Abstract
Regulatory T cells play a major role in induction and maintenance of immune tolerance and immunological homeostasis. A variety of strategies have been attempted to induce regulatory T cells for control of unwanted, adverse immunity in autoimmune diseases, transplantation as well as gene transfer. We recently reported efficient induction of immune tolerance to coagulation factor IX (FIX) following intramuscular AAV1 gene transfer. In the current study, we performed a systematic and comprehensive examination of the role and function of regulatory T cells in induction and maintenance of FIX tolerance in the context of intramuscular AAV1 gene transfer. We observed no significant upregulation of regulatory T cells in the FIX-tolerant mice. In addition, adoptive transfer of splenocytes from FIX-tolerant mice did not suppress anti-hFIX immunity in recipient mice. Both in vitro and in vivo depletion of regulatory T cells failed to reverse FIX tolerance. These observations revealed that regulatory T cells do not play a significant role in the maintenance/protection of the established FIX tolerance. Our results provide critical insight into the role and function of regulatory T cells in induction and maintenance/protection of immune tolerance in gene transfer, complementing the current paradigm of immune tolerance mechanism.
Introduction
Induction of adaptive, antigen-specific immune tolerance to prevent and control unwanted immunity is of considerable importance for the treatment of autoimmune diseases and organ transplantation.1,2,3 It is also of great interest to induce tolerance to therapeutic protein in treatment of a variety of deficiency diseases,4 such as tolerance to coagulation factor IX (FIX) in hemophilia treatment.5 Peripheral immune tolerance is maintained by means of recessive and dominant mechanisms.1,3 The recessive tolerance is usually developed by deletion and/or anergy of the reactive T-cell clones in the immature thymus or other lymphoid organs. For instance, injection of high doses of soluble peptides can lead to a state of T-cell unresponsiveness (referred to as anergy) owing to a block in T-cell proliferation and/or interleukin-2 (IL-2) production, or results in activation of induced cell death after T-cell restimulation with the cognate peptide.2,6,7 The dominant mechanism complements recessive tolerance by executing suppression on the reactive T cells that escape deletion/anergy or are generated after thymus maturation.1,3 Dominant immune tolerance functions through the suppressive regulatory T cells. CD4+CD25+FoxP3+ regulatory T cells are the major type of the regulatory T cells.1,2,3
Gene therapy is emerging as an effective, alternative treatment for genetic diseases. On one hand, the control of unwanted, adverse cellular, and humoral immune responses subsequent to gene transfer poses an immense challenge for the successful application of gene therapy.8 On the other hand, conceptually, gene transfer can be exploited to induce immune tolerance. Induction of regulatory T cells was reported as the primary mechanism that mediates immune tolerance following gene transfer approaches.9,10 For example, FIX tolerance induced in hepatic adeno-associated virus (AAV) hemophilia gene transfer was reported to be mediated by upregulation of regulatory T cells.10
We found that expression of high levels of FIX is critical to induction of FIX tolerance following intramuscular injection of AAV.11,12,13 Our preliminary investigation found no upregulation of regulatory T cells in the high-dose AAV1-injected, FIX-tolerant mice, suggesting that regulatory T cells may not play a major role in the FIX tolerance induced by intramuscular injection of AAV1.13 In the current study, we performed a more systematic and comprehensive examination of the role and function of regulatory T cells in induction and maintenance of FIX tolerance induced by intramuscular injection of AAV1. Our results revealed that depletion of regulatory T cells was not able to rescue the in vitro proliferation activity of the anergized FIX-specific T cells induced by intramuscular injection of AAV1. Depletion of regulatory T cells also could not reverse the established, in vivo FIX tolerance induced by intramuscular injection of AAV1. This is different from the induction of regulatory T-cell-mediated FIX tolerance following hepatic AAV gene transfer and supports an essential function of T-cell anergy for achieving peripheral tolerance in gene therapy protocols. Our results provide critical insight into the role of regulatory T cells in induction and maintenance of FIX tolerance following muscular AAV1 gene transfer.
Results
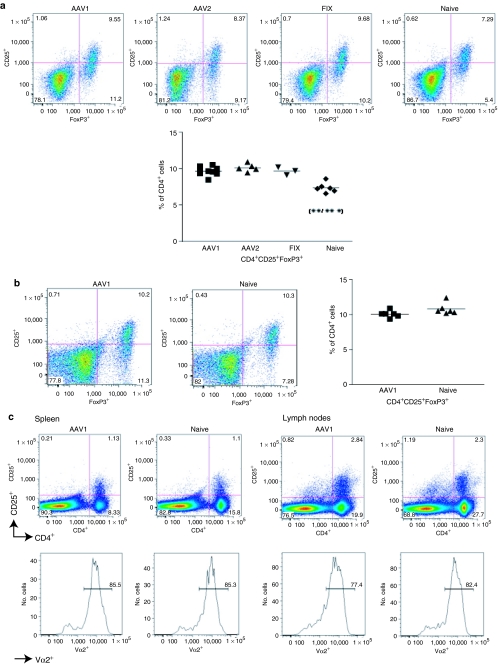
Comparable number of regulatory T cells among AAV1-injected FIX-tolerant mice, AAV2-injected FIX-immunized mice, and naive, untreated mice
We previously reported detection of an equivalent number of CD4+CD25+FoxP3+ regulatory T cells in FIX-tolerant C57BL/6 mice that received intramuscular injection of AAV1 compared to naive, untreated congenic mice, suggesting that regulatory T cells may not play a major role in induction of immune tolerance to FIX by intramuscular injection of AAV1.13 In order to further validate our previous observation, in the current study, we performed an extended investigation on regulatory T cells following intramuscular injection of AAV1, AAV2, and congenic mice immunized by FIX/complete Freund's adjuvant (CFA) in hemostatically normal mice. We also examined regulatory T cells following intramuscular injection of AAV1 in hemophilia B (FIXKO) mice. Cohorts of 10-week-old C57BL/6 normal mice and FIX knockout (FIXKO) mice were given intramuscular injection of AAV1 [1 × 1011 vg (vector genomes) per mouse, n = 9 for normal C57BL/6, n = 6 for FIXKO], AAV2 (6 × 1010 vg per mouse, n = 5 for normal C57BL/6), or FIX (n = 3 for normal C57BL/6) as described. Blood was collected from the mice every 4 weeks after AAV injection, to measure human FIX (hFIX) antigen and anti-hFIX antibodies by enzyme-linked immunosorbent assay (ELISA) as described. Immune tolerance to hFIX was induced in the AAV1-injected mice, demonstrated by sustained expression of high level of hFIX antigen with background level of anti-hFIX antibodies. Formation of anti-hFIX antibodies was observed in the AAV2-injected mice (mean = 15,500.84 ± SEM = 10,964 ng/ml, n = 5). Three to four months after AAV injection, upon proof of FIX tolerance or formation of anti-FIX antibodies, we evaluated regulatory T cells in the mice. Isogenic naive mice were used as control (n = 6 for normal C57BL/6, n = 6 for FIXKO). Similar numbers of regulatory T cells (CD4+CD25+FoxP3+) were observed in the spleen among the AAV1-injected FIX-tolerant, AAV2-injected FIX-immunized, and FIX/CFA-immunized mice (Figure 1a). We did note a slight, however, statistically significant increase in the numbers of regulatory T cells in all the treated C57BL/6 normal mice when compared to naive mice (Figure 1a). The age difference between the treated mice (28 weeks at analysis) and the naive control mice (12 weeks old at analysis) likely accounts for the discrepancy in the number of the regulatory T cells.14 The regulatory T-cell numbers that we detected in FIXKO mice were about the same as that observed in the normal mice (Figure 1b). There is no significant difference between the numbers of regulatory T cells of the AAV1-injected, tolerant FIXKO mice and the age-matched naive control mice (Figure 1b). Our flow cytometry method identified a similar number of regulatory T cells in naive mice in comparison with literature reports.13 This has proven the dependability of our methods in detecting regulatory T cells. Our competence to detect an increase of regulatory T cells in mice that received AAV2 gene transfer (Supplementary Figure S1) further validated the sensitivity and reliability of our flow cytometry method in measuring number of regulatory T cells in the current study.
Figure 1.
Comparable number of regulatory T cells among FIX-tolerant, FIX-immunized, and naive mice. (a) Regulatory T cells in C57BL/6 normal mice: 8- to 10-week-old C57BL/6 mice received intramuscular injection of 1 × 1011 viral genomes (vg) of AAV1-hFIX (AAV1, n = 9), 6 × 1010 vg of AAV2-hFIX (AAV2, n = 5), or hFIX + CFA (FIX, n = 3). Lymphocytes were collected from spleen of the relevant mice 3–4 months later and evaluated for regulatory T cells by flow cytometry as described. Ten to twelve-week-old mice (naive, n = 6) were used as control. The naive mice used for these set of experiments were 12–16 weeks younger than the experimental mice. There is no considerable difference in the number of regulatory T cells among the various treatment groups. A slight upregulation of regulatory T cells in the treated mice is observed in comparison to naive mice (P = 0.0015 and P < 0.0001). This figure depicts results compiled from three independent experiments. (b) Regulatory T cells in C57BL/6 FIXKO mice: 8- to 10-week-old C57BL/6 FIXKO mice that received intramuscular injection of 1 × 1011 vg of AAV1 (AAV1, n = 6) were analyzed 3–4 months after AAV injection. Control mice were naive, age-matched congenic mice (naive, n = 6). There is no significant difference in regulatory T-cell number between the AAV1-injected, FIX-tolerant, and naive mice (P = 0.0903). This figure depicts data compiled from two independent experiments. (c) No increase of single antigen-specific regulatory T cells following intramuscular AAV1 gene transfer: 8- to 10-week-old OT-II mice that received intramuscular injection of 5 × 1010 vg of AAV1 expressing hFIX-OVA (AAV1, n = 5) were analyzed 2 months after AAV injection. OT-II specific T cells were detected using anti-V2α chain antibody (BD Pharmingen, San Jose, CA). Control mice were naive, age-matched congenic mice (naive, n = 5). The figure depicts typical results of one mouse from the cohorts.
We then moved on to explore whether intramuscular AAV1 gene transfer may increase single antigen-specific regulatory T cells despite no increase in total regulatory T cells. We injected a strain of OVA (ovalbumin) T-cell receptor transgenic mice (OT-II, n = 5)15 with 1 × 1011 vg of AAV1 expressing a fusion protein of hFIX and the OT-II-specific OVA immunodominant epitope. Comparable numbers of OVA-specific regulatory T cells were detected in the AAV1-injected mice and untreated congenic mice (Figure 1c).
These results corroborated our previous, preliminary observation, strongly suggesting that FIX tolerance following intramuscular injection of AAV1 is not the result of upregulation of regulatory T cells.
FIX tolerance induced in the context of intramuscular AAV1 gene transfer is not driven/mediated via an active/dominant mechanism
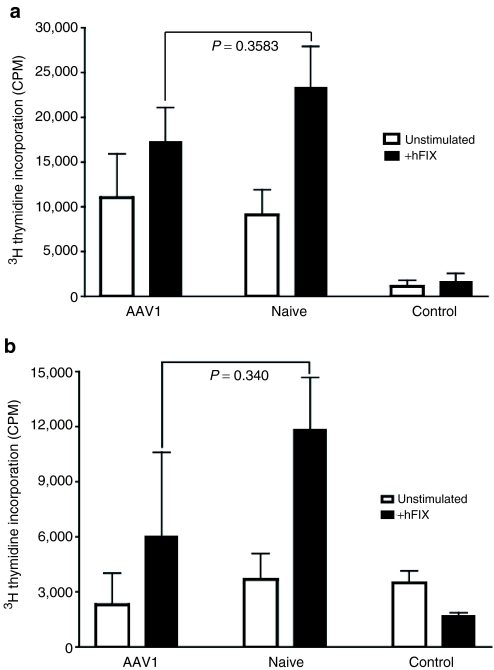
Regulatory T-cell-mediated immune tolerance is reported to be the dominant and active mechanism, thereby transportable upon transfer of the regulatory T cells.3 We previously demonstrated that transfer of CD4+ T cells did not carry the FIX tolerance to the recipient mice.13 This evidence did not support (albeit it could not completely exclude) regulatory T cells as the principal mechanism for induction of FIX tolerance by intramuscular injection of AAV1. It is conceivable that transfer of only CD4+ T cells may not be adequate to completely deliver the activity of regulatory T cells.16 To further validate our hypothesis and previous observation regarding the role of regulatory T cells on the induction of the FIX tolerance by intramuscular injection of AAV1, we examined the immune response to hFIX in mice that received transfer of whole splenocytes from AAV1-injected, FIX-tolerant mice. Eight- to ten-week-old C57BL/6 wild-type mice (n = 4) and 14-week-old C57BL/6 FIXKO mice (n = 3) were given intramuscular injection of ≥2 × 1010 vg of AAV1-hFIX vector as described. Induction of immune tolerance to hFIX in the mice was proven upon observation of high levels of hFIX antigen and low anti-hFIX antibody levels by ELISA [normal C57BL/6 mice (n = 4) at 12 weeks after AAV1-FIX injection, FIX antigen, mean = 1,557.59 ± SEM = 1,739.48 ng/ml; anti-FIX IgG antibodies, mean = 78.33 ± SEM = 156.7 ng/ml]. Two to four months after intramuscular injection of AAV1, splenocytes were isolated from the tolerant mice and infused into isogenic naive recipient mice of the same major histocompatibility complex background. As depicted in Figure 2, transfer of whole splenocytes from the AAV1-injected mice (C57BL/6 normal as well as FIXKO) could not suppress formation of anti-hFIX antibodies (normal C57BL/6 mice, n = 4, anti-FIX IgG antibodies in the mice receiving AAV1 T cells: mean = 26,474 ± SEM = 12,760 ng/ml versus the mice receiving naive T cells: mean = 23,329 ± SEM = 12,728 ng/ml) or proliferation of hFIX-specific reactive T cells (Figure 2a, C57BL/6 normal; Figure 2b, FIX KO mice) upon immunization of the recipient mice with hFIX protein in CFA. These results evidently demonstrate that FIX tolerance induced following intramuscular injection of AAV1 is nontransferable.
Figure 2.
Transfer of whole splenocytes from AAV1-treated, FIX-tolerant mice does not suppress anti-hFIX immunity in recipient mice. AAV1, recipient mice that received cells from congenic AAV1-treated, FIX-tolerant mice; naive, recipient mice that received cells from congenic naive mice; control, congenic naive mice of matching homeostatic background without any treatment as a negative control for T-cell proliferation. Results are shown as mean ± SEM. (a) FIX-specific T-cell proliferation in normal C57BL/6 recipient mice. AAV1, n = 4; naive, n = 5; control, n = 4. Closed bars, stimulation with 10 µg/ml of rhFIX; open bars, mock stimulation, without hFIX. All the samples were performed in triplicate. This figure depicts data compiled from three independent experiments. (b) FIX-specific T-cell proliferation in C57BL/6 FIXKO recipient mice. AAV1, n = 3; naive, n = 3; control, n = 2. Closed bars, stimulation with 10 µg/ml of rhFIX; open bars, mock stimulation without hFIX. All the samples were performed in triplicate. CPM, counts per minute.
In vitro depletion of regulatory T cells does not reverse the T-cell tolerance to FIX
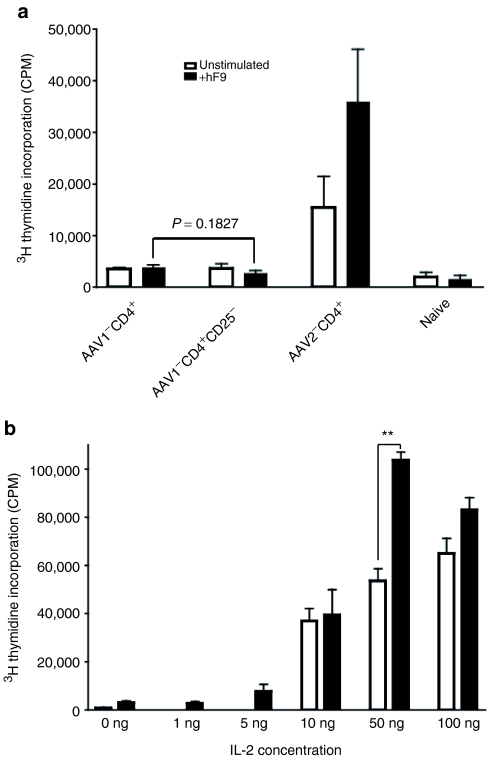
Development of anti-FIX antibodies is T-cell dependent. We have established that the absence of anti-FIX antibodies following intramuscular injection of AAV1-hFIX is the result of T-cell tolerance, although the status of hFIX-specific B cells remains to be characterized.13 A large body of literature has proven that the critical role of regulatory T cells is not only in induction, but also maintenance and protection of immunological tolerance and homeostasis.1,3,17,18 We first investigated the role of regulatory T cells in protection and maintenance of the established FIX T-cell tolerance, by examining the status of FIX T-cell immune tolerance upon depletion of regulatory T cells in vitro. As depicted in Figure 3a, we did not observe retrieval of proliferation activity of hFIX-specific T cells upon depletion of regulatory T cells. It has been reported that retrieval of T-cell proliferation activity occurs upon depletion of regulatory T cells.17,18 In our case, we observed a constant suppression in the hFIX-specific T-cell proliferation in the AAV1-injected mice regardless of depletion of regulatory T cells (Figure 3a).13 These results provide evidence that regulatory T cells may not be the major mechanism involved in the maintenance and protection of T-cell tolerance.
Figure 3.
In vitro assessment of the status of hFIX-specific T cells in AAV1-injected, FIX-tolerant mice. (a) Human FIX–specific T-cell proliferation with depletion of CD4+CD25+ cells. AAV1-CD4+, hFIX-specific proliferation of CD4+ T cells isolated from 1 × 1011 AAV1-hFIX-injected, FIX-tolerant mice (n = 3); AAV1-CD4+CD25‐, hFIX-specific proliferation of CD4+CD25‐ T cells isolated from 1 × 1011 AAV1-hFIX-injected, FIX-tolerant mice (n = 3); AAV2-CD4+, hFIX-specific proliferation of CD4+ T cells isolated from 6 × 1010 AAV2-hFIX-injected, FIX-immunized mice (n = 3); naive, congenic naive mice of matching homeostatic background without any treatment used as negative control (n = 3). Closed bars, stimulation with 10 µg/ml of rhFIX; open bars, mock stimulation without hFIX. Results are shown as mean ± SEM. All the samples were performed in triplicate. This figure depicts the results of one typical 3H-thymidine incorporation (CPM) experiment of four performed. (b) Human FIX–specific T-cell proliferation with IL-2 stimulation. Escalating doses of IL-2 (0–100 ng) were added to the cultures of hFIX-specific T-cell proliferation reaction. Closed bars, 1 × 1011 vg AAV1-hFIX-injected, FIX-tolerant mice (n = 6); open bars, congenic naive mice (n = 3). Results are shown as mean ± SEM. All the samples were performed in triplicate. This figure depicts results compiled from two independent experiments. CPM, counts per minute. **P = 0.0033.
IL-2 dose-dependent rescue of proliferation of hFIX-specific T cells in AAV1-injected FIX-tolerant mice
In order to clearly illuminate the status of the hFIX-specific T-cell clone (deletion or anergy) in the context of FIX tolerance subsequent to intramuscular injection of AAV1, we tested revitalization of the hFIX-specific T cells isolated from AAV1-injected, FIX-tolerant mice, upon stimulation with increasing doses of IL-2. As depicted in Figure 3b, we observed an IL-2 dose-dependent recovery of proliferation of hFIX-specific T cells (P = 0.0033 at IL-2 dose of 50 ng). This pattern of IL-2 dose-dependent recovery of proliferation of hFIX-specific T cells is consistent with literature reports of T-cell anergy.7,19 This clearly indicates that anergy rather than clonal deletion of the hFIX-specific T cells is the main mechanism in the FIX tolerance following intramuscular injection of AAV1.7,19
In vivo depletion of regulatory T cells does not reverse the T or B tolerance to FIX
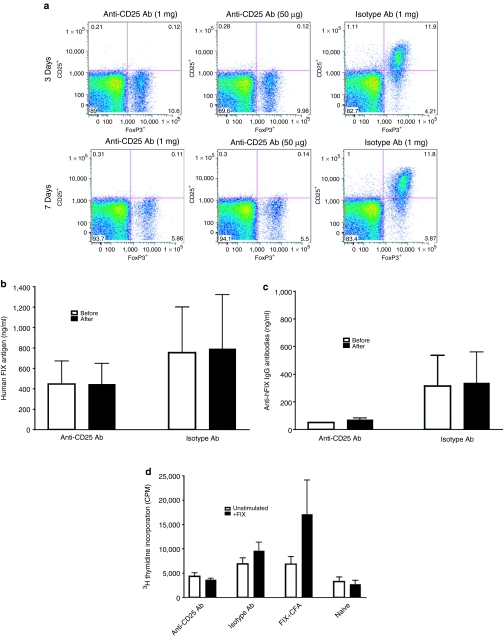
To further elucidate how regulatory T cells interact with, and influence, induction and protection of FIX tolerance following intramuscular injection of AAV1, we examined the status of FIX T- and B-cell immune tolerance upon in vivo depletion of regulatory T cells. Nine-week-old C57BL/6 wild-type mice (n = 8) were given intramuscular injection of high dose of AAV1-hFIX vector as described. Induction of immune tolerance to hFIX in the mice was proven upon observation of high levels of hFIX antigen and low anti-hFIX antibody levels by ELISA. Five to nine months after AAV1 injection, the mice were separated into two cohorts and given intraperitoneal injections of anti-CD25 antibody (n = 4) or isotype control antibody (n = 4) as described. The mice were immunized with hFIX antigen in CFA, 3 days after injection of the anti-CD25 or isotype antibody. A second dose of the anti-CD25 antibody was administrated on day 7 to extend depletion of the regulatory T cells. hFIX immunization of the mice was further boosted by subcutaneous injection of hFIX in IFA (incomplete Freund's adjuvant) 10 days after the initial antibody injection. We have shown in Figure 4a that one-dose injection of 50 µg of the anti-CD25 antibody is sufficient to completely deplete the CD4+CD25+FoxP3+ regulatory T cells in naive mice for at least 7 days. AAV1-injected FIX-tolerant mice also exhibited a complete depletion of regulatory T cells following anti-CD25 antibody injection (data not shown). However, complete removal of the regulatory T cells was unable to reverse the FIX-specific tolerance in the AAV1-injected mice (Figure 4b–d). Specifically, we observed no change in the levels of hFIX antigen or anti-hFIX antibodies, before and after depletion of regulatory T cells (Figure 4b,c). We also observed a continued suppression of hFIX-specific T-cell tolerance in the FIX-tolerant mice injected with anti-CD25 antibody (Figure 4d). These findings further support our hypothesis that regulatory T cells may not be involved in the protection or maintenance of immune tolerance to FIX in the context of intramuscular AAV gene transfer.
Figure 4.
In vivo depletion of regulatory T cells fails to reverse FIX tolerance. (a) Efficient in vivo depletion of CD4+CD25+FoxP3+ regulatory T cells subsequent to administration of anti-CD25 antibody (Ab). C57BL/6 mice received one-dose intraperitoneal injection of anti-CD25 or isotype control Ab. Splenocytes were collected 3 or 7 days later, and evaluated for regulatory T cells by flow cytometry. (b) No change in hFIX antigen level in AAV1-injected, FIX-tolerant mice before and after in vivo depletion of regulatory T cells. Anti-CD25 Ab, FIX-tolerant mice that received anti-CD25 Ab (n = 4); isotype Ab, FIX-tolerant mice that received IgG2b isotype control Ab (n = 4). Open bars, human FIX antigen level before Ab administration; closed bars, human FIX antigen level after Ab administration. Results are shown as mean ± SEM. All the samples were performed in duplicate. This figure depicts results compiled from two independent experiments. (c) No change of anti-hFIX Ab titer in AAV1-injected, FIX-tolerant mice before and after in vivo depletion of regulatory T cells. Anti-CD25 Ab, FIX-tolerant mice that received anti-CD25 Ab (n = 4); isotype Ab, FIX-tolerant mice that received IgG2b isotype control Ab (n = 4). Open bars, anti-hFIX Ab before Ab administration; closed bars, anti-hFIX Ab after Ab administration. Results are shown as mean ± SEM. All the samples were performed in duplicate. This figure depicts results compiled from two independent experiments. (d) In vivo depletion of regulatory T cells fails to recover proliferation activity of hFIX-specific T cells in AAV1-injected, FIX-tolerant mice. C57BL/6 FIX-tolerant mice were given injections of anti-CD25 Ab (anti-CD25, n = 4) or IgG2b isotype (isotype, n = 4) as described. CD4+ T cells were collected from spleens of the relevant mice for T-cell proliferation assay as described. C57BL/6 mice immunized by hFIX/CFA were used as positive control (FIX, n = 3); naive, cells from naive C57BL/6 mice used as negative control (n = 3). Closed bars, stimulation with 10 µg/ml of rhFIX; open bars, mock stimulation without hFIX. All the samples were performed in triplicate.
Discussion
In the current study, we comprehensively investigated the role and function of regulatory T cells in induction and maintenance (protection) of immune tolerance to FIX following intramuscular AAV gene transfer. The results generated in this study evidently demonstrate that regulatory T cells are not a primary, dominant mechanism in induction or protection of immune tolerance to FIX subsequent to intramuscular AAV gene transfer.
A large body of literature in the recent decade has established the paradigm that regulatory T cells function as a major, fundamental, dominant mechanism in induction and maintenance of immune tolerance and immunological homeostasis.1,2,3,10,17,20,21,22 It has also been reported that induction of regulatory T cells in immune tolerance occurs subsequent to gene transfer, as well as FVIII/FIX tolerance in hemophilia treatment.9,10,23,24,25,26 A variety of strategies were also attempted and proved successful in inducing regulatory T cells in order to control the unwanted, adverse immunity subsequent to gene transfer and hemophilia treatment.9,10,23,24,25,26
Induction of regulatory T cells was reported as a primary mechanism mediating immune tolerance to FIX subsequent to hepatic AAV gene transfer.10 In this study, we found that regulatory T cells may not be the major mechanism involved in the induction as well as protection of FIX tolerance following intramuscular AAV gene transfer. We detected ~7.74% (6.56–9.77%, mean 7.865 ± 0.1765, n = 19 in total, Figure 1 and ref. 13) of CD4+ T cells are CD4+CD25+FoxP3+ regulatory T cells in naive mice, consistent with literature reports.1,10,20 Furthermore, by using the same protocol, we detected an increase of CD4+CD25+Foxp3+ Tregs in mice that received AAV2-hFVIII gene transfer in comparison with naive congenic mice (Supplementary Figure S1). These results validate the sensitivity and reliability of our method to detect a regulatory T-cell response when such a response is present. Similar numbers of CD4+CD25+FoxP3+ regulatory T cells were detected in >30 mice irrespective of the status of FIX tolerance or immunity (Figure 1 and ref. 13), strongly suggesting that upregulation of CD4+CD25+FoxP3+ regulatory T cells is not the primary mechanism for induction of FIX tolerance in the context of intramuscular AAV gene transfer. Inefficiency in suppression of anti-FIX immunity in the recipient mice by adoptive transfer of CD4+ and whole splenocytes further confirms that the FIX tolerance is not mediated via a (extrinsic) dominant mechanism (Figure 2).1,3,13
FIX tolerance in the context of intramuscular AAV gene transfer is dependent on the dose of AAV1 and the consequential high levels of the FIX antigen.13,27 Although it is not well understood how the regulatory T cells are generated or regulated,2,3 accumulating evidence supports that prolonged provision of low-dose antigen typically favors induction/expansion of regulatory T cells, whereas high-dose antigen usually leads to recessive tolerance, resulting from clonal deletion or anergy (unresponsiveness) of the reactive antigen-specific T cells.6,7,20,22,28,29 Therefore, our finding of the minimal role of regulatory T cells in induction of FIX tolerance following intramuscular AAV1 gene transfer is not surprising. Although the FIX tolerance induced in the context of hepatic AAV gene transfer is also high-level FIX antigen-dependent, the unique immune tolerogenic nature of the liver may account for upregulation of regulatory T cells as a primary mechanism underlying the hepatic FIX tolerance.21
The fundamental and critical role of regulatory T cells is to protect the established immune tolerance and to maintain the immunological homeostasis.1,2,3,23 It is well established that depletion of regulatory T cells can disrupt the immunological homeostasis, or reverse the established immune tolerance, thus lead to a variety of autoimmune diseases.10,17,18,23,30 We thereby looked into the role and authority of regulatory T cells in protection and maintenance of the induced FIX tolerance. The FIX-specific reactive T cells in the context of FIX tolerance induced by intramuscular injection of AAV are likely anergized, therefore certainly not being deleted (Figure 3). According to the current paradigm and mechanism of immune tolerance, protection of the FIX-specific tolerance by regulatory T cells in this scenario should be essential and indispensable.1,2,3,17,23,30 It is, however, surprising as well as intriguing that we have failed to observe retrieval of the proliferative activity of the FIX-specific T cells, upon complete depletion of the regulatory T cells from the FIX-tolerant mice in vitro. Such observations suggest that protection of FIX tolerance (at least in the context of intramuscular AAV1 gene transfer) is not dependent on the dominant protective mechanism executed by regulatory T cells, as proposed in the theory and paradigm of immune tolerance and homeostasis of modern immunology.
We then moved on to examine and test the effect of regulatory T cells on the FIX tolerance in vivo. We observed that neither FIX T-cell tolerance nor FIX B-cell tolerance could be reversed upon complete depletion of regulatory T cells in the FIX-tolerant mice that received intramuscular injection of AAV1 (Figure 4). It is understandable that we did not observe recurrence of anti-FIX antibodies upon in vivo depletion of regulatory T cells. It was reported that inactivation/depletion of regulatory T cells may cause organ-specific autoimmunity in which T cells are the principal pathologic mediators.1,17,30 It is actually more complicated and elusive to determine the role of regulatory T cells in tolerance where B cells and antibodies are the prominent instrument.30,31 Regulatory T cells have been shown to exert suppression of antibody production at the initiation.30,31 This may imply a complicated, multiple level–regulated mechanism in the FIX tolerance induction following AAV gene transfer. A recent report illustrated induction of regulatory T-cell-mediated FIX tolerance subsequent to hepatic AAV2 gene transfer.10 In that report, complete deletion of regulatory T cells from the onset of AAV injection only resulted in a delayed, very modest level of anti-FIX antibodies. This level of the antibody was merely a small fraction of what was detected in FIX protein or intramuscular injection of AAV2-immunized mice.10
It is intriguing, however, that we even did not observe recovery of FIX T-cell proliferation upon complete in vivo depletion of regulatory T cells (Figure 4a,d). This observation suggests the insignificance of regulatory T cells in maintenance or protection of T-cell immune tolerance.
We would like to point out that gene therapy immunobiology is different from and also more complicated than that of the traditional protein replacement. In the gene therapy setting, transgene products such as FIX are presented to the host immune system in a different way from the traditional protein infusion–based treatment. For example, following AAV1 gene transfer, FIX is presented to the host immune system at a more constant, steady, intensive, and “endogenous” manner compared to a classical bolus injection/provision of protein. In addition, the immune interactions occurring during gene therapy are more complicated because of the involvement of the viral vector, the characteristics of the target tissue/organ and the extent of transgene expression, in addition to the genetic and acquired status of the hosts. Such unique nature of gene therapy may (at least partially) account for discrepancy of some immunological observations in gene therapy compared to knowledge and reports in classical immunology.
Persistence and strength of antigen stimulation are critical for adaptive T-cell anergy.28,29,32 In the context of intramuscular AAV gene transfer, high levels of FIX antigen are steadily and constantly presented to stimulate the T cells, thus inducing and arresting the FIX-specific T-cell clone at the status of “profound” anergy. Such a “profound” T-cell anergy in the context of persistent, constant and intense antigen presentation, is conceivably more difficult to be reversed upon depletion of regulatory T cells, than the “regular” anergy induced by antigen presentation via bolus injection. This suggests that the intrinsic factor such as anergy extent of the T cells, in comparison to the extrinsic factor, such as regulatory T cells, may be more critical for maintenance and protection of the established tolerance (at least in the context of intramuscular AAV1 gene transfer). Our findings thus may complement the current paradigm of immune tolerance mechanism.
In summary, we reported that regulatory T cells may not play a major role in the induction as well as maintenance/protection of FIX tolerance in the context of AAV gene transfer. Further testing and verification of our findings in other antigen and disease models is eagerly warranted.
Materials and Methods
AAV vector production, animal care and procedures. The AAV vectors expressing hFIX (AAV-hFIX) or hFIX-OVA were produced using the protocol of transient co-transfection of three plasmids as previously described.12,13 Wild-type C57BL/6 and OT-II mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6 FIXKO mice were gifts from Paul Monahan (University of North Carolina, Chapel Hill, NC). All the mice were maintained in pathogen-free animal facilities at Mount Sinai School of Medicine and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine. AAV injections, animal procedures, and plasma sample collection were conducted as previously described.12,13 FIX immunizations were carried out by subcutaneous injection of 2 units (1 unit = 5 µg) of rhFIX (recombinant hFIX) protein emulsified in CFA (Sigma, St Louis, MO). All injections and blood collections were preceded by Forane (Baxter, Deerfield, IL) inhaled anesthesia.
Detection of hFIX antigen and anti-hFIX antibodies. hFIX antigen was measured by ELISA as previously described.12,13 Anti-hFIX antibody-specific ELISAs were performed to detect anti-hFIX antibodies as previously described.12,13 All samples were performed in duplicates.
Lymphocyte preparation and purification. Lymphocytes were isolated from the spleens and/or lymph nodes of the pertinent mice as previously described.13 CD4+ T cells were purified by negative selection using MACS CD4+ T-cell Isolation Kit (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Analysis by flow cytometry showed ~87.5% purity of the CD4+ T cells with ~2% of CD8+ T cells and 2% of B cells. The CD4+/CD25‐ T-cell sub-population was isolated using MACS CD4+CD25+ Regulatory T-Cell Isolation Kit, according to the manufacturer's instructions. Irradiated whole splenocytes (3,000 rad) from congenic naive mice were used as APCs (antigen-presenting cells) for T-cell proliferation assay.
Flow cytometry analysis. Single-cell suspensions were obtained from the spleen of the pertinent mice as described above. The cells were then suspended in 100 µl of FACS (fluorescence-activated cell sorting) staining buffer (5 ml phosphate-buffered saline, 100 µl each of normal mouse, rabbit, and human serum, 333 µl 30% bovine serum albumin, 5 ml HBSS complete with bovine serum albumin and 100 mmol/l EDTA), and then stained with the following fluorochrome-conjugated antibodies: CD4-APC (1:300) (eBioscience, San Diego, CA) and CD25-PerCP-Cy5.5 (1:300) (BD Pharmingen, San Diego, CA). The cells were incubated with the antibody mixture (cell surface antibodies) for 30 minutes on ice. The cells were washed twice by the addition of 1 ml FACS buffer. The cell pellet was resuspended in 1 ml freshly prepared fixation/permeabilization reagent (FoxP3 staining buffer set; eBioscience) and incubated for 30 minutes on ice. Cells were washed twice by addition of 1 ml of 1× permeabilization buffer followed by incubation with the intracellular antibody [FoxP3-PE (1:200)] (eBioscience) for 30 minutes on ice. They were washed twice with 1× permeabilization buffer, and then were stored in 1× formalin buffer prior to analysis. Data were acquired on BD LSR II flow cytometer equipped with the BD FACSDiva software (Becton Dickinson, San Jose, CA). The data were analyzed using FlowJo analysis software (TreeStar, Ashland, OR).
In vitro culture and T-cell proliferation assay. CD4+ T cells (2 × 105/well) plus APCs (1 × 105/well), CD4+CD25‐ T cells (2 × 105/well) plus APCs (1 × 105/well), were cultured in 96-well plates in 200 µl/well of RPMI medium, with or without stimulation of immunogen (10 µg/ml of rhFIX; Genetics Institute, Cambridge, MA) at 37 °C for 4 days. Recombinant mouse IL-2 (R&D Systems, Minneapolis, MN) was added at the doses from 0 to 100 ng in the cultures for the T-cell anergy assay. For T-cell proliferation assay, cells were labeled with 1 µCi 3H-thymidine/well (GE Healthcare, Piscataway, NJ) 18 hours before harvest. Lymphocyte (T-cell) proliferation was measured by scintillation count of 3H-thymidine incorporation using a Beta Reader (1450 Microbeta Plus; Wallac, Waltham, MA). All samples were performed in triplicate.
Adoptive T-cell transfer. Whole splenocytes isolated from naive or AAV-injected normal C57BL/6 and FIXKO mice (9 × 107–1.3 × 108 cells per mouse) were collected and adoptively transferred into 8- to 14-week-old congenic naive mice by retro-orbital injection. Recipient mice were immunized by subcutaneous injection of hFIX in CFA 24 hours after cell transfer, as described. Anti-hFIX T-cell responses and antibody formation were examined, as described, 14 days after hFIX immunization.
In vivo depletion of regulatory T cells. In vivo depletion of regulatory T cells was performed by intraperitoneal injection of 1 mg of LEAF purified anti-mouse CD25 antibody (Clone PC61; BioLegend, San Diego, CA) on day 0 followed by a subsequent dose of 200 µg on day 7. Control mice were injected with LEAF Purified Rat IgG2b isotype intraperitoneally at the same frequency. Mice were immunized and boosted with 2 units of rhFIX in CFA or IFA on days 3 and 10, respectively. Naive congenic mice were immunized with the same dose and frequency to serve as a positive control. Plasma and splenocytes were collected from the mice for testing on day 15.
Statistical analysis. The data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Statistical differences between the various experimental groups were evaluated by two-tailed, unpaired t-test. P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Detection of increase of regulatory T cells. Increase of CD4+CD25+FoxP3+ regulatory T cells was detected in C57BL/6 hemophilia A mice that received AAV2-FVIII gene transfer (AAV2), in comparison to congenic, age-matched naïve hemophilia A mice (Naïve) (p=0.0113, two-tailed T test). The mice at age of 8–10 weeks received intravenous injection of 1×1011 vector genomes of AAV2 vectors expressing B domain deleted human FVIII. Lymphocytes were collected from spleen of the mice four months later and evaluated for regulatory T cell by flow cytometry as described. Results are shown as mean +/- SEM.
Supplementary Material
Detection of increase of regulatory T cells.
Increase of CD4+CD25+FoxP3+ regulatory T cells was detected in C57BL/6 hemophilia A mice that received AAV2-FVIII gene transfer (AAV2), in comparison to congenic, age-matched naïve hemophilia A mice (Naïve) (p=0.0113, two-tailed T test). The mice at age of 8–10 weeks received intravenous injection of 1×1011 vector genomes of AAV2 vectors expressing B domain deleted human FVIII. Lymphocytes were collected from spleen of the mice four months later and evaluated for regulatory T cell by flow cytometry as described. Results are shown as mean +/- SEM.
Acknowledgments
H.C. designed the study, analyzed the data, and wrote the paper. M.K. and A.S.B. performed the experiments, summarized the data, and participated in data analysis and paper writing. F.T. contributed to the development of experimental protocol and data analysis. This work is supported by NIH (National Institutes of Health) RO1-HL076699 to H.C. H.C. is a NHLBI (National Heart, Lung, and Blood Institute)/National Hemophilia Foundation (NHF) researcher.
REFERENCES
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Vukmanovic-Stejic M, Taams LS., and , Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- Rudensky AY, Gavin M., and , Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell. 2006;126:253–256. doi: 10.1016/j.cell.2006.07.005. [DOI] [PubMed] [Google Scholar]
- De Groot AS., and , Moise L. Prediction of immunogenicity for therapeutic proteins: state of the art. Curr Opin Drug Discov Devel. 2007;10:332–340. [PubMed] [Google Scholar]
- Berntorp E, Shapiro A, Astermark J, Blanchette VS, Collins PW, Dimichele D, et al. 2006Inhibitor treatment in haemophilias A and B: summary statement for the 2006 international consensus conference Haemophilia 12suppl. 6): 1–7. [DOI] [PubMed] [Google Scholar]
- Critchfield JM, Racke MK, Zúñiga-Pflücker JC, Cannella B, Raine CS, Goverman J, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Fathman CG., and , Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- Hackett NR, Kaminsky SM, Sondhi D., and , Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL., and , Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Monahan PE, Liu Y, Samulski RJ., and , Walsh CE. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol Ther. 2001;4:217–222. doi: 10.1006/mthe.2001.0449. [DOI] [PubMed] [Google Scholar]
- Cohn EF, Zhuo J, Kelly ME., and , Chao HJ. Efficient induction of immune tolerance to coagulation factor IX following direct intramuscular gene transfer. J Thromb Haemost. 2007;5:1227–1236. doi: 10.1111/j.1538-7836.2007.02522.x. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Zhuo J, Bharadwaj AS., and , Chao H. Induction of immune tolerance to FIX following muscular AAV gene transfer is AAV-dose/FIX-level dependent. Mol Ther. 2009;17:857–863. doi: 10.1038/mt.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Iida R, Yamazaki S., and , Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR., and , Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Annoni A, Battaglia M, Follenzi A, Lombardo A, Sergi-Sergi L, Naldini L, et al. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
- McHugh RS., and , Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Noelle RJ., and , Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly B, Kang SM, Lenardo MJ., and , Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L., and , von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC., and , von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Skupsky J, Su Y, Lei TC., and , Scott DW. Tolerance induction by gene transfer to lymphocytes. Curr Gene Ther. 2007;7:369–380. doi: 10.2174/156652307782151443. [DOI] [PubMed] [Google Scholar]
- Waters B, Qadura M, Burnett E, Chegeni R, Labelle A, Thompson P, et al. Anti-CD3 prevents factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113:193–203. doi: 10.1182/blood-2008-04-151597. [DOI] [PubMed] [Google Scholar]
- Madoiwa S, Yamauchi T, Kobayashi E, Hakamata Y, Dokai M, Makino N, et al. Induction of factor VIII-specific unresponsiveness by intrathymic factor VIII injection in murine hemophilia A. J Thromb Haemost. 2009;7:811–824. doi: 10.1111/j.1538-7836.2009.03314.x. [DOI] [PubMed] [Google Scholar]
- Zhang TP, Jin DY, Wardrop RM, 3rd, Gui T, Maile R, Frelinger JA, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007;14:429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- Friedman A., and , Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K., and , Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, et al. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- Ding C., and , Yan J. Regulation of autoreactive B cells: checkpoints and activation. Arch Immunol Ther Exp (Warsz) 2007;55:83–89. doi: 10.1007/s00005-007-0011-0. [DOI] [PubMed] [Google Scholar]
- Singh NJ., and , Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of increase of regulatory T cells.
Increase of CD4+CD25+FoxP3+ regulatory T cells was detected in C57BL/6 hemophilia A mice that received AAV2-FVIII gene transfer (AAV2), in comparison to congenic, age-matched naïve hemophilia A mice (Naïve) (p=0.0113, two-tailed T test). The mice at age of 8–10 weeks received intravenous injection of 1×1011 vector genomes of AAV2 vectors expressing B domain deleted human FVIII. Lymphocytes were collected from spleen of the mice four months later and evaluated for regulatory T cell by flow cytometry as described. Results are shown as mean +/- SEM.