 4 µm. This is small enough to prevent entrapment within the pulmonary capillary bed (ranging from 5 to 8 µm in diameter), but big enough to entrap and protect viral vectors such as Ad from the environment.
We previously demonstrated the feasibility of site-specific gene delivery–mediated by diagnostic US using Ad-GFP encapsulated in commercially available US contrast agents in vitro and in vivo.12 An additional goal of our previous study was to determine whether incubation of the MBs with complement could improve specificity of viral transgene transduction to the target tissue/organ allowing a simplified approach to encapsulation of the viral vectors with commercially available contrast agents. In the current investigation, we tested a US contrast agent provided by Targeson (San Diego, CA) and the portable SonoSite Micro-Maxx US platform (SonoSite, Bothell, WA) equipped with an L25 linear array transducer. Targeson's agents are lipid-encapsulated perfluorocarbon MBs with a mean diameter of 2.5 µm that can be used in a wide variety of animal models, and are compatible with virtually all US scanners. The gas-filled microspheres effectively lower the energy threshold for nonthermal cavitation. This allows diagnostic transducers operating within the energy levels mandated by the US Food and Drug Administration to be used for drug/gene delivery. US-targeted MB destruction enables focal release of entrapped materials as well as the creation of small shock waves that increase cellular permeability.19 In addition, the MBs protect the viruses from rapid degradation by the immune system, thus allowing for intravenous injection rather than direct target organ delivery by catheter-based approaches or operative bed injection.12,17 This is particularly important in cancer gene therapy of potentially inaccessible tumors because the MBs may also limit the amount of inflammatory response to the viruses and may allow repeated injections.
The ultimate goal of our research programs is to develop efficacious therapies for cancer. A primary focus is to engineer effective and safe delivery systems for viruses, chemotherapeutic agents, and small molecule drugs. In this study, we provide proof-of-principle for two essential components of this process, a site-specific gene delivery approach mediated by diagnostic US generated by a portable platform that works efficiently in vivo in combination with Ads delivering a highly effective, broad-based cancer gene therapeutic mda-7/IL-24. Evidence is provided that this combination has profound effects in animal models containing therapy-resistant human PC cells.
Results
Targeson MBs and a SonoSite portable Micro-Maxx US platform efficiently targets Ad-GFP viruses to tumors
We previously documented the feasibility of in vivo gene delivery mediated by diagnostic US using Ad-GFP encapsulated in a series of commercially available US contrast agents.12 In this investigation, we tested a different US contrast agent available from Targeson and the portable SonoSite Micro-Maxx US platform (SonoSite) equipped with an L25 linear array transducer. Targeson's agents are lipid-encapsulated perfluorocarbon MBs with a mean diameter of 2.5 µm that can be used in a wide variety of animal models, and are compatible with virtually all US scanners.20 Targeson agents are normally sold as already reconstituted contrast agents that are stable for 3 months from arrival, and for this study we obtained a custom-made freeze-dried Targeson contrast agent (perfluorocarbon MBs, encapsulated by a lipid monolayer and poly(ethyleneglycol) stabilizer) to be reconstituted with the viruses as previously described.12
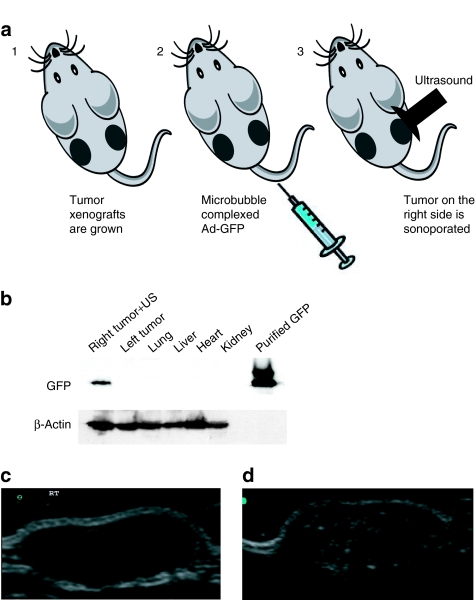
To confirm the ability of the lyophilized Targeson US contrast agent to deliver viruses efficiently and specifically to defined sites in vivo, we performed a pilot study in which tumor xenografts were established in both flanks of athymic nude mice by injecting each site with 2 × 106 DU-145 human prostate carcinoma cells (Figure 1a). The DU-145 tumor-bearing nude mice (n = 10) were then injected in their tail vein with 100 µl of US contrast agent that was reconstituted with Ad-GFP or water as control. A portable SonoSite Micro-Maxx US platform (SonoSite) equipped with an L25 linear array transducer set at 0.7 Mechanical Index, 1.8 MPa for 10 minutes was used to sonoporate only the tumor implanted on the right side (Figure 1a). Mice were killed 96 hours after treatment and tumors (right and left side), lung, heart, liver, and kidney were harvested and snap frozen. Figure 1b shows the specific delivery to the right tumor as evidenced by expression of the green fluorescent protein (GFP) in an immunoblot in which total protein extracts were run on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. As a GFP control, we ran a glutathione-S-transferase–GFP fusion protein. Protein gel loading was normalized using β-actin as a control.
Figure 1.
(a) Schematic representation of the microbubble delivery of Ad-GFP complexes and ultrasound (US) release in a tumor target site of the mouse. (b) Western blot analysis of Ad-GFP/microbubble–transduced DU-145 tumor xenografts. Immunoblot showing the expression levels of green fluorescent protein (GFP) in DU-145 cells following ultrasound-targeted microbubble/Ad transduction of GFP at 96 hours. Only the tumor on the right flank was sonoporated for 10 minutes resulting in the delivery and expression of GFP. The left tumor, heart, lung, liver, and kidney were negative for GFP expression. Purified glutathione-S-transferase–GFP was used as a positive control. Protein gel loading was normalized using β-actin as a control. (c) Ultrasound imaging and US contrast enhancement of in vivo transduced DU-145 tumor xenografts. B-mode US imaging of a tumor before MB contrast agent injection. (d) B-mode US imaging of the same tumor depicted in c following injection of microbubbles/Ad-GFP complexes. MBs cavitation within the targeted tumor dramatically enhances the tumor image within the US field of view. Ad, adenovirus, MB, microbubbles.
US-targeted MB destruction enables focal release of entrapped materials as well as the creation of small shock waves that are visualized as an enhancement of the image on the US scanner. Figure 1c depicts the B-mode US imaging of a sonoporated tumor before injection with the MB/Ad-GFP complex contrast agent. Figure 1d shows the B-mode US imaging of the same sonoporated tumor following MB/Ad-GFP complex injection. The image enhancement of the targeted tumor from cavitation of the MBs within the US field of view is clearly discernable indicating that the US settings are efficient in targeting MB destruction.
MB-assisted Ad.mda-7 gene delivery inhibits DU-145 human PC growth in vivo
In vitro and in vivo Ad-mediated gene transfer of the human mda-7/IL-24 gene (Ad.mda-7) potently suppresses the growth of human cancer cells with no apparent toxicity to normal cells.6,8,9,21,22,23,24,25,26,27,28,29,30 Repeated intratumoral administration of Ad.mda-7 to tumor xenografts of various histological origin results in growth suppression via induction of apoptosis and antiangiogenic mechanisms.4,5,6,7,8,9,25,28,31 Additionally, mda-7/IL24 induces a profound “bystander” antitumor effect resulting in tumor growth suppression not only in the treated tumors, but also in untreated distant tumors.6,8,9,10,11,22,23,25,27,32,33,34,35,36 Although these results have been encouraging, this approach is limited as systemic delivery of Ad for treatment of disseminated cancer have not shown significant efficacy.
We have employed a novel systemic delivery approach to target Ad release in a site-specific manner that consists of Ad incorporated in MBs combined with diagnostic US.12 Proof-of-principle for this strategy comes from studies using Ad to systemically deliver the GFP gene in a tissue-specific manner.12 Because mda-7/IL-24 has shown significant potential as a selective and effective anticancer agent in multiple animal model studies and in a Phase I intratumoral gene therapy trial in patients with advanced solid cancers,6,7,8,9,21,22,23,28 we tested the capacity of this approach to deliver Ad expressing this novel cytokine in prostate adenocarcinoma nude mouse xenograft models. For these studies, we used DU-145 human prostate carcinoma cells and DU-145 cells genetically engineered to express elevated levels of Bcl-xL (DU-Bcl-xL),4 which is a common event in advanced PC and provokes resistance to multiple chemotherapeutic agents and to mda-7/IL-24 (refs. 2,3,4,5). The therapeutic arm of this work included two different viral constructs to deliver mda-7/IL-24, Ad.mda-7, a nonreplicating Ad similar to the one used in Phase I clinical trials,26 and the CTV, a CRCA capable of expressing mda-7/IL-24 that has been previously shown to completely eradicate not only primary breast, prostate, and melanoma tumors but also distant tumors by intratumoral injections in a nude mouse model.11,33,34,37
To test this new therapeutic approach for tumor delivery, DU-145 or DU-Bcl-xL tumor xenografts were established on both flanks of nude mice by injecting 2 × 106 cells in each side of the animals. DU-145 and DU-Bcl-xL tumor-bearing nude mice (n = 10 in each group) were then injected in their tail vein with 100 µl of US contrast agent that was reconstituted with Ad-GFP or water as control. Additional DU-145 and DU-Bcl-xL tumor control nude mice (n = 10 in each group) were injected in the tail vein with 100 µl of Ad.mda-7 or the CTV (Ad.PEG-E1A-mda-7) without US contrast agent. Alternatively, tumor-bearing animals were injected in their tail vein with 100 µl of US contrast agent that was reconstituted with Ad.mda-7 or the CTV. A portable SonoSite Micro-Maxx US platform (SonoSite) equipped with an L25 linear array transducer set at 0.7 Mechanical Index, 1.8 MPa for 10 minutes was used to sonoporate the tumor implanted on the right side. In this study, gene therapy treatments were started 10 weeks after the injection of the cell lines, when tumors reached an approximate volume of 150–200 mm3. Mice were injected once a week for 4 weeks for a total of four treatments. Mice were killed 2 weeks after the end of the treatments to determine whether tumor suppression was reversible or irreversible. At the end of the study, tumors (right and left flank), lung, heart, liver, and kidney were harvested and snap frozen using liquid nitrogen.
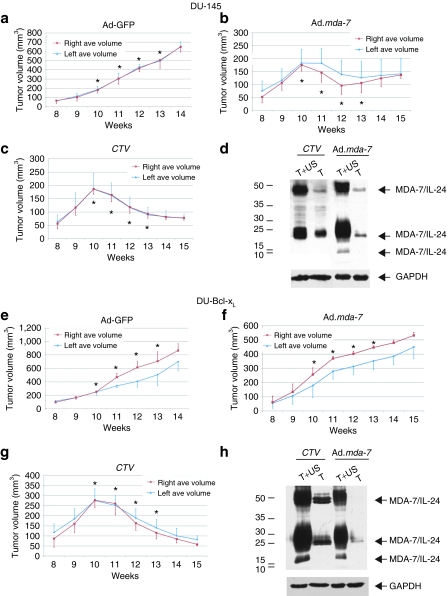
US of Ad-GFP/MB complexes in the right side tumor resulted in progressive growth of the tumors on both flanks (Figure 2a,e). The results shown in Figure 2 represent the average tumor volumes measured in a minimum of seven mice for each mda-7/IL-24 group and a minimum of five mice for each control GFP group. All the mice were injected in the tail vein with the MB/Ad complexes and only the tumor on the right flank was sonoporated. Interestingly, we observed that MB-mediated Ad.mda-7 gene therapy inhibited the growth of DU-145 prostate tumor xenografts during the treatment regimen (Figure 2b), while the CTV/MB-mediated gene therapy resulted in a steady progressive tumor regression that lasted an additional 2 weeks post-treatment (Figure 2c).
Figure 2.
Growth curves and western blot analysis of large DU-145 and DU-Bcl-xL tumor xenografts treated with microbubble encapsulated Ad-GFP, Ad.mda-7, or cancer terminator virus (CTV) (Ad.PEG-E1A-mda-7) and treated with ultrasound (US) in the right tumor. Subcutaneous tumor xenografts from DU-145 and DU-Bcl-xL were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumor treatments were initiated when tumors reached a size of 250–350 mm3. Arrows point at tumors and asterisks point at treatment times. (a) Measurement of green fluorescent protein (GFP)-treated DU-145 tumor volumes. The data represent mean ± SD with at least 5 mice in each group. (b) Measurement of Ad.mda-7-treated DU-145 tumor volumes. The data represent mean ± SD with at least 7 mice in each group. (c) Measurement of CTV-treated DU-145 tumor volumes. The data represent mean ± SD with at least 7 mice in each group. (d) Western blot analysis of protein extracts from representative DU-145 tumor samples treated with Ad.mda-7 or CTV. The immunoblot was reacted with anti-MDA-7/IL-24. Arrowheads point at the various glycosylated forms of MDA-7/IL-24. Protein gel loading was normalized using anti-GAPDH as a control. (e) Measurement of GFP-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with at least 5 mice in each group. (f) Measurement of Ad.mda-7-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with at least 7 mice in each group. (g) Measurement of CTV-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with at least 7 mice in each group. (h) Western blot analysis of protein extracts from representative DU-Bcl-xL tumor samples treated with Ad.mda-7 or CTV. The immunoblot was reacted with anti-MDA-7/IL-24. Arrowheads point at the various glycosylated forms of MDA-7/IL-24. Protein gel loading was normalized using anti-GAPDH as a control. Ad, adenovirus; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
As predicted from previous studies,4,5,11 Ad.mda-7 was ineffective in causing a therapeutic response in tumor xenografts on either flank developed from DU-Bcl-xL cells (Figure 2f). In contrast, the conditionally replication-competent CTV (Ad.PEG-Prom-mda-7) elicited a sustained growth inhibition of the therapy resistant DU-Bcl-xL tumor xenografts (Figure 2g). A western blot analysis of total protein extracts from the harvested tumors showed expression of MDA-7/IL-24 protein in both the tumor samples implanted on the right and left flank (Figure 2d,h) validating the “bystander” effects of MDA-7/IL-24 previously reported.11,33,35,36,37 In the case of CTV, this amplified expression of MDA-7/IL-24 in the noninjected left tumor may also reflect secondary viral infection by CRCA.11 GAPDH expression was used to confirm equal loading of the gel. No tumor regression was observed in mice bearing DU-145 and DU-Bcl-xL control tumors when injected intravenously with comparable doses of unprotected Ad.mda-7 and CTV viruses (Supplementary Figures S1 and S2).
MB-assisted CTV gene delivery eradicates PC growth in vivo
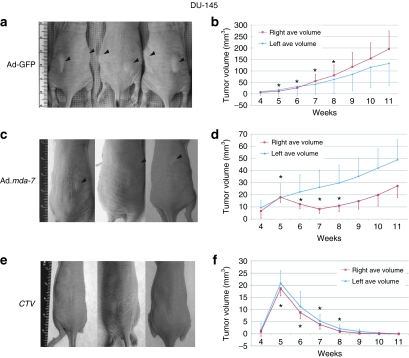
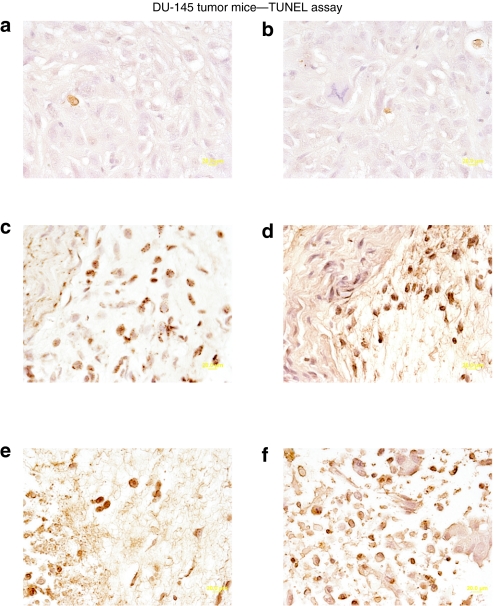
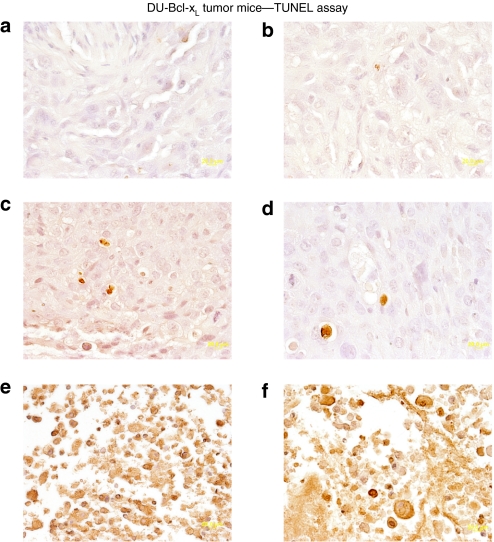
Based on the initial positive results obtained with the CTV/MB delivery approach, further experiments were performed in nude mice. Tumor xenografts were again established on both flanks of nude mice by injecting 1.5 × 106 DU-145 or DU-Bcl-xL cells. After the development of palpable tumors in 5 weeks (25–50 mm3), four injections of the various MB/Ad complexes into the tail vein once a week (for a total of four weeks) with US for 10 minutes on the tumor on the right side were performed. No treatment was performed on the tumor xenografted on the left flank. Injection of Ad-GFP-MB and US in the right tumor plus US did not significantly impact tumor growth on either flank (Figure 3a,b). The data are presented as the average tumor volumes measured in at least nine mice for each active experimental group, with six mice in the control Ad-GFP group. In the case of CTV/MB-treated animals, the experiment was terminated after 6 weeks because DU-145 tumors on both sides showed regression after two injections, and with four injections tumors were completely eradicated (Figure 3e,f). Additionally, control GFP-transduced DU-145 tumor xenografts reached tumor volumes of 250–300 mm3 after similar treatment. Although Ad.mda-7-MB treatment visibly inhibited the growth of tumors on the sonoporated flank, it had some inhibitory effect on tumors on the left side, which was not statistically significant (Figure 3c,d). Interestingly, treatments with the CTV/MB complexes resulted in disruption of the tumor cytoarchitecture, as visualized by hematoxylin and eosin staining (data not shown), which correlated with an increase in the number of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-positive tumor cells in both sonoporated (Figure 4e) and nonsonoporated tumors (Figure 4f). Control tumors treated with Ad-GFP/MB complexes were mostly TUNEL negative, showing a few cells positive to the TUNEL colorimetric reaction as depicted in Figure 4a (sonoporated tumor on the right flank) and Figure 4b (untreated tumor). Additionally, DU-145 tumor xenografts treated with Ad.mda-7-MB complexes showed a higher percentage of TUNEL-positive tumor cells (Figure 4c) than the untreated left flank (Figure 4d).
Figure 3.
Growth curves of DU-145 tumor xenografts treated with microbubble/ultrasound-guided Ad-GFP, Ad.mda-7, or cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-145 cells were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumor treatments were initiated when tumors reached a size of 25–35 mm3. (a,c,e) Photographs of animals representative of each group. Black arrows indicate tumors. (b) Measurement of GFP-treated DU-145 tumor volumes. The data represent mean ± SD with at least 6 mice in each group. (d) Measurement of Ad.mda-7-treated DU-145 tumor volumes. The data represent mean ± SD with at least 9 mice in each group. (f) Measurement of CTV-treated DU-145 tumor volumes. The data represent mean ± SD with at least 9 mice in each group. Asterisks indicate treatment times. Ad, adenovirus; GFP, green fluorescent protein.
Figure 4.
Colorimetric TUNEL assay of DU-145 tumor xenografts treated with microbubble/ultrasound (US)-guided Ad-GFP, Ad.mda- 7, or cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-145 cells were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumors were removed, fixed, sectioned, and stained to determine levels of double-stranded DNA breaks (TUNEL). Microscopy for TUNEL sections was under visible light at ×40 magnification (a representative of three separate tumors). (a,b) TUNEL staining of left and right side DU-145 tumors following systemic injection of Ad-GFP-microbubble plus US treatment of the tumor on the right side. (c,d) TUNEL staining of left and right side DU-145 tumors following systemic injection of Ad.mda-7-microbubble plus US treatment of the tumor on the right side. (e,f) TUNEL staining of left and right side DU-145 tumors following systemic injection of CTV/microbubble plus US treatment of the tumor on the right side. Ad, adenovirus; GFP, green fluorescent protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Experiments were next performed in the therapy resistant DU-Bcl-xL xenografted mice (Figure 5). In this study, mice with established xenografts on both flanks were injected in the tail vein with the MB/Ads complexes, and only the tumors on the right flank were sonoporated. Animals receiving the Ad-GFP/MB complexes plus US treatment showed no statistically significant effect on the growth of DU-Bcl-xL tumors (Figure 5a,b). The data presented are the average tumor volumes of nine mice receiving therapeutic gene treatment and six mice receiving Ad-GFP treatment. The experiment was terminated after 6 weeks with injections of CTV/MB complexes because DU-Bcl-xL tumors on both sides showed regression after only two injections, and within four injections profound tumor regression was evident (Figure 5e,f). Additionally, 3 weeks after the last injections tumors were completely eradicated. Control Ad-GFP/MB complex treated DU-Bcl-xL tumor xenografts reached tumor volumes of 350–400 mm3 requiring the mice to be killed as per our animal protocol.
Figure 5.
Growth curves of therapy resistant DU-Bcl-xL tumor xenografts treated with microbubble/ultrasound-guided Ad-GFP, Ad.mda-7, or cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-Bcl-xL cells were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Arrows point at tumors and asterisks point at treatment times. (a,c,e) Photographs of animals representative of each group. Black arrows point at tumors. (b) Measurement of Ad-GFP-microbubble-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with a minimum of 6 mice in each group. (d) Measurement of Ad.mda-7-microbubble-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with a minimum of 9 mice in each group. (f) Measurement of CTV-microbubble-treated DU-Bcl-xL tumor volumes. The data represent mean ± SD with a minimum of 9 mice in each group. Ad, adenovirus; GFP, green fluorescent protein.
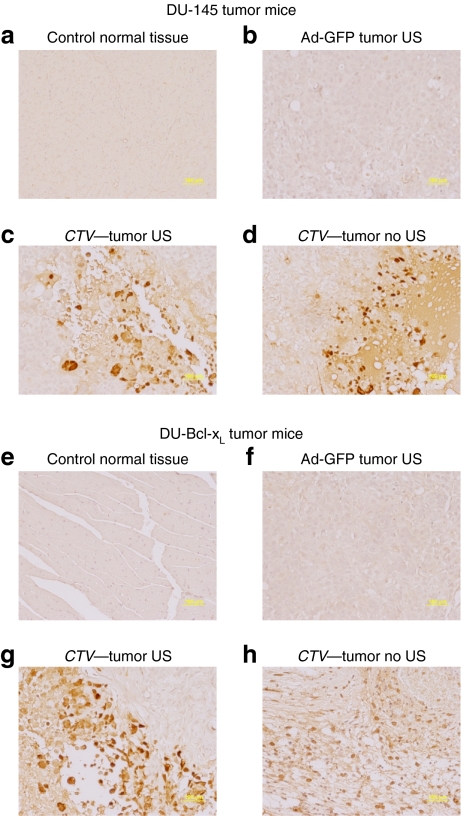
As previously observed with direct intratumoral injection, Ad.mda-7/MB complexes plus US was ineffective in reducing the growth of therapy resistant DU-Bcl-xL tumor xenografts (Figure 5c,d).11 In contrast, treatments with CTV/MB complexes plus US disrupted the tumor cytoarchitecture, which correlated with an increase in the number of TUNEL-positive tumor cells in both sonoporated right (Figure 6e) and untreated left tumors (Figure 6f). Control tumors treated with Ad-GFP/MB complexes were TUNEL negative, whereas tumors treated with Ad.mda-7/MB complexes showed small numbers of TUNEL-positive cells Figure 6c (sonoporated tumor on the right flank) and Figure 6d (nonsonoporated tumor). As previously shown using intratumoral injection11 the CTV virus when delivered by the MB plus US replicated in the treated tumor tissue as reflected by positive staining using antibody reacting with Ad E1A protein (Figure 7). Additionally, as seen using the CTV and intratumoral injection of tumors on one flank of the animal, positive staining against E1A protein was found not only in the sonoporated tumor on the right flank but also on the nonsonoporated tumor on the left flank. Negative staining was found in the control tissues, GFP-transduced, and normal tissues from a CTV-transduced and US-treated mouse. The observation that MB/Ad-mediated gene therapy of CTV/MB complexes plus US completely eradicated the primary and the distant tumor (comparable to a metastasis) provides confidence that this strategy may prove amenable for successfully treating aggressive cancers for which intratumoral direct injection of the CTV virus is not possible due to location, number of metastases, and spread of the malignancy to distant organ sites.
Figure 6.
Colorimetric TUNEL assay of therapy resistant DU-Bcl-xL tumor xenografts treated with microbubble/ultrasound (US)-guided Ad-GFP, Ad.mda-7, or cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-Bcl-xL cells were established in athymic nude mice in both right and left flanks, and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumors were removed fixed, sectioned, and stained to determine levels of double-stranded DNA breaks (TUNEL). Microscopy for TUNEL sections was under visible light at ×40 magnification (a representative of three separate tumors). (a,b) TUNEL staining of left and right side DU-Bcl-xL tumors following Ad-GFP/microbubble treatment. (c,d) TUNEL staining of left and right side DU-Bcl-xL tumors following Ad.mda-7-microbubble treatment. (e,f) TUNEL staining of left and right side DU-Bcl-xL tumors following CTV/microbubble treatment. Ad, adenovirus; GFP, green fluorescent protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 7.
Immunohistochemical analysis of DU-145 and therapy resistant DU-Bcl-xL tumor xenografts treated with microbubble/ultrasound (US)-guided Ad-GFP, Ad.mda-7, or cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-145 or DU-Bcl-xL cells were established in athymic nude mice in both right and left flanks, and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumors were removed fixed, sectioned, and immunostained to determine levels of E1A expression. (a) E1A immunohistochemical staining of control normal tissue from a DU-145 mouse treated with CTV/microbubble. (b) E1A immunohistochemical staining of control tumor tissues from a DU-145 mouse treated with Ad-GFP/microbubble and US. (c,d) E1A immunohistochemical staining of left and right side DU-145 tumors following CTV/microbubble treatment and US treatment of the tumor on the right side. (e) E1A immunohistochemical staining of control normal tissues from a DU-Bcl-xL mouse treated with CTV microbubbles. (f) E1A immunohistochemical staining of control tumor tissues from a DU-Bcl-xL mouse treated with Ad-GFP microbubbles and US. (g,h) E1A immunohistochemical staining of left and right side DU-Bcl-xL tumors following CTV/microbubble treatment of the tumor on the right side. Ad, adenovirus; GFP, green fluorescent protein.
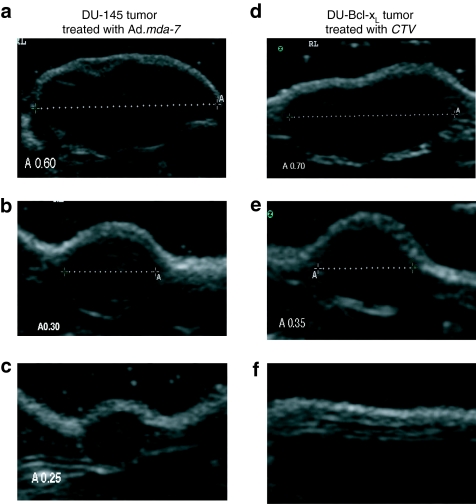
In our study, the size of the tumor was measured twice a week by caliper as well as by B-mode US scanning. Figure 8a shows the US image and measurements of a DU-145 tumor before treatment with Ad.mda-7/MB complexes. Figure 8b,c demonstrates the volume reduction in the same tumor after 2 and 4 weeks of treatments with Ad.mda-7/MB complexes and US. Figure 8d shows the B-mode scan image and measurements of a DU-Bcl-xL tumor before treatment with CTV/MB complexes. Figure 8e,f emphasizes the dramatic volume reduction in the same tumor after 2 and 4 weeks of treatments with CTV/MB complexes and US leading to the eradication of the tumor xenograft. Additionally, no tumor regrowth in the primary or distant sites was evident CTV/MB complex and US-treated DU-Bcl-xL animals after an additional 3 weeks post-treatment. To investigate whether the tumor would reappear after a longer period of time following the last treatment, 3 out of 10 animals initially treated with CTV/MB complexes were not killed at the end point of the study and were maintained for an additional 3 months. The mice were then killed and dissected to look for potential tumor recurrence and/or eventual tumor spread. We did not observe any local tumor reappearance or distant metastasis in the lungs or liver in these mice that were treated with CTV/MB complexes and US indicating that this therapeutic approach could be suitable to target CRCAs to prostate tumors causing the eradication of localized as well as distant metastatic tumors. Future studies testing this approach in immune-competent tumor-bearing and transgenic animals would provide definitive support for exploring this strategy in the context of a Phase I clinical trial.
Figure 8.
B-mode ultrasound (US) imaging of DU-145 tumor xenografts treated with microbubble/US-guided Ad.mda-7 and therapy resistant DU-Bcl-xL tumor xenografts treated with microbubble/US-guided cancer terminator virus (CTV). Subcutaneous tumor xenografts from DU-145 and DU-Bcl-xL cells were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail-vein injection of the indicated microbubble/Ad complexes during a course of 4 weeks. Tumor volumes were determined by measuring twice a week the tumors with either a caliper or by US measurements of the tumor axes. (a) Ultrasound image and measurement of a DU-145 tumor before treatment with Ad.mda-7/microbubble complexes and US. (b) US image and measurement of the same DU-145 tumor 2 weeks following treatments with Ad.mda-7/microbubble complexes and US. (c) US image and measurement of the same DU-145 tumor 4 weeks following treatments with Ad.mda-7/microbubble complexes and US. (d) US image and measurement of a DU-Bcl-xL tumor before treatment with CTV/microbubble complexes and US. (e) US image and measurement of the same DU-Bcl-xL tumor 2 weeks following treatments with the CTV/microbubble complexes and US. (f) US image and measurement of the same DU-Bcl-xL tumor 4 weeks following treatments with CTV/microbubble complexes and US. Complete eradication of the DU-Bcl-xL tumor occurs 4 weeks after initiating the therapeutic treatment protocol. Ad, adenovirus; GFP, green fluorescent protein.
Discussion
MBs have been used to protect viruses from rapid degradation by the immune system, thus allowing intravenous injection rather than direct target organ delivery by catheter-based approaches or operative bed injection.38 However, variable levels of nontargeted gene expression have been noted in other organs such as the liver and lungs.38 We have recently shown that US imaging and US contrast agents can increase target specificity of Ads to tumors, achieving transient transgene expression with strict image-guided site specificity by selecting MBs that completely enclosed the Ads in their gas-filled core.12 In our previous experience, US-mediated MB destruction improved the efficacy and reduced the nonspecific expression of gene therapy vectors providing a useful tool for manipulating gene expression in the living animal.
Genetic therapies for PC represent promising strategies for the treatment of this neoplasm. The prostate gland is accessible by US, and potential therapeutic genes can be directed to this organ using portable diagnostic US platforms such as the SonoSite Micro-Maxx (SonoSite) after a simple intravenous injection. Importantly, because PC is commonly a relatively slow-growing disease, it may be necessary to use repeated gene therapy applications, with single or multiple genes, over the life span of the patient. In these contexts, gene therapy protocols that delimit virus exposure to the immune system and can be administered multiple times during a patient's lifetime are appealing. This possibility will need to be explored in the future using tumor-bearing immune-competent animals. In this work, we explored the ability of US-mediated MB destruction to specifically deliver in prostate adenocarcinoma xenografts the mda-7/IL-24 gene,39,40 that has been successfully employed in a Phase I clinical trial in patients with advanced solid tumors.6,7,8,9,21,22,23,28
Potentially useful approaches for treating prostate and other cancers involve the use of a replication-incompetent Ad (Ad.mda-7) or a CRCA (Ad.PEG-E1A-mda-7; CTV) to administer the therapeutic cytokine mda-7/IL-24 to induce targeted therapy of tumors.6,7,8,9,11,33,34,37,41 Although very effective in PC cell lines, no therapeutic benefit is observed with Ad.mda-7 in the context of PC cells displaying elevated expression of Bcl-2 and/or Bcl-xL.4,11 In contrast, administration of the CTV by direct intratumoral delivery in nude mice containing xenografted Bcl-xL overexpressing DU-145 cells implanted on both flanks of the animal results in tumor eradication in both the primary injected tumor and the distant untreated tumor.11 CRCAs, which induce oncolysis by cancer-specific replication, have been evaluated in several PC clinical trials.42,43 Most currently employed CRCAs are based on the ONYX-015 backbone, which is dependent on the p53 status of the cancer cells and have shown only minimal objective clinical responses, thus limiting their universal applicability for the treatment of prostate or other cancers.44 To this end, the novel CTV CRCA that employs the progression-elevated gene-3 (PEG-3) promoter that functions in all types of cancer cells,11,34,37,45,46 irrespective of their p53 or Rb/retinoblastoma gene status, with very limited to no activity in normal cells has been constructed. In the CTV, Ad replication through the E1A gene is driven by the cancer-specific promoter of progression-elevated gene-3 (PEG- 3) (ref. 47), which results in concomitant production of mda-7/IL-24 from the E3 region of the Ad. This CTV generates large quantities of MDA-7/IL-24 as a function of Ad replication uniquely in cancer cells that not only has cancer-selective apoptosis-inducing properties but also displays a plethora of indirect antitumor “bystander” activities, including distant tumor growth suppression and apoptosis, immune modulation, and antiangiogenesis.6,7,8,9,22,32,35,37,41
A limiting factor in effective gene therapy when employing intravenous viral delivery and when using CRCA is the effect of the immune system in neutralizing Ads.13 In this context, a means of shielding the initial viral delivery vector using MBs in principle permits enhanced delivery of the viral payload to tumors when coupled with US.12 In this study, we have employed this strategy using CTV/MB complexes coupled with US to treat both DU-145 and therapy-resistant DU-Bcl-xL established tumor xenografts on both flanks in nude mice. Systemic administration of the CTV/MB with US on the established right-sided tumor resulted in robust transgene expression and apoptosis induction with complete eradication of both the injected right side primary and distant (opposite flank; potentially representative of metastasis) human PCs. However, no tumor regression was observed instead in mice bearing DU-145 and DU-Bcl-xL control tumors when injected intravenously with unprotected Ad.mda-7 and CTV viruses (see Supplementary Figures S1 and S2), indicating that comparable doses of untargeted, unprotected viruses injected directly intravenous failed to elicit an antitumoral response. An exciting finding was that this protocol resulted in the indication of an enduring response in which no tumor regrowth occurred 3 months after cessation of the therapy protocol in the treated or untreated tumor site and additionally these animals had no signs of metastatic spread to the lungs or liver. Previous studies have indicated that the CTV when injected intratumorally will enter into the circulation, replicate and generate MDA-7/IL-24 protein in the primary and distant tumors in the nude mouse, predicting induction of a potential immune response.11,34,37,41,45 Further studies are planned in the context of immune-competent animals, which would be an important step toward developing clinical trials with the CTV/MB approach and US.
Obvious questions are why mda-7/IL-24 serves as such an effective antitumor agent and why the CTV is superior to Ad.mda-7 as a viral-based therapeutic for primary and disseminated cancers? A noteworthy reason for the robustness of mda-7/IL-24 is the ability of this secreted cytokine to elicit a potent “bystander” anticancer effect.6 mda-7/IL-24 can directly induce apoptosis when expressed inside cancer cells and can also induce growth suppression, apoptosis, and endogenous MDA-7/IL-24 protein expression and secretion when added as a purified protein through interactions with the IL-20R1/IL-20R2 and IL-22R1/IL-20R2 cell surface receptors.6,8,32,35 As a secreted cytokine, MDA-7/IL-24 also induces an array of potent immunomodulatory proteins from immune cells, including IL-6, interferon-γ, tumor necrosis factor-α, IL-1β, IL-12, and granulocyte macrophage colony–stimulating factor.32 These cytokines secreted by peripheral blood mononuclear cells can activate antigen-presenting cells to present tumor antigens, thereby triggering an antitumor immune response.48 These observations have been recapitulated in a Phase I clinical trial involving intratumoral injection of Ad.mda-7 (INGN 241) in patients with advanced carcinomas and melanomas.8,21,28 In principle, the “bystander” effects elicited by MDA-7/IL-24 are concentration dependent35 and large amounts of this cytokine generated by the CTV would be predicted to have an enhanced therapeutic impact in the patient. Moreover, the immunomodulatory functions of mda-7/IL-24 would be particularly significant in a patient with an intact immune system where the generation of robust amounts of mda-7/IL-24 by the CTV might result in an amplified immune response against the cancer cells. The potent activity of the CTV compared to Ad.mda-7 suggests a need for only limited administration of this CRCA,11,34,37,41 which would work extremely well in the context of MB/Ad complexes. In principle, the MB approach would further minimize the activation of the immune system against the Ad that would normally promote viral clearance. We presently confirm for the first time that MB-assisted delivery of the CTV can serve as a valuable therapeutic tool to combat therapy resistant PC. As previously emphasized, the CTV/MB-injected US-treated mice appeared to be disease free 3 months after therapy cessation suggesting that a cure was established. We are planning to conduct long-term animal studies to verify this provocative observation in both immune-incompetent and immune-competent animals.
In summary, the present studies support the proposition that US-directed delivery of CTV/MB complexes might provide a nontoxic and effective alternative or complement to conventional adjuvant treatment modalities for patients with primary and metastatic PC. Based on previous studies of combinatorial therapy preclinical-trials in cell culture and in animal models, a combination of CTV/MB approach employing US with localized low-dose radiotherapy might promote an even more profound effect, potentially further enhancing mice survival.9,49 We are currently studying the effects of chemotherapy and/or radiation therapy combined with MB-enhanced delivery of the CTV on promoting a cure in a preclinical setting of PC.
Materials and Methods
Cell lines, cell culture, and adenoviral production. The DU-145 (human prostate adenocarcinoma), cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and the DU-Bcl-xL cell line, which constitutively expresses elevated levels of Bcl-xL has been described previously.4 The cell lines were grown at 37 °C, in a 5% CO2/95% atmosphere, in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum from Hyclone (Logan, UT). Ad-GFP, which expresses the GFP gene under the strong cytomegalovirus (CMV) constitutive promoter was generated using the AdEasy system (Carlsbad, CA); the conditionally replication-competent CTV (Ad.PEG-E1A-mda-7) (refs. 11,34,37) and Ad.mda-7 (ref. 26) were amplified and purified with the BD Adeno-X virus purification kit (BD Biosciences, Mountain View, CA) following manufacturer's directions. Viral titers were determined by a plaque assay and the titer was adjusted to 1.2 × 1012 plaque-forming units/ml as described.26
Preparation of MBs and US platform. Targeson (Targeson) custom synthesis US contrast agent (perfluorocarbon MBs, encapsulated by a lipid monolayer and poly(ethyleneglycol) stabilizer) were prepared following manufacturer's instructions. MBs were reconstituted in the presence or absence of 1 ml of 1.2 × 1012 plaque-forming units of Ads and unenclosed, surface-associated Ads were inactivated as previously described.12 For in vivo experiments US exposure was achieved with a Micro-Maxx SonoSite (SonoSite) US machine equipped with the transducer L25 set at 0.7 Mechanical Index, 1.8 MPa for 10 minutes.
Antibodies and western blot analysis. DU-145 cells were transduced with 50 multiplicity of infection of Ad.mda-7 or Ad-CMV as a control and 24 or 48 hours post-transduction 50 µg of total cell extracts were subjected to western blot analysis using a mouse monoclonal anti-MDA-7/IL-24 (GenHunter, Nashville, TN) (1:2,000; incubation for 1 hour) or the mouse monoclonal anti-GAPDH sc-0411 (1:5,000; incubation for 1 hour) (SantaCruz, Santa Cruz, CA), as control. Western blot analysis was also conducted on protein extracts from MB/US-assisted in vivo transfer of Ad-GFP or mda-7/IL-24 using antibodies that specifically recognized GFP sc-53882 (SantaCruz), MDA-7/IL-24 (GenHunter), and β-actin sc-47778 (SantaCruz). Briefly, 96 hours following targeted MB/US-assisted in vivo transfer of Ad-GFP, mice were killed and fresh tumor (right and left flank), heart, lung, liver, and kidney tissues were harvested and snap frozen in liquid nitrogen. Mice receiving mda-7/IL-24 gene-MB/US-guided therapy were killed at the end point of the study (5–6 weeks after gene therapy injections). Tissues were homogenized and equal amounts of proteins were run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was then incubated with the monoclonal anti-GFP 1:2,000 for 1 hour at room temperature and then washed three times in TBS-T (Tris-buffered saline Tween-20). Monoclonal anti-MDA-7/IL-24 (GenHunter) was incubated 1:2,000 for 1 hour at room temperature and then washed three times in TBS-T. Monoclonal anti β-actin (1:5,000) was incubated 1 hour at room temperature and then washed three times in TBS-T. Appropriate secondary horseradish peroxidase–conjugated antibodies 1:20,000 were incubated 45 minutes at room temperature and washed three times with TBS-T. Signals were developed on an X-ray film after reaction with an Electrogenerated ChemiLuminescence Supersignal kit (Pierce, Rockford, IL).
Animal study and ultrasonic bubble destruction. Animal studies were performed in accordance with National Institutes of Health recommendations and the approval of the institutional animal research committee. Animal care and humane use and treatment of mice were in strict compliance with (i) institutional guidelines, (ii) the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC, 1996), and (iii) the Association for Assessment and Accreditation of Laboratory Animal Care International (Rockville, MD, 1997). All the animals used in these studies were 8- to 12-week-old female/male congenitally athymic BALB/c nude mice, homozygous for the nu/nu allele, bred in our laboratory. The colony of the mice was developed from breeding stock obtained from Charles Rivers Laboratories, Wilmington, MA. The mice were maintained in isolation in autoclaved cages with polyester fiber filter covers, under germ-free conditions; all food, water, and bedding were sterilized. A total of about 420 nude mice (n = 10 each experimental point) were implanted with the human prostate adenocarcinoma cell lines (DU-145 or DU-Bcl-xL) as a xenograft model (injecting 1.5 × 106 or 2.5 × 106 cancer cells on each flank of the animal). After ~30-days, mice were sedated in an IMPAQ6 anesthesia apparatus (VetEquip, Pleasanton, CA) that was saturated with 3–5% isofluorane and 10–15% oxygen with the aid of a precision vaporizer (VetEquip) to deliver the appropriate amount of anesthetic and to induce anesthesia. The mice were placed on a warmed mat with 37 °C circulating water for the entire procedure. A 27-gauge needle with a heparin lock was placed within a lateral tail vein for administration of contrast material. The nude mice received injections of 100 µl of MBs with/without Ads through the tail vein once a week for 5 weeks. The mice were split into two control groups (one control group receiving 100 µl of MBs and US, and another control group receiving both MBs/Ad-GFP and US) and eight active groups of 10 mice each (all receiving MBs and Ad.mda-7 or CTV and US). Six additional control groups were set up which received direct intravenous injections of 100 µl of the Ads (Ad-GFP, Ad.mda7/IL-24, or CTV) in the presence or not of US. Grayscale US imaging was performed with a SonoSite scanner (SonoSite) equipped with the transducer L25 set at 0.7 Mechanical Index, 1.8 MPa for 10 minutes. US images were recorded as digital clips. In every experiment, 10 animals for each treatment or control group were used to study tumor regression. Every experiment was repeated at least twice. Tumor volumes were determined by measuring the tumors twice a week with either a caliper or by US measurements of the tumor axes. Tumor volumes were determined using the following formula: V = (π/8)a × b2, where V is the tumor volume, a is the maximum tumor diameter, and b is the diameter at 90° to a (ref. 50). The mice were humanely killed by placing them in a CO2 gas jar placed in a ventilated fume hood. The tumors (right and left flank), heart, lungs, kidneys, and liver were harvested. Tissues to be sectioned were dry snap frozen or placed either in OCT (Sakura Finetek USA, Torrance, CA), frozen in liquid nitrogen, and stored at −80 °C or were preserved in neutral buffered formalin at 4 °C before embedding in paraffin for immunohistochemical analysis.
Determination of E1A immunohistochemical staining. For immunohistochemical analysis, formalin-fixed and paraffin-embedded specimens were sectioned 3–4 µm thick. Sections were deparaffinized, rehydrated, and then quenched in 3% H2O2 for 20 minutes. Sections were washed with phosphate-buffered saline (PBS) and blocked in PBS containing 1% bovine serum albumin for 20 minutes at 37 °C. Monoclonal anti-E1A (M73) antibodies (sc-25; Santa Cruz), 1:2,000, were incubated with slides for 3 hours at room temperature and then washed 3× in PBS. Sections were incubated with an avidin–biotin–peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) and then washed 2× in PBS. The immunoreactivity was determined using diaminobenzidine as the final chromogen. Finally, sections were counterstained with Meyer's hematoxylin, dehydrated through a sequence of increasing concentrations of alcohol solutions, cleared in xylene, and mounted with Permount medium (Fisher Scientifics, Pittsburgh, PA). During the immunohistochemical assay proof slides were coupled with negative control slides on which the primary antibody was omitted. Sections were also processed for hematoxylin and eosin staining.
Determination of apoptotic cells by TUNEL assay. A TUNEL method was used for the detection of apoptotic cells. For this purpose, we used the DeadEnd Colorimetric TUNEL Assay kit (Promega, Madison, WI) according to the manufacturer's instructions. Briefly, paraffin-embedded slides were deparaffinized and rehydrated. Pre-equilibrated slides were labeled with a labeling DNA-strand breaks solution containing a biotinylated nucleotide mix (60 minutes at 37 °C). After several washes in 2× SSC and PBS, slides were blocked with hydrogen peroxide (3–5 minutes at room temperature). After several washes in PBS, the slides were incubated with streptavidin/horseradish peroxidase–conjugated antibodies diluted in PBS (30 minutes at room temperature). Diaminobenzidine was used as the final chromogen and hematoxylin was used as a counterstaining procedure. Apoptotic cells on the slides were observed under an Olympus light microscope (×400 magnification; Olympus, Center Valley, PA) in randomly chosen fields.
Statistical analysis. All statistical analyses were performed by using SAS, version 9.1. Comparisons of tumor volumes were done separately three times: before the treatment, 2 weeks after the treatment, and at the end of the study. Statistical analyses for comparisons of different types of treatments were done using one-way analysis of variance followed by Tukey–Kramer multiple adjusted pair-wise tests. P value <0.05 was considered significant.
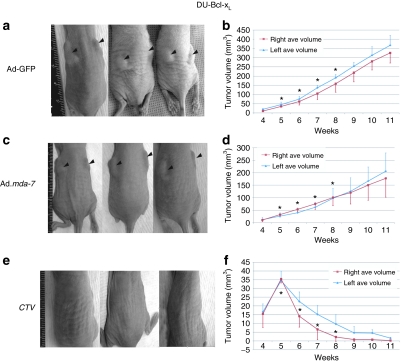
SUPPLEMENTARY MATERIALFigure S1. Growth curves of control DU-145 tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-145 were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.Figure S2. Growth curves of control DU-Bcl-xL tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-Bcl-xL were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.
Supplementary Material
Growth curves of control DU-145 tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-145 were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.
Growth curves of control DU-Bcl-xL tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-Bcl-xL were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.
Acknowledgments
We gratefully acknowledge the Marshall University Biochemistry and Microbiology & Surgery Departments for their support. This study was supported in part by the awards number CA131395 and CA140024 from the National Cancer Institute, and in part by NIH-COBRE 5P20RR020180, WV-INBRE 5P20RR016477, and the Cell Differentiation and Development Center (CDDC), Marshall University (to P.P.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institute of Health. We also acknowledge support from: the Goldhirsh Foundation and the Dana Foundation (to D.S.); NIH grants P01 CA104177, R01 CA35675, and R01 CA097318 (to P.B.F.); the Samuel Waxman Cancer Research Foundation (to P.B.F.); and the National Foundation for Cancer Research (NFCR) (to P.B.F.). S.K. is the recipient of a fellowship from WV NASA Space Grant Consortium. We also gratefully acknowledge SonoSite, Inc. for loaning the Micro-Maxx, SonoSite ultrasound portable platform and Targeson, Inc. for the custom synthesis of the ultrasound contrast agent that were used in this study. D.S. is a Harrison Endowed Scholar in Cancer Research, VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research, VCU Massey Cancer Center.
REFERENCES
- Damber JE., and , Aus G. Prostate cancer. Lancet. 2008;371:1710–1721. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- Moon C, Park JC, Chae YK, Yun JH., and , Kim S. Current status of experimental therapeutics for prostate cancer. Cancer Lett. 2008;266:116–134. doi: 10.1016/j.canlet.2008.02.065. [DOI] [PubMed] [Google Scholar]
- Shi XB, Gumerlock PH., and , deVere White RW. Molecular biology of prostate cancer. World J Urol. 1996;14:318–328. doi: 10.1007/BF00184605. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, et al. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138–8144. [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer. Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. 2003mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic Cancer Biol Ther 24 suppl 1): S23–S37. [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (review) Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, et al. mda-7/IL-24: exploiting cancer's Achilles' heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Dent P, Curiel DT., and , Fisher PB. Acquired and innate resistance to the cancer-specific apoptosis-inducing cytokine, mda-7/IL-24: not insurmountable therapeutic problems. Cancer Biol Ther. 2008;7:109–112. doi: 10.4161/cbt.7.1.5693. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, et al. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA., and , Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Z, Serra D, Frank MM., and , Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC., and , Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- Ng KY., and , Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22:204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- Larina IV, Evers BM., and , Esenaliev RO.2005Optimal drug and gene delivery in cancer cells by ultrasound-induced cavitation Anticancer Res 251A): 149–156. [PubMed] [Google Scholar]
- Howard CM.2004The role of ultrasound contrast agents in gene therapy Appl Radiol 33suppl): 126–135. [Google Scholar]
- Goldberg BB, Liu JB., and , Forsberg F. Ultrasound contrast agents: a review. Ultrasound Med Biol. 1994;20:319–333. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Pitt WG, Husseini GA., and , Staples BJ. Ultrasonic drug delivery—a general review. Expert Opin Drug Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton PA., and , Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front Biosci. 2007;12:5124–5142. doi: 10.2741/2553. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Eager R, Harle L., and , Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8:1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, et al. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC, et al. mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol Ther. 2007;15:287–294. doi: 10.1038/sj.mt.6300035. [DOI] [PubMed] [Google Scholar]
- Ramesh R, Ito I, Saito Y, Wu Z, Mhashikar AM, Wilson DR, et al. Local and systemic inhibition of lung tumor growth after nanoparticle-mediated mda-7/IL-24 gene delivery. DNA Cell Biol. 2004;23:850–857. doi: 10.1089/dna.2004.23.850. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara I, Miyake K, Hanawa H, Kurai T, Hirai Y, Ishizaki M, et al. Systemic cancer gene therapy using adeno-associated virus type 1 vector expressing MDA-7/IL24. Mol Ther. 2007;15:1805–1811. doi: 10.1038/sj.mt.6300225. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003;2:623–632. [PubMed] [Google Scholar]
- Zerbini LF, Czibere A, Wang Y, Correa RG, Otu H, Joseph M, et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66:11922–11931. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Ramesh R, Munshi A, Chada S., and , Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–828. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet' for cancer therapy. Expert Opin Biol Ther. 2007;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P., and , Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, et al. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Beeri R, Guerrero JL, Supple G, Sullivan S, Levine RA., and , Hajjar RJ. New efficient catheter-based system for myocardial gene delivery. Circulation. 2002;106:1756–1759. doi: 10.1161/01.cir.0000035240.92015.e4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI., and , Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS., and , Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ., and , Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5:1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD., and , Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Randolph A, Valerie K, et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Sarkar D, Emdad L, Duigou GJ, Young CS, Ware J, et al. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc Natl Acad Sci USA. 2005;102:1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Shi Y., and , Fisher PB. Subtraction hybridization identifies a transformation progression-associated gene PEG-3 with sequence homology to a growth arrest and DNA damage-inducible gene. Proc Natl Acad Sci USA. 1997;94:9125–9130. doi: 10.1073/pnas.94.17.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, et al. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68:3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Hamed H, Emdad L, Dos Santos W, Gupta P, Broaddus WC, et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7:917–933. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, et al. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther. 1998;9:2481–2492. doi: 10.1089/hum.1998.9.17-2481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth curves of control DU-145 tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-145 were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-145 tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.
Growth curves of control DU-Bcl-xL tumor xenografts injected i.v. using unprotected Ad-GFP, Ad.mda‐7, or CTV (Ad.PEG-E1A-mda‐7) and treated or not with US. Subcutaneous tumor xenografts from DU-Bcl-xL were established in athymic nude mice in both right and left flanks and only tumors on the right side were sonoporated following tail vein injection of the indicated Ads during a course of 4 wks. Tumor treatments were initiated when tumors reached a size of 150 – 200 mm3. Asterisks point at treatment times.A) Measurement of CTV-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.B) Measurement of CTV-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.C) Measurement of Admda- 7-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Admda- 7-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.E) Measurement of Ad.GFP-injected and sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.D) Measurement of Ad.GFP-injected, but not sonoporated DU-Bcl-xL tumor volumes. The data represent mean ± s.d. with at least 5 mice in each group.