Abstract
Oncolytic adenoviruses (Ads) constitute a promising new class of anticancer agent. They are based on the well-studied adenoviral vector system, which lends itself to concept-driven design to generate oncolytic variants. The first oncolytic Ad was approved as a drug in China in 2005, although clinical efficacy observed in human trials has failed to reach the high expectations that were based on studies in animal models. Current obstacles to the full realization of efficacy of this class of anticancer agent include (i) limited efficiency of infection and specific replication in tumor cells, (ii) limited vector spread within the tumor, (iii) imperfect animal models and methods of in vivo imaging, and (iv) an incomplete understanding of the interaction of these agents with the host. In this review, we discuss recent advances in the field of oncolytic Ads and potential ways to overcome current obstacles to their clinical application and efficacy.
Oncolytic Adenoviruses are a New Class of Anticancer Agents
Oncolytic replication–selective viruses are a new class of anticancer agents with great therapeutic potential.1,2,3,4 The selective replication of the viruses in cancer cells amplifies the initial viral inoculum, leading to destruction of the infected cells by virus-mediated cytolysis. The viral progenies are thereby released and can spread through the tumor mass to infect neighboring cancer cells, resulting in self-perpetuating cycles of infection, replication, and oncolysis. Past cancer gene therapy clinical trials have defined major limitations of replication-defective vectors for cancer gene therapy; unable to infect the majority of the cells within a clinically presented three-dimensional solid tumor mass. Replication-selective viruses are designed to overcome such clinical limitations of cancer gene therapy by virus replication/spread whereas the restriction of their replication to tumor cells embodies the safety of oncolytic viruses for clinical usage.2,3
Although several oncolytic viruses have been identified to date,5,6,7 replication-selective adenoviruses (Ads) based on human serotype 5 of species C possess a number of advantages.1,2,3,4 Human serotype 5 Ads, which are associated with relatively mild diseases, are well characterized, their genomes can be manipulated with relative ease, and they can be purified to high titer.2,8 Moreover, the long history of usage as replication-defective adenoviral vectors for cancer gene therapy has defined the strategies for cancer targeting, including targeting based on binding and infection as well as the strategies to restrict the replication of Ads to tumor cells.1,2,3,4 The latter can be achieved via placing the expression of viral genes, most commonly the E1A gene, under the control of tumor- or tissue-specific promoters, or via the complete or partial deletion of viral genes required for replication in normal cells, but not in tumor cells.1 In this sense, Ad is one of a few systems which are capable of concept-based design of the vector structure in the context of oncolytic viruses.
In 2005, State Food and Drug Administration in China approved mutation-based oncolytic Ad (H101; Sunway Biotech, Shanghai, P.R. China) as a drug for head and neck squamous cell carcinoma for local injection based on good responses observed in clinical trials.9 This brings the hope that oncolytic Ads can be used in patients in the countries other than China.
Replicating Ads are Promising but have Shown Limited Efficacy in Human Clinical Trials So Far
In recognition of their therapeutic potential, replication-selective Ads have been rapidly translated into human clinical trials in patients with advanced cancer,10,11 where their safety has been demonstrated. In this regard, a phase I clinical trial of an intraperitoneally administered replication-selective Ad has been conducted in patients with recurrent/refractory ovarian cancer.12 In this trial, the maximum tolerated dose was not reached at 1011 plaque-forming units, and patients did not experience significant toxicity with this dose of administration.12 Hence, there is a precedent for the safe use of replication-selective Ads in ovarian cancer patients.
However, in spite of their promise as selective cancer therapeutics, replicating Ads have shown limited efficacy in the clinical setting.3 In this regard, there was no clear-cut evidence of clinical or radiologic response in any of the 16 patients with recurrent/refractory ovarian cancer who received an intraperitoneal replication–selective Ad in the phase I trial.12 Similarly, phase I and II clinical trials in which patients with recurrent squamous cell carcinoma of the head and neck received direct intratumoral injection of a replicating Ad, ONYX-015, resulted in clinical benefit in <15% of cases.13,14 Only when combined with standard chemotherapy did this oncolytic Ad cause an objective response (at least a 50% reduction in tumor size) in 19 of 30 cases, with 8 complete responses.15,16 This indicates a need for implementation of novel strategies to improve the efficacy of replicating Ads while assuring safety in normal cells for clinical application in the treatment of patients with cancer.
Effective Infection of Cancer Cells
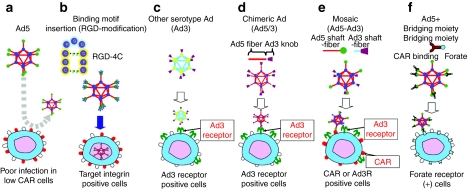
As noted, there is no conditionally replicative adenovirus (CRAd) which shows clinical efficacy as a single therapy agent so far. This highlights the recognition that further augmentation of antitumor effects is the most crucial issue in CRAd development. Although Ad has been used for cancer gene therapy due to its exceptionally high in vivo transduction efficiency, many cancers (including gastrointestinal cancers, pancreatic cancer, ovarian cancer, and hormone-refractory prostate cancer) do not express Ad primary receptor (coxsackie adenovirus receptor, CAR).2,3,17,18,19 Without a strategy for infecting target cells via CAR-independent pathways, achieving sufficient antitumor efficiency is difficult with the initial generation CRAd systems (Figure 1a). On this basis, various strategies have been developed to address CRAd infectivity. Specific strategies for Ad infectivity enhancement are:
Figure 1.
Modification of adenovirus (Ad) to achieve coxsackie adenovirus receptor (CAR)-independent transduction. To achieve CAR-independent transduction, several modification strategies have been employed in Ad. (a) Poor infectivity of CAR negative cells with conventional Ad system, (b) fiber modification, (c) switching serotypes, (d) chimeric, (e) mosaic, and (f) bridging molecule-based targeting (see detail in text).
Fiber modification
The Ad fiber region contains the “knob” domain which binds to the primary adenoviral receptor (CAR) on the surface of target cells. Because this position is the natural binding locale, a number of fiber-knob modifications have been endeavored to increase viral infectivity for CAR negative cancer cells. One of the most effective fiber modification is based upon infectivity enhancement via incorporation of an arginine–glycine–aspartic acid-4C (RGD-4C) motif into the HI-loop of the fiber-knob region20,21 (Figure 1b). The RGD-4C motif is a partial peptide sequence of fibronectin identified by phage library screening. When it was incorporated into the surface exposed HI-loop of the fiber-knob region, the Ad vector with this motif showed CAR-independent infection of the target cells. Also, oncolytic Ads with this motif showed an augmented cytocidal effect in CAR negative cancer cell lines in vitro and in vivo.18 However, identification of new peptide motifs for Ad modification is nontrivial. Most attempts to incorporate preidentified peptide coding sequence have failed because of a lack of production of fully assembled virus, or the incorporated motif did not show sufficient affinity to the binding counterpart on the surface of the target cells. Screening of the ligand in the form presented in the fiber-knob region is logical direction but there remains the issue of adequate library diversity.
Switching serotypes
Historically, Ad vectors have been derived based on subtype 2 or 5. This is the reason that CAR deficiency on the target cell is a major issue for Ad-based cancer gene therapy. Interestingly, other Ad serotype vectors do not necessarily use CAR as the primary receptor. For example, Ad35 uses CD46 for initial binding, and thus the infection is CAR independent.22,23 There are basically two approaches for incorporating other subtypes' tropism into adenoviral vectors. One approach is to make a vector fully based on alternate subtype vectors (Figure 1c); the other is to design an Ad2/5-based vector with an alternate subtype's binding domain incorporated (chimeric) (Figure 1d). The first method has the advantage that all parts of the capsid consist of alternate subtype Ad proteins, thus the distribution is assumed to be completely the same as the parental virus. However, in this approach there is no guarantee that the CRAd replication processes after viral entry are as efficient as those of Ad2/5. In recognition of this fact, chimeric approaches are more frequently adaptable to oncolytic Ads, which require efficient post-transduction processes for efficient replication.19,24,25
Mosaic
Mosaic vectors are vectors with multiple binding moieties derived from different parental viruses and/or targeting peptides (Figure 1e). Such vectors not only embody a wider infectious range, due to multiple binding motifs, but also show higher infectivity compared to singly targeted vectors. Because enhanced infection is a crucial aspect of CRAd functionality and efficacy, such modifications have been incorporated into CRAds, which thereby show an improved cytocidal effect.26 Such mosaic vectors can be derived by either pseudotyping, with 293 cells expressing different binding motifs, or by incorporating more than one fiber into the vector genome.26,27 Because the progeny virus needs the same efficient binding, genetic incorporation is preferred in CRAds.
Bridging molecule-based targeting
This method can achieve the precise selectivity embodied by employing a high affinity/specificity antibody (Ab), or a specific binding motif for the target moiety expressed on the surface of the cells28 (Figure 1f). Such bridging can be realized by anti-knob Ab fused with targeting ligands/Abs28,29 or incorporation of immunoglobulin binding domains into the viral fiber.30 Although this kind of targeting is functional only for initial entry, the targeting ability is high. Thus, there is a good possibility that such a bridging moiety can be applied for CRAds, especially for systemic administration. Although transductional targeting by these modifications is very useful for improving the efficacy of CRAd therapy, it does not provide sufficient levels of selectivity to the progeny viruses. This is because the transduction-based CRAd selectivity is currently imprecise and because efficient incorporation of the extrinsic targeting moiety into the intratumorally produced progeny virus is uneasy. At this time, bridging moiety-based targeting is used as a way to increase initial infectivity in CAR negative cancer cells,29 or a way to deliver the systemically administered virus to the tumor locale.31
Replication Specificity
The second key issue in CRAd development is a replication control mechanism for selectivity. Aforementioned various methods have been utilized for controlling viral replication in a tumor-specific manner. However, some replication control systems may not function as originally designed. For example, ONYX-015 was originally introduced as a virus that specifically replicates in cancer cells with mutation of the p53 tumor suppressor gene.32 Although this virus was shown to be safe in early phase clinical trials, there are studies suggesting that the selectivity of this agent is based on p14ARF status in p53 functional cells.33 Another report states that replication of the same virus does not depend on both p53 or p14ARF status.34 Likewise, the CRAd agent Adδ24 has also shown incomplete specificity, allowing replication in pRb intact cells in some experimental systems.35,36 Thus, additional deletions have been performed to confer more stringent selectivity.35,36,37 These approaches are designed because selectivity becomes particularly important in CRAds displaying an augmented antitumor effect, whereby sufficient clinical safety must be maintained. In addition, the fact that each tumor context may present different requirements for selectivity highlights the need to develop more variety of precise control mechanisms for future CRAd design.
In the process of the development of CRAds, strict control of selectivity has historically been considered the key issue relevant to developmental realization. However, wild-type Ad5 had been injected into cervical cancers in the 1950s, causing no severe toxicity.38 More recently, agents without additional control mechanisms (ING007) have been approved for phase I clinical trial.39,40 These are based on the data of intrinsic cancer selectivity of wild-type Ad5. However, in the context of tropism-modified Ads, the US Food and Drug Administration still has concerns vis-à-vis replicative selectivity due to possibility of infection of a wider range of normal cells as a consequence of infectivity enhancement.
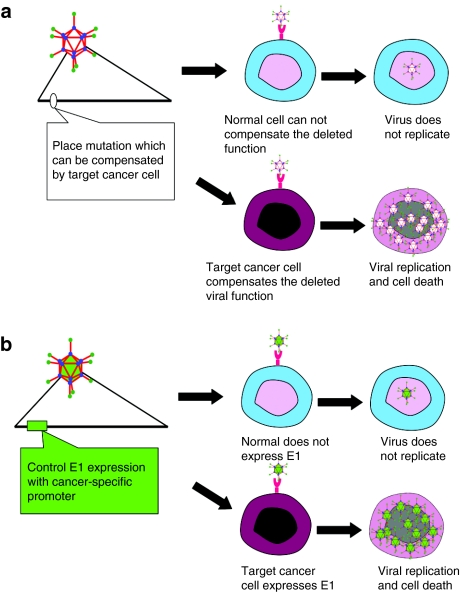
With respect to the achievement of replicative selectivity, two general strategies have been endeavored. The first type is based upon mutations in the viral genome which are essential for viral replication in normal cells but can be selectively compensated by particular defects in cancer cells (Figure 2a). In this regard, the dl1520 (or ONYX-015) Ad lacks the E1B region and was originally designed to achieve selective replication in cancer cells with mutated p53.32 Adδ24 has a mutation in the E1A region which theoretically restricts replication to cancer cells with mutated pRb.41 The second type of CRAds relies on cancer-specific, promoter-controlled transcription of the E1 region (Figure 2b). Because the E1A protein is necessary for the replication of Ad, these viruses can replicate only in cells where the controlling promoter is active. In this regard, CN706 has a prostate-specific antigen promoter–driven E1 expression cassette which enables selective replication in prostate cancer cells in an androgen-dependent manner.42
Figure 2.
Control mechanisms of oncolytic adenovirus. (a) Deletion-type conditionally replicative adenoviruses (CRAds): this type of CRAd has a mutation/deletion in a region crucial for viral replication. While cancer cells possess the cellular environment to compensate the function of the deleted viral gene, normal cells do not have that capability. For example, ONYX-015 (dl1520) and Adδ24 were designed to replicate only in p53 and pRb mutated cells, respectively. (b) Selective promoter-based CRAds (i.e., RGDCRAdCOX2F, CN706, CV739): a tumor/tissue-specific promoter controls the expression of viral genes crucial for replication. As a result, the virus can replicate only in cells in which the promoter is active. By using a promoter with a tumor-ON/normal cell-OFF profile, the replication can be limited to cancer cells.
As an emerging strategy for CRAd replication control, post-transcriptional control can be also used. Micro-RNAs are short RNAs which are expressed in the cells and determine many aspects of the cell characteristics. By placing micro-RNA binding sequence into the context of Ad E1A, replication can be restricted to the target cells (e.g., cancer cells).43 Also, the 5′- or 3′-untranslated region placed on E1A can embody cancer specificity.44,45
Lateral Spread
CRAd infection and its productive replication would ideally result in the dissemination of progeny virions throughout the target tumor.1,4 This local amplification of viral inocolum constitutes the basis of the expected multiplicative effects of CRAds, which is the definitive difference of CRAds from nonreplicative Ad agents.2,3 Full exploitation of this amplified local viral mass, however, requires effective lateral spread of the virus via infection of the neighboring cancer cells. In fact, both cellular and tissue barriers render this process as a limiting factor in CRAd physiology.1,4 On this basis, strategies have been proposed to enhance target cell killing by CRAds in order to enhance effective escape and release of progeny virions from tumor cells. Such strategies have sought to facilitate the apoptotic killing function of CRAd action by configuring into the Ad genome human tumor suppressor genes, such as p53,46,47 or viral genes involved in the native process of target cell senescence, such as the Ad death protein.48,49 Each of these approaches has yielded so-called “armed” CRAds with enhanced potencies exhibited in model systems. Other strategies for CRAd arming have exploited toxin genes previously studied in the context of replication-defective Ad-based molecular chemotherapy in cancer gene therapy schemas. Cautionary reports have highlighted that selected antitumor genes may actually operate at cross-purpose with Ad replicative physiology, yielding armed CRAd agents with reduced potency compared to their unarmed parental counterparts. On this basis, recent arming strategies tend to exploit encoded transgenes with antitumor activities with potential synergy with CRAd replicative physiology. These approaches have sought to utilize antiangiogenesis genes50,51 and other factors directed at tumor microenvironment biology.52 In addition, immunostimulatory genes have been configured into CRAds to induce antitumor immune reaction.53
Model Systems
In addition to vector engineering, valid in vivo experimental systems need to be developed for further understanding CRAd functionality. In particular, a convenient in vivo experimental system for the analyses of CRAd replication/toxicity and virus–host interaction is urgently needed. To date, most in vivo experiments have been performed with human cancer cell xenografts in immunodeficient mice. However, the stringent species selectivity of adenoviridae replication does not permit human Ad to replicate in most rodents cells including mice and rats. This biology greatly limits the ability to conduct virus replication–related studies in one of the most useful experimental animals. Cotton rat and Syrian hamster permit productive human Ad replication,54 however, it is not yet clear how closely viral replication in this system resembles that in humans. The fact that this is the only small animal model system permissive for human Ad replication highlights the importance of this model especially in the context of toxicological studies.39 In addition, syngeneic models have been proposed to better understand the biology of replicative Ad in the matched host settings. One current model is based upon hamster cancer cell line syngeneic graft in Syrian hamsters.55 Another approach is to employ conditionally replicative canine Ads to treat spontaneous dog osteosarcoma.56,57 This unique model would provide valuable information about an oncolytic agent in its natural host, and such data would be uniquely translatable to human context. However, experiments with nonhuman, nonmouse models still have relevance vis-à-vis analyzing host-specific phenomena e.g., immunity.
Imaging
The development of a noninvasive monitoring system to track treatment is another challenge. Although the ultimate goal of CRAd therapy is to achieve an antitumor effect, determination of CRAd functionality requires interval end-point assays to monitor the progress of treatment. These assays should be informative yet minimally invasive and would also be valuable for maintaining safety in clinical trials.2 For example, if the expression of the TK toxin gene is detected in normal organs, administration of the prodrug could be halted to prevent adverse event. In the context of CRAds, such therapy monitoring has heretofore been performed by immunoblotting or immunohistochemistry of biopsy specimens.11 Although biopsy examination is informative, this procedure is considerably invasive. Also, the data obtained reflect only one time point when the specimen was taken: the monitoring the dynamic replication/spread of CRAds would require biopsies at multiple time points. In vivo imaging has been pursued to monitor transgene expression after vector administration.58,59,60
Initial localization of the vector can be determined by conventional labeling of the viral capsid with tracers (e.g., fluorophore, I131), or placing a replication-independent expression cassette of noninvasively detectable marker gene in the CRAds.61 However, in the latter case, the expression of the marker increases along with viral genome copy number upon viral amplification, and thus results in the representation of viral replication.
Imaging of viral replication requires the marker gene expression in a replication-dependent manner. When we placed the marker gene into the E3 region of CRAds under the control of major late promoter, the reporter gene was expressed only when the virus is replicating.62 This is because major late promoter is closely linked to the Ad replication cycle. We also placed the reporter as a fusion protein with the viral capsid protein pIX, which is exposed to the outside of the virion.63 The capsid of the virus with pIX-RFP fusion protein was fluorescently labeled. Interestingly, the virus showed a signal corresponding to viral replication. In this instance, the representation of the replication status in this vector is linked to the property of the pIX promoter in activation after early gene expression.
At this time, optical imaging methods which are most commonly used for in vivo imaging cannot be utilized directly for human application. Luciferase-based imaging requires substrate injection upon imaging and detection of fluorescence. In addition, this method is limited by issues of detection sensitivity and background autofluorescence. Thus, the radionuclide-based imaging reporters have greater possibility for human application due to their ability for imaging of tumors, established imaging protocols, as well as established safety profiles.64,65 Table 1 shows the most commonly used radionuclide-based imaging techniques in conjunction with gene therapy. The realization of such replication monitoring strategy is awaited for safer and more efficient CRAd therapy.
Table 1.
Typical radionuclide-based imaging reporters for gene therapy application
Systemic Versus Local Therapy
The overwhelming majority of CRAd applications, both in model systems and in early phase human clinical trials, have been for the context of local disease. This reflects the fact that Ad administered via the systemic route localizes principally to the liver. The consequences of this biology are that liver localization may limit desired delivery of Ad to tumor targets and may also elicit hepatotoxicities.28 This biology has thus restricted the employment of Ad for any clinical context involving systemic delivery schemas. In addition, preformed Abs as the result of community acquired infection with parental Ad or prior treatment with similar vectors could neutralize Ad administered in this manner, confounding the goals of effective agent delivery to tumor cells.66 Nonetheless, some early phase human clinical trials have administered CRAd agents via the vascular route without any noted untoward effects, indicating that the pre-existing Ab does not compromise the safety of the CRAds. Recently, various targeting strategies have realized effective tumor transduction with decent selectivity with modified Ads delivered systemically.2,3,4 Some of these targeting methods allow effective mitigation of viral particle sequestration in the liver.67 In addition to the efforts to eliminate the natural tropism by viral capsid modification, a number of efforts have been reported to avoid the contact of the vector with unwanted cells by making the structure stealth. Coating with polymer or polyethylene glycol is reported to not only evade the immune system but also increase the delivery to the tumor locale.68,69 Alternatively, usage of various cells as a vehicle for the delivery of oncolytic Ad has been exploited as a way to increase tumor targeting upon systemic delivery.70,71
The lack of effective treatments for disseminated disease suggests the desirability of application of Ad-based virotherapy for the metastatic disease context. Particularly, CRAd-based therapeutics require far lower initial tumor transduction for its functionality compared to those with nonreplicative vectors thanks to local multiplication of the vector. Thus, the aforementioned improvements of targeting have made systemic CRAd delivery more realistic.
Liver Sequestration and Coagulation Factors
As widely known, Ad released into systemic circulation sequesters in the liver in mice. Initially, such distribution was thought to be mediated by high CAR expression of liver parenchymal cells. However, analyses with CAR binding–ablated viruses and/or penton base RGD-deleted viruses indicated that neither CAR binding nor the integrin binding can fully explain liver sequestration.72 Cationic repeat (KKTK) in the fiber-shaft region was also suggested for explanation. However, analyses with chimeric Ad5 with Ad35 fiber suggest that liver distribution can be observed without KKTK in the shaft and that platelet depletion virtually eliminated the liver sequestration.73 More recently, Ad hexon particularly hypervariable region 5 has been shown to be crucial for viral binding to a coagulation factor (factor X).74,75,76 This indicates that the liver sequestration observed in mice may be altered by mutating hypervariable region in adenoviral hexon protein. However, there is still a question whether this understanding can be generalized to other species, including humans, as mice are known to show extremely high liver sequestration of human Ad compared to other mammals.
Host Immunological Reactions
It is widely recognized that most adults have neutralizing Ab against most common vector strains (Ad2 or 5). Thus, pre-existing immunity is understood to be a major issue for CRAd functionality. However, clinical trials based upon local administration of CRAd in prostate cancer have shown no correlation between neutralizing Ab levels and the effect on prostate-specific antigen.11 The same phenomenon was observed in syngeneic tumor models in immunocompetent hamsters.77 This recognition suggests that pre-existing neutralizing Ab may not be a major factor affecting therapeutic outcomes in CRAd local administration. However, pre-existing Ab may still be a major obstacle in the context of systemic administration of CRAd because Ab may neutralize the CRAds before it reaches the target site.
The effect of cellular immune response to antitumor effect is supposed to be affected by a delicate balance between induction of antitumor response and elimination of virus itself.78 A recent publication indicates that the enhanced in vivo antitumor effect in the combination of E3B-deleted Ad (dl309) and paclitaxel was observed only in immunocompetent mice,79 indicating the interaction of viral function and host immune system.
On the other hand, innate immune response has been a major issue in Ad vector system in general. This is particularly valid as the one and only lethal adverse effect reported with Ad vector is understood to be due to innate immune response showing cytokine storm. Recently, Toll-like receptor (TLR) has been reported to play a major role in innate immune response against DNA viruses including Ads via TLR9 as a response to double-strand DNA introduction.80,81 However, Di Paolo et al. recently reported that there is another cascade independent of TLR9 or NLRP3 inducing innate immune response against double-stranded DNA.82 The IL-1α-mediated anti-Ad response required a selective interaction of virus RGD motifs with macrophage β3 integrins. So far, oncolytic Ad development has been taking advantage of the low requirement of initial administration dose as a way to avoid innate immune response. In addition, the above-mentioned elucidation of the antiviral responses may lead to vector design which can efficiently mitigate the innate immune response issue in this field.
A variety of immunomodulators have been endeavored in oncolytic virus systems including CRAds, and cyclophosphamide is the most widely tested in animal models.77,83 In syngeneic tumor models in immunocompetent hamster, cyclophosphamide induced prolonged local viral replication and enhanced the antitumor effect.84 In other oncolytic virus models, other mechanisms of enhancement (e.g., suppression of regulatory T cells) are reported for cyclophosphamide.83,85 In this regard, the application of immunomodulators upon CRAd therapy will continue to be an important subject for enhancing the effect of CRAd therapies.
Toward Clinical Usage
As mentioned earlier, H101 (E1b55K-deleted oncolytic Ad) has been approved as a drug in P.R. China.9 In the United States, there are several clinical trials with oncolytic Ads including ONYX-015 (Onyx Pharmaceuticals, Emeryville, CA) with similar structure, but none of them has been approved for the treatment of patients. The response to H101 plus chemotherapy in phase III was 79% (41/52),9 which is significantly higher than ONYX-015 phase II data.14 Although there is some discussion doubting the fairness of tumor response evaluation,86 such points will be elucidated through the accumulation of cases in real clinical settings. Another possible difference is the refractoriness of the disease between the studies. While ONYX-015 trial was only for refractory patients, the phase III H101 trial includes many patients without prior treatment history. This may mean that the patients with earlier disease may be a better target of oncolytic virus therapy compared to those with advanced disease. The other possible branching factor may be the cost for phase III and IV studies. Practically, the costs of later phase clinical studies are astronomical, and thus many small to mid-size pharmaceutical companies are forced to reduce the number of research projects when one candidate is entering phase III. This prioritization issue might have practical impact on the decision-making for later phase clinical studies.
Other Considerations
A cell population responsible for resistance to chemotherapy and disease relapse, called cancer stem (-like) cell, has been gathering a lot of attention. This population shows a low proliferation rate. Ad which shows high transduction in nonproliferating cells is a suitable choice of vector for that population.87,88
Recently, many new vectors as well as improvements of existing oncolytic virus systems have been reported.89 Thus, it will be necessary to fairly compare the effect of various vectors in a reasonable in vivo system. Comparing them in humans is ideal because all vectors are intended for usage in humans, although impractical. In addition, it is not that easy to compare different vectors in vivo models because the tropism of each virus replication is highly stringent. At this time, finding acceptable in vivo experimental models for comparison of different viral system is very difficult.
Kuhn et al. reported generation of more potent chimeric viruses by coinfection of multiple serotype vectors.90 This method has a potential to generate a lot of vectors with novel tropism, but the issue of long-term stability may need to be addressed for future clinical usage of resultant vectors.
From a practical vector production stand point, vector manufacturing of oncolytic Ads requires special consideration. Compared to E1-deleted vectors, the oncolytic Ad with intact E1 region has a much higher possibility for recombination generating the left end structure of wild-type Ad. Although the Food and Drug Administration accepts a little bit higher level of contaminating wild-type vector for oncolytic Ads compared to that in replication-deficient vectors, the effort to reduce wild-type contamination is crucial. We have been using target cancer cell lines without E1 gene (e.g., A549 lung cancer cell for cyclooxygenase-2 promoter–driven virus) for amplification and getting good yield at high quality.
Conclusion
In this review, we discussed advances in the field of oncolytic Ads as well as current obstacles and the directions for overcoming them. Oncolytic Ad is an efficient and interesting material for cancer therapeutics development because accumulated knowledge and technique enable rationale-driven vector design. We believe that future advances in adenovirology and its application will further advance the field of oncolytic Ad toward full realization of the potential of oncolytic Ads.
Acknowledgments
We thank Leonard Armstrong for suggestions and editorial help. This work is supported by the National Institutes of Health grants 5R01 CA121187 (D.T.C.), 2R01 CA094084 (M.Y.), and 5R01 DK063615 (M.Y.).
REFERENCES
- Alemany R, Balagué C., and , Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Conditionally replicative adenovirus for gastrointestinal cancers. Expert Opin Biol Ther. 2004;4:1241–1250. doi: 10.1517/14712598.4.8.1241. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., and , Curiel DT. Cancer gene therapy. Technol Cancer Res Treat. 2005;4:315–330. doi: 10.1177/153303460500400402. [DOI] [PubMed] [Google Scholar]
- Curiel DT. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395–3399. [PubMed] [Google Scholar]
- Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9:961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- Mohr I. To replicate or not to replicate: achieving selective oncolytic virus replication in cancer cells through translational control. Oncogene. 2005;24:7697–7709. doi: 10.1038/sj.onc.1209053. [DOI] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., and , Curiel DT. Nonreplicating DNA viral vectors for suicide gene therapy: the adenoviral vectors. Methods Mol Med. 2004;90:61–70. doi: 10.1385/1-59259-429-8:61. [DOI] [PubMed] [Google Scholar]
- Yu W., and , Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Alvarez RD., and , Curiel DT. A phase I study of recombinant adenovirus vector-mediated intraperitoneal delivery of herpes simplex virus thymidine kinase (HSV-TK) gene and intravenous ganciclovir for previously treated ovarian and extraovarian cancer patients. Hum Gene Ther. 1997;8:597–613. doi: 10.1089/hum.1997.8.5-597. [DOI] [PubMed] [Google Scholar]
- Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- Lamont JP, Nemunaitis J, Kuhn JA, Landers SA., and , McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7:588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, et al. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V., and , Yamamoto M. Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004;64:4319–4327. doi: 10.1158/0008-5472.CAN-04-0064. [DOI] [PubMed] [Google Scholar]
- Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N., and , Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V., and , Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP., and , Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirena D, Lilienfeld B, Eisenhut M, Kälin S, Boucke K, Beerli RR, et al. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang H, Yumul R, Gao W, Gambotto A, Morita T, et al. Transduction of liver metastases after intravenous injection of Ad5/35 or Ad35 vectors with and without factor X-binding protein pretreatment. Hum Gene Ther. 2009;20:621–629. doi: 10.1089/hum.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Heim A, Nettelbeck DM, Steinstraesser L., and , Wildner O. Evaluation of twenty human adenoviral types and one infectivity-enhanced adenovirus for the therapy of soft tissue sarcoma. Hum Gene Ther. 2007;18:51–62. doi: 10.1089/hum.2006.132. [DOI] [PubMed] [Google Scholar]
- Takayama K, Reynolds PN, Short JJ, Kawakami Y, Adachi Y, Glasgow JN, et al. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology. 2003;309:282–293. doi: 10.1016/s0042-6822(03)00067-9. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ugai H, Everts M, Belousova N, Yamamoto M., and , Curiel D.2008A simplified method for the generation of a fiber mosaic adenoviral vector Mol Ther 16suppl. 1): S307 [Google Scholar]
- Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann NY Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M., and , Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Huang J, Hirai S, Kuroki M, Kuroki M, Watanabe N, et al. Carcinoembryonic antigen-targeted selective gene therapy for gastric cancer through FZ33 fiber-modified adenovirus vectors. Clin Cancer Res. 2006;12:3803–3813. doi: 10.1158/1078-0432.CCR-06-0024. [DOI] [PubMed] [Google Scholar]
- Li HJ, Everts M, Yamamoto M, Curiel DT., and , Herschman HR. Combined transductional untargeting/retargeting and transcriptional restriction enhances adenovirus gene targeting and therapy for hepatic colorectal cancer tumors. Cancer Res. 2009;69:554–564. doi: 10.1158/0008-5472.CAN-08-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD., and , Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Yang CT, You L, Uematsu K, Yeh CC, McCormick F., and , Jablons DM. p14(ARF) modulates the cytolytic effect of ONYX-015 in mesothelioma cells with wild-type p53. Cancer Res. 2001;61:5959–5963. [PubMed] [Google Scholar]
- Edwards SJ, Dix BR, Myers CJ, Dobson-Le D, Huschtscha L, Hibma M, et al. Evidence that replication of the antitumor adenovirus ONYX-015 is not controlled by the p53 and p14(ARF) tumor suppressor genes. J Virol. 2002;76:12483–12490. doi: 10.1128/JVI.76.24.12483-12490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Manzano C, Balague C, Alemany R, Lemoine MG, Mitlianga P, Jiang H, et al. A novel E1A-E1B mutant adenovirus induces glioma regression in vivo. Oncogene. 2004;23:1821–1828. doi: 10.1038/sj.onc.1207321. [DOI] [PubMed] [Google Scholar]
- Balagué C, Noya F, Alemany R, Chow LT., and , Curiel DT. Human papillomavirus E6E7-mediated adenovirus cell killing: selectivity of mutant adenovirus replication in organotypic cultures of human keratinocytes. J Virol. 2001;75:7602–7611. doi: 10.1128/JVI.75.16.7602-7611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JA, Demers GW, Johnson DE, Neugebauer SE, Perry ST, Vaillancourt MT, et al. Evaluation of E1-mutant adenoviruses as conditionally replicating agents for cancer therapy. Mol Ther. 2000;2:485–495. doi: 10.1006/mthe.2000.0206. [DOI] [PubMed] [Google Scholar]
- Huebner RJ, Rowe WP, Schatten WE, Smith RR., and , Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV, et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009;16:644–654. doi: 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 2009;16:625–637. doi: 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW., and , Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- Ylösmäki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R., and , Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82:11009–11015. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Nedeljkovic-Kurepa A, DeBenedetti A, Li XL, Odaka Y, et al. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res Treat. 2008;108:43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Thompson J, Emiliusen L, Murphy S, Beauchamp RD, Suzuki K, et al. A conditionally replicating adenovirus targeted to tumor cells through activated RAS/P-MAPK-selective mRNA stabilization. Nat Biotechnol. 2003;21:771–777. doi: 10.1038/nbt835. [DOI] [PubMed] [Google Scholar]
- Mitlianga PG, Sioka C, Vartholomatos G, Goussia A, Polyzoidis K, Rao JS, et al. p53 enhances the Delta-24 conditionally replicative adenovirus anti-glioma effect. Oncol Rep. 2006;15:149–153. [PubMed] [Google Scholar]
- van Beusechem VW, van den Doel PB, Grill J, Pinedo HM., and , Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–6171. [PubMed] [Google Scholar]
- Suzuki K, Alemany R, Yamamoto M., and , Curiel DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res. 2002;8:3348–3359. [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE., and , Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- Gupta V, Wang W, Sosnowski BA, Hofman FM., and , Chen TC. Fibroblast growth factor-2-retargeted adenoviral vector for selective transduction of primary glioblastoma multiforme endothelial cells. Neurosurg Focus. 2006;20:E26. [PubMed] [Google Scholar]
- Jin F, Xie Z, Kuo CJ, Chung LW., and , Hsieh CL. Cotargeting tumor and tumor endothelium effectively inhibits the growth of human prostate cancer in adenovirus-mediated antiangiogenesis and oncolysis combination therapy. Cancer Gene Ther. 2005;12:257–267. doi: 10.1038/sj.cgt.7700790. [DOI] [PubMed] [Google Scholar]
- Seth P, Wang ZG, Pister A, Zafar MB, Kim S, Guise T, et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factor-beta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther. 2006;17:1152–1160. doi: 10.1089/hum.2006.17.1152. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Kuppuswamy MN, Wold WS., and , Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde WA., Jr Experimental models for study of common respiratory viruses. Environ Health Perspect. 1980;35:107–112. doi: 10.1289/ehp.8035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF., and , Wold WS. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- Hay JG. “Man's best friend”: a new model system for cancer therapeutics. Mol Ther. 2003;7:144–145. doi: 10.1016/s1525-0016(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri TR, Rogers BE, Buchsbaum DJ, Mountz JM., and , Zinn KR. A noninvasive reporter system to image adenoviral-mediated gene transfer to ovarian cancer xenografts. Gynecol Oncol. 2001;83:432–438. doi: 10.1006/gyno.2001.6333. [DOI] [PubMed] [Google Scholar]
- Chaudhuri TR, Mountz JM, Rogers BE, Partridge EE., and , Zinn KR. Light-based imaging of green fluorescent protein-positive ovarian cancer xenografts during therapy. Gynecol Oncol. 2001;82:581–589. doi: 10.1006/gyno.2001.6297. [DOI] [PubMed] [Google Scholar]
- Dingli D, Russell SJ., and , Morris JC.3rd (2003In vivo imaging and tumor therapy with the sodium iodide symporter J Cell Biochem 901079–1086. [DOI] [PubMed] [Google Scholar]
- Le LP, Le HN, Nelson AR, Matthews DA, Yamamoto M., and , Curiel DT. Core labeling of adenovirus with EGFP. Virology. 2006;351:291–302. doi: 10.1016/j.virol.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono HA, Le LP, Davydova JG, Gavrikova T., and , Yamamoto M. Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res. 2005;65:10154–10158. doi: 10.1158/0008-5472.CAN-05-1871. [DOI] [PubMed] [Google Scholar]
- Le LP, Le HN, Dmitriev IP, Davydova JG, Gavrikova T, Yamamoto S, et al. Dynamic monitoring of oncolytic adenovirus in vivo by genetic capsid labeling. J Natl Cancer Inst. 2006;98:203–214. doi: 10.1093/jnci/djj022. [DOI] [PubMed] [Google Scholar]
- Herschman HR. Molecular imaging: looking at problems, seeing solutions. Science. 2003;302:605–608. doi: 10.1126/science.1090585. [DOI] [PubMed] [Google Scholar]
- Herschman HR. Noninvasive imaging of reporter gene expression in living subjects. Adv Cancer Res. 2004;92:29–80. doi: 10.1016/S0065-230X(04)92003-9. [DOI] [PubMed] [Google Scholar]
- Hedley SJ, Chen J, Mountz JD, Li J, Curiel DT, Korokhov N, et al. Targeted and shielded adenovectors for cancer therapy. Cancer Immunol Immunother. 2006;55:1412–1419. doi: 10.1007/s00262-006-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P, Dmitriev I., and , Curiel D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther. 1999;6:1336–1339. doi: 10.1038/sj.gt.3300941. [DOI] [PubMed] [Google Scholar]
- Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- Doronin K, Shashkova EV, May SM, Hofherr SE., and , Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT., and , Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Pereboeva L, Komarova S, Mikheeva G, Krasnykh V., and , Curiel DT. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21:389–404. doi: 10.1634/stemcells.21-4-389. [DOI] [PubMed] [Google Scholar]
- Alemany R., and , Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S., and , Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D, Spencer JF, Toth K., and , Wold WS. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol Ther. 2009;17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M, Kawamura K, Shimozato O, Ma G, Li Q, Suzuki N, et al. Virology- and immunology-based gene therapy for cancer. Cancer Immunol Immunother. 2006;55:1420–1425. doi: 10.1007/s00262-006-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SC, Wang Y, Meng JH, Hill R, Sweeney K, Kirn D, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KJ., and , Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kaisho T., and , Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ., and , Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH. The end of the beginning: oncolytic virotherapy achieves clinical proof-of-concept. Mol Ther. 2006;13:237–238. doi: 10.1016/j.ymthe.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wu CL, Shieh GS, Chang CC, Yo YT, Su CH, Chang MY, et al. Tumor-selective replication of an oncolytic adenovirus carrying oct-3/4 response elements in murine metastatic bladder cancer models. Clin Cancer Res. 2008;14:1228–1238. doi: 10.1158/1078-0432.CCR-07-1047. [DOI] [PubMed] [Google Scholar]
- Short JJ., and , Curiel DT. Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther. 2009;8:2096–2102. doi: 10.1158/1535-7163.MCT-09-0367. [DOI] [PubMed] [Google Scholar]
- Pandha H, Melcher A, Harrington K., and , Vile R. Oncolytic viruses: time to compare, contrast, and combine? 5th international meeting on replicating oncolytic virus therapeutics. Banff, Alberta, Canada, 18–22 March 2009. Mol Ther. 2009;17:934–935. doi: 10.1038/mt.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, Thorne S, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]