Abstract
RNA interference (RNAi)–mediated knockdown of gene expression offers a novel treatment strategy for human immunodeficiency virus (HIV) infection. However, the major hurdle for clinical use is a practical strategy for small interfering RNA (siRNA) delivery to the multiple immune cell types important in viral pathogenesis. We have developed a novel immunoliposome method targeting the lymphocyte function–associated antigen-1 (LFA-1) integrin expressed on all leukocytes and evaluated it for systemic delivery of siRNA in a humanized mouse model. We show that in vivo administration of the LFA-1 integrin–targeted and stabilized nanoparticles (LFA-1 I-tsNPs) results in selective uptake of siRNA by T cells and macrophages, the prime targets of HIV. Further, in vivo administration of anti-CCR5 siRNA/LFA-1 I-tsNPs resulted in leukocyte-specific gene silencing that was sustained for 10 days. Finally, humanized mice challenged with HIV after anti-CCR5 siRNA treatment showed enhanced resistance to infection as assessed by the reduction in plasma viral load and disease-associated CD4 T-cell loss. This study demonstrates the potential in vivo applicability of LFA-1-directed siRNA delivery as anti-HIV prophylaxis.
Introduction
Sequence-specific gene silencing by RNA interference (RNAi) is being explored as a novel therapeutic strategy in a variety of diseases.1 Despite the availability of highly active antiretroviral therapy, there is a particularly strong interest in developing RNAi as a therapeutic option for human immunodeficiency virus (HIV) because of the significant practical problems such as toxicity and development of drug resistance associated with lifelong treatment. A number of studies have demonstrated the potential of RNAi targeting cellular receptor/co-receptors and viral genes to inhibit HIV replication in vitro.2,3,4 However, an effective delivery system to target relevant cells in vivo will have to be developed for exploiting the technology for antiviral therapy.3,5
Recently, cell-surface receptor-specific ligands attached to positively charged proteins or peptides that bind to small interfering RNA (siRNA) by charge interaction have been used for in vivo delivery of siRNA to immune cells.3,5,6 We have shown that a single chain antibody directed at the pan-T-cell surface molecule CD7 conjugated to polyarginine peptide could deliver siRNA to T cells and suppress HIV-1 infection in humanized mice.3 Although this served as a convincing proof of principle that siRNA can be used for treating HIV infection, CD7 expression is confined to T cells only, which limits its usefulness for targeting other relevant HIV-susceptible cell types. We have recently shown that an antibody-protamine fusion protein directed to the human lymphocyte function–associated antigen-1 (LFA-1), the predominant integrin present on all leukocytes, could selectively deliver siRNAs to multiple immune cell types, including T cells, macrophages, and dendritic cells that play key roles in HIV infection and pathogenesis.6 Thus, LFA-1 integrin antibody could be harnessed as a versatile tool for RNAi-based therapy for HIV.
For actual clinical application, a more optimal delivery vehicle will have to be developed taking into consideration the siRNA payload capability, serum stability, and pharmaceutical scalability. To this end, liposomal nanoparticles are promising delivery vehicles for siRNA because of their nano-dimension, enhanced payload, and protection of encapsulated siRNA from external environments. In a previous study, we described a novel integrin-targeted and stabilized nanoparticle (I-tsNP) formulation for siRNA delivery that uses neutral phospholipids to circumvent the potential toxicity common to cationic lipids and polymers used for systemic siRNA delivery.7 Systemic delivery of siRNA to β7-integrin+ leukocytes by this strategy effectively blocked cyclin D1 expression in vivo, thereby suppressing gut inflammation in mice.7 Here, we have used this I-tsNP approach with an LFA-1 integrin–targeted antibody for delivery of anti-HIV siRNAs to human lymphocytes and monocytes. We silenced leukocyte-specific HIV co-receptor CCR5 expression in the bone marrow liver thymic (BLT) mice, transplanted with human fetal hepatic CD34+ cells. The chemokine receptor CCR5 that functions as a co-receptor for macrophage-tropic strains of HIV is an attractive target for therapeutic ablation as its natural mutation is well tolerated and provides protection from HIV.1,3 Our data show that silencing CCR5 expression by nanoparticle (LFA-1 I-tsNPs)-mediated siRNA delivery protects mice from HIV challenge in vivo, suggesting that this could be developed as a novel intracellular immunization strategy for clinical application.
Results
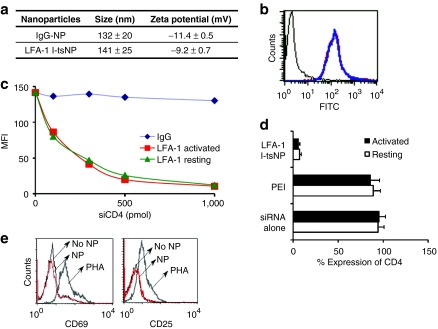
LFA-1-conjugated liposomal nanoparticles selectively deliver siRNAs to LFA-1-expressing human leukocytes
We generated LFA-1 I-tsNP, surface-modified neutral liposomes with the size of ~100 nm and zeta potential of −9.2 mV (Figure 1a) that efficiently bound to primary human lymphocytes independent of their activation status (Figure 1b), which is important as lymphocyte activation is implicated in facilitating HIV infection.3 To test whether we can use LFA-1-conjugated I-tsNPs to deliver siRNA to target cells to induce gene silencing, we transduced anti-CD4 siRNA (siCD4) into primary human CD3+ T cells using LFA-1 I-tsNPs. LFA-1 I-tsNP was able to transduce siCD4 and silence CD4 expression in vitro in a dose-dependent manner with efficiencies of nearly 95% in both resting and activated T cells (Figure 1c). In contrast, treatment with siRNA alone or with a conventional transfection reagent PEI (polyethylenimine) showed negligible silencing effects (Figure 1d) as did graded concentrations of siCD4 encapsulated in isotype control mAb-coated nanoparticles (IgG-NPs) (Figure 1c). To exclude the possibility that LFA-1-targeted nanoparticles should induce aberrant activation of lymphocytes, freshly isolated cord blood mononuclear cells (which are a stringent source of naive T cells) were incubated in the absence or presence of LFA-1 I-tsNP and stained with activation makers CD69 and CD25. Despite the efficient siRNA delivery with LFA-1 I-tsNPs, the binding of the immunoliposomes to the cells did not induce activation of the cells (Figure 1e). However, the PHA (phytohemagglutinin)-stimulated positive control showed highly elevated expression of both activation markers. These results show, at least in the current experimental setting, that siRNA delivery with LFA-1 I-tsNP does not perturb the resting status of naive T cells.
Figure 1.
siRNA delivery to resting and activated T cells using nanoparticles targeted to LFA-1. (a) The size and zeta potential of carrier nanoparticles. (b) Binding of LFA-1 I-tsNPs to activated (blue) and naive (red) human primary lymphocytes. (c) CD4 silencing tested with LFA-1 I-tsNP at different concentrations of CD4 siRNA. (d) LFA-1 I-tsNP-mediated CD4 knockdown in resting and activated T cells. (e) Expression of the activation markers on LFA-1 I-tsNP-treated and nontreated primary lymphocytes. FITC, fluorescein isothiocyanate; I-tsNP, integrin targeted and stabilized nanoparticle; LFA-1, lymphocyte function–associated antigen-1; MFI, mean fluorescence intensity; NP, nanoparticle; PEI, polyethylenimine; PHA, phytohemagglutinin.
Intravenous administration of siRNA-entrapped LFA-1 I-tsNP silences target gene expression in T cells of humanized mice
To examine the therapeutic feasibility of LFA-1 I-tsNPs-directed siRNA delivery in vivo, we investigated selective silencing of target gene expression in two different humanized mouse models. We first used the NOD/SCID/IL2rγnull-based Hu-PBL mouse model where adoptively transferred human T cells undergo a short-term expansion after xenogeneic stimulation. In Hu-PBL mice injected intravenously with LFA-1 I-tsNP/siCD4 complexes, CD4 expression on peripheral blood T cells was reduced by >50% compared to isotype control IgG-NP/siCD4-treated mice accompanied by a reciprocal increase in CD3+CD4−CD8− T-cell population (Figure 2a–c). Similar results were observed in T cells from liver and spleen. As the repopulated T cells in Hu/PBL mice are of an activated phenotype, the model precludes testing of siRNA delivery to naive/resting T cells. Thus, we also studied the ability of LFA-1 I-tsNP to silence gene expression in NOD/SCID/IL2rγnull BLT mice engrafted with human hematopoietic stem cells. In the BLT mouse model, human fetal liver and thymus tissue are surgically implanted under the kidney capsule to provide an optimal human microenvironment for systemic reconstitution of all major human hematopoietic lineages, including T, B, monocyte/macrophage, and dendritic cells, that are particularly relevant to HIV infection. In contrast to the PBL model, here the T cells are of a naive phenotype. A substantial reduction in CD4 expression was also observed in BLT mice treated with LFA-1 I-tsNP/siCD4 (Figure 2d,e). The treatment did not change CD8 expression in either Hu-PBL or BLT mice, which proves that the silencing was specific to CD4. Thus, LFA-1 I-tsNP can deliver siRNA and mediate specific gene silencing in vivo not only in activated but also in naive and resting human T cells that are particularly refractory to nucleic acid uptake.3
Figure 2.
Nanoparticle-mediated in vivo gene silencing in humanized mice. (a) LFA-1 I-tsNP-mediated in vivo silencing of CD4 expression in liver, spleen, and blood of Hu-PBL mice. Representative dot plots from one mouse. (b) Percent ratio of CD3+CD4−CD8− population and (c) percent reduction of surface CD4 expression monitored by flow cytometry. Cumulative data from three mice are shown. (d) LFA-1 I-tsNP-mediated in vivo silencing of CD4 expression in liver, spleen, and blood of BLT mice. Representative histogram plots from one mouse. (e) Percent reduction of surface CD4 expression. Cumulative data from three mice. I-tsNP, integrin targeted and stabilized nanoparticle; LFA-1, lymphocyte function–associated antigen-1.
LFA-1-conjugated liposomal nanoparticles selectively deliver siRNAs to LFA-1-expressing human leukocytes in vivo
We tested the uptake of CCR5 siRNA (siCCR5) in various subsets of leukocytes isolated from BLT mice by real-time PCR, 24 hours after intravenous administration of LFA-1 I-tsNPs/siCCR5. siCCR5 was selectively detected in human T cells (CD3+), B cells (CD19+), and monocytes (CD14+), but not in mouse-derived CD45+ cells and brain cells that served as controls (Figure 3a). Quantitative PCR analysis of immunomagnetically isolated peripheral blood mononuclear cell (PBMC) populations before treatment revealed CCR5 expression in CD14+ monocytes but not in T cells presumably because of their naive status.8 Remarkably, a single intravenous dose of LFA-1 I-tsNPs/siCCR5 potently reduced CCR5 mRNA levels in CD14+ monocytes at 3 days after treatment, with the silencing lasting for at least 10 days as compared to siLuc-treated control mice (Figure 3b). CCR5 expression was undetectable in CD14− flow through in both control and test samples.
Figure 3.
Systemic delivery of siCCR5 with LFA-1 I-tsNPs silences CCR5 expression in vivo. (a) siCCR5 uptake was monitored using RT-PCR in peripheral blood T cells (CD3+), B cells (CD19+), and monocytes (CD14+) in humanized mice. n.d., not detected. (b) The CCR5 levels in peripheral blood CD14+ monocytes were measured by RT-PCR before, and 3 and 10 days after siRNA treatment from blood collected. Reduction in CCR5 expression was calculated as a percentage of initial expression level before siRNA injection. siCCR5 (open bar) and siLuc (solid bar). (c) Expression of IFN responsive genes relative to β-actin were analyzed by quantitative RT-PCR in PBMC treated with siLuc delivered as indicated. Poly (I:C) was used as a positive control to induce IFN responses. (d) Freshly isolated PBMCs were treated with LFA-1 I-tsNP-encapsulated siLuc, siCCR5, or siβgal, and TNF-α release was determined after 24 hours of stimulation. Lipofectamine 2000–encapsulated siβgal was used as a positive control. Empty liposomes and naked siRNA did not induce detectable cytokines. Values are mean ± SD of triplicate cultures. IFN, interferon; LFA-1, lymphocyte function–associated antigen-1; TNF-α, tumor necrosis factor-α.
As liposomally encased siRNAs can potently stimulate both murine and human innate immune systems by engaging endosomally localized TLRs,9 we measured induction of cellular mRNA levels of IFN-β (interferon-β) and the key interferon-responsive molecules 2′,5′-oligoadenylate synthetase (OAS1) and STAT1 (ref. 5) in PBMC after exposure to tsNP/siRNA complexes. In contrast to the known IFN inducer poly (I:C), siLuc-encapsulated tsNPs did not induce expression of any of the interferon-responsive genes indicating that the LFA-1 I-tsNP does not trigger nonspecific IFN responses (Figure 3c). LFA-1 I-tsNP/siCCR5 also did not induce secretion of the inflammatory cytokine TNF-α (tumor necrosis factor-α) from PBMC in contrast to a known immunostimulatory siRNA (Figure 3d),10 indicating that this formulation can be evaluated for antiretroviral activity in an in vivo setting.
Systemic delivery of LFA-1 I-tsNP/siCCR5 complex protects BLT mice from HIV infection
To directly evaluate protection from HIV infection, BLT mice were treated with siCCR5 complexed LFA-1 I-tsNPs and challenged with macrophage-tropic HIVBaL intraperitoneally as depicted (Figure 4a). LFA-1 I-tsNPs mediated delivery of siCCR5 but not of the control siLuc prevented CD4 T-cell depletion and greatly reduced HIV viral load (Figure 4b–d). As early as 11 days after infection, CD4 T-cell levels declined in control siLuc-treated mice, with CD3+CD4+ T-cell percentages dropping to as low as 31% and CD3+CD8+ percentages concomitantly increasing to over 58% (Figure 4b,d). In contrast, CD4 T-cell levels were sustained up to 8 weeks tested after infection in LFA-1 I-tsNPs/siCCR5-treated mice (mean level 80%). The mean CD4 levels in the LFA-1 I-tsNPs/siCCR5-treated mice were comparable to those in uninfected mice (Figure 4b,d). Consistent with CD4 T-cell levels, plasma viral load was at least two log units lower in siCCR5-treated mice (3.4 × 103 copies/ml plasma) as compared to siLuc-treated control mice (1.1 × 105 copies/ml plasma) (Figure 4c) demonstrating that LFA-1 I-tsNP-mediated systemic delivery of siCCR5 could prevent HIV infection in BLT mice.
Figure 4.
Systemic delivery of siCCR5 with LFA-1 I-tsNPs protects BLT mice from HIV infection. (a) BLT mice were treated intravenously with either siLuc (n = 3) or siCCR5 (n = 3) complexed to LFA-1 I-tsNPs, infected with HIVBaL, and monitored for viral load and CD4 counts according to protocol described. (b) Representative dot plots from one siLuc-treated and one siCCR5-treated mouse are shown. (c) Viral copy numbers in plasma measured by the Amplicor test and (d) CD3+CD4+ T-cell percentages monitored by flow cytometry at various times are shown. CD4 T-cell ratios were calculated as a ratio of the entire CD3 population (CD3+CD4+/CD3+). Individual animals in each group are represented by distinct symbols. HIV, human immunodeficiency virus; I-tsNP, integrin targeted and stabilized nanoparticle; LFA-1, lymphocyte function–associated antigen-1.
Discussion
Delivery to appropriate cells and tissues in vivo remains a major hurdle for harnessing the potent gene silencing ability of siRNA molecules for treating human diseases such as HIV infection.3 Inducing RNAi in leukocytes, which are the prime targets of HIV, has been particularly challenging, as these cells are difficult to transduce even by conventional transfection methods.7 In previous studies, we have used scFvs directed at LFA-1 present on all leukocytes or CD7 on T cells for siRNA delivery to immune cells by linking them to positively charged proteins/peptide that bind nucleic acids by charge interactions.3,5,6 Compared to CD7 scFv that targets only T cells, scFv to LFA-1 provides a particularly versatile tool for RNAi-based therapy for HIV as it targets multiple immune cell types such as T cells, macrophages, and dendritic cells that play crucial roles in viral infection and pathogenesis. The novel immunoliposomal delivery platform that we have developed in this study improves upon LFA-1-directed siRNA delivery for therapeutic applications as it combines the same targeting advantage but with improved pharmacokinetics and high cargo capacity. Unlike cationic nanoparticles that can aggregate or get opsonized in the blood because of their excessive positive charge, tsNPs have a hyaluronan modification that confers a net neutral charge so that the blood circulation time is prolonged.11 Moreover, the siRNA is well protected as it is encapsulated within the liposome and can be delivered at high concentrations to achieve therapeutic efficacy without unintended off-target effects in nonleukocyte populations that do not express LFA-1. Further, this formulation was not immunostimulatory or inflammatory in nature. In fact, we have successfully used this stabilized nanoparticle approach to target cyclin D1 siRNA to gut-homing leukocytes expressing the integrin β7 (β7 I-tsNPs) in a mouse model of gut inflammation, where a significant beneficial effect was achieved at a low dose of 2.5 mg/kg (ref. 7). Although this remains to be formally tested, liposomal packaging of siRNA should also reduce the amount of antibody required for targeting compared to approaches that we and others have used where the antibody is tagged to a positively charged peptide/protein that has to be used at high molar concentrations for siRNA binding. A direct head-to-head comparison of different siRNA delivery technologies will be required to clarify whether tsNPs do have a distinct advantage for use as a potential tool for therapeutic delivery of siRNA.
Although initial experimentation for evaluating silencing efficacy utilized the CD4 T-cell antigen as a target, these are more proof-of-principle studies considering the important role this molecule plays in immune function. A more attractive and therapeutically relevant target is the chemokine receptor CCR5 that functions as a co-receptor for most strains of HIV for entry into target cells (mostly activated T cells and macrophages) during the early stages of infection. Further, natural mutations in the CCR5 are well tolerated and provide protection from HIV.12,13,14 Many novel experimental HIV drugs called entry inhibitors are being investigated to inhibit the utilization of CCR5 as a co-receptor by HIV-1 (refs. 15,16). Conceptually, the delivery strategy can be expanded to target both cellular and viral genes important in HIV replication. However, for preventing new infection, targeting a host receptor for viral entry may be more useful as many studies indicate that the incoming viral genome may be protected from RNAi attack that allows it to undergo reverse transcription and integrate into the host genome. Further, the propensity of the virus to mutate its sequences also necessitates targeting highly conserved viral regions to prevent the emergence of RNAi escape mutants. On the other hand, given the virus' potential to shift its co-receptor usage, an optimal therapeutic approach may be to target simultaneously the host cellular receptor and multiple conserved viral sequences.
The LFA-1 I-tsNP was tested successfully in two different animal models (Hu-PBL mouse model and BLT mouse model) for HIV. The Hu-PBL mouse model, where adoptively transferred human T cells undergo a short-term expansion after xenogeneic stimulation and the BLT model, where human fetal liver and thymus implanted under the renal capsule provide an improved human microenvironment for systemic reconstitution of all major human hematopoietic lineages relevant to HIV infection, including T, B, monocyte/macrophage, and dendritic cells. Although the Hu-PBL model allowed us to demonstrate LFA-1 I-tsNP-induced silencing in activated human lymphocytes, it is unsuitable for testing siRNA delivery to resting T cells and monocyte/macrophages that are latent reservoirs of HIV and a major impediment to eliminating HIV from the body.3 This limitation was overcome in the BLT mice engrafted with human hematopoietic stem cells. In contrast to T cells from Hu-PBL mice, which display a predominantly activated phenotype, the T cells in this model are predominantly naive unactivated (CD45RAhi, CCR7hi, CD62Lhi, CD27hi, and CCR5lo).17 In BLT mice, the conformation-insensitive antibody to LFA-1 integrin (TS1/22) could induce potent gene silencing in all leukocytes independently of activation status. These findings overcome a critical barrier of in vivo delivery, significantly enhancing the prospect of siRNA-based therapeutics for HIV infection. In a therapeutic setting, it is important to deliver siRNA to uninfected naive and resting T cells to ensure that siRNA is present in cells at the time of activation when they become most vulnerable to infection and to protect them from infection. This is also important for suppressing viral resurgence from latently infected resting T cells harboring integrated provirus.3 Thus, the control of HIV infection in BLT mice attests to the successful delivery to naive/resting T cells as well as monocytes that suggests that the strategy may be practical for clinical application.
After in vivo administration of anti-CCR5 siRNA encapsulated in LFA-1 I-tsNP, carrier-specific gene silencing in CD14+ monocytes was sustained for up to 10 days. Normal half-life for siRNAs in actively dividing cells in culture as well as in vivo can range from <1 week to as long as 10 days depending on the kinetics of target cell division.18 Our previous study demonstrated a loss in silencing effects by about 3 days after initiating siRNA treatment in Hu-PBL mice that are repopulated predominantly by activated rapidly dividing T cells. However, in light of the fact that most T cells in the BLT humanized mouse model are of the resting/naive phenotype, and that nondividing cells like macrophages can harbor functional siRNAs for >3 weeks,16 it is quite likely that knockdown effects in T cells can be sustained for extended periods of time in the BLT mouse model, and perhaps even in a physiological situation.
Systemic delivery of siCCR5 was able to protect BLT mice from HIV challenge. Although both T cells and macrophages that express CCR5 are likely to contribute to viral load in this model, this has to be reconciled with our finding of undetectable CCR5 expression in freshly isolated peripheral blood T cells of BLT mice tested prior to siRNA treatment. One plausible explanation is that activated T cells that express CCR5 were present but in too few numbers to permit detection. As T cells are known to upregulate CCR5 expression after activation, the protection observed with LFA-1 I-tsNP-mediated siCCR5 delivery to naive T cells may reflect a prophylactic effect in blocking CCR5 expression when the cells become activated subsequently. In nonreplicating cells such as macrophages, siRNA is known to be retained over a long period of time. This allows sustained gene silencing and antiviral effects, which is important for a chronic infection such as HIV.19 Although the durability of gene silencing is not comparable with gene therapy where CCR5 ablated progeny can be generated from engineered hematopoietic stem cells, at the current stage of development, the siRNA approach may be more practical in terms of the target hits that can be achieved.3,20,21 Moreover, the use of siRNA as a drug provides the flexibility for changing target sequences to cope with HIV mutations and shift in co-receptor usage.
Despite the promising data with this leukocyte-targeted siRNA delivery method in small animal models and the apparently noninflammatory nature of this formulation, there are some considerations that need to be addressed for therapeutic application of the leukocyte-targeted siRNA delivery approach. LFA-1 antibody can block leukocyte adhesion and result in silencing of proinflammatory molecules with inhibition of LFA-1-mediated cell adhesion.22 Therefore, the immunomodulatory effect of LFA-1-integrin binding by the antibody on HIV infection needs further study.22 Use of nonfunction blocking LFA-1 mAb (e.g., TS2/4) could be considered to avoid the problem. In addition, anti-LFA-1 mAb is being tested in clinical trial for HIV infection, based on the hypothesis that LFA-1-mediated cytotoxic T lymphocyte killing of CD4 T cells might be involved in CD4 depletion. In this way, there is also a possibility that blocking LFA-1 might be partly beneficial for treating HIV infection.23,24 The possibility of generating immune responses to the antibody used as a delivery vehicle is also a concern that needs to be addressed. However, humanized antibodies can be used to reduce potential immunogenicity. Off-targeting effects of synthetic siRNA is also a concern, but this can be minimized by the judicious choice of siRNA sequences and by introducing asymmetry in their design to favor the uptake of the antisense strand into RNA-induced silencing complex.25,26
In summary, this study suggests that LFA-1-directed systemic delivery of siRNA is a promising strategy for the in vivo targeting of multiple immune cell types for potential RNAi-based HIV therapeutics.
Materials and Methods
siRNAs. siRNAs targeting firefly luciferase (siLuc),27 the human T-cell receptor CD4 (ref. 4), and co-receptor CCR5 were purchased from Dharmacon (Lafayette, CO). siRNA sequences were as follows: siLuc, 5′-UCGAAGUACUCAGCGUAAGdTdT-3′ (sense)/5′-CUUACGCUGAG UACUUCGAdTdT-3′ (antisense); siCD4, 5′-GAUCAAGAGACUCCUC AGUdTdT-3′ (sense)/5′-ACUGAGGAGUCUCUUGAUCdTdT-3′ (antisense); siCCR5, 5′-GUCAGUAUCAAUUCUGGAAdTdT-3′ (sense)/ 5′-UUCCAGAAUUGAUACUGACdTdT-3′ (antisense); siβgal, 5′-CUAC ACAAAUCAGCGAUUU-3′ (sense)/5′-AAAUCGCUGAUUUGUGUAG-3′ (antisense).
Preparation of I-tsNPs and siRNA entrapment. I-tsNPs were prepared and surface-modified with LFA-1 mAbs as described.7 Briefly, multilamellar vesicle liposomes composed of soybean PC (phosphatidylcholine), DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine), and Chol (cholesterol) at a molar ratio of 3:1:1 (all from Avanti Polar Lipids, Alabaster, AL) were prepared by a lipid-film method.28 Resulting multilamellar vesicles were extruded into small unilamellar vesicles with an extrusion device (Lipex Thermobarrel Extruder System; Northern Lipids, Vancouver, British Columbia, Canada), and small unilamellar vesicles were surface-modified with high molecular weight HA (hyaluronan, molecular weight 751 kd, intrinsic viscosity 16 dl/g; Genzyme, Cambridge, MA) as described.29 HA-modified small unilamellar vesicles (PC:Chol:DPPE; 25 mmol total lipid, 75 µg HA/µmol lipid) were coupled to mAbs [TS1/22 (against human integrin LFA-1) or isotype control (mouse IgG1)] using an amine-coupling method. Resulting I-tsNP and IgG-sNP were purified by using a size exclusion column and lyophilized using α1-2 LD plus lyophilizer (Christ, Osterode, Germany). To entrap siRNA in I-tsNPs, siRNA was mixed with full-length human recombinant protamine (Abnova, Taipei City, Taiwan) in a molar ratio of 1:5 (siRNA:protamine) and incubated for 30 minutes at room temperature to form protamine-condensed siRNA complexes.6 For entrapment, lyophilized liposome nanoparticles (1–2.5 mg lipids) were rehydrated by adding 0.2 ml DEPC (diethylpyrocarbonate)-treated water containing protamine-condensed siRNAs (1–3.5 nmol). The entrapment procedure was performed immediately before use.7
Particle hydrodynamic diameter and zeta potential measurements. The diameter of the I-tsNPs was measured on a Malvern Zetasizer Nano ZS zeta potential and dynamic light scattering instrument (Malvern Instruments, Southborough, MA) using the automatic algorithm mode and analyzed with the PCS 1.32a. All measurements were done in 0.01 mol/l NaCl, pH 6.7, at room temperature.
Generation of humanized mice. Immunodeficient NOD.cg-PrkdcscidIL2rgtm/Wjl/Sz (NOD/SCID/IL2rγnull) mice at 6–10 weeks of age were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free microisolator cage. Hu-PBL mice were generated as described previously.12 In brief, 107 PBMCs freshly isolated from HIV-seronegative donors were injected intraperitoneally into NOD/SCID/IL2rγnull mice. Cell engraftment was tested 3–5 days after transplantation by staining of the mouse PBMCs for human CD45+, CD3+, CD4+, and CD8+ cells. For BLT mice, humanized mice preparation was performed as previously described.30 Human fetal thymus and liver tissues of gestational age of 17–20 weeks were obtained from Advanced Bioscience Resources (Alameda, CA). Mice were conditioned with sublethal (2–3 Gy) whole-body irradiation. Human fetal thymus and liver fragments measuring about 1 mm3 were then implanted under the recipient kidney capsule. After implantation, mice received CD34+ fetal liver cells (1–5 × 105/mouse, intravenously) purified from the same donor on the day of human thymus/liver implantation. CD34+ fetal liver cells were isolated by the magnetic-activated cell sorter separation system using anti-CD34 microbeads (Miltenyi Biotec, Auburn, CA). Transplanted mice were tested for engraftment 12 weeks later as described above. Levels of human hematopoietic cells in the mice were determined by multicolor flow cytometric analysis using various mAbs: antihuman CD3, CD4, CD8, CD11c, CD19, and CD45; anti-mouse CD45; and isotype control mAbs (all purchased from BD Pharmingen, San Diego, CA). Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA). Protocols involving the use of human tissues and animals were approved by the Institutional Review Board at the Immune Disease Institute.
In vitro and in vivo silencing. To test CD4 silencing in vitro, both resting and activated primary human T cells were treated with siCD4/LFA-1 I-tsNPs. For in vivo siRNA delivery, mice were injected with siRNAs (50 µg/injection) encapsulated in 100 µl I-tsNPs through tail vein. In HIV infection experiments, BLT mice were intraperitoneally injected with 30,000 TCID50 of HIVBaL in a 100 µl volume 24 weeks after reconstitution. PBMCs recovered from the mice at different times were analyzed by flow cytometry for gene silencing and antiviral effects. Viral loads in EDTA-treated plasma samples were determined with the Roche Amplicor Monitor assay (Roche Diagnostics, Indianapolis, IN).
TNF-α assay. Freshly isolated PBMCs (2 × 105 cells/well cultured in triplicate in 96-well plates) were mock treated or treated with LFA-1 I-tsNP entrapping siLuc, siCCR5, or siβgal (2 µmol/l). Naked siRNA and Lipofectamine 2000–complexed βgal siRNAs were used as controls. Empty liposomes and transfection reagents alone were used as vehicle controls. Culture supernatants were collected after 24 hours and assayed for TNF-α by sandwich ELISA (enzyme-linked immunosorbent assay) using the human TNF-α/TNFSF1A Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
Analysis of cellular siRNA levels. CD3+ T cells, CD19+ B cells, and CD14+ monocytes were isolated from the spleen, liver, and peripheral bloods of BLT mice given single administration of LFA-1 I-tsNPs/siCCR5 (50 µg siRNA/injection, intravenous) with the Dynal magnetic beads (Dynal, Lake Success, NY), and small RNAs were extracted with the miRNeasy mini kit (Qiagen, Valencia, CA). The small RNAs were poly(A) tailed by A-plus poly(A) polymerase tailing kit (Epicentre, Madison, WI) and reverse-transcribed by Superscript III (Invitrogen, Carlsbad, CA) with miR RT-oligo dT (5′-GCGAGCACAGAATTAATACGACTCACTATAGGT(20) VN-3′) primer. Resulting complementary DNAs were subjected to 3′RACE RT-PCR-based real-time PCR with reverse primer (5′-GCGAGCACAGAATTAATACGAC-3′) and siCCR5-specific (5′-TTCCAGAATTGATACTG-3′) or U6B RNA-specific (5′-ATGACACGCAAATTCGTGAAGC-3′) primers as described.3,31
Quantitative RT-PCR. CCR5 mRNA levels in CD14+ and CD14− PBMC populations of BLT mice were evaluated by Quantitative RT-PCR using SYBR Premix Ex Taq Polymerase (Takara, Otsu, Shiga, Japan) and ABI prism 7000 as previously described.3 The following primer pairs were used: CCR5, 5′-GGCAGGGCTCCGATGTATAA-3′ (forward)/5′-CATCCGTTCCCCTACAAGAA-3′ (reverse); GAPDH (glyceraldehyde 3-phosphate dehydrogenase), 5′-AATGAAGGGGTCATTGATGG-3′ (forward)/5′-AAGGTGAAGGTCGGAGTCAA-3′ (reverse). PCR parameters consisted of 5 minutes of Taq activation at 95 °C, followed by 40 cycles of PCR at 95 °C for 5 seconds, 60 °C for 20 seconds, and 72 °C for 31 seconds. All reactions were done in a 20 µl reaction volume in triplicate. Standard curves were generated, and the relative amount of target gene mRNA was normalized to GAPDH mRNA. Quantitative RT-PCR for IFN-β, OAS1, and STAT1 was carried out as described.7
Acknowledgments
This work was supported by grants from NIH (National Institutes of Health), AI071882 (P.S.) and AI063421 (M.S.); the Leukemia & Lymphoma Society (M.S.); the KRF (Korea Research Foundation) and MOEHRD (Ministry of Education and Human Resource Development), KRF-2006-352-D00070 (S.-S.K.); and the CFAR (Center for AIDS Research) fellowship P30 A060354 (P.K.). We thank Erica Nakajima from the Yale School of Medicine for technical assistance and critical reading of the manuscript.
REFERENCES
- Rossi JJ.2006RNAi as a treatment for HIV-1 infection Biotechniquessuppl.): 25–29. [DOI] [PubMed]
- Lee SK, Dykxhoorn DM, Kumar P, Ranjbar S, Song E, Maliszewski LE, et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood. 2005;106:818–826. doi: 10.1182/blood-2004-10-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, et al. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- Peer D, Zhu P, Carman CV, Lieberman J., and , Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc Natl Acad Sci USA. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, Carman CV., and , Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski MA, Chun TW, Justement SJ, Motola I, Spinelli MA, Adelsberger J, et al. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT., and , Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and , MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Peer D., and , Margalit R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer. 2004;108:780–789. doi: 10.1002/ijc.11615. [DOI] [PubMed] [Google Scholar]
- Nakata H, Maeda K, Miyakawa T, Shibayama S, Matsuo M, Takaoka Y, et al. Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/ GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model. J Virol. 2005;79:2087–2096. doi: 10.1128/JVI.79.4.2087-2096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, Lapenta C, Santini SM, Spada M, Parlato S, Logozzi M, et al. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J Virol. 1999;73:6453–6459. doi: 10.1128/jvi.73.8.6453-6459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges BK, Wheat WH, Palmer BE, Connick E., and , Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacovina A., and , New M. Chemokine (C-C motif) receptor 5 entry inhibitors: new class of drugs for acquired immune deficiency syndrome. Mt Sinai J Med. 2008;75:297–298. doi: 10.1002/msj.20050. [DOI] [PubMed] [Google Scholar]
- Qian K, Morris-Natschke SL., and , Lee KH. HIV entry inhibitors and their potential in HIV therapy. Med Res Rev. 2009;29:369–393. doi: 10.1002/med.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice. J Virol. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DW., and , Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N, Kumar P, Lee SK., and , Shankar P. Interfering antiviral immunity: application, subversion, hope. Trends Immunol. 2006;27:328–335. doi: 10.1016/j.it.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci USA. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Shimaoka M, Salas A., and , Springer TA. The binding sites for competitive antagonistic, allosteric antagonistic, and agonistic antibodies to the I domain of integrin LFA-1. J Immunol. 2004;173:3972–3978. doi: 10.4049/jimmunol.173.6.3972. [DOI] [PubMed] [Google Scholar]
- Giguère JF., and , Tremblay MJ. Statin compounds reduce human immunodeficiency virus type 1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule 1 and its natural cell surface ligand LFA-1. J Virol. 2004;78:12062–12065. doi: 10.1128/JVI.78.21.12062-12065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioe CE, Chien PC, Jr, Lu C, Springer TA, Wang XH, Bandres J, et al. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J Virol. 2001;75:1077–1082. doi: 10.1128/JVI.75.2.1077-1082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Rogoff HA., and , Li CJ. Asymmetric RNA duplexes mediate RNA interference in mammalian cells. Nat Biotechnol. 2008;26:1379–1382. doi: 10.1038/nbt.1512. [DOI] [PubMed] [Google Scholar]
- Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N., and , Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Peer D., and , Margalit R. Physicochemical evaluation of a stability-driven approach to drug entrapment in regular and in surface-modified liposomes. Arch Biochem Biophys. 2000;383:185–190. doi: 10.1006/abbi.2000.2046. [DOI] [PubMed] [Google Scholar]
- Peer D, Florentin A., and , Margalit R. Hyaluronan is a key component in cryoprotection and formulation of targeted unilamellar liposomes. Biochim Biophys Acta. 2003;1612:76–82. doi: 10.1016/s0005-2736(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S., and , Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, et al. miRNA profiling of naïve, effector and memory CD8 T cells. PLoS ONE. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]