Abstract
Recently, gene-based cytokine treatment has been actively pursued as a new promising approach in treating cancer. In an effort to augment the efficiency of antitumor effect by cytokine-mediated immunotherapy, we selected both interleukin (IL)-12 and 4-1BB ligand (4-1BBL) as suitable cytokines to fully activate the type-1 immune response. Coexpression of IL-12 and 4-1BBL mediated by oncolytic adenovirus (Ad) greatly enhanced the antitumor effect. Further, synergistic enhancement in interferon (IFN)-γ levels were seen in mice treated with oncolytic Ad expressing both IL-12 and 4-1BBL. Next, to improve the overall antitumor immune response, we coadministered IL-12- and 4-1BBL-coexpressing oncolytic Ad with dendritic cells (DCs). Combination treatment of IL-12- and 4-1BBL-coexpressing oncolytic Ad and DCs elicited greater antitumor and antimetastatic effects than either treatment alone. Moreover, enhanced type-1 antitumor immune response and higher migratory abilities of DCs in tumors were also observed in the combination arms. The nature of the enhanced antitumor immune response seems to be mediated through the enhanced cytolytic activity of cytotoxic T lymphocytes (CTLs) and IFN-γ-releasing immune cells. Taken together, these data highlight the potential therapeutic benefit of combining IL-12- and 4-1BBL-coexpressing oncolytic Ad with DCs and warrants further evaluation in the clinic.
Introduction
Recent focus on the development of gene-based cancer therapy has been on utilizing cytokines, tumor suppressor, and apoptosis-related genes as therapeutic genes.1,2 Central to this strategy, however, is the engineering of vectors that can efficiently and uniformly deliver the therapeutic gene of interest to solid tumors. By far, oncolytic adenoviral vector–mediated gene therapy is one of the most promising approaches, as confirmed by clinical observations and studies performed using animal tumor models.3,4,5 Recently, we have reported that oncolytic adenoviruses (Ads), which express multiple cytokines can elicit potent antitumor efficacy by coupling the lytic function of an oncolytic Ad with its ability to amplify harboring cytokine genes.6,7 Replicating oncolytic Ads were able to infect and deliver therapeutic genes to adjacent cells as well as to those infected initially. Further, they also induced high therapeutic gene expression through increased adenoviral replication, and more importantly restricted the expression of therapeutic genes to cancer cells.
Interleukin (IL)-12 is a heterodimeric cytokine, which strongly promotes the differentiation of naive CD4+ T cells to type-1 T helper (Th1) phenotype and suppresses the expression of Th2 cytokines; and recently has been demonstrated to be one of the most efficient antitumor cytokines in experimental animal models.8 For example, oncolytic Ad–mediated local expression of IL-12 alone or with B7.1 co-stimulatory molecule generated tumor-specific immune responses and resulted in enhanced antitumor activity and higher incidences of tumor regressions.7 Moreover, coexpression of B7.1 with IL-12 showed synergistic responses in promoting tumor-specific immunity, highlighting the importance of co-stimulatory factor in full activation of T cells.
4-1BB ligand (4-1BBL) is a well-characterized co-stimulatory molecule expressed on antigen-presenting cells, including dendritic cells (DCs), macrophages, and B cells. Engagement of 4-1BBL on antigen-presenting cells by its natural receptor, 4-1BB, a member of the tumor necrosis factor receptor superfamily, promotes Th1 cell development and leads to increased expansion, cytokine production, and the development of cytolytic effector function of human T cells.9,10 Recent studies in vitro have demonstrated that agonistic anti-4-1BB Abs and 4-1BBL co-stimulated proliferation and cytokine secretion in both CD4+ and CD8+ T cells.11 In addition, the treatment of anti-4-1BB Abs has also been shown to eradicate well-established tumors in mice.12
DCs are highly efficient and specialized antigen-presenting cells with a remarkable ability to stimulate naive T cells and generate memory T cells. As such DC-based therapies have emerged as a promising anticancer therapy. DCs pulsed with tumor-associated antigens in various forms, including whole-cell lysates, peptides, proteins, RNA, or DNA, have proven effective in eliciting protective and therapeutic tumor antigen-specific immunity in murine models.13 DC-based vaccines have also been applied clinically for advanced-stage cancers, such as B-cell lymphoma, melanoma, prostate cancer, and renal cell carcinoma.14 However the overall clinical benefit of these vaccines has been low; possibly owing to the impairment of transferred DCs' function in cancer-bearing patients.15,16 Interestingly, the expression of mature DCs and major histocompatibility complex (MHC) class II markers was found to be significantly lower in breast cancer patients. In addition, DCs from cancer patients exhibited reduced ability to stimulate cytotoxic T lymphocytes (CTLs).15
In this report, we explored the potential benefit of combining IL-12- and 4-1BBL-coexpressing oncolytic Ad with DCs in the treatment of established tumors. We show that combination therapy of IL-12- and 4-1BBL-coexpressing oncolytic Ad and DCs elicited potent and synergistic antitumor effects. Further, enhanced Th1 antitumor immune response and higher DCs migratory abilities in tumors were also observed. These data highlight the potential therapeutic benefit of combining IL-12- and 4-1BBL-coexpressing oncolytic Ad with DCs preclinically and lays the foundation for examining these newly engineered Ads in the clinic.
Results
Characterization of IL-12 and/or 4-1BBL-expressing oncolytic Ads
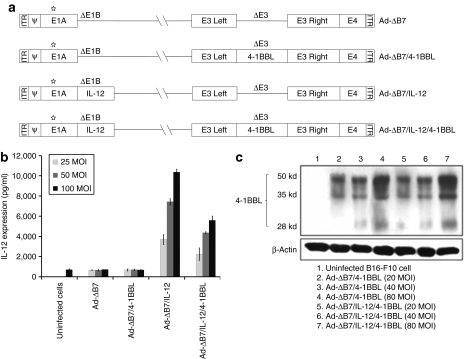
Cultured mouse melanoma B16-F10 cells were infected with Ad-ΔB7/4-1BBL, Ad-ΔB7/IL-12, or Ad-ΔB7/IL-12/4-1BBL, along with control oncolytic Ad, Ad-ΔB7. Ad-ΔB7 is an oncolytic Ad with mutation in retinoblastoma binding sites of E1A and deletion in E1B 19 kd and E1B 55 kd, showing significant cancer-specific cytotoxicity and viral replication.17 The supernatant from infected cells was collected to assess IL-12 levels by enzyme-linked immunosorbent assay (ELISA). As shown in Figure 1b, a dose-dependent increase in IL-12 was found in cells infected with either Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL, whereas no IL-12 production was found in cells infected with Ad-ΔB7. High levels of IL-12 were obtained even at a multiplicity of infection (MOI) of 25, and a higher expression was achieved until the MOI was increased up to 100. Interestingly, the level of IL-12 expression by Ad-ΔB7/IL-12 is noticeably higher than those induced by Ad-ΔB7/IL-12/4-1BBL, suggesting that additional expression of 4-1BBL in the E3 region of Ad may negatively affect the expression of IL-12. The expression of 4-1BBL was also dose (MOI)-dependently enhanced in cells infected with either Ad-ΔB7/4-1BBL or Ad-ΔB7/IL-12/4-1BBL (Figure 1c). However, the expression of 4-1BBL was not adversely affected by additional expression of IL-12 in the E1 region, showing equivalent expression of 4-1BBL.
Figure 1.
Schematic representation and kinetics of IL-12 and 4-1BBL expression of oncolytic adenoviruses expressing IL-12 and/or 4-1BBL. (a) Ad-ΔB7 contains mutated E1A (open star, mutation at Rb protein–binding site), but has deleted E1B 19 kd, E1B 55 kd (ΔE1B), and E3 region (ΔE3); The expression cassette for murine IL-12 and murine 4-1BBL was inserted into the E1 and E3 region of the Ad genome, respectively. Expression kinetics of (b) IL-12 and (c) 4-1BBL in B16-F10 cells after infection with Ad-ΔB7, Ad-ΔB7/4-1BBL, Ad-ΔB7/IL-12, or Ad-ΔB7/IL-12/4-1BBL at different MOIs. After 48 hours, supernatant and cell lysate was collected for the measurement of IL-12 by enzyme-linked immunosorbent assay and 4-1BBL by western blot analysis, respectively. Data represent the mean ± SE of three experiments. 4-1BBL, 4-1BB ligand; IL, interleukin; MOI, multiplicity of infection.
Therapeutic efficacy of oncolytic Ads expressing IL-12 or/and 4-1BBL in established tumor model
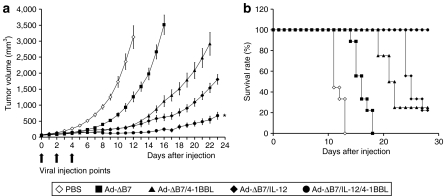
To evaluate the therapeutic efficacy of IL-12- or/and 4-1BBL-expressing oncolytic Ads in vivo, we intratumorally injected established B16-F10 melanoma tumors with Ad-ΔB7 [1 × 1010 virus particles (VP)], Ad-ΔB7/4-1BBL (1 × 1010 VP), Ad-ΔB7/IL-12 (5 × 109 VP), or Ad-ΔB7/IL-12/4-1BBL (5 × 109 VP). The reason why we have used half-dose of Ad for Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL group relative to Ad-ΔB7 or Ad-ΔB7/4-1BBL group is that the antitumor effect of IL-12-expressing two oncolytic Ad were much better than either Ad-ΔB7 or Ad-ΔB7/4-1BBL. Mice injected with phosphate-buffered saline (PBS) rapidly formed large tumors (over 3,000 mm3) and consequently were killed on day 12. In contrast, as shown in Figure 2a, all oncolytic Ads caused a significant inhibition of tumor growth (P < 0.01 versus PBS). At 12 days post-treatment, the mean tumor volume for the tumors treated with Ad-ΔB7, Ad-ΔB7/IL-12, Ad-ΔB7/4-1BBL, or Ad-ΔB7/IL-12/4-1BBL were 1,265 ± 155, 383 ± 27, 354 ± 71, and 136 ± 22 mm3, respectively, showing 60, 88, 89, and 96% tumor growth inhibition in comparison to PBS-treated group. In relative comparison, the antitumor efficacy of Ad-ΔB7/IL-12/4-1BBL was superior to that of tumors treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL (P < 0.05 versus Ad-ΔB7/IL-12; P < 0.01 versus Ad-ΔB7/4-1BBL). Moreover, all mice in the Ad-ΔB7/IL-12/4-1BBL group survived >30 days after initial viral injection (P < 0.01 versus Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL), whereas only 20% of the animals treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL were viable in the same time period (Figure 2b). This further supports the rationale of combining IL-12 and 4-1BBL to achieve maximal antitumor efficacy.
Figure 2.
Evaluation of therapeutic effect on established tumor. Mice with B16-F10 subcutaneous tumors were intratumorally injected with PBS, Ad-ΔB7 [1 × 1010 virus particles (VP)], Ad-ΔB7/IL-12 (5 × 109 VP), Ad-ΔB7/4-1BBL (1 × 1010 VP), or Ad-ΔB7/IL-12/4-1BBL (5 × 109 VP) three times. (a) Mean tumor volume following treatment with oncolytic adenoviruses. Data points represent the mean ± SE of the tumor size in each group (n = 8–9 mice) at the indicated time points. The antitumor efficacy of Ad-ΔB7/IL-12/4-1BBL was superior to those of tumors treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL (P < 0.05 versus Ad-ΔB7/IL-12; P < 0.01 versus Ad-ΔB7/4-1BBL). (b) Survival of animals after treatment with oncolytic adenoviruses. The survival advantage for the Ad-ΔB7/IL-12/4-1BBL group was significant compared to Ad-ΔB7, Ad-ΔB7/IL-12, or Ad-ΔB7/4-1BBL (P < 0.01). *P < 0.05; **P < 0.01. 4-1BBL, 4-1BB ligand; PBS, phosphate-buffered saline; VP, virus particles.
Upregulated IFN-γ expression in tumor tissues
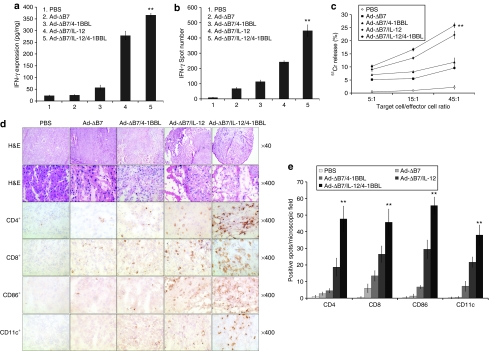
To assess whether interferon (IFN)-γ contributed to the observed antitumor efficacy induced by the oncolytic Ads expressing IL-12 or/and 4-1BBL, its concentration in tumor tissues were measured by ELISA at day 5 post-treatment. As shown in Figure 3a, the level of IFN-γ was increased in the Ad-ΔB7/4-1BBL-, Ad-ΔB7/IL-12-, and Ad-ΔB7/IL-12/4-1BBL-treated groups. In relative comparison, significantly higher IFN-γ levels were observed in tumors treated with Ad-ΔB7/IL-12/4-1BBL than those treated with Ad-ΔB7/4-1BBL or Ad-ΔB7/IL-12 (P < 0.01), implying that elevated IFN-γ maybe the reason for the observed enhanced antitumor effect.
Figure 3.
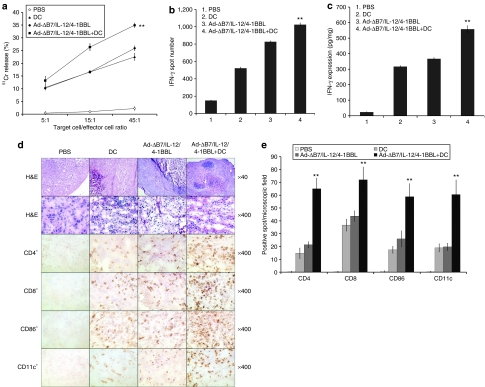
Generation of tumor-specific immune response by oncolytic adenoviruses expressing IL-12 and/or 4-1BBL. (a) Evaluation of IFN-γ expression in tumor tissues after treatment of IL-12- and/or 4-1BBL-expressing oncolytic adenoviruses. The level of IFN-γ was significantly increased in the Ad-ΔB7/IL-12/4-1BBL-treated mice than those in the mice treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL (P < 0.01). Data points represent mean ± SE of three independent experiments. IFN-γ ELISpot (b) and Direct cytotoxicity (c) of splenocytes from B16-F10 melanoma-bearing mice treated with PBS, Ad-ΔB7, Ad-ΔB7/4-1BBL, Ad-ΔB7/IL-12, or Ad-ΔB7/IL-12/4-1BBL. Tumor-specific IFN-γ secreting splenocytes were detected by IFN-γ ELISpot assay. Ad-ΔB7/IL-12/4-1BBL-treated group showed higher number of IFN-γ spots than Ad-ΔB7/IL-12- or Ad-ΔB7/4-1BBL-treated groups (P < 0.01). Cytotoxicity was measured by a standard 4 hours 51Cr-release assay. CTL activity of Ad-ΔB7/IL-12/4-1BBL-treated group was significantly increased than other groups (P < 0.01). Data points represent mean ± SE of three independent experiments. (d) Histological and immunohistochemical analysis. At 5 days following final administration of viruses, mice were killed and sections of paraffin embedded or frozen tumor tissue from each group were stained with hematoxylin and eosin (H&E) (top two rows) and analyzed for T cells with anti-CD4 monoclonal antibody (mAb) (third row) and anti-CD8 mAb (fourth row) or antigen presenting cells with anti-CD86 mAb (fifth row) and anti-CD11c mAb (bottom row). Necrotic area of Ad-ΔB7/IL-12- or Ad-ΔB7/IL-12/4-1BBL-treated groups were larger compared to that of other treatment groups. CD4+, CD8+, CD86+, and CD11c+ cells infiltrated deeply into tumors treated with Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL compared with other adenovirus-treated groups. (e) The average number ± SE of CD4+, CD8+, CD86+, and CD11c+-positive cells in microscopic field (×400). **P < 0.01 versus Ad-ΔB7/IL-12 group. 4-1BBL, 4-1BB ligand; CTL, cytotoxic T lymphocytes; ELISpot, enzyme-linked immunosorbent spot; IFN, interferon; IL, interleukin.
Generation of tumor-specific immune response by oncolytic Ads expressing IL-12 and/or 4-1BBL
To further assess whether IL-12- or/and 4-1BBL-expressing oncolytic Ads could induce tumor-specific immune responses against B16-F10 melanoma cells, IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay and 51Cr-release assay were independently performed. Splenocytes isolated from mice in all groups on day 5 after last viral injection were stimulated in vitro with irradiated B16-F10 cells for 5 days. Then the number of IFN-γ-secreting cells and the CTL activity was assessed by IFN-γ ELISpot assay and a standard 51Cr-release assay, respectively. As shown in Figure 3b, splenocytes obtained from mice treated with Ad-ΔB7/IL-12/4-1BBL group yielded significantly higher number of IFN-γ spots (P < 0.01) than those treated with either Ad-ΔB7/4-1BBL or Ad-ΔB7/IL-12. Significant CTL activity was also observed in mice receiving the IL-12-expressing oncolytic Ads (Ad-ΔB7/IL-12 and Ad-ΔB7/IL-12/4-1BBL; P < 0.01 versus Ad-ΔB7), whereas only a modest but reproducible CTL activity was detected in Ad-ΔB7- or Ad-ΔB7/4-1BBL-treated group (Figure 3c). In addition, the CTL activity of splenocytes from mice treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL was specific to B16-F10 tumors, as no CTL activity could be detected when Mus dunni mouse fibroblast cells were used as target cells in the 51Cr-release assay (data not shown). These results indicate that the potent antitumor effect seen with Ad-ΔB7/IL-12/4-1BBL in B16-F10 established tumor model (Figure 2) correlated positively with the tumor-specific immune response elicited by IL-12 and 4-1BBL expressed by the oncolytic Ad.
Histological and immunohistochemial assessment of tumor tissues treated with oncolytic Ads expressing IL-12 and/or 4-1BBL
To better understand the mechanism behind IL-12- and/or 4-1BBL-expressing Ad-induced antitumor effect, histological and immunohitochemical analysis were performed. Tumors treated with either Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL were visibly smaller and had significantly higher immune cell infiltration (Figure 3d). Disruption of the tumor as a result of tumor necrosis was markedly evident in the tumors treated with Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL as seen in the hematoxylin and eosin–stained sections. Immunohistochemical (IHC) analysis also confirmed the presence of both CD4+ and CD8+ T lymphocytes in the tumors treated with Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL. Minimal to moderate level of CD4+ and CD8+ T lymphocytes were also detected in tumors treated with Ad-ΔB7 or Ad-ΔB7/4-1BBL. Moreover, CD86+ and CD11c+ DC population was substantially enhanced in sections derived from Ad-ΔB7/IL-12/4-1BBL-treated animals.
Injection of both Ad-ΔB7/IL-12/4-1BBL and DCs induces robust antitumor effect in vivo
With potential clinical applications in mind, we next compared whether oncolytic Ad expressing IL-12 and 4-1BBL would be more effective in combination with DCs. Quality control for DC preparation is mandatory in order to obtain reproducible and comparable study results. Therefore, the DC phenotype was first studied to determine maturation after the culture of DCs with tumor cell lysate and activation by lipopolysaccharide. As shown in Supplementary Figure S1a, the bone marrow–derived DCs cultured for 8 days exhibited the veiled dendrite morphology typical of DCs, showing the pricking and dendritic eminences on the cell surface. A representative phenotype for these DCs assessed by fluorescence-activated cell sorting analysis is shown in Supplementary Figure S1b. Activated DCs expressed high levels of maturation markers such as CD11c, CD40, CD80, CD86, MHC class I, and MHC class II, but negative for CD3ε (T-cell marker), CD19 (B-cell marker), and CD14 (monocyte marker).
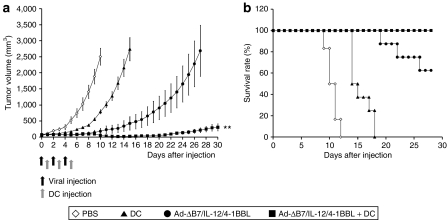
The therapeutic efficacy of activated DCs in combination with Ad-ΔB7/IL-12/4-1BBL was next tested in established B16-F10 tumor model (Figure 4). The tumor-bearing mice were treated with 2.5 × 109 VP of Ad-ΔB7/IL-12/4-1BBL, 1 × 106 DCs, or combination of Ad and DCs. By day 15, tumors from mice treated with combination of Ad-ΔB7/IL-12/4-1BBL and DCs were significantly smaller in size than DCs- or Ad-ΔB7/IL-12/4-1BBL-treated mice (all P < 0.01). Mice were killed when mean tumor volumes exceeded 3,000 mm3 and all mice treated with PBS or DCs succumbed to euthanasia by day 18. All mice receiving Ad-ΔB7/IL-12/4-1BBL oncolytic Ad alone exhibited significant tumor growth delay, with three out of eight mice reaching its end point by day 28. In contrast, 100% of tumor-bearing mice treated with combination therapy of Ad-ΔB7/IL-12/4-1BBL and DCs were viable on day 28 (P < 0.01 versus DCs or Ad-ΔB7/IL-12/4-1BBL alone). Further, three out of eight mice achieved a complete remission and survived over 180 days. These data suggest that intratumoral injection of Ad-ΔB7/IL-12/4-1BBL plus DCs elicited potent antitumor effect in a murine model with pre-established B16 melanoma.
Figure 4.
Enhanced therapeutic efficacy of combined therapy of Ad-ΔB7/IL-12/4-1BBL and DCs. B16-F10 tumors were grown subcutaneously in the abdomen of C57BL/6 mice and were subsequently injected with PBS (open diamonds), DCs (filled triangles), Ad-ΔB7/IL-12/4-1BBL (filled circles), or Ad-ΔB7/IL-12/4-1BBL plus DCs (filled squares). Virus was injected intratumorally three times (2.5 × 109 VP/time) with 2 days interval. DCs (1 × 106) were also injected itratumorally three times between the virus injections with 2-day interval. Tumor growth was monitored every day after each treatment. (a) Combined treatment group showed markedly enhanced antitumor effect compared with either Ad-ΔB7/IL-12/4-1BBL or DCs alone group (P < 0.01). Ad-ΔB7/IL-12/4-1BBL or DCs alone group also showed antitumor effect (P < 0.01 versus PBS group). (b) Survival percentage of B16-F10 melanoma-bearing mice in different groups. Survival was significantly improved by Ad-ΔB7/IL-12/4-1BBL plus DCs compared with either Ad-ΔB7/IL-12/4-1BBL or DCs alone group (P < 0.01). **P < 0.01. 4-1BBL, 4-1BB ligand; DC, dendritic cell; PBS, phosphate-buffered saline.
Tumor-specific immune response is associated with a treatment of Ad-ΔB7/IL-12/4-1BBL and DCs
To better understand the nature of the observed antitumor effects, splenocytes were collected from animals treated with PBS, DCs, Ad-ΔB7/IL-12/4-1BBL, or Ad-ΔB7/IL-12/4-1BBL plus DCs. As shown in Figure 5a, splenocytes from mice treated with Ad-ΔB7/IL-12/4-1BBL plus DCs displayed significantly stronger tumor-specific CTL activity against B16-F10 cells than those from either DCs- or Ad-ΔB7/IL-12/4-1BBL-treated mice (P < 0.01). The presence of tumor-specific immune response was also studied using an IFN-γ ELISpot assay. Splenocytes from the mice treated with Ad-ΔB7/IL-12/4-1BBL plus DCs showed significantly higher number of IFN-γ producing immune cells than those obtained from DCs- or Ad-ΔB7/IL-12/4-1BBL-treated mice (P < 0.01), suggesting that combination therapy with Ad-ΔB7/IL-12/4-1BBL and DCs enhanced the Th1 immune response against B16-F10 tumor cells. Moreover, IFN-γ expression level in the tumor tissue treated with Ad-ΔB7/IL-12/4-1BBL plus DCs was higher than those of either DCs or Ad-ΔB7/IL-12/4-1BBL alone (P < 0.01). It is also worth noting that systemic levels of IFN-γ were undetectable, a finding that correlates with the absence of toxic effects in animals receiving the treatment.
Figure 5.
Induction of tumor-specific immune response. Splenocytes obtained from mice treated with PBS, DCs, Ad-ΔB7/IL-12/4-1BBL, Ad-ΔB7/IL-12/4-1BBL plus DCs were evaluated tumor-specific CTL activity and tumor-specific IFN-γ secreting ability. (a) Tumor-specific CTL activity performed by 51Cr-release assay. Significantly enhanced CTL activity was observed in mice treated with Ad-ΔB7/IL-12/4-1BBL plus DCs than those treated with either DCs or Ad-ΔB7/IL-12/4-1BBL alone (P < 0.01). The effector:target (E:T) ratio indicated. Values are the mean ± SE of triplicate wells. (b) Tumor-specific IFN-γ secreting splenic immune cells. Mice treated with Ad-ΔB7/IL-12/4-1BBL plus DCs showed significantly increased number of IFN-γ producing immune cells than those treated with either DCs or Ad-ΔB7/IL-12/4-1BBL (P < 0.01). Results are representative of three independent experiments. (c) Evaluation of IFN-γ expression in tumor tissue after treatment of Ad-ΔB7/IL-12/4-1BBL and/or DCs. Significantly enhanced IFN-γ expression level was detected in tumor tissue treated with Ad-ΔB7/IL-12/4-1BBL plus DCs than those from other groups (P < 0.01). Data points represent mean ± SE of three independent experiments. (d) Histology and Immunohistochemistry with H&E and immune cell markers. Massive necrotic areas were seen in tumors treated with combination therapy compared to other groups. Also large number of CD4+, CD8+, CD86+, and CD11c+ lymphocyte was infiltrated into tumor treated with Ad-ΔB7/IL-12/4-1BBL plus DCs compared to DC- or Ad-ΔB7/IL-12/4-1BBL-treated groups. The results indicate that more effective antitumor immune response was induced in tumors treated with Ad-ΔB7/IL-12/4-1BBL plus DCs than other groups. (e) The average number ± SE of CD4+, CD8+, CD86+, and CD11c+-positive cells in microscopic field (×400). **P < 0.01 versus Ad-ΔB7/IL-12/4-1BBL group. 4-1BBL, 4-1BB ligand; CTL, cytotoxic T lymphocyte; DC, dendritic cell; H&E, hematoxylin and eosin; IFN, interferon.
Increased immune cell infiltration in tumor tissues treated with oncolytic Ads expressing IL-12 and/or 4-1BBL and DCs
To gain insight into the potential mechanism of synergy between Ad-ΔB7/IL-12/4-1BBL oncolytic Ad and DCs, we analyzed infiltration of immune cells in the treated tumors. Histological examination revealed that a large number of immune cells were found within and around tumors in mice when intratumorally injected with Ad-ΔB7/IL-12/4-1BBL, DCs, or Ad-ΔB7/IL-12/4-1BBL plus DCs (Figure 5d, hematoxylin and eosin staining). In contrast, small number of immune cell infiltration was present in tumors treated with PBS. IHC analysis confirmed the presence of both CD4+ and CD8+ T lymphocytes at the periphery of the excised tumor tissues treated with DCs alone, whereas significant lymphocyte infiltration inside of tumors treated with Ads alone or Ads plus DCs. Moreover, tumors treated with Ad-ΔB7/IL-12/4-1BBL plus DCs showed a significantly higher number of CD11c+ and CD86+ DCs than tumors treated with either Ad-ΔB7/IL-12/4-1BBL or DCs alone.
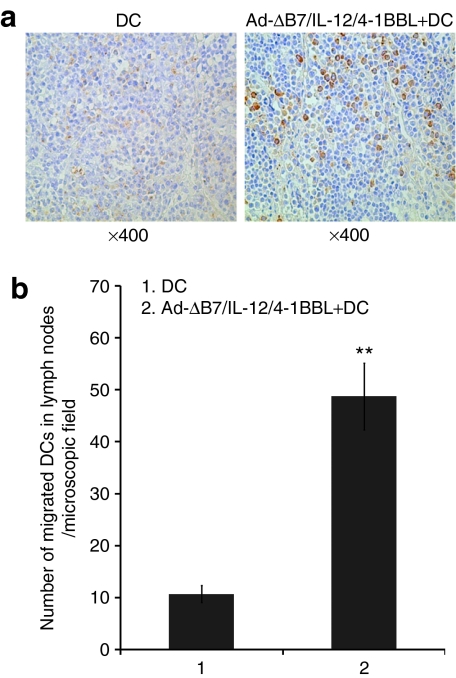
To further determine DC migration to regional lymph nodes in vivo, we injected 1 × 106 green fluorescent protein (GFP)–expressing DCs alone or in combination with Ad-ΔB7/IL-12/4-1BBL into pre-established B16-F10 tumor nodules 24 hours after viral injection. The draining lymph nodes (DLNs) were then harvested 2 days after DC injection. The lymph node was prepared and stained with GFP-specific antibody (Ab). As shown in Figure 6, more GFP-expressing DCs were detected in the DLNs of tumor-bearing mice treated with combination therapy of DCs and Ad-ΔB7/IL-12/4-1BBL compared to DCs alone-treated group (P < 0.01), suggesting greater DC migratory proficiency in mice treated with combination of DCs and Ad-ΔB7/IL-12/4-1BBL.
Figure 6.
DC migration activity in mice treated with combination of Ad-ΔB7/IL-12/4-1BBL and DCs. Two groups of mice were used. In the first group (DCs alone), B16-F10 tumors were intratumorally injected with 1 × 106 DCs alone three times every 2 days. In the second group (DCs plus Ad-ΔB7/IL-12/4-1BBL), tumors were intratumorally injected with 5 × 109 VP of Ad-ΔB7/IL-12/4-1BBL oncolytic adenovirus. One day later, 1 × 106 DCs were injected intratumorally. (a) Two days after final DC administration, draining lymph nodes were harvested and green fluorescent protein (GFP) expression of DCs was determined. Original magnification ×400. (b) The mean number of migrated GFP+ DCs in the draining lymph nodes in microscopic files (×400). One representative experiment of three independent experiments is shown. **P < 0.01. 4-1BBL, 4-1BB ligand; DC, dendritic cell.
Potent inhibition of tumor metastasis by treatment with Ad-ΔB7/IL-12/4-1BBL and DCs
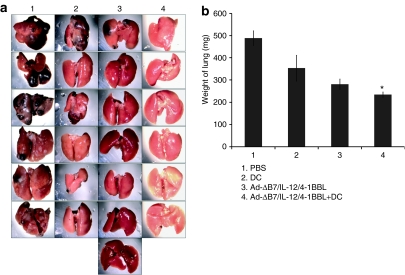
The antimetastatic effect of combination therapy with Ad-ΔB7/IL-12/4-1BBL and DCs was next evaluated using a spontaneous metastasis model. B16BL6 melanoma cells were implanted on the footpad subcutaneously and the primary tumors were subsequently removed at 9 days postimplant, and tumor metastases to the lung were then measured. As shown in Figure 7a, B16BL6 metastatic tumor volume in the lung was smaller in response to treatment with combination of Ad-ΔB7/IL-12/4-1BBL and DCs in comparison with Ad-ΔB7/IL-12/4-1BBL or DCs alone. The average relative lung weight from mice treated with Ad-ΔB7/IL-12/4-1BBL alone, DCs alone, or combination of Ad-ΔB7/IL-12/4-1BBL and DCs was 353 ± 58, 281 ± 23, and 235 ± 12 mg, respectively, compared to PBS-treated control group (488 ± 33 mg) (P < 0.05, Ad-ΔB7/IL-12/4-1BBL plus DCs versus Ad-ΔB7/IL-12/4-1BBL alone) (Figure 7b). Taken together, the marked inhibition of spontaneous lung metastasis obtained with combination of Ad-ΔB7/IL-12/4-1BBL and DCs suggests that intratumoral injection of oncolytic Ad expressing both IL-12 and 4-1BBL along with injection of DCs at the primary tumor site greatly reduced the formation of metastatic lesions at distal sites in this model system.
Figure 7.
Therapeutic efficacy of combining Ad-ΔB7/IL-12/4-1BBL and DCs in spontaneous pulmonary metastasis model. (a) Representative lung macroscopic view of each group. Considerably less metastatic lesions were visible in lungs of mice treated with Ad-ΔB7/IL-12/4-1BBL and DCs. (b) Comparison of lung weight from each group. Significant inhibition of spontaneous lung metastasis was observed with combination of Ad-ΔB7/IL-12/4-1BBL and DCs (P < 0.05 versus Ad-ΔB7/IL-12/4-1BBL alone). DC, dendritc cell; PBS, phosphate-buffered saline.
Discussion
Recently, gene-based cytokine treatment has been actively pursued as a new promising approach in treating cancer. Among the different cytokines, IL-12 is of particular interest because of its critical role in the stimulation of cell-mediated immune response. As systemic administration of IL-12 has been shown to induce dose-dependent and schedule-related systemic toxicity in both animal studies18 and in clinical trials,19,20 alternative strategies based on gene transduction within tumor cells with IL-12 genes have been investigated.21,22,23 Local delivery of IL-12 expressing fibroblast cells has been shown to suppress tumor growth and animals developed antitumor immune response.24 In addition, intratumoral transfer of recombinant Ad expressing IL-12 resulted in substantial antitumor effect as well as increased survival rate.22 More recently, we have shown the utility of IL-12-expressing oncolytic Ad in a cancer cell-restricted manner without accompanying toxicities.7
In an effort to augment the efficiency of antitumor effect by cytokine-mediated immunotherapy, we selected both IL-12 and 4-1BBL as suitable immunostimulants to fully activate Th1 immune response. 4-1BBL has been shown to mediate co-stimulation and expansion of CD8+ T cells greater than CD28 signaling.25 It is also been shown to strongly promote the differentiation of naive CD4+ T cells to Th1 phenotype and suppress the expression of Th2 cytokines. Moreover, 4-1BBL has also been demonstrated to activate T cells in the absence of B7.1/CD28 interactions.26,27
This study demonstrates that coexpression of IL-12 and 4-1BBL mediated by oncolytic Ad greatly enhanced the antitumor effect, compared with oncolytic Ads expressing IL-12 or 4-1BBL alone (Figure 2), suggesting that tumor-specific T-cell activation was involved in this increased antitumor efficacy. We also report synergistically enhanced IFN-γ levels, a Th1 cytokine that is important for cell-mediated immunity, in mice treated with oncolytic Ad expressing both IL-12 and 4-1BBL (Figure 3a). The induction of IFN-γ after treatment with Ad-ΔB7/IL-12/4-1BBL Ad indicates biologically active production of IL-12 by tumor cells and is exerting its characteristic immunoregulatory functions. Moreover, increased CTL activity and IFN-γ-expressing immune cells were detected in splenocytes taken from mice receiving intratumoral injection of Ad-ΔB7/IL-12/4-1BBL Ad relative to either Ad-ΔB7/IL-12 or Ad-ΔB7/4-1BBL Ad (Figure 3b,c). These findings suggest that precursor CD4+ T cells become Th1 cells by the effective antigen presentation of tumor cells that express 4-1BBL and that precursor cytotoxic T cells (CD8+) are activated by Th1 cells. Consistent with these findings, IHC analysis also revealed a massive infiltration of both CD4+ and CD8+ T lymphocytes into tumors treated with Ad-ΔB7/IL-12/4-1BBL oncolytic Ad. In addition, we also observed a greater number of activated DCs infiltrated into these tumors (Figure 3d). This finding is in good agreement with recent reports by Pan et al., in which they demonstrate that 4-1BB activation in conjunction with IL-12 gene delivery increased DC proliferation and tumor infiltration and enhanced the surface expression of MHC class II and co-stimulatory molecules such as intercellular adhesion molecule-1, CD40, CD86, and 4-1BBL (ref. 28). Further these investigators also demonstrated that IL-12-activated natural killer cells upregulated 4-1BB expression by DCs through an IFN-γ-dependent pathway and that triggering of 4-1BB on DCs by injecting anti-4-1BB Ab resulted in an enhancement of tumor infiltration and maturation of DCs.28 These observations imply that high expression of IFN-γ in the Ad-ΔB7/IL-12/4-1BBL oncolytic Ad-treated tumors appeared to upregulate 4-1BB activation on DCs through an IFN-γ-dependent pathway and the subsequent activation and tumor infiltration of DCs.
In recent clinical trials, DC-based therapies have yielded some objective responses and mechanistically tumor-specific immune responses were seen but the overall clinical outcomes have been disappointing. One of the reasons for this limitation maybe due to functional impairment of the DCs in the tumor microenvironment that failed to induce a full antitumor immune response.29 Research into optimization of protocols to provide effective in vivo activation stimuli for DCs was therefore actively pursued.30 In an attempt to improve the overall antitumor immune response, we examined a combination strategy in which IL-12- and 4-1BBL-coexpressing oncolytic Ad was administered in conjunction with DCs. We show that this combination therapy significantly improved the therapeutic efficacy as well as survival. In particular, low dose (2.5 × 109 VP) of Ad-ΔB7/IL-12/4-1BBL oncolytic Ad elicited much better long-term survival (complete remission in three out of eight mice; Figure 4) when combined with DCs than double dose (5 × 109 VP) of Ad-ΔB7/IL-12/4-1BBL oncolytic Ad alone treatment (no remission in eight treated mice; Figure 2). The nature of the enhanced antitumor immune response seems to be mediated through the enhanced cytolytic activity of CTLs and IFN-γ-releasing immune cells, in agreement with recent study of Christopher et al. reporting that combination treatment of oncolytic herpes simplex virus 1 and DCs exhibits potent antitumor effect via enhancement of antitumor immunity.31 IL-12 has been shown to stimulate Th1-mediated cellular immune response, promote proliferation and maturation of CTLs and natural killer cells, and enhance the cytolytic function of these cells at least in part via the production of IFN-γ. It has also been reported that murine lymphoid DCs stimulated with IL-12 can release IFN-γ.32 These observations suggest that the observed increase in IFN-γ seen in this study was mediated by both IL-12 expressed from Ad-ΔB7/IL-12/4-1BBL oncolytic Ad as well as the activated DCs. The activation of IL-12 receptor on DCs by IL-12 has been shown to promote nuclear localization of NF-κB, which increases the expression of MHC and co-stimulatory molecules. IL-12 also regulates DC immunostimulatory function. Thus, IL-12 production from oncolytic Ad may serve as a positive stimulus to correct tumor-induced dysfunction in DCs.
Recently it has been demonstrated that activated natural killer cells can kill immature DCs,33 but also can induce the maturation of these DCs as well.34 In our study, it is possible that the high concentration of IFN-γ in tumor microenvironment induced by the combination treatment may have activated natural killer cells, which may have induced further maturation of DCs. Studies have also shown that the functionally impaired DCs found in tumor-bearing animals and in advanced cancer patients correlated with the production of tumor-derived soluble factors, such as vascular endothelial growth factor, IL-10, and IL-6 that affect DC activation.35 IL-12 has been known to exhibit antiangiogenic effects that are mediated through IFN-γ induced production of monokine (by monocytes) and IFN-inducible protein 10 (by a variety of cells including endothelial cells).36 This suppression of angiogenic cascade in tumor milieu and suppression of VEGF expression induced by E1A in oncolytic Ad would further increase DC activation in tumor microenvironment.37
DCs exhibit an inherent migratory capability that is tightly linked to their activation/maturation status and seem to correlate interestingly with T-cell proliferation. As the mobilization of DCs from tumor tissues and their homing to DLNs is an essential component in activating a full antitumor immune response, the effect of IL-12 and 4-1BBL expressed by oncolytic Ad in tumor microenvironment on the migratory potential of the DCs was further assessed. Injected DCs in tumors treated with combination therapy showed markedly enhanced migration toward the DLNs (Figure 6), indicating that the enhanced DC migratory properties induced by the combination therapy facilitates their aimed movement toward and into the DLNs where DCs are recruited to activate T cells and initiate antitumor immune responses. This finding concurs with a recent report showing that genetic modification of DCs with tumor-associated antigen-specific CD40 chimeric receptor enhances the ability of DCs in migrating to the DLNs.38 Moreover, Lapteva et al., also reported that transduction of immature DCs with a synthetic ligand-inducible CD40 receptor (iCD40) enhanced DC migration,39 implying that DC maturation is a key parameter of effective DC function.
Taken together, our study provides a proof of principle for the feasibility of using combined cytokine-expressing oncolytic Ads with DCs. Oncolytic Ad-induced tumor cell lysis would provide tumor antigens in an immunogenic form for presentation to injected DCs and the ability of 4-1BBL to mobilize DCs together with the high local concentration of antitumor immune cytokines such as IL-12 would provide an ideal tumor environment for the creation of potent antitumor immune response, resulting in the full activation of antitumor T-cell responses.
Materials and Methods
Cell lines and cell culture. Human embryonic kidney cell line 293, murine melanoma cell lines B16-F10 and B16BL6 (a metastatic variant of B16 melanoma cells), and mouse fibroblast cell line Mus dunni were purchased from the American Type Culture Collection (Manassas, VA). All cell lines were cultured at 37 °C in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco) and antibiotics, except for B16BL6 cells, which were cultured in modified Eagle's medium (Gibco) supplemented with 5% fetal bovine serum, modified Eagle's medium vitamin solution (1 mmol/l, product 11120-052; Gibco), penicillin (500 IU/ml), and streptomycin (50 µg/ml). All have tested negative for Mycoplasma using Hoeschst dye (MP Biomedicals, Irvine, CA), cell culture, and PCR.
Mice. C57BL/6 mice were obtained from SLC (Japan SLC, Tokyo, Japan). Mice were used at 6–7 weeks of age, and all animal studies were performed according to institutionally approved protocols at Yonsei University College of Medicine.
Ad vectors. Ad-ΔB7 is an Ad5-based E1B/E3-deleted oncolytic Ad with substitution in retinoblastoma binding sites of E1A (ref. 17). To generate an oncolytic Ad that expresses IL-12 at the E1 region of Ad-ΔB7, pCA14/E1AE1B19-IL-12 (ref. 6) E1 shuttle vector was linearized with NdeI digestion and then homologous recombination was induced in Escherichia coli BJ5183 with BstBI-digested Ad-ΔB7 to generate Ad-ΔB7-IL-12. To generate Ads that express 4-1BBL at the E3 region of Ad-ΔB7, the full-length mouse 4-1BBL complementary DNA was first cloned by reverse transcriptase-PCR using total RNA from bone marrow–derived activated DCs. The 4-1BBL complementary DNA (53–982 nucleotides of National Center for Biotechnology Information L15435) was generated using following primer pairs (sense: 5′-ATGGATCCACCATGGACCAGCACACACTTG-3′ antisense: 5′-AGATAAGC TTTCATTCCCATGGGTTGTC-3′). The resulting PCR product was digested with BamHI/HindIII and cloned into the BamHI/HindIII-digested pSP72ΔE3/CMV-polA (ref. 40) Ad E3 shuttle vector, generating pSP72-E3/4-1BBL E3 shuttle vector. This newly constructed pSP72-E3/4-1BBL E3 shuttle vector was then recombined with Ad-ΔB7 and Ad-ΔB7-IL-12, generating Ad-ΔB7-4-1BBL and Ad-ΔB7-IL-12/4-1BBL, respectively. All viral vectors were produced and propagated following standard procedures.41 Viral particle numbers were calculated from measurements of absorbance at 260 nm (A260), where 1 absorbency unit is equivalent to 1012 VP/ml, and infectious titers were determined by limiting dilution in 293 cells.
Expression of 4-1BBL and IL-12. The expression of 4-1BBL was determined by western blot analysis. At 48 hours after infection of B16-F10 cells with Ad-ΔB7-4-1BBL or Ad-ΔB7-IL-12/4-1BBL at various MOIs, proteins in the cell extracts were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane. The membrane was incubated with primary monoclonal goat anti-mouse 4-1BBL Ab (R&D Systems, Minneapolis, MN) and anti-β-actin Ab for 1 hour. After incubation with secondary rabbit anti-goat IgG (H+L)-horseradish peroxidase Ab (Southern Biotech, Birmingham, AL) for 45 minutes at room temperature, filters were developed with the enhanced chemiluminescence system (Santa Cruz Biotechnology, Santa Cruz, CA). The expression of IL-12 was determined by ELISA as previously described.7 Briefly, B16-F10 cells were infected with Ad-ΔB7-IL-12 or Ad-ΔB7-IL-12/4-1BBL at various MOIs. At 48 hours after infection, IL-12 in the supernatant was determined with ELISA using anti-mouse IL-12 purified monoclonal Ab (ENDOGEN, Woburn, MA) and biotin-conjugated anti-mouse IL-12 monoclonal Ab (ENDOGEN).
Generation of bone marrow–derived DC. Femur and tibia marrow cells from C57BL/6 mice were depleted of erythrocytes using red blood cell lysis buffer (Sigma, St Louis, MO). Cells were then cultured in complete RPMI 1640 media containing 10% fetal bovine serum, GM-CSF (10 ng/ml; ENDOGEN), and IL-4 (10 ng/ml; ENDOGEN). On day 2, floating cells were discarded and the adherent cells were replenished with fresh complete media. At day 4, culture supernatant was collected and centrifuged, and the cell pellet was resuspended in fresh RPMI 1640 containing cytokines and returned to the original plate. On day 6, adherent DCs were incubated with B16-F10 cell lysate (50 µg/ml) for 24 hours and then matured by addition of 1 µg/ml of lipopolysaccharide (Sigma) for 24 hours. Mature DCs were harvested and phenotypic markers of DC was confirmed by fluorescence-activated cell sorting analysis.
Established tumor models for in vivo antitumor effect. B16-F10 cells (5 × 105) were injected subcutaneously into the right abdomen of 6- to 7-week-old male C57BL/6 mice. When the tumor volume reached around 100 mm3, animals were sorted into groups with similar mean tumor volumes and different doses of oncolytic Ads (Ad-ΔB7 or Ad-ΔB7/4-1BBL at 1 × 1010 VP; Ad-ΔB7/IL-12 or Ad-ΔB7/IL-12/4-1BBL at 5 × 109 VP) were injected intratumorally three times every other day in a volume of 20 µl diluted in PBS. Tumor growth was monitored everyday by measuring two perpendicular tumor diameters using calipers. Tumor volume was calculated by the following formula: volume = 0.523 LW2, where L is length and W is width. Animals with tumors that were >3,000 mm3 were killed for ethical reasons.
IFN-γ detection in tissue homogenates. Tumor tissues were obtained from intratumoral treated mice by presented various viruses. After 5 days of final injection of viruses, tumors were grinded and liquefied in the PBS with protease inhibitor cocktail (cat no. P8340; Sigma). IFN-γ expression of treated tumors was determined by IFN-γ ELISA kit (ENDOGEN) according to the manufacturer's instructions. Each experiment was carried out three to four times with three replicates in each group.
Tumor-specific IFN-γ ELISpot assay. The ELISpot assay was used to determine the B16-F10 tumor-specific IFN-γ-secreting T cells. As responder cells, splenocytes from mice in all groups on day 5 after final treatment were stimulated with irradiated B16-F10 in medium supplemented with recombinant human IL-2 (100 units/ml) for 5 days. Splenocytes were then serially diluted from 2 × 104 to 8 × 105 cells per well and incubated in anti-IFN-γ monocolonal Ab–coated plates. After 12 hours of incubation, plates were developed as previously described.7 After overnight drying at room temperature in the dark, plates were evaluated. Each experiment was carried out three to four times with three replicates in each group.
CTL assay. Viable splenocyte samples were isolated from Ad-ΔB7/IL-12/4-1BBL and/or DCs treatment animals, and the cytolytic activity of CTL was determined as described previously.7 Percent-specific cytotoxicity was determined from the formula: % Specific cytotoxicity = [(experimental release − spontaneous release)/(maximum release − spontaneous release) × 100].
Histological and IHC staining. After treatment by Ad and/or DCs, tumors were harvested, snap-frozen, and 10-µm sections were prepared in Tissue Tek (Sakura Finetec, Torrance, CA). Tumor sections were blocked with 4% PBS–bovine serum albumin (Sigma) for 1 hour and incubated for 2 hours with appropriate dilution of biotin-labeled CD4 (purified rat anti-mouse CD4 monoclonal Ab; Pharmingen, San Diego, CA), CD8 (purified rat anti-mouse CD8 monoclonal Ab; Pharmingen), CD86 (purified rat anti-mouse CD86 monoclonal Ab; Pharmingen), or CD11c (purified hamster anti-mouse CD11c monoclonal Ab; Pharmingen) in 1% PBS–bovine serum albumin. Diaminobenzidine/hydrogen peroxidase (Dako, Carpinteria, CA) was used as the chromogen substrate. All slides were counterstained with Meyer's hematoxylin.
Analysis of DC migration in vivo. To investigate the ability of DCs to migrate into regional lymph nodes in vivo, activated DCs were first transduced with GFP-expressing Ad at an MOI of 300. After 48 hours of transduction, 1 × 106 DCs alone or in combination with Ad-ΔB7/IL-12/4-1BBL oncolytic Ad (5 × 109 VP) were intratumorally injected into established B16-F10 tumors three times every other day, in a sequence of Ad followed by DCs. At 48 hours after final intratumoral DC injection, the DLNs were harvested and stained with GFP-specific Ab for the analysis of the presence of DCs.
Quantification of spontaneous pulmonary metastasis. The B16BL6 spontaneous metastasis model was initiated by subcutaneously inoculation of 1.5 × 105 tumor cells in 50 µl of Hank's buffered salt solution into the right footpad. When tumors reached a volume of 200 mm3, the primary tumors were treated with PBS, DCs (1 × 106 cells), Ad-ΔB7/IL-12/4-1BBL (5 × 109 VP), or Ad-ΔB7/IL-12/4-1BBL plus DCs. Ad-ΔB7/IL-12/4-1BBL oncolytic Ad was intratumorally injected on days 1, 3, and 5, and DCs were injected into the intrapleural cavity on days 2, 4, and 6. On day 9, the primary tumor was then surgically excised by amputating the right hind leg below the knee under mild anesthesia. At 3 weeks after final treatment, lungs were harvested for assessment of metastatic tumor lesions.
Statistical analysis. The data were expressed as mean ± SE. Statistical analyses of the data were performed using the two-tailed Student's t-test (SPSS 10.0 software; SPSS, Chicago, IL). P values of <0.05 were considered statistically significant (*P < 0.05; **P < 0.01). Analysis of variance was used for multiple group comparison on antitumor effect examination.
SUPPLEMENTARY MATERIALFigure S1. Microscopy and flow cytometry analysis of bone marrow derived DCs.
Supplementary Material
Microscopy and flow cytometry analysis of bone marrow derived DCs.
Acknowledgments
This work was supported by grants from the Ministry of Commerce Industry and Energy (10030051, C.-O.Y.), the Korea Science and engineering foundation (R04-2004-000-10011-0, R01-2006-000-10084-0, R15-2004-024-02001-0, M10416130002-04N1613-00210, C.-O.Y.), and through its Nuclear Research & Development Program (2007-00299, C.-O.Y.) of the Korea Science and Engineering Foundation. J.-H.H., S.-N.Z., and K.-J.C are graduate students sponsored by the Brain Korea 21 Project for Medical Science, and I.-K.C is a graduate student sponsored by the Graduate Program for Nanomedical Science, Yonsei University College of Medicine, Seoul, South Korea.
REFERENCES
- Wadhwa PD, Zielske SP, Roth JC, Ballas CB, Bowman JE., and , Gerson SL. Cancer gene therapy: scientific basis. Annu Rev Med. 2002;53:437–452. doi: 10.1146/annurev.med.53.082901.104039. [DOI] [PubMed] [Google Scholar]
- Podhajcer OL, Lopez MV., and , Mazzolini G. Cytokine gene transfer for cancer therapy. Cytokine Growth Factor Rev. 2007;18:183–194. doi: 10.1016/j.cytogfr.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Alemany R, Balagué C., and , Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- Dummer R, Hassel JC, Fellenberg F, Eichmüller S, Maier T, Slos P, et al. Adenovirus-mediated intralesional interferon-γ gene transfer induces tumor regressions in cutaneous lymphomas. Blood. 2004;104:1631–1638. doi: 10.1182/blood-2004-01-0360. [DOI] [PubMed] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H, et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin Cancer Res. 2006;12:5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- Colombo MP., and , Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- Vinay DS., and , Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Wen T, Bukczynski J., and , Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Proudfoot O, Pouniotis D, Sheng KC, Loveland BE., and , Pietersz GA. Dendritic cell vaccination. Expert Rev Vaccines. 2007;6:617–633. doi: 10.1586/14760584.6.4.617. [DOI] [PubMed] [Google Scholar]
- Fong L., and , Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- Gervais A, Levêque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L, et al. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005;7:R326–R335. doi: 10.1186/bcr1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V, Hermans IF., and , Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim JH, Choi KJ, Kim PH., and , Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum Gene Ther. 2007;18:773–786. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- Orange JS, Salazar-Mather TP, Opal SM, Spencer RL, Miller AH, McEwen BS, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Zitvogel L, Campbell R, Robbins PD, Elder E, Haluszczak C, et al. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann N Y Acad Sci. 1996;795:440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- Meko JB, Yim JH, Tsung K., and , Norton JA. High cytokine production and effective antitumor activity of a recombinant vaccinia virus encoding murine interleukin 12. Cancer Res. 1995;55:4765–4770. [PubMed] [Google Scholar]
- Caruso M, Pham-Nguyen K, Kwong YL, Xu B, Kosai KI, Finegold M, et al. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci USA. 1996;93:11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J., and , Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, Sondel PM, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci USA. 1996;93:6291–6296. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Johnson BD., and , Orentas RJ. Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4-1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumour-reactive effector cells in vivo. Immunology. 2004;112:105–116. doi: 10.1111/j.1365-2567.2004.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedette MA, Shahinian A, Mak TW., and , Watts TH. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- Bukczynski J, Wen T., and , Watts TH. Costimulation of human CD28− T cells by 4-1BB ligand. Eur J Immunol. 2003;33:446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- Pan PY, Gu P, Li Q, Xu D, Weber K., and , Chen SH. Regulation of dendritic cell function by NK cells: mechanisms underlying the synergism in the combination therapy of IL-12 and 4-1BB activation. J Immunol. 2004;172:4779–4789. doi: 10.4049/jimmunol.172.8.4779. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Zhong H, Shurin MR., and , Han B. Optimizing dendritic cell-based immunotherapy for cancer. Expert Rev Vaccines. 2007;6:333–345. doi: 10.1586/14760584.6.3.333. [DOI] [PubMed] [Google Scholar]
- Farrell CJ, Zaupa C, Barnard Z, Maley J, Martuza RL, Rabkin SD, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008;14:7711–7716. doi: 10.1158/1078-0432.CCR-08-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, et al. Interleukin 12-dependent interferon-γ production by CD8α+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G., and , Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke I., and , Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. 2006;35:459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, et al. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol. 1998;64:384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhou RR, Guan H, Bucana CD., and , Kleinerman ES. E1A gene therapy inhibits angiogenesis in a Ewing's sarcoma animal model. Mol Cancer Ther. 2003;2:1313–1319. [PubMed] [Google Scholar]
- Wei H, Wang H, Lu B, Li B, Hou S, Qian W, et al. Cancer immunotherapy using in vitro genetically modified targeted dendritic cells. Cancer Res. 2008;68:3854–3862. doi: 10.1158/0008-5472.CAN-07-6051. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Seethammagari MR, Hanks BA, Jiang J, Levitt JM, Slawin KM, et al. Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 2007;67:10528–10537. doi: 10.1158/0008-5472.CAN-07-0833. [DOI] [PubMed] [Google Scholar]
- Yun CO, Kim E, Koo T, Kim H, Lee YS., and , Kim JH. ADP-overexpressing adenovirus elicits enhanced cytopathic effect by induction of apoptosis. Cancer Gene Ther. 2005;12:61–71. doi: 10.1038/sj.cgt.7700769. [DOI] [PubMed] [Google Scholar]
- Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A., and , Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microscopy and flow cytometry analysis of bone marrow derived DCs.