Abstract
Metastatic cancer remains an incurable disease in the majority of cases and thus novel treatment strategies such as oncolytic virotherapy are rapidly advancing toward clinical use. In order to be successful, it is likely that some type of combination therapy will be necessary to have a meaningful impact on this disease. Although it may be tempting to simply combine an oncolytic virus with the existing standard radiation or chemotherapeutics, the long-term goal of such treatments must be to have a rational, potentially synergistic combination strategy that can be safely and easily used in the clinical setting. The combination of oncolytic virotherapy with existing radiotherapy and chemotherapy modalities is reviewed along with novel biologic therapies including immunotherapies, in order to help investigators make intelligent decisions during the clinical development of these products.
Introduction
Oncolytic viruses (OVs) are biological machines that kill cancer cells while sparing normal cells. Often they utilize sophisticated gene products to facilitate immune evasion, allow recognition and penetration of cells, co-opt cellular biosynthetic machinery and ultimately manipulate cell death programs. Interestingly, many of the biological pathways that viruses manipulate are the same ones that tumor cells deregulate during their malignant evolution and, as a consequence, these same pathways have become the targets for anticancer drug development. It seems reasonable to expect that certain kinds of chemical, radiological or biological therapy could be used to synergize with OVs and enhance tumor killing.
Broadly speaking, there have been three strategies for the creation of combination therapy approaches. The first is to simply combine an oncolytic virus with the current standard of care therapies, an approach which one could argue is the most likely to have immediate clinical relevance. The second strategy is to identify barriers that are limiting to oncolytic virus activity and select therapies that target that barrier. The third approach is to combine OVs, which may act to induce some level of antitumoral immunity as a byproduct of oncolysis, with some form of immunotherapy to achieve a synergistic immune response against the tumor.
Although the quickest route to the clinic may be to combine oncolytic therapy with the existing standard treatments, we know that in some cases certain chemotherapeutics1 and radiation modalities2 may have a negative influence on viral replication. Hence, this review will attempt to provide some insight into the types of combinations that rationally should be chosen for further development. These combinations will be discussed in detail.
OVs Combined with Conventional Therapies
OVs and external beam radiotherapy
Efficacy of combination therapy in preclinical models. The appeal of combining OVs with radiation therapy continues to grow as the relationship between these two therapies is better understood. Through either radiation-mediated enhancement of viral oncolysis or virus-mediated sensitization of cells to radiation therapy, combination of these two treatments has resulted in synergistic antitumor effects in numerous preclinical models.
Combined oncolytic adenovirus therapy and external beam radiotherapy (XRT) has shown significantly improved results over individual therapies in preclinical models.3,4,5,6,7,8,9 Treatment with ONYX-015 (E1B-55k deletion),6 AdΔ24 (24-bp deletion in E1A region rendering the virus ineffective in cells with intact Rb pathways),4 AdΔ24-p53 (ref. 4) or AdΔ24RGD5 in combination with radiation in a subcutaneous (s.c.) glioma model resulted in 50–100% long-term survival (alive at 120 days either tumor-free or without increased tumor size over initial levels). Conversely, in a study of intracranial delivery of AdΔ24RGD in an orthotopic glioma model, neither combination with total body irradiation nor whole brain irradiation resulted in a significantly improved antitumor effect relative to virus alone.10 This somewhat disappointing finding highlights a critical shortcoming in the design of the majority of the studies discussed here. The natural environment of any given tumor can significantly impact the efficacy of a therapy. Although orthotopic models tend to pose more significant challenges compared to s.c. models in terms of monitoring tumor growth or response and defining end points, their value is unquestionable.
The prostate-specific adenovirus CV706 combined with XRT resulted in synergistic inhibition of tumor growth in a prostate cancer xenograft model at all time points from 7 to 42 days post-treatment.8 Furthermore, it reduced the prostate-specific antigen levels at 6 weeks to 1% of baseline which was significantly better than virus alone (86% of baseline) or radiation alone (139% of baseline). XRT combined with CV787 (also prostate specific) resulted in significant mean tumor volume regression (34% of baseline), complete regression (CR) in 80% of mice (up to 8 weeks postinfection) and a significant reduction in serum prostate-specific antigen (11% of baseline) relative to either single therapy.9
Significant improvements in disease outcomes have also been observed with combination herpes simplex virus (HSV) virotherapy and XRT in preclinical models. In two different studies, NV1066 (ICP0/ICP4/γ34.5 deletions) combined with irradiation was shown to significantly reduce tumor volume compared to either treatment alone for nonsmall cell lung cancer11 and malignant mesothelioma.12 Complete eradication of cervical cancer, determined by histology, was achieved by 30 days post-treatment in 42% of mice treated with G207 (ICP6/γ34.5 deletions) and XRT compared to 0% in all other treatment groups.13 Comparison of single versus multiple doses of R3616 (inactivated γ34.5) for glioblastoma14 found that virus administered on three consecutive days and combined with fractionated XRT over 2 days resulted in a greater number of CRs (90%) compared to XRT combined with a single virus dose (56.5%). Furthermore, variance modeling showed that the effects of combination R3616 and XRT were greater than the additive effects of the individual therapies.14 The relationship of NV1023 (γ34.5/UL24/UL56/US11/ICP47 deletions) in combination with XRT was investigated in three models of cholangiocarcinoma generated using different cell lines.15 Combination therapy showed a synergistic reduction in tumor volume in one model (at two different virus doses), whereas the effect was additive or not significantly better than individual therapies in the other models.15 Despite the promising results described above, the potential for combination therapy remains to be seen in a variety of other cancers. For example in the only study to look at herpes virotherapy and XRT in prostate cancer models, combination therapy was not significantly better then virus therapy alone in both immunocompetent and immunocompromised models.16
With the exception of a handful of studies the majority of in vivo studies discussed above use s.c. tumor models combined with intratumoral OV delivery. This approach is simple and practical from an experimental point of view however perhaps less pragmatic in a clinical setting. Indeed, OV clinical trials have for the most part been restricted to intratumoral virus administration (largely due to safety concerns) however the focus should be to demonstrate safety and efficacy following systemic delivery. This will allow treatment of solid tumors inaccessible by a needle and targeting of metastases. Similarly, fractionated XRT is used widely in the clinic however used much less frequently in preclinical studies. Investigators would do well to strongly consider the value of the knowledge gained through use of orthotopic models combined with clinically relevant methods of OV and XRT delivery.
Mechanisms of synergy. The relationship between herpes virus and radiation is perhaps the most well studied and well characterized. Radiation exposure increases HSV titers in a variety of different types of cancer cell lines in vitro11,12,15,17 and in vivo.14,17 Evidence suggests that the increase seen in viral titers is both virus and radiation dose-dependent in some but not all cell lines.11,15
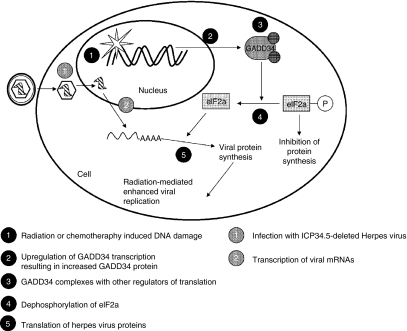
The underlying mechanism of increased viral replication in the presence of XRT is widely hypothesized due to a radiation-mediated increase in cellular GADD34 expression (Figure 1). GADD34 is a DNA damage- and growth arrest-inducible gene that helps protect cells against genetic insults such as those caused by radiation. A region of the GADD34 protein shows significant structural homology to the HSV-1 γ34.5 protein.18 γ34.5, in combination with other cellular proteins, dephosphorylates the cellular translational initiation factor eIF-2a leading to continued protein synthesis and cell survival. Deletion of γ34.5 (a common modification in many oncolytic herpes viruses) significantly reduces virus-induced neurotoxicity,19 however, it also significantly attenuates viral replication and cytotoxicity in tumor cells.20 GADD34 also acts to prevent phosphorylation of eIF-2a. Therefore, radiation-mediated upregulation of cellular GADD34 can functionally replace γ34.5 resulting in increased viral replication without the risk of neurovirulence. Exposure to XRT resulted in a 1.12- to 5.04-fold increase in cellular GADD34 mRNA by real-time reverse transcriptase PCR in human nonsmall cell lung cancer cell lines in vitro.11 Similar results were observed in human cholangiocarcinoma cells15 and confirmed by western blot in human malignant mesothelioma cells.12 In all cases, upregulation of GADD34 was associated with increased viral titers and improved cytotoxicity.
Figure 1.
Model of the mechanism of synergy between γ34.5-deleted herpes viruses and conventional cancer therapies. γ34.5-deleted herpes viruses are favored for oncolytic virotherapy because they show reduced neurotoxicity. Deletion of the γ34.5 gene also results in attenuation of viral replication in tumor cells. Interestingly, γ34.5 shows significant structural homology to a portion of human GADD34, a protein involved in the cells response to DNA damage. γ34.5 is responsible for dephosphorylation of the eukaryotic translation initiation factor eIF-2a, which is required for translation of both host and viral proteins. Radiation or chemotherapy-induced upregulation of GADD34 functionally replaces the γ34.5 protein in infected tumor cells leading to increased viral protein synthesis and production of infectious virus particles.
Many studies looking at combination adenovirus and XRT therapy have hypothesized that the radiation-mediated increase in oncolysis is due in part to an increase in viral replication rates. Unfortunately, little information about the underlying molecular mechanism has been uncovered. When compared to cells treated with virus alone, viral titers were significantly increased 24 hours postinfection in human prostate cancer cells treated with the prostate-specific adenoviruses CV787 (ref. 9) or CV706 (ref. 8) followed by XRT. Increased viral titers correlated with a synergistic cytotoxic effect in vitro. In vivo combination therapy significantly inhibited tumor growth relative to individual therapies.8,9 Histological analysis of prostate cancer xenografts found a significant decrease in the number of CD31+ cells (a marker of angiogenesis)9 and/or blood vessels8,9 in mice treated with combination CV706 (ref. 8) or CV787 (ref. 9) and XRT, relative to either therapy alone. Decreased blood flow to the tumor, resulting from combination therapy, likely contributed to the high levels of necrosis and eventual scar formation found at the tumor site. In another study, two out of three human glioblastoma cell lines supported increased viral replication and release when irradiated 6 hours prior to infection with ONYX-015 (ref. 7). However, in vivo there was no significant difference in ONYX-015 replication in tumors in the presence or absence of total body irradiation.6
An increase in adenovirus uptake, due to upregulated CAR (coxsackie adenovirus receptor) and/or integrin expression levels following radiation exposure, has widely been postulated as one of the mechanisms that leads to increased viral titers and oncolysis but there are conflicting reports. Flow cytometry of human glioblastoma and malignant glioma cell lines 24 hours after exposure to radiation showed no significant increase in expression of CAR and/or αvβ3 and αvβ5 integrins.6,7,10 Conversely, CAR expression levels were found to be increased 24 and 48 hours following irradiation of a head and neck cancer and a colorectal cancer cell line.21 It has also been shown that a radiation-mediated increase in dynamin 2, a GPTase required for endocytosis of the virus, can act in a CAR-independent manner to increase viral uptake in colon, brain, breast, and pancreatic cell lines.22,23
Depending on the mechanism of synergy, the timing and order of the treatment regime could have significant effects on treatment efficacy. If viral oncolysis is enhanced through radiation-induced changes in the host cell then it would be beneficial to deliver the virus after radiation therapy, at a time when such cellular changes are maximized. Conversely, if radiation is acting on the virus directly, therapy would be most effective when the virus is delivered prior to radiation. Evidence from a CV706 study suggests that in the long-term (8 weeks) the difference in tumor volume between groups receiving radiation 24 hours prior to or 24 hours after virus was negligible.8 However, a decreased antitumor effect was observed when radiotherapy was administered 7 days postinfection compared to 1 or 4 days postinfection. This supports the notion that the relative timing could strongly impact therapy efficacy.
Genetic modifications to enhance synergy. In order to increase the combinatorial effects of oncolytic virotherapy and radiotherapy, many viruses have been modified to include promoters that are activated by exposure to radiation or genes that sensitize cells to radiation-induced cell death. Nandi et al. tested the response of several mammalian promoters to XRT and found marked increases in survivin mRNA.3 When this promoter was used to drive adenovirus E1 expression, as with the CRAd-S-pk7 virus, combination with XRT in vivo significantly delayed glioma tumor growth at 6 days postinfection compared to either single treatment or a combination of XRT and wild-type adenovirus. Furthermore, viral titers in tumors 6 days postinfection were increased by 100-fold when combined with XRT. This strategy may prove useful in overcoming one of the major challenges facing current clinical usage of OVs: inadequate viral replication and spread.
In another attempt to improve the radiosensitivity of infected cells, the tumor-specific adenovirus, AdΔ24 (E1A-deleted),24 was modified to express the tumor suppressor gene p53 (ref. 4). Studies have shown that adenovirus-mediated cell lysis is more effective in cells that express p53 (ref. 25), however lack of functional p53 expression is common in malignancy. Also, introduction of functional p53 into p53-negative cells resulted in increased sensitivity to radiation-induced cell death.26 In vitro, AdΔ24-p53 was significantly more cytotoxic than AdΔ24 in human glioma cell lines and synergistic cytotoxicity was observed for both viruses when combined with XRT at viral doses as low as MOI 0.001 (ref. 4). Higher levels of apoptosis were observed in cells treated with AdΔ24-p53 plus XRT relative to those treated with XRT alone or in combination with AdΔ24. Considering that infection alone resulted in minimal levels of apoptosis, it is likely that the increased apoptosis is due to radiosensitization of AdΔ24-p53 infected cells. In immunocompromised s.c. glioma models, AdΔ24 + XRT and AdΔ24-p53 + XRT each resulted in 50% long-term survival which was significantly improved relative to either virus alone (11 and 22% for AdΔ24 and AdΔ24-p53, respectively).4
OVs and targeted radionuclide therapy
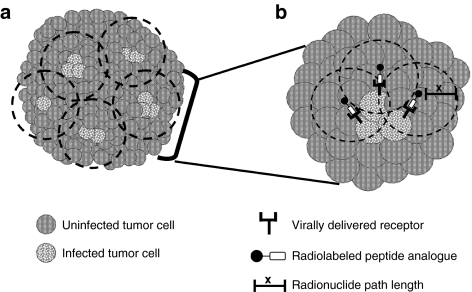
Targeted radionuclide therapy is a treatment used in a subset of cancers that are known to over-express specific cell-surface receptors. Radiolabeled iodine is used to treat thyroid tumors which express the sodium iodide symporter (NIS), and radiolabeled somatostatin ligands are used in patients with somatostatin receptor-positive neuroendocrine tumors. Previously, targeted radiotherapy was limited to receptor-positive tumors. Today oncolytic virotherapy with viral-mediated delivery of specific receptors makes targeted radiotherapy possible, irrespective of the endogenous receptor status. With this approach, damaging radiation can be delivered specifically to infected cells (as well as neighboring cells) (Figure 2) thereby sparing the collateral damage typically sustained by normal cells during XRT.
Figure 2.
Model of tumor killing following treatment with combination oncolytic virotherapy and targeted radionuclide therapy. Initial sites of virus infection often occur in distinct foci surrounding blood vessels (if delivered intravenously) or along the needle track (if delivered intratumorally). Preclinical data indicates that spreading of the virus from these initial sites may be limited. Incomplete transduction of large tumors remains a barrier to effective oncolytic virotherapy. (a) Combination oncolytic virotherapy and targeted radionuclide therapy overcomes the barrier of incomplete transduction due to the radiation cross-fire effect. Uninfected cells falling within the area of the path length (depicted by the dashed lines) will be exposed to radiation resulting in DNA damage and subsequent cell death. (b) Enlarged view of the mechanism of killing at each foci of infection. Infected cells express the virally encoded receptor (for example, vvDD-SSTR2) at the cell surface. The receptors are specifically bound by their cognate radiolabeled peptide analogue from which radiation is emitted. Radiation is emitted in all three-dimensions from the site of origin with a maximum tissue penetration distance defined by the path length (x). Virally induced oncolysis and radiation-induced apoptosis will result in significantly increased tumor cell death relative to either therapy alone.
A tumor-specific vaccinia virus (VV) expressing the human somatostatin receptor (vvDD-SSTR2) resulted in specific uptake of the radiolabeled somatostatin analogue 111In-pentetreotide in vitro.27 111In-pentetreotide delivered systemically to mice bearing s.c. colon cancer tumors localized specifically to the tumors of vvDD-SSTR2 but not vvDD-GFP treated mice.
Several OVs have been designed to encode the hNIS gene. Ad5-yCD/mutTKSR39rep-hNIS is an oncolytic adenovirus vector that is currently being investigated in clinical trials.28 In addition to hNIS, it expresses a highly efficient fusion protein of the catalytic domains of yeast cytosine deaminase (yCD) and herpes virus thymidine kinase (mutTKSR39) that activate the prodrugs 5-fluorocytosine (5-FC) and ganciclovir (GCV), respectively. This virus was demonstrated to result in specific accumulation of 99mTc in a canine model of spontaneous soft tissue sarcoma.29 NanoSPECT/CT imaging was used to demonstrate dose-dependent tumor accumulation of 99mTc in mice bearing s.c. colon cancer tumors treated with the hNIS-expressing oncolytic adenovirus AdAM6 (ref. 30). Accumulation was no longer detectable at 5–6 days postinfection, however it was not shown whether this was due to loss of functional NIS expression or loss of virus replication. Treatment of mice bearing s.c. and intraperitoneal ovarian cancers with a measles virus expressing NIS significantly decreased the tumor burden and increased survival, respectively, relative to saline treated controls.31 Expression of NIS was used to image infected tumors by gamma camera imaging using 99mTc. The efficacy of combination therapy was investigated in both s.c. and orthotopic models of multiple myeloma.32 Combining an attenuated vesicular stomatitis virus (VSV) lacking the ability to block interferon (IFN) production and expressing NIS (VSV(Δ51)-NIS) with 131I radiotherapy prolonged survival relative to treatment with virus alone in a multiple myeloma model.32 These studies are proof of principle that OVs encoding receptors result in tumor-specific accumulation of radiolabeled ligands. Further studies will be required to determine the efficacy of this combination therapy.
OVs and chemotherapy
Although many believe that OVs have the potential to be used as frontline therapies, immediate clinical applications will require that they are at least compatible with current chemotherapeutics. OVs have been investigated in combination with various standard chemotherapeutics that can be organized based on their mechanism of action. Some of the most common drugs fall into the categories of alkylating agents [cisplatin, cyclophosphamide (CPA), and mitomycin C (MMC)], DNA intercalators (doxorubicin), nucleotide analogues (5-fluorouracil (5-FU) and GCV), modifiers of the cellular cytoskeleton (paclitaxel and docetaxel) and cytostatic agents (rapamycin). Regardless of their mechanism of action, the effects of chemotherapy drugs are not specific to tumor cells but instead to all rapidly dividing cells. Consequently, chemotherapy is often associated with high levels of toxicity and significant side effects. OVs have a higher level of tumor-specificity relative to chemotherapy drugs due to both an innate preference for tumor cells and specificity-enhancing genetic modifications. Given that the antitumor effects of OVs and chemotherapy drugs are mediated by different pathways, many investigators have hypothesized that in combination they may act synergistically. Although this is not the case for all chemotherapy and virus combinations, many combinations do exert synergistic cytotoxicity, typically in a cell line-dependent manner.
Alkylating agents. CPA is a common chemotherapy drug used primarily for the treatment of lymphoma, chronic lymphocytic leukemia and breast, ovarian and bladder cancers. CPA is converted into its active metabolites, 4-hydroxycyclophosphamide and aldophosphamide by liver oxidases. Only a small fraction of aldophosphamide is converted into the toxic metabolite phosphoramide mustard that causes DNA cross-linking leading to apoptosis. There have been two main strategies for combining CPA with OVs. First, CPA is used as an immunosuppressant to enhance viral infectivity, replication and spread. The exact nature of the immunosuppressive effects of CPA leading to enhanced OV efficacy is not entirely clear however they have been shown to be both global (nonspecific) and in some cases virus-specific. Second, viruses engineered to encode cytochrome P450 (CYP2B1), which converts CPA to its active metabolites, are used to concentrate the toxic metabolites in virus-infected cells. Both strategies have proven to be very successful at enhancing the antitumor effects of OVs.
Use of CPA as an immunosuppressant to enhance viral oncolysis has improved virotherapy efficacy in combination with HSV,33,34,35 adenoviruses,36 measles virus,37 reovirus,38,39 and VV.40 Reovirus virotherapy of a melanoma lung metastasis model resulted in CR in 5/8 animals treated with combination therapy38 and survival was further enhanced with interleukin-2 treatment.39 In a syngeneic model of murine colon cancer, intratumoral injection of measles virus combined with CPA resulted in 100% survival at 90 days post-treatment and CR in 9/10 animals compared to 30% CR with either therapy individually.37
The immune-modulating effects of CPA are complex, affecting humoral and cellular mediators of both the innate and acquired immune responses. Initial infection, particularly for systemically delivered viruses, requires that the virus evade innate antiviral factors present in the serum. Serum complement and immunoglobulin M have been shown to significantly decrease the infectivity of the herpes virus hrR3 (inactivated ribonucleotide reductase); whereas serum from animals treated with CPA show neutralizing antibody titers below the limit of detection and reduced inhibition of viral infection.34 Furthermore, in vivo depletion of complement significantly improved survival of HSV and CPA treated tumor-bearing rats.33 CPA was also reported to result in global immunosuppression, including significant decreases in total white blood cell, lymphocyte, neutrophil, and monocyte counts in tumor-bearing mice.36 This was accompanied by significantly improved survival and decreased tumor volume in mice treated with both adenovirus and CPA relative to treatment with either therapy alone. Numerous studies have shown that CPA significantly reduces virus-induced infiltration of immune cells into tumors. Infiltration by hematopoietic cells40,41,42 and macrophages42,43,44 as well as levels of phagocytosis43 were all decreased with CPA treatment relative to virus alone. The combined immunosuppressive effects of CPA correlated with increased viral transgene expression,40,42 replication34,36,39,40,41,42,43 and spread35 in a variety of tumor models. To further support immune modulation as a key mechanism by which CPA enhances oncolytic virus therapy, experiments performed in nonobese diabetes/severe combined immunodeficient mice (lacking functional T and B lymphocytes) or in vitro showed no effect of CPA on immune cell infiltrates, viral replication or viral transgene expression.42,44,45
Although high-dose CPA can cause widespread immune suppression in humans, administration of low-dose CPA (<100 mg/kg) to mice resulted in a significant reduction in regulatory T cell (Treg) frequency and function.46 In a tumor vaccine study, tumor cells infected ex vivo with an oncolytic adenovirus did not induce a significant antitumor immune response.47 Low-dose CPA significantly reduced the percent of splenic and tumor Tregs and resulted in a significant delay in tumor growth, prolonged survival and increased tumor-specific T-cell responses when combined with the infected tumor cell vaccine. Oncolytic reovirus in combination with low-dose CPA had numerous immune-modulating effects. CPA decreased the function of Tregs, induced natural killer cell expression of matrix metalloproteinase-2 when combined with interleukin-2, and significantly decreased the level of circulating neutralizing antibodies.39 Decreases in neutralizing antibodies as a result of CPA treatment have also been reported as early as 8 days and as late as 41 days post-treatment with herpes virus,34 adenovirus,36 measles virus,37 or reovirus.38 Combined, these effects are hypothesized to decrease immune sensitization to tumor cells, increase viral spread through tumors due to degradation of the extracellular matrix, and decrease antibody-mediated inhibition of viral infection.
One of the potential pitfalls of CPA-mediated immune suppression is that in addition to promoting tumor oncolysis, it may also lead to increased virus dissemination throughout the body. Immunocompetent hamsters with s.c. renal cell tumors treated with intratumoral adenovirus showed CPA-induced increases in blood and liver titers that ultimately lead to viremia in several animals.36 CPA also induced the spread of HSV into normal brain tissue following treatment of an orthotopic glioma tumor.45 When combined with intravenous reovirus, CPA increased viral titers in the lung, blood, liver, spleen, intestine, brain, heart and bone marrow, and induced cardiac toxicity.38 Interestingly, when metronomic dosing was used (CPA given 1 day prior to virus with a total of three doses) the survival benefit of CPA was maintained whereas the virus titer in the heart and associated toxicity was significantly reduced.38 These data suggest that a balance must be achieved wherein the immune response is suppressed sufficiently to allow enhanced viral oncolysis but not to the point of widespread viral dissemination and toxicity.
Insertion of the CYP2B1 gene into the UL39 locus of herpes virus hrR3 resulted in a virus (rRp450) which can convert CPA into its active metabolites. In the absence of viral replication, treatment of rRp450-infected glioma cells with GCV and CPA resulted in synergistic cytotoxicity.48 This is thought to be due to GCV-mediated inhibition of DNA repair following CPA-induced DNA damage. Furthermore, extracellular accumulation of the toxic CPA metabolites mediated bystander killing of uninfected colon cancer cells.49 Treatment of s.c. tumors with rRp450 combined with CPA significantly reduced tumor growth relative to virus alone48,50,51 and addition of GCV resulted in a further reduction in tumor volume.48
Cisplatin is another alkylating agent that binds and cross-links cellular DNA leading to apoptosis when DNA is not repaired. Cisplatin has been investigated in combination with oncolytic adenovirus,52,53,54,55,56,57,58,59,60,61,62 HSV,63,64,65 parvovirus,66 VV67 and VSV.68
As with radiation therapy, viruses have been genetically modified in order maximize the combinatorial effects with cisplatin. Comparison of adenoviruses Ad-ΔE1B55 and Ad-ΔE1B19/55 showed that deletion of the E1B 19kD protein significantly increased cell susceptibility to cisplatin in vitro and in vivo.52 This is not surprising given that the E1B 19kD protein is a BCL-2-related apoptosis inhibitor homolog. Mice treated with Ad-ΔE1B19/55 and cisplatin showed 96% reduction in s.c. tumor volume relative to PBS treated mice and CR without recurrence at 6 months in two of six mice. Two other E1B 55kD-deleted adenoviruses, encoding activators of apoptosis (ZD55-TRAIL and ZD55-SMAC), also showed increased cytotoxicity in tumor cells when combined with cisplatin.53,54 The combination therapy showed dose-dependent cytotoxicity in normal cell lines; however it was not significantly greater than the drug-induced cytotoxicity. Comparison of several E3B-deleted adenoviruses in a panel of different tumor cell lines found that dl309 (ΔE3B) and dl704 (ΔE3gp19kD) resulted in synergistic cytotoxicity in combination with cisplatin or paclitaxel in 4/7 cell lines and antagonistic effects in the remaining three cell lines.55 Replication of wild-type adenovirus or dl309 in tumors was significantly increased by cisplatin relative to virus alone in an immunocompetent model of nonsmall cell lung cancer but not in an immunocompromised model. Increased viral replication was accompanied by a synergistic reduction in tumor volume. Interestingly, an increase in in vitro E1A protein expression was detected following combination treatment relative to virus alone. Previous studies have reported that upregulation of E1A may sensitize cells to cytotoxic drugs.69
Three adenoviruses derived from the same backbone but encoding antisense cDNA for cell cycle regulating proteins (chk1, chk2, plk-1), showed a significant increase in in vitro apoptosis of tumor cells but not normal cells when combined with cisplatin, compared to the parental virus with cisplatin or either treatment alone.56,58,61 When combined with cisplatin in vivo these viruses showed significantly improved tumor regression, reduction of metastases and increased survival relative to parental virus plus cisplatin or cisplatin alone in s.c. and orthotopic tumor models.
In an important controlled phase 2 clinical trial combining ONYX-015 with cisplatin and 5-FU for treatment of recurrent head and neck cancer patients, a clear benefit was observed in tumors receiving virus injections compared to those treated with chemotherapy alone.62 Objective (>50% reduction in tumor volume) responses in virus injected tumors occurred (>50% reduction in tumor volume) in 63% of patients (19 out of 30) including 8 complete responses and 11 partial responses. Furthermore, the time to progression in the virus injected tumors was significantly greater then that in the uninjected tumors.
The role of p53-status in cisplatin combination therapy was examined in a study using the wild-type H-1 parvovirus.66 A human hepatocellular carcinoma cell line with wild-type p53 was transduced with a stable dominant-negative p53 mutant. Combination therapy was significantly better than individual therapies only in the p53-negative cell line. These findings are particularly interesting given the evidence that cisplatin induces apoptosis or cell cycle arrest through p53-dependent mechanisms.69 Therefore, this study suggests parvovirus can sensitize p53-negative cells to the cytotoxic effects of these drugs.
In a comprehensive study of the interactions between the herpes virus NV1066 and cisplatin, moderate to strong synergy was observed in 7 out of 10 tumor cell lines tested.63 The mechanism of synergy between herpes virus and cisplatin may be similar to that with radiation. Cisplatin significantly increased in vitro viral titers and resulted in a marked increase in GADD34 mRNA and protein expression. Inhibition of GADD34 using siRNA resulted in a loss of combination therapy cytotoxicity.63 Cisplatin has also been shown to improve VV oncolytic virotherapy.67 In a s.c. pancreatic tumor model, intravenous GLV-1h68 (F14.5L/J2R/A56R deleted, Lister strain vaccinia virus) combined with cisplatin resulted in faster growth inhibition, significant reduction in tumor volume and CR in seven of eight mice (compared to CR in one of eight virus alone-treated mice).
MMC is a DNA cross-linking antibiotic with antineoplastic properties. MMC and the γ34.5-deleted HSV-1716 showed synergistic cytotoxicity in two of five nonsmall cell lung cancer cell lines and additive effects in the remaining three.70 Combined with NV1066 there was synergistic cytotoxicity in two human transitional cell carcinoma cell lines allowing for dose reductions of up to 10.4-fold and 156-fold for the virus and drug, respectively.71 MMC had no effect on viral replication in nonsmall cell lung cancer cells70 however it was shown to increase both GADD34 mRNA levels and viral titers in a human gastric cancer cell line.72 Similarly, temozolomide (TMZ), another DNA alkylating agent, was shown to synergistically enhance cytotoxicity when combined with G207 (γ34.5-deleted HSV) in glioma cell lines.73 In this study, synergy was dependent on TMZ-induced upregulation of GADD34 and ribonuclease reductase, with significantly higher levels of virus found in GADD34 expressing cells. As with radiation and cisplatin, upregulation of GADD34 can functionally replace the deleted γ34.5 causing increased cytotoxicity (Figure 1). To further support this mechanism, siRNA inhibition of GADD34 reduced viral titers and cytotoxic synergy in gastric cancer combined with MMC72 and glioma cells combined with TMZ.73 In vivo, combination herpes virus and MMC significantly improved therapeutic effects in models of gastric carcinomatosis72 and nonsmall cell lung cancer70 whereas combination with TMZ improved survival in immunosuppressed models of glioma.73
Oncolytic viral therapy combined with TMZ for the treatment of glioma is particularly attractive as some viruses have been shown to downregulate DNA repair proteins, in particular O6-methylguanine DNA methyl transferase (MGMT) which is involved in glioma resistance to TMZ therapy.74 In human glioma cells, Ad-Δ24RGD downregulated TMZ-induced MGMT expression and synergistically enhanced in vitro cytotoxicity and in vivo survival in glioma xenograft models.74 Similarly, inhibition of MGMT expression resulted in a loss of synergy with G207.73 TMZ has also been shown to significantly improve survival when combined with the adenovirus ICOVIR-5 in glioma xenografts.75
DNA intercalating agents. Doxorubicin is an anthracycline antibiotic that intercalates into DNA and prevents the action of topoisomerase II. Doxorubicin was synergistically cytotoxic when combined with an oncolytic adenovirus in several osteosarcoma cell lines and one patient sample in a viral replication-independent mechanism.76 In cells where synergism was observed, a concomitant increase in G2/M phase arrest was also detected. Given that adenovirus infection is enhanced by an increased percentage of cells in G2 (ref. 77), this provides a possible mechanism through which synergy is achieved. Combination of adenovirus and doxorubicin resulted in synergistic in vitro cytotoxicity71 and significantly reduced in vivo tumor growth78 relative to either therapy alone in hepatocellular carcinoma models. ONYX-015 was successfully combined with MAP (MMC, doxorubicin, and cisplatin) chemotherapy in a phase 1–2 clinical trial for treatment of advanced sarcomas.57 Therapy was well tolerated in all six patients and a partial response occurred in one patient.
Nucleotide analogues. GCV is a widely used antiviral agent originally developed for the treatment of cytomegalovirus infections. GCV is a guanosine analogue prodrug that upon phosphorylation by herpes virus thymidine kinase (TK) competes with cellular deoxyguanosine-5′- triphosphate for incorporation into DNA resulting in elongation termination. Viruses encoding the HSV TK gene lead to an accumulation of toxic GCV metabolites in tumor cells which interfere with cellular DNA synthesis leading to apoptosis.79 Early studies combining viruses with GCV focused on the use of viruses solely as vectors for gene therapy; therefore these vectors were predominately nonreplicating. There has been a shift toward using replicating oncolytic viruses as they have the potential to improve therapy both due to direct oncolysis and a more disseminated distribution of gene delivery. Targeted oncolytic HSVs in combination with GCV significantly improved survival in models of human ovarian cancer80 and rat gliosarcoma.81 Bystander killing of uninfected cells has been reported and is likely mediated by upregulation of gap junctions through which triphosphorylated GCV can travel.82 Adenoviruses83 and sindbis viruses,84 engineered to express the HSV TK gene, also show enhanced antitumor activity when combined with GCV. Intraperitoneally delivered sindbis virus combined with GCV significantly reduced the peritoneal ovarian tumor burden compared to virus alone. In addition to its function as an enzyme for prodrug therapy, TK can also be a target for 18F-labeled fluoro-ethyl-arabinosyluridine, which can be detected using PET imaging. Tseng et al., used this noninvasive and clinically relevant approach to confirm tumor-specific localization of TK activity following treatment with a TK-expressing sinbis virus.84
In addition to TK, Ad5-yCD/mutTKSR39rep-ADP also encodes yCD which converts the prodrug 5-FC into 5-FU. Combination of this virus with radiation and dual prodrug therapy significantly improved the survival in a model of human pancreatic cancer.85 In a phase 1 trial for the treatment of prostate cancer, Ad5-yCD/mutTKSR39rep-ADP in combination with radiation had limited toxicity and showed some therapeutic effect in intermediate risk patients.86 In contrast, Ad5/3-Δ24-TK-GFP which was highly cytotoxic in ovarian cancer cells, showed decreased viral replication in vitro87 and no improved therapeutic effect in vivo when combined with GCV.87,88
CD/5-FC enzyme/prodrug therapy has also proven successful in combination with oncolytic virotherapy. 5-FU is a pyrimidine analogue that inhibits the synthesis of thymidine. The antitumor activity of two different VVs expressing CD was significantly enhanced when combined with 5-FC therapy in immunocompetent ovarian cancer89 and immunosuppressed colon cancer models.1,90 Interestingly, 5-FU also showed antiviral activity,1 and may also provide a safety mechanism for uncontrolled viral replication. M012, a recently described HSV expressing CD and designed for treatment of primary brain tumors showed little neurotoxicity and significantly inhibited growth of s.c. neuroblastoma tumors when combined with 5-FC.91
Cytoskeleton modifiers. Taxanes are a class of chemotherapy drugs, including paclitaxel and docetaxel, which cause stabilization of cellular microtubules thereby preventing function of the cellular cytoskeleton, a requirement for mitosis. Combination of docetaxel or paclitaxel with an urothelium- or prostate-targeted adenovirus significantly reduced in vivo tumor volume and resulted in synergistic in vitro cytotoxicity.92,93 One effect of paclitaxel is an upregulation of TRAIL receptors which sensitize cells to TRAIL-mediated apoptosis. Pretreatment of tumor-bearing mice with paclitaxel and TRAIL prior to HSV injection significantly retarded tumor growth and increased viral spread in the tumors.94 Taxanes combined with other HSV-recombinants, demonstrated synergistic cytotoxicity in prostate cancer cells95 and significantly improved survival in a colon carcinomatosis model96 and lung cancer xenograft model97 relative to either therapy alone.
Cytostatic agents. Rapamycin (sirolimus) is an immunosuppressant commonly used in transplant patients, however it has also been shown to significantly enhance the oncolytic effects of the poxviruses myxoma and VV.40,98,99,100 Rapamycin inhibits the cellular serine/threonine kinase mTOR (mammalian target of rapamycin) which is critical to numerous pathways contributing to cell growth, proliferation, differentiation and survival.97 Myxoma virus infection is permissive in a limited range of cells, dependent on the cells endogenous activation levels the cellular seronine/threonine kinase Akt. Cells with high levels of Akt (type I) are permissive to infection whereas cells with very low levels of Akt activation are not permissive to infection (type III). In cells with moderate levels of Akt (type II), myxoma virus-induced Akt phosphorylation through a mechanism dependent on the viral M-T5 protein.98 mTOR is a downstream mediator of Akt activation and although rapamycin alone decreased mTOR phosphorylation, it also resulted in increased Akt activation likely due to a positive feedback loop. When combined with myxoma virus, rapamycin-induced increases in Akt phosphorylation relative to virus alone correlated with increases in viral replication rates and cell-to-cell spread in type II cells.98,100 The in vitro mechanisms behind the increased activity of combination rapamycin and VV therapy are under investigation, however in vivo, rapamycin appears to influence the antiviral immune response with a decrease of tumor infiltrating natural killer cells.40 In preclinical models of medulloblastoma100 and melanoma,99 rapamycin has significantly improved virotherapy resulting in increased survival rates and decreased tumor burdens. The rapamycin analogue RAD001 has also been shown to significantly improve adenovirus virotherapy in models of glioblastoma.101,102 In vitro it was demonstrated that RAD001 (refs. 75,101,102) as well as temozolimide75,102 significantly increased virus-induced autophagy96,97 and resulted in synergistic cytotoxicity.75,101,102
The prototypical proteosome inhibitor MG-132 enhanced cellular CAR expression in Lovo colon carcinoma cells, which was accompanied with enhanced adenovirus target gene expression and oncolysis.103
OVs Combined with Biologic Therapies
Small molecules
Overcoming innate immune barriers to tumor infection. Mammals have evolved a variety of natural or innate barriers to rampant virus spread throughout the body and many of these pose an impediment to virus infection of tumors, particularly when these have been purposely attenuated for the purpose of virotherapy. Of notable importance are barriers such as the cellular antiviral response (e.g., IFN signaling), the innate immune response (e.g., neutralizing antibodies, complement, scavenging macrophages), and physico-chemical barriers (e.g., blood flow, hypoxia/pH). Although many have tried to tackle these problems through viral engineering, others are finding success in combining both experimental and well established chemotherapeutics that target one or more of these barriers.
HDAC inhibitors and drugs that target the innate antiviral response. Histone deacetylase (HDAC) inhibitors (HDIs) have been explored for their use as anticancer drugs since the 1990s and have been recently approved for the treatment of lymphoma.104 Although their immediate targets are known (HDACs), HDI effects are pleitropic because HDACs are prime controllers of transcriptional regulation. HDIs are known to both up- and downregulate a panoply of genes (up to 10% of the transcriptome), which typically leads to cell cycle arrest and apoptosis preferentially in cancer cells. HDIs have also been reported to have antiangiogenic and immuno-modulatory properties (reviewed in refs. 105,106).
Valproic acid, a low potency HDAC inhibitor was found to enhance CAR expression in cervix, breast, and bladder cancer cells in vitro and in ex vivo cervical cancer samples. This was accompanied by increased infection by adenoviral vectors.107
HDIs have been shown by several groups to suppress the innate cellular antiviral response, at least in part by downregulating IFN and the IFN-stimulated genes.108,109,110 This has led to the combination of HDIs with various OVs. Trichostatin A, a pan-HDAC inhibitor was found to modestly enhance HSV oncolysis in squamous cell carcinoma cells. In this study, it was proposed that the modest enhancement of HSV replication may be due to effects on viral replication induced by NF-κB activation and cell cycle inhibition.111 However, in a more recent study, valproic acid convincingly enhanced HSV oncolysis in human glioma cells, suggesting the effects on HSV replication may be cell or tissue specific.112 In another study, the antitumor effect of a telomerase-specific, replication-selective adenovirus (OBP-301) in human lung cancer cells was enhanced by the lesser known HDI FR901228 (ref. 113). Nguyên et al, have shown that several HDIs can synergize with the oncolytic VSV-Δ51, an attenuated oncolytic VSV-mutant which is incapable of blocking IFN production.109 Combination treatment with HDIs resulted in synergistic cell killing, likely due to both enhanced induction of cell death and increased viral output (typically over 100-fold). Enhanced spread of VV and semliki forest virus was also observed using HDIs. Perhaps most interestingly, the replication of VSV in SW620 colon carcinoma xenografts in vivo could be halted by interrupting treatment with HDIs and resumed once HDIs were resupplied. This brings forth the interesting possibility that HDIs can be used as molecular switches to control viral replication.109
Other drugs that may target the cellular antiviral response include Jun N-terminal kinase inhibitors. In one study, Jun N-terminal kinase-deficiency (genetic or induced by Jun N-terminal kinase inhibitors) could enhance oncolytic VV replication. This was suggested to occur by preventing the activation of double-stranded RNA-dependent protein kinase.114
Modulators of cell death and other oncolytic virus barriers. There are likely to be many barriers to oncolytic virotherapy, some of which have yet to be discovered. In parallel to the cellular antiviral responses and innate immune barriers, the ability of OVs to reach cancer cells and to kill them efficiently can likely be manipulated by small molecules. Tumilasci et al., were able to enhance the efficacy of oncolytic VSV against chronic lymphocytic leukemia cells by combination therapy with the BCL-2 inhibitor EM20-25 (ref. 115). Other less specific modulators of cell death have also been shown to enhance oncolysis. As mentioned previously, HDIs may function at least partially by increasing cell death induced by virus, although the details of how this occurs remain elusive.109 Apoptosis induced by measles virus was also enhanced by cotreatment with heat shock protein inhibitors in vitro. This was potentially mediated by the effects of HSP inhibitors on rhoA expression, important for measles-induced cell fusion.116 Some studies suggest that certain drug/OV combinations induce autophagic cell death and that intact autophagy pathways are required for the observed combined effect.75,101,102
With respect to using combination therapy to enhance virus spread to and within tumor sites, one group showed that a single dose of angiostatic cRGD peptide treatment before oncolytic virus treatment enhanced the antitumor efficacy of oncolytic HSV.41,117 These results are somewhat surprising, because restricted bloodflow appeared to be beneficial to the virus, contrary to what might be expected. This was found to be associated with decreased tumor production of IFN-γ and decreased infiltration of immune cells within the tumor. It will be interesting to assess whether antiangiogenic drugs can enhance the oncolytic ability of other viruses such as VSV, that induce vascular shutdown118 instead of permeabilization as is observed for HSV. Finally, coadministration of various OVs with proteases such as hyaluronidase has been performed intratumorally in order to increase access of virus to tumors and enhance viral spread.94,119,120,121,122
Immunotherapies
The idea that OVs exert their effects not only directly through lysis of tumor cells but also through induction of an immune response is intriguing and has garnered much attention; both in understanding this immune response on its own and to use other forms of immune modulation to try to enhance it. It is likely that OVs induce some level of antitumoral immunity as a byproduct of oncolysis. As pathogens, viruses elicit toll-like receptor signaling through a variety of TLRs present on a variety of cells including antigen-presenting cells.123,124,125 In the course of replicating within the tumor, OVs generate pathogen-associated molecular patterns which are ligands for these receptors thus providing two of the major requirements for initiating and enhancing antitumor immune responses: they supply tumor antigens to local dendritic cells through the direct oncolysis of tumor cells, while providing the “danger signals” necessary to promote localized inflammation and dendritic cell activation.
There are numerous examples of OVs initiating antitumor immune responses. This effect has been best demonstrated in studies using oncolytic HSVs where cured mice generally resist subsequent rechallenge with the same tumor cell line.126,127,128,129,130,131,132 Several reports from Toda et al. demonstrate the establishment of antitumor immunity in various rodent models using oncolytic HSV. The treatment of immunocompetent mice bearing CT26 s.c. tumors, or orthotopic colon cancer with liver metastases, using the conditionally replicating HSV G207 virus led to regression of the primary injected tumors, and of metastases or contralateral untreated tumors.126,127 Importantly, this effect was absent in athymic nude mice, arguing in favor of the induction of antitumoral immunity following viral oncolysis.126,128 In an ovarian carcinoma model, Benencia et al. reported the induction of antitumor immune responses postoncolytic viral therapy through the induction of proinflammatory signals and tumor antigen presentation133 and similar effects were reported following treatment of melanoma using H-1 parvovirus.134 This enhancement of antitumoral immunity has also been demonstrated in a phase 1 dose-escalation clinical trial using oncolytic measles virus in cutaneous T-cell lymphoma patients where biopsies displayed heightened IFN-γ mRNA in infiltrating CD4+ and CD8+ T lymphocytes and an overall expansion of the CD8+ T-cell population.135 Overall, the process by which viral oncolysis leads to induced or enhanced antitumoral immunity is poorly understood and has not really been studied in detail.
Many groups are actively exploring strategies to enhance or directly generate antitumoral immunity with OVs. One promising approach is through the use of fusogenic OVs.126,127,131,132,136 The formation of multinucleated syncytia following infection is a property of certain viruses, including some being developed as oncolytics such as measles virus. Viruses that are not naturally fusogenic can be engineered to have this property. The fusion of tumor cells within a solid tumor appears to generate two desirable effects, one being the enhanced spread of the virus through the tumor and increased oncolysis along with an enhanced ability to generate an antitumoral immune response. The syncytial, oncolytic FusOn-H2 HSV-2 induced strong T-cell responses against primary and metastatic 4T1 breast tumors in immune-competent mice while adoptive transfer of splenocytes from the treated mice to naive mice prevented metastasis of 4T1 in the recipients.131 Additional studies using this oncolytic herpes virus have demonstrated antitumor immune responses following treatment of both neuroblastoma and colon cancer.126,128,132 The induction of antitumor immunity by fusogenic OVs has been suggested to be the result of killing large numbers of tumors cells by nonapoptotic means leading to the production of a mass of inflammatory tumor tissue.
Another approach to enhancing the immunogenicity of OVs has been to engineer them to express various immunostimulatory cytokines.137,138,139,140,141,142,143,144,145 Many candidate cytokines and immunomodulatory factors have been tested in this setting and have shown promise in preclinical testing. As many of the vectors being used are likely to induce some level of proinflammatory immune mediators, it is likely that most of these strategies can only lead to enhanced production of particular mediators. It is also very difficult to predict and control the amount of cytokine produced and there is a theoretical risk of excessive cytokine production if a tumor is heavily infected. The potential immunostimulatory benefits of producing proinflammatory cytokines in the tumor microenvironment justifies this approach.
An emerging strategy for the combination of viral oncolysis with immunotherapy consists of enhancing tumor killing through adoptive cellular therapy. Adoptive transfer of tumor-specific CD8+ T cells into naive recipients showed some efficacy in the B16/OVA melanoma model, and this was heightened by further intratumoral treatment with VSV, providing the necessary local tumor inflammation to recruit and maintain activation of adoptively transferred T cells.146 One variation on this approach has been to transfer dendritic cells during oncolysis to encourage presentation of the unidentified tumor antigens provided by the viral-mediated destruction of tumor.147 An alternative approach is to load an oncolytic virus into or onto tumor-specific lymphocytes and use these cells to enhance targeting of the virus to the tumor while potentially providing additional immune-mediated tumor destruction along with viral oncolysis.148,149,150 This is a complex arena as the cells harboring virus may be cytotoxic themselves (i.e., cytokine-induced killer cells)148 or act to target the virus to tumor or shield it from neutralizing antibodies. This has been recently extensively reviewed by Willmon et al.151 The partnering of oncolytic viral therapy with adoptive transfer of immune cells holds significant potential, as viral oncolysis may be able to enhance and drive the occasionally efficacious effects of cellular therapies. By feeding transferred antigen-presenting cells and/or enhancing viral delivery while attracting tumor-specific effector cells into the tumor, OVs may partner very well with cellular therapies in the clinic, an area that has not yet been explored to any extent.
Other viruses
It has long been proposed that OVs should be used as combination agents with other viruses. In the setting where an immune response to one virus develops, an appealing idea is to use a second virus to continue the therapy. From a practical point of view, this is far from the clinic as obtaining regulatory approval for two separate experimental modalities will be a challenge. But the challenge may indeed be worth it: a recent article out of the Bell lab (F. Le Beouf, J.-S. Diallo, J.A. McCart, S. Thornc, T. Falls, M. Stanford et al., manuscript submitted.) has shown that the powerful immune evasion genes of VV render cells otherwise resistant to killing by VSV, uniquely sensitive when VSV is used as the second agent. In this case VV is acting as a biologic therapy to suppress the innate antiviral immune system and permit VSV to infect and kill the cells. This is an excellent example of the synergy that can be seen when combining modalities that utilize different mechanisms to kill cells and should guide future design of combination therapies.
Challenges to Combination Therapies
The use of relevant animal tumor models is a concern for all investigators, however it is of particular importance for those studying OVs. Recent studies that have focused on elucidating the role of the immune system in OV therapy found that both the innate118 and adaptive immune responses152 contribute significantly to overall efficacy of the OV. Furthermore, antiviral immune responses are both implicated in hindering OV efficacy by inhibiting early infection and required for eventual clearance of the virus. Many of the studies discussed in this review were performed in xenograft models using immunocompromised mice. This is appealing because it allows testing in human tumors (as opposed to murine tumors) however this comes at a significant cost. Adding to the limitation of these models is the widespread use of tumor cell lines and the scarcity of data using fresh human tumor samples. Although use of human tumors in xenograft models is useful, it is perhaps more clinically relevant to demonstrate OV efficacy in immunocompetent hosts. It will be important to consider these factors when choosing the best combination therapy regimens to develop clinically.
Discussion and Future Directions
As we have discussed, as OVs move toward clinical use, it is likely that in order to be successful, some type of combination therapy will need to be employed. Rather than simply combining existing modalities, we have attempted to show how certain combinations make the most sense and have a high likelihood of acting in a synergistic manner. Although initially we expect that OVs will be combined with standard validated therapeutics (i.e., chemo and radiotherapy), overall we anticipate that novel combinations with other biologics (including other OVs) or immunotherapeutics will yield better results. It is critical to understand the interplay between the virus and the combination of choice. As we have described earlier, some chemotherapy and radiotherapy modalities1,2 may inhibit viral replication, and as such could be used to improve the safety of the virus rather than influencing its efficacy. Studies using OVs are now demonstrating an immune-mediated component to successful therapy in addition to direct oncolysis. Importantly, the induction of an antitumoral immune response during oncolysis has the potential to provide prolonged tumor control long after the oncolytic virus has been cleared. Future studies will need to further elucidate the role that the immune system is playing in OV therapy and disease-specific combinations will need to be defined.
The race is on to move these novel therapeutics to the clinic. Although combining OVs with the current standards of care may be appealing both from a cost-effective point of view (no need to “develop” the second arm of the therapy) and from a regulatory point of view (getting two novel therapeutics approved for one trial), we hope that this review will challenge the reader to pause and consider whether what he/she is proposing to take to the clinic is rational and will provide the best possible outcome for the patients.
REFERENCES
- McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, et al. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–1223. doi: 10.1038/sj.gt.3301237. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Vongpunsawad S, Bergert ER, Kyle RA, et al. Interaction of measles virus vectors with Auger electron emitting radioisotopes. Biochem Biophys Res Commun. 2005;337:22–29. doi: 10.1016/j.bbrc.2005.08.261. [DOI] [PubMed] [Google Scholar]
- Nandi S, Ulasov IV, Tyler MA, Sugihara AQ, Molinero L, Han Y, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idema S, Lamfers ML, van Beusechem VW, Noske DP, Heukelom S, Moeniralm S, et al. AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J Gene Med. 2007;9:1046–1056. doi: 10.1002/jgm.1113. [DOI] [PubMed] [Google Scholar]
- Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Lecluse Y, et al. Potentiation of radiation therapy by the oncolytic adenovirus dl1520 (ONYX-015) in human malignant glioma xenografts. Br J Cancer. 2003;89:577–584. doi: 10.1038/sj.bjc.6601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieler A, Mantwill K, Holzmüller R, Jürchott K, Kaszubiak A, Stärk S, et al. Impact of radiation therapy on the oncolytic adenovirus dl520: implications on the treatment of glioblastoma. Radiother Oncol. 2008;86:419–427. doi: 10.1016/j.radonc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453–5460. [PubMed] [Google Scholar]
- Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- Lamfers ML, Idema S, Bosscher L, Heukelom S, Moeniralm S, van der Meulen-Muileman IH, et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin Cancer Res. 2007;13:7451–7458. doi: 10.1158/1078-0432.CCR-07-1265. [DOI] [PubMed] [Google Scholar]
- Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, et al. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Ann Thorac Surg. 2005;80:409–16; discussion 416. doi: 10.1016/j.athoracsur.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli PS, Chan MK, Hezel M, Yu Z, Stiles BM, Chou TC, et al. Radiation-induced cellular DNA damage repair response enhances viral gene therapy efficacy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol. 2007;14:258–269. doi: 10.1245/s10434-006-9127-4. [DOI] [PubMed] [Google Scholar]
- Blank SV, Rubin SC, Coukos G, Amin KM, Albelda SM., and , Molnar-Kimber KL. Replication-selective herpes simplex virus type 1 mutant therapy of cervical cancer is enhanced by low-dose radiation. Hum Gene Ther. 2002;13:627–639. doi: 10.1089/10430340252837224. [DOI] [PubMed] [Google Scholar]
- Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Zager JS, Hezel M, Stanziale SF, Adusumilli PS, Gonen M, et al. Treatment of cholangiocarcinoma with oncolytic herpes simplex virus combined with external beam radiation therapy. Cancer Gene Ther. 2006;13:326–334. doi: 10.1038/sj.cgt.7700890. [DOI] [PubMed] [Google Scholar]
- Jorgensen TJ, Katz S, Wittmack EK, Varghese S, Todo T, Rabkin SD, et al. Ionizing radiation does not alter the antitumor activity of herpes simplex virus vector G207 in subcutaneous tumor models of human and murine prostate cancer. Neoplasia. 2001;3:451–456. doi: 10.1038/sj.neo.7900193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Wong RJ, Kooby DA, Carew JF, Adusumilli PS, Patel SG, et al. Combination of mutated herpes simplex virus type 1 (G207 virus) with radiation for the treatment of squamous cell carcinoma of the head and neck. Eur J Cancer. 2005;41:313–322. doi: 10.1016/j.ejca.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Chou J., and , Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Kern ER, Whitley RJ., and , Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Kramm CM, Chase M, Herrlinger U, Jacobs A, Pechan PA, Rainov NG, et al. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- Hingorani M, White CL, Zaidi S, Merron A, Peerlinck I, Gore ME, et al. Radiation-mediated up-regulation of gene expression from replication-defective adenoviral vectors: implications for sodium iodide symporter gene therapy. Clin Cancer Res. 2008;14:4915–4924. doi: 10.1158/1078-0432.CCR-07-4049. [DOI] [PubMed] [Google Scholar]
- Qian J, Yang J, Dragovic AF, Abu-Isa E, Lawrence TS., and , Zhang M. Ionizing radiation-induced adenovirus infection is mediated by Dynamin 2. Cancer Res. 2005;65:5493–5497. doi: 10.1158/0008-5472.CAN-04-4526. [DOI] [PubMed] [Google Scholar]
- Egami T, Ohuchida K, Mizumoto K, Onimaru M, Toma H, Nishio S, et al. Radiation enhances adenoviral gene therapy in pancreatic cancer via activation of cytomegalovirus promoter and increased adenovirus uptake. Clin Cancer Res. 2008;14:1859–1867. doi: 10.1158/1078-0432.CCR-07-0933. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Hall AR, Dix BR, O'Carroll SJ., and , Braithwaite AW. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med. 1998;4:1068–1072. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- Badie B, Kramar MH, Lau R, Boothman DA, Economou JS., and , Black KL. Adenovirus-mediated p53 gene delivery potentiates the radiation-induced growth inhibition of experimental brain tumors. J Neurooncol. 1998;37:217–222. doi: 10.1023/a:1005924925149. [DOI] [PubMed] [Google Scholar]
- McCart JA, Mehta N, Scollard D, Reilly RM, Carrasquillo JA, Tang N, et al. Oncolytic vaccinia virus expressing the human somatostatin receptor SSTR2: molecular imaging after systemic delivery using 111In-pentetreotide. Mol Ther. 2004;10:553–561. doi: 10.1016/j.ymthe.2004.06.158. [DOI] [PubMed] [Google Scholar]
- Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, Lu M, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F, Barton KN, Stricker HJ, Steyn PF, Larue SM, Karvelis KC, et al. Design considerations for incorporating sodium iodide symporter reporter gene imaging into prostate cancer gene therapy trials. Hum Gene Ther. 2007;18:312–322. doi: 10.1089/hum.2006.131. [DOI] [PubMed] [Google Scholar]
- Merron A, Peerlinck I, Martin-Duque P, Burnet J, Quintanilla M, Mather S, et al. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14:1731–1738. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ., and , Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- Goel A, Carlson SK, Classic KL, Greiner S, Naik S, Power AT, et al. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood. 2007;110:2342–2350. doi: 10.1182/blood-2007-01-065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- Kambara H, Saeki Y., and , Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ., and , Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther. 2007;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Okegawa T, Lombardi DP, Frenkel EP., and , Hsieh JT. Enhanced transgene expression in androgen independent prostate cancer gene therapy by taxane chemotherapeutic agents. J Urol. 2002;167:339–346. [PubMed] [Google Scholar]
- Wakimoto H, Fulci G, Tyminski E., and , Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J., and , Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- Di Paolo NC, Tuve S, Ni S, Hellström KE, Hellström I., and , Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghi M, Chou TC, Suling K, Breakefield XO., and , Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865. [PubMed] [Google Scholar]
- Pawlik TM, Nakamura H, Mullen JT, Kasuya H, Yoon SS, Chandrasekhar S, et al. Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the CYP2B1 transgene. Cancer. 2002;95:1171–1181. doi: 10.1002/cncr.10776. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Petros WP, Ludeman SM, Fangmeier J, Hochberg FH, Colvin OM, et al. Intraneoplastic polymer-based delivery of cyclophosphamide for intratumoral bioconversion by a replicating oncolytic viral vector. Cancer Res. 2001;61:864–868. [PubMed] [Google Scholar]
- Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon AR, Kim JH, Lee YS, Kim H, Yoo JY, Sohn JH, et al. Markedly enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in combination with cisplatin. Hum Gene Ther. 2006;17:379–390. doi: 10.1089/hum.2006.17.379. [DOI] [PubMed] [Google Scholar]
- Pan Q, Liu B, Liu J, Cai R, Wang Y., and , Qian C. Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell Biochem. 2007;304:315–323. doi: 10.1007/s11010-007-9514-6. [DOI] [PubMed] [Google Scholar]
- Pan QW, Zhong SY, Liu BS, Liu J, Cai R, Wang YG, et al. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol Sin. 2007;28:1996–2004. doi: 10.1111/j.1745-7254.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- Cheong SC, Wang Y, Meng JH, Hill R, Sweeney K, Kirn D, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhou J, Gao Q, Huang X, Li K, Zhuang L, et al. Oncolytic adenovirus-mediated transfer of the antisense chk2 selectively inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 2006;13:930–939. doi: 10.1038/sj.cgt.7700967. [DOI] [PubMed] [Google Scholar]
- Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhou J, Huang X, Chen G, Ye F, Lu Y, et al. Selective targeting of checkpoint kinase 1 in tumor cells with a novel potent oncolytic adenovirus. Mol Ther. 2006;13:928–937. doi: 10.1016/j.ymthe.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hsieh JL, Lee CH, Teo ML, Lin YJ, Huang YS, Wu CL, et al. Transthyretin-driven oncolytic adenovirus suppresses tumor growth in orthotopic and ascites models of hepatocellular carcinoma. Cancer Sci. 2009;100:537–545. doi: 10.1111/j.1349-7006.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KF, Wu CL, Huang SC, Hsieh JL, Huang YS, Chen YF, et al. Conditionally replicating E1B-deleted adenovirus driven by the squamous cell carcinoma antigen 2 promoter for uterine cervical cancer therapy. Cancer Gene Ther. 2008;15:526–534. doi: 10.1038/cgt.2008.37. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gao Q, Chen G, Huang X, Lu Y, Li K, et al. Novel oncolytic adenovirus selectively targets tumor-associated polo-like kinase 1 and tumor cell viability. Clin Cancer Res. 2005;11:8431–8440. doi: 10.1158/1078-0432.CCR-05-1085. [DOI] [PubMed] [Google Scholar]
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Adusumilli PS, Chan MK, Chun YS, Hezel M, Chou TC, Rusch VW, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya H, Nishiyama Y, Nomoto S, Goshima F, Takeda S, Watanabe I, et al. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther. 2007;14:533–542. doi: 10.1038/sj.cgt.7701049. [DOI] [PubMed] [Google Scholar]
- Mace AT, Harrow SJ, Ganly I., and , Brown SM. Cytotoxic effects of the oncolytic herpes simplex virus HSV1716 alone and in combination with cisplatin in head and neck squamous cell carcinoma. Acta Otolaryngol. 2007;127:880–887. doi: 10.1080/00016480601075381. [DOI] [PubMed] [Google Scholar]
- Sieben M, Herzer K, Zeidler M, Heinrichs V, Leuchs B, Schuler M, et al. Killing of p53-deficient hepatoma cells by parvovirus H-1 and chemotherapeutics requires promyelocytic leukemia protein. World J Gastroenterol. 2008;14:3819–3828. doi: 10.3748/wjg.14.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YA, Galanis C, Woo Y, Chen N, Zhang Q, Fong Y, et al. Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol Cancer Ther. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CK, Choi B, Wanna G, Genden EM, Woo SL., and , Shin EJ. Combined VSV oncolytic virus and chemotherapy for squamous cell carcinoma. Laryngoscope. 2008;118:237–242. doi: 10.1097/MLG.0b013e3181581977. [DOI] [PubMed] [Google Scholar]
- Liao Y, Zou YY, Xia WY., and , Hung MC. Enhanced paclitaxel cytotoxicity and prolonged animal survival rate by a nonviral-mediated systemic delivery of E1A gene in orthotopic xenograft human breast cancer. Cancer Gene Ther. 2004;11:594–602. doi: 10.1038/sj.cgt.7700743. [DOI] [PubMed] [Google Scholar]
- Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR., and , Molnar-Kimber KL. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Hum Gene Ther. 1999;10:3013–3029. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- Mullerad M, Bochner BH, Adusumilli PS, Bhargava A, Kikuchi E, Hui-Ni C, et al. Herpes simplex virus based gene therapy enhances the efficacy of mitomycin C for the treatment of human bladder transitional cell carcinoma. J Urol. 2005;174:741–746. doi: 10.1097/01.ju.0000164730.38431.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JJ, Adusumilli P, Petrowsky H, Burt BM, Roberts G, Delman KA, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- Aghi M, Rabkin S., and , Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK., and , Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Gomez-Manzano C, Jiang H, Bekele NB, Piao Y, Yung WK, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, et al. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Steinwaerder DS, Ni S, Li ZY, Roffler SR., and , Lieber A. Enzyme-activated Prodrug Therapy Enhances Tumor-specific Replication of Adenovirus Vectors. Cancer Res. 2002;62:6089–6098. [PubMed] [Google Scholar]
- Ryan PC, Jakubczak JL, Stewart DA, Hawkins LK, Cheng C, Clarke LM, et al. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004;11:555–569. doi: 10.1038/sj.cgt.7700735. [DOI] [PubMed] [Google Scholar]
- Tomicic MT, Thust R., and , Kaina B. Ganciclovir-induced apoptosis in HSV-1 thymidine kinase expressing cells: critical role of DNA breaks, Bcl-2 decline and caspase-9 activation. Oncogene. 2002;21:2141–2153. doi: 10.1038/sj.onc.1205280. [DOI] [PubMed] [Google Scholar]
- Nawa A, Nozawa N, Goshima F, Nagasaka T, Kikkawa F, Niwa Y, et al. Oncolytic viral therapy for human ovarian cancer using a novel replication-competent herpes simplex virus type I mutant in a mouse model. Gynecol Oncol. 2003;91:81–88. doi: 10.1016/s0090-8258(03)00417-7. [DOI] [PubMed] [Google Scholar]
- Kramm CM, Rainov NG, Sena-Esteves M, Barnett FH, Chase M, Herrlinger U, et al. Long-term survival in a rodent model of disseminated brain tumors by combined intrathecal delivery of herpes vectors and ganciclovir treatment. Hum Gene Ther. 1996;7:1989–1994. doi: 10.1089/hum.1996.7.16-1989. [DOI] [PubMed] [Google Scholar]
- Luo C, Mori I, Goshima F, Ushijima Y, Nawa A, Kimura H, et al. Replication-competent, oncolytic herpes simplex virus type 1 mutants induce a bystander effect following ganciclovir treatment. J Gene Med. 2007;9:875–883. doi: 10.1002/jgm.1085. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Abei M, Ugai H, Kawashima R, Seo E, Wakayama M, et al. E1A, E1B double-restricted replicative adenovirus at low dose greatly augments tumor-specific suicide gene therapy for gallbladder cancer. Cancer Gene Ther. 2009;16:126–136. doi: 10.1038/cgt.2008.67. [DOI] [PubMed] [Google Scholar]
- Tseng JC, Zanzonico PB, Levin B, Finn R, Larson SM., and , Meruelo D. Tumor-specific in vivo transfection with HSV-1 thymidine kinase gene using a Sindbis viral vector as a basis for prodrug ganciclovir activation and PET. J Nucl Med. 2006;47:1136–1143. [PubMed] [Google Scholar]
- Freytag SO, Barton KN, Brown SL, Narra V, Zhang Y, Tyson D, et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol Ther. 2007;15:1600–1606. doi: 10.1038/sj.mt.6300212. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- Raki M, Hakkarainen T, Bauerschmitz GJ, Särkioja M, Desmond RA, Kanerva A, et al. Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther. 2007;14:1380–1388. doi: 10.1038/sj.gt.3302992. [DOI] [PubMed] [Google Scholar]
- Hakkarainen T, Hemminki A, Curiel DT., and , Wahlfors J. A conditionally replicative adenovirus that codes for a TK-GFP fusion protein (Ad5Delta24TK-GFP) for evaluation of the potency of oncolytic virotherapy combined with molecular chemotherapy. Int J Mol Med. 2006;18:751–759. [PMC free article] [PubMed] [Google Scholar]
- Chalikonda S, Kivlen MH, O'Malley ME, Eric Dong XD, McCart JA, Gorry MC, et al. Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther. 2008;15:115–125. doi: 10.1038/sj.cgt.7701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y, et al. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- Guffey MB, Parker JN, Luckett WS, Jr, Gillespie GY, Meleth S, Whitley RJ, et al. Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors. Cancer Gene Ther. 2007;14:45–56. doi: 10.1038/sj.cgt.7700978. [DOI] [PubMed] [Google Scholar]
- Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H, et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- Zhang J, Ramesh N, Chen Y, Li Y, Dilley J, Working P, et al. Identification of human uroplakin II promoter and its use in the construction of CG8840, a urothelium-specific adenovirus variant that eliminates established bladder tumors in combination with docetaxel. Cancer Res. 2002;62:3743–3750. [PubMed] [Google Scholar]
- Nagano S, Perentes JY, Jain RK., and , Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–3802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD., and , Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–560. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama S, Goshima F, Teshigahara O, Kasuya H, Kodera Y, Nakao A, et al. Enhanced efficacy of herpes simplex virus mutant HF10 combined with paclitaxel in peritoneal cancer dissemination models. Hepatogastroenterology. 2007;54:1038–1042. [PubMed] [Google Scholar]
- Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- Stanford MM, Barrett JW, Nazarian SH, Werden S., and , McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81:1251–1260. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MM, Shaban M, Barrett JW, Werden SJ, Gilbert PA, Bondy-Denomy J, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther. 2008;16:52–59. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF, et al. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16:487–493. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Iwado E, Kondo Y, Aoki H, Hayashi Y, Georgescu MM, et al. Autophagy-inducing agents augment the antitumor effect of telerase-selve oncolytic adenovirus OBP-405 on glioblastoma cells. Gene Ther. 2008;15:1233–1239. doi: 10.1038/gt.2008.98. [DOI] [PubMed] [Google Scholar]
- Zhang NH, Song LB, Wu XJ, Li RP, Zeng MS, Zhu XF, et al. Proteasome inhibitor MG-132 modifies coxsackie and adenovirus receptor expression in colon cancer cell line lovo. Cell Cycle. 2008;7:925–933. doi: 10.4161/cc.7.7.5621. [DOI] [PubMed] [Google Scholar]
- Garber K. HDAC inhibitors overcome first hurdle. Nat Biotechnol. 2007;25:17–19. doi: 10.1038/nbt0107-17. [DOI] [PubMed] [Google Scholar]
- Bruserud O, Stapnes C, Ersvaer E, Gjertsen BT, Ryningen A. Histone deacetylase inhibitors in cancer treatment: a review of the clinical toxicity and the modulation of gene expression in cancer cell. Curr Pharm Biotechnol. 2007;8:388–400. doi: 10.2174/138920107783018417. [DOI] [PubMed] [Google Scholar]
- Minucci S., and , Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Segura-Pacheco B, Avalos B, Rangel E, Velazquez D., and , Cabrera G. HDAC inhibitor valproic acid upregulates CAR in vitro and in vivo. Genet Vaccines Ther. 2007;5:10. doi: 10.1186/1479-0556-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Paulson M, Holko M, Rice CM, Williams BR, Marié I, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci USA. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyên TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinzon I., and , Horvath CM. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc Natl Acad Sci USA. 2003;100:14742–14747. doi: 10.1073/pnas.2433987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura T, Iwai S, Ota Y, Shimizu H, Ikuta K., and , Yura Y. The effects of trichostatin A on the oncolytic ability of herpes simplex virus for oral squamous cell carcinoma cells. Cancer Gene Ther. 2009;16:237–245. doi: 10.1038/cgt.2008.81. [DOI] [PubMed] [Google Scholar]
- Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hioki M, Fujiwara T, Nishizaki M, Kagawa S, Taki M, et al. Histone deacetylase inhibitor FR901228 enhances the antitumor effect of telomerase-specific replication-selective adenoviral agent OBP-301 in human lung cancer cells. Exp Cell Res. 2006;312:256–265. doi: 10.1016/j.yexcr.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Hu W, Hofstetter W, Guo W, Li H, Pataer A, Peng HH, et al. JNK-deficiency enhanced oncolytic vaccinia virus replication and blocked activation of double-stranded RNA-dependent protein kinase. Cancer Gene Ther. 2008;15:616–624. doi: 10.1038/cgt.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumilasci VF, Olière S, Nguyên TL, Shamy A, Bell J., and , Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol. 2008;82:8487–8499. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Erlichman C, McDonald CJ, Ingle JN, Zollman P, Iankov I, et al. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008;15:1024–1034. doi: 10.1038/gt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H., and , Bell JC. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M., and , Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin Cancer Res. 2008;14:3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- Kuriyama N, Kuriyama H, Julin CM, Lamborn KR., and , Israel MA. Protease pretreatment increases the efficacy of adenovirus-mediated gene therapy for the treatment of an experimental glioblastoma model. Cancer Res. 2001;61:1805–1809. [PubMed] [Google Scholar]
- Mok W, Boucher Y., and , Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67:10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- O'Neill L. Toll-like receptors and the danger hypothesis. Trends Immunol. 2001;22:421. doi: 10.1016/s1471-4906(01)02019-1. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Endo T, Toda M, Watanabe M, Iizuka Y, Kubota T, Kitajima M, et al. In situ cancer vaccination with a replication-conditional HSV for the treatment of liver metastasis of colon cancer. Cancer Gene Ther. 2002;9:142–148. doi: 10.1038/sj.cgt.7700407. [DOI] [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and , Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Toda M, Iizuka Y, Kawase T, Uyemura K., and , Kawakami Y. Immuno-viral therapy of brain tumors by combination of viral therapy with cancer vaccination using a replication-conditional HSV. Cancer Gene Ther. 2002;9:356–364. doi: 10.1038/sj.cgt.7700446. [DOI] [PubMed] [Google Scholar]
- Nakamori M, Fu X, Rousseau R, Chen SY., and , Zhang X. Destruction of nonimmunogenic mammary tumor cells by a fusogenic oncolytic herpes simplex virus induces potent antitumor immunity. Mol Ther. 2004;9:658–665. doi: 10.1016/j.ymthe.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Hummel JL, Safroneeva E., and , Mossman KL. The role of ICP0-Null HSV-1 and interferon signaling defects in the effective treatment of breast adenocarcinoma. Mol Ther. 2005;12:1101–1110. doi: 10.1016/j.ymthe.2005.07.533. [DOI] [PubMed] [Google Scholar]
- Li H, Dutuor A, Fu X., and , Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J Gene Med. 2007;9:161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- Li H, Dutuor A, Tao L, Fu X., and , Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–322. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- Benencia F, Courrèges MC, Fraser NW., and , Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther. 2008;7:1194–1205. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- Moehler MH, Zeidler M, Wilsberg V, Cornelis JJ, Woelfel T, Rommelaere J, et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum Gene Ther. 2005;16:996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H., and , Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Bayer W., and , Wildner O. Therapeutic immune response induced by intratumoral expression of the fusogenic membrane protein of vesicular stomatitis virus and cytokines encoded by adenoviral vectors. Int J Mol Med. 2007;20:673–681. [PubMed] [Google Scholar]
- Fukuhara H, Ino Y, Kuroda T, Martuza RL., and , Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- Su C, Peng L, Sham J, Wang X, Zhang Q, Chua D, et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-gamma gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol Ther. 2006;13:918–927. doi: 10.1016/j.ymthe.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T., and , Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–265. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Wanna GB, Choi B, Aguila D, 3rd, Ebert O, Genden EM, et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope. 2007;117:210–214. doi: 10.1097/01.mlg.0000246194.66295.d8. [DOI] [PubMed] [Google Scholar]
- Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang T, Park BH, Bell J., and , Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Zhao H, Janke M, Fournier P., and , Schirrmacher V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 2008;136:75–80. doi: 10.1016/j.virusres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Zaupa C, Barnard Z, Maley J, Martuza RL, Rabkin SD, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008;14:7711–7716. doi: 10.1158/1078-0432.CCR-08-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Negrin RS., and , Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A., and , Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]