Abstract
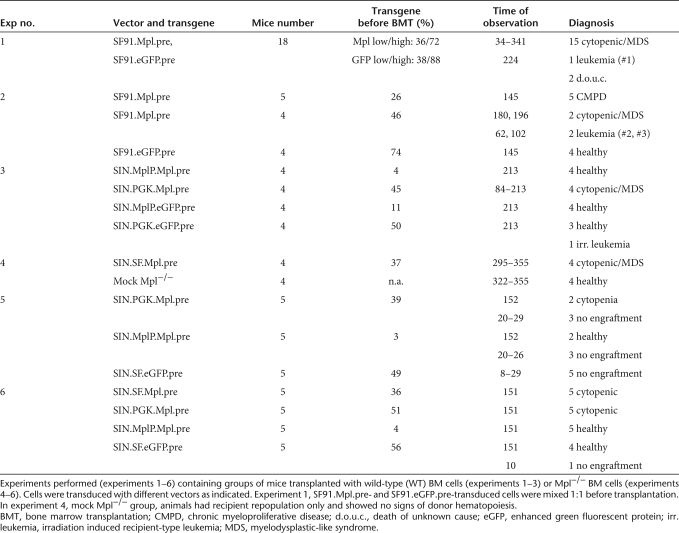
Signaling of the thrombopoietin (THPO) receptor MPL is critical for the maintenance of hematopoietic stem cells (HSCs) and megakaryocytic differentiation. Inherited loss-of-function mutations of MPL cause severe thrombocytopenia and aplastic anemia, a syndrome called congenital amegakaryocytic thrombocytopenia (CAMT). With the aim to assess the toxicity of retroviral expression of Mpl as a basis for further development of a gene therapy for this disorder, we expressed Mpl in a murine bone marrow transplantation (BMT) model. Treated mice developed a profound yet transient elevation of multilineage hematopoiesis, which showed morphologic features of a chronic myeloproliferative disorder (CMPD) with progressive pancytopenia. Ten percent of mice (3/27) developed erythroleukemia, associated with insertional activation of Sfpi1 and Fli1. The majority of transplanted mice developed a progressive pancytopenia with histopathological features of a myelodysplastic syndrome (MDS)–like disorder. To avoid these adverse reactions, improved retroviral vectors were designed that mediate reduced and more physiological Mpl expression. Self-inactivating γ-retroviral vectors were constructed that expressed Mpl from the phosphoglycerate kinase (PGK) or the murine Mpl promoter. Mice that received BM cells expressing Mpl from the Mpl promoter were free of any previously observed adverse reactions.
Introduction
Cytokine receptors and their ligands are the major regulators of cell proliferation and maturation in the hematopoietic system. The thrombopoietin (THPO)/myeloproliferative leukemia virus oncogene (MPL) system is one of the essential signaling pathways in hematopoiesis.1 Binding of THPO to its receptor MPL activates defined signaling pathways like JAK-STAT, MAPK-ERK1/2, and PI3K-AKT.1,2 MPL activation regulates megakaryocyte proliferation, maturation, and platelet production.3 MPL also plays a role in erythropoiesis and is a key player in hematopoietic stem cell (HSC) self-renewal.4,5,6 Deficiency of Mpl (Mpl−/−) or Thpo (Thpo−/−) in mice leads to thrombocytopenia and a reduction in HSC and progenitor cells of all hematopoietic lineages.7,8 The Thpo/Mpl axis is of great importance for HSC maintenance and quiescence.5,6 Mpl is expressed on long-term repopulating HSC in the bone marrow (BM) and the lack of Mpl signaling in Thpo−/− mice induces the entry of HSC from G0 into the G1 and S/G2/M phase of the cell cycle.5 Long-term downregulation of Thpo/Mpl signaling may induce proliferation and exhaustion of HSC. In contrast, Mpl signaling in progenitor cells induces cell proliferation and differentiation. Expression of chemically inducible Mpl in hematopoietic cells of mice and dogs was shown to be a tool for in vivo expansion of progenitor but not stem cells.9,10
Inactivating mutations of the MPL receptor in patients cause thrombocytopenia and aplastic anemia in early years of life, frequently leading to early death of patients. This syndrome is known as congenital amegakaryocytic thrombocytopenia (CAMT).11,12 Today, the only curative therapy is allogeneic bone marrow transplantation (BMT) which, however, bears substantial risks with potentially lethal adverse reactions.13 Because CAMT is a monogenetic defect, it is a classical target for gene therapy and could be cured by expression of an intact copy of the MPL gene. However, MPL is also a proto-oncogene. Mutations resulting in constitutive MPL activation are involved in myeloproliferative disease (MPD) in patients and MPD-like leukemia in mice.14
Mpl was discovered as homologue of the viral oncogene v-Mpl15,16 that is expressed as a fusion protein with the env gene and is responsible for the oncogenicity of the murine myeloproliferative leukemia virus.16 The myeloproliferative leukemia virus env region encoded env-mpl (v-Mpl) fusion polypeptide is a transmembrane protein and results in constitutive activation of the Mpl signaling pathways.17 Furthermore overexpression of Mpl in mice was shown to induce leukemias or hyperproliferative erythropoiesis18,19 probably induced by enhanced Mpl signaling. However, the reported phenotypes differed significantly between the studies probably due to variations in the experimental setup.
With the aim to address potential adverse reactions induced by ectopic Mpl expression, we used retroviral vectors in a BMT model in wild-type (WT) C57BL/6J mice and performed competitive repopulation studies with unmodified or control-vector-transduced cells. Due to the higher sensitivity of Mpl−/− mice to pretransplant conditioning, we did not use the Mpl−/− mice as recipients of hematopoietic cells transduced with Mpl vectors. Until today, only one study reports the successful engraftment of WT BM cell in Mpl−/− mice following nonmyeloablative conditioning (specific CXCR4 antagonist AMD3100) with a very low chimerism (~5%; ref. 20), while others present only very short follow-ups (8–9 days) because mice became moribund.8 Our study therefore focuses on the induction of potential adverse reactions in the WT BMT mouse model and how to overcome toxicity of ectopic Mpl expression by using vectors that express Mpl under the control of the low-expressing cellular phosphoglycerate kinase (PGK) promoter or a 2-kb fragment of the upstream region of the endogenous Mpl gene (MplP).21 As a subsequent step, we studied the potential of the Mpl vectors to restore defective megakaryopoiesis in vitro and the potential of Mpl-transduced Mpl−/− BM cells to repopulate the HSC compartment of WT mice, using the CD45.1/CD45.2 chimerism model. Our experiments demonstrate that ectopic Mpl expression induces potentially lethal adverse reactions that can be avoided by improved vector design.
Results
Mpl expression confers a transient growth advantage to murine BM cells
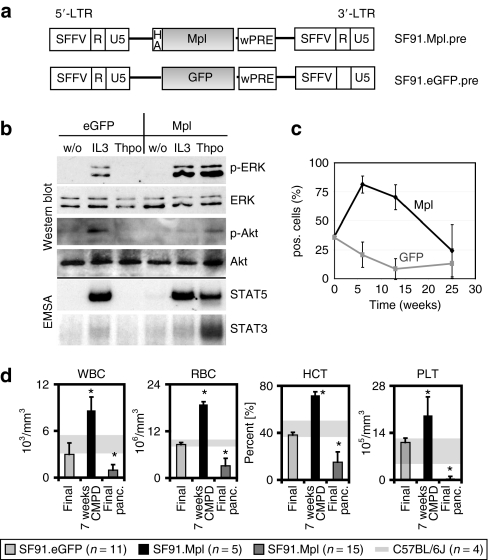
To assess the consequences of ectopic Mpl expression, we constructed γ-retroviral vectors expressing murine Mpl under the control of the retroviral enhancer–promoter of the spleen focus-forming virus (SFFV) in the vectors' long-terminal repeats (LTRs; SF91.Mpl.pre) (Figure 1a). A hemagglutinin tag was added after the signal peptide 5′ of the Mpl protein. SF91.Mpl.pre rendered the interleukin-3-dependent myeloid cell line 32D responsive to Thpo. These cells grew in Thpo-containing medium without the addition of interleukin-3 (Supplementary Figure S1). Western blot and electromobility shift assay analysis showed phosphorylation and activation of major Mpl downstream pathways after Thpo stimulation like ERK1/2, Akt, STAT3 and STAT5 (Figure 1b).
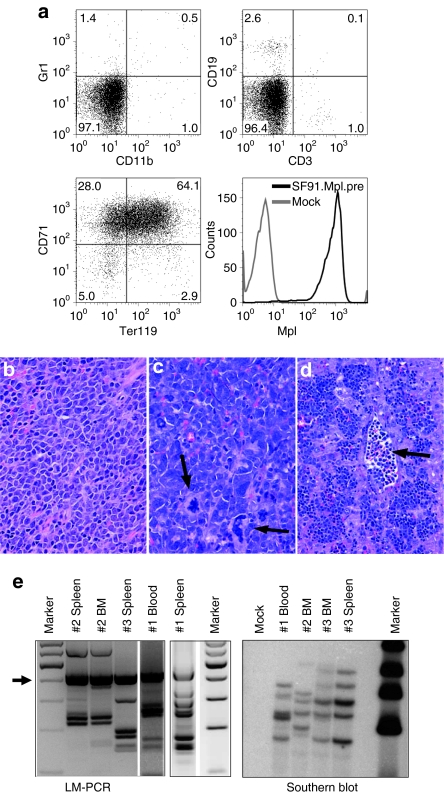
Figure 1.
Vectors and transplantation side effects. (a) γ-Retroviral vectors used in experiments 1 and 2. Long-terminal repeat (LTR, containing the spleen focus-forming virus enhancer/promoter (SFFV), R, and U5 region), HA (hemagglutinin tag), Mpl (wild-type Mpl), enhanced green fluorescent protein (eGFP), posttranscriptional regulatory element of woodchuck hepatitis virus (wPRE). (b) Western blot and electromobility shift assay analysis of protein lysates prepared from Mpl-transduced 32D cells. Cells were analyzed for ERK and Akt phosphorylation and STAT3 and STAT5 activation. (c) Percentage of transgene-positive cells in PB samples. Mice received an 1:1 mix of SF91.Mpl.pre- and SF91.GFP.pre-transduced BM cells in a competition assay. SF91.Mpl.pre-transduced cells showed an initial growth advantage compared to SF91.eGFP.pre-expressing cells (week 7, P ≤ 0.001, Wilcoxon signed rank test). (d) Peripheral blood (PB) cell counts. Mice in chronic myeloproliferative disease state (black, n = 5, experiment 2) showed increased PB counts [white blood cell (WBC), red blood cell (RBC), hematocrit (HCT) and platelet (PLT)] compared to SF91.eGFP.pre control mice (eGFP light grey, n = 11, experiments 2 and 3). Mice in pancytopenic state at final analysis presented decreased PB counts (medium grey, n = 15, experiments 1 and 2). Arrow bars indicate SD; grey shaded areas indicate mean ± SD of PB counts from normal donor C57BL/6J mice (n = 4); *P ≤ 0.03.
Because constitutive active Mpl signaling is involved in human MPD, we first addressed the potential toxicity of ectopic Mpl overexpression in BM cells of WT C57BL/6J mice. In our first dose finding experiment, we transplanted four groups of mice with BM cells that were transduced with SF91.Mpl.pre or SF91.eGFP.pre at different multiplicities of infection (multiplicity of infection 3 and 10). Cells were then mixed 1:1 resulting in four groups of five mice each (Figure 1c and Supplementary Figure S2). Two mice died unattended and were not included in the study. In total, 18 mice were observed long term in experiment 1.
We monitored transgene expression in peripheral blood (PB) cells at 7, 13, and 25 weeks after BMT. In all mice except two, until week 13 the percentage of Mpl+ cells increased, while enhanced green fluorescent protein–positive (eGFP+) cells decreased in all mice (Wilcoxon signed rank test P ≤ 0.001 at week 7) (Figure 1c). Mice that received high Mpl-transduced BM cells reached up to 87% Mpl+ cells in the PB, and the low level Mpl-transduced cells increased to 71% Mpl+ cells (P ≤ 0.01 at 7 and 13 weeks post-BMT; Supplementary Figure S2). However, at week 25 after BMT the percentage of Mpl+ cells in the PB decreased, while surprisingly the percentage of eGFP+ cells did not increase. Mice rather developed life-threatening peripheral pancytopenia (Figure 1d), indicating that the eGFP+ competitor cells were unable to rescue the loss of Mpl-expressing cells. Untransduced donor-derived BM cells also failed to support hematopoiesis.
Careful analysis of PB parameters displayed elevated erythrocyte (red blood cells), white blood cell, and thrombocyte (platelet) counts at 7 weeks post-BMT (Figure 1d) and the strongly increased hematocrit (80%) resulted in a striking reddish appearance of nose and paws. Therefore, ectopic Mpl expression induced proliferation of major hematopoietic lineages. However, this increase was transient. Beginning at 13 weeks, when the percentage of Mpl+ cells was still very high, mice developed cytopenia affecting major hematopoietic lineages. White blood cell counts of pancytopenic mice dropped to 1.0 ± 0.7 × 103/µl (~20% of normal) at final analysis. Red blood cell counts and hematocrit were as low as ~20–40% of normal counts. Platelet counts were more than tenfold reduced. Mice had to be killed due to severe symptoms of illness.
Mpl overexpression resulted in a transient MPD that converted to a myelodysplastic syndrome–like disorder
For more precise analysis of the disease phenotypes that developed due to Mpl overexpression in mice, a new BMT experiment (experiment 2, Table 1) was performed and groups of mice were killed and analyzed by histopathology at the different time points during the rise and fall of hematopoiesis. One group of transplanted mice (n = 5) was killed at 4 months after BMT when the PB cell parameters were still high, a second group was observed long term until they developed severe pancytopenia (>28 weeks). As a control, we transplanted mice with BM cells that were transduced with the vector SF91.eGFP.pre (n = 4). All eGFP mice were healthy after long-term observation (>21 weeks) and confirmed that the development of the pancytopenia was not due to our transplantation conditions.
Table 1.
Summary of all experiments
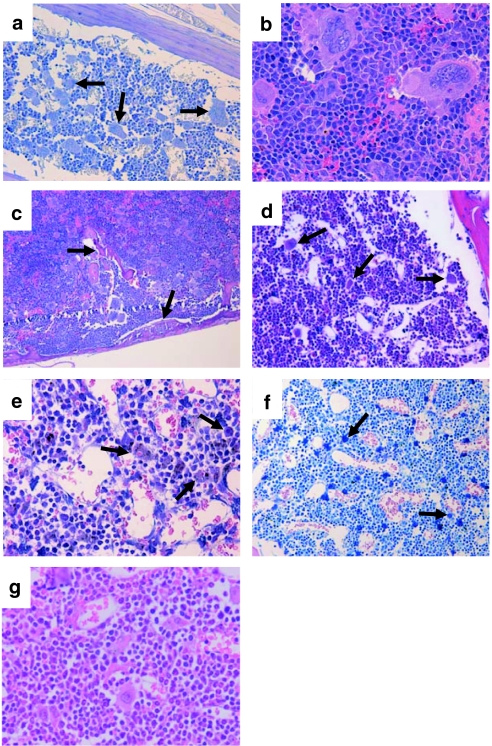
Histopathology of mice at 4 months after BMT (rise of hematopoiesis) uncovered morphologic features of a chronic MPD: (i) The erythropoiesis was significantly increased in the spleen (weights from 150 to 454 mg, mean 291 ± 116 mg, Supplementary Figure S3d) and BM (Figure 2a–c). (ii) The number of mature megakaryocytes was elevated within the BM and spleen with numerous atypical giant megakaryocytes (Figure 2a,b, arrows). One animal presented atypical neoformation of trabecular bone surrounded by myeloproliferative megakaryopoiesis in the spleen resembling osteosclerosis in human chronic idiopathic myelofibrosis (Figure 2c, arrows).
Figure 2.
Histopathology and immunophenotyping of transplanted animals. (a–c) Histopathology of mice transplanted with SF91.Mpl.pre-transduced bone marrow (BM) cells 20 weeks post-transplantation in high proliferation state [chronic myeloproliferative disease (CMPD)–like disorder]. Increase of atypical giant megakaryocytes in the (a, arrows) BM, and (b) spleen. (c, arrows) Atypical neoformation of bone in the spleen. (d–f) BM samples from the sternum of SF91.Mpl.pre-transplanted mice in pancytopenic state [myelodysplastic-like syndrome (MDS)–like disorder]. Dysmegakaryopoiesis with reduction of mature megakaryocytes and relatively increased number of (d, arrows) atypical micro–megakaryocytes, (e, arrows) histiocytes with erythrocytophagocytosis, and (f, arrows) atypical micro–megakaryocytes, atypical mast cell proliferation. In (g) healthy mice and in (d–f) those with an MDS-like disorder, the majority of hematopoietic cells in spleen (data not shown) and (d–g) BM were part of the granulocytic/monocytic lineage (Gr1+, CD11b+). In mice with a CMPD-like disorder, ≥50% of hematopoietic precursors were part of the erythropoietic lineage (Ter119/CD71+; a–c and Supplementary Figure S3). Magnification and staining: (a) ×200, periodic acid-Schiff; (b) ×400, hematoxylin–eosin (HE); (c) ×100, HE; (d) ×200, HE; (e) ×400, HE; (f) ×200, Giemsa, (g) ×400, HE.
Furthermore, we analyzed histology samples of mice from experiment 2 which were observed long term and of all the pancytopenic mice from experiment 1 where tissues were available (n = 16). In summary, the morphological alterations were classified as a myelodysplastic syndrome (MDS)–like disorder (Figure 2d–f and Supplementary Figure S3, detailed histopathological description in the supplements). As human MDS usually arises from a single neoplastic clone, we analyzed the BM of pancytopenic Mpl-expressing mice for their clonality by ligation mediated-PCR. The situation was poly- to oligoclonal and similar to the eGFP expressing control group (Supplementary Figure S4) arguing against a major influence of insertional mutagenesis in the development of the MDS-like disorder.
High-level and ectopic Mpl expression disturbs Thpo/Mpl homeostasis
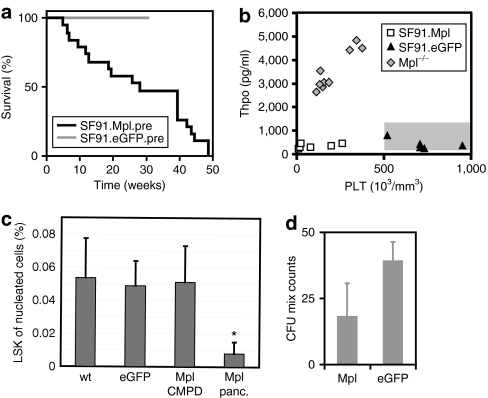
The onset of pancytopenia was variable but all mice eventually succumbed due to severe symptoms (Figure 3a). Mpl transgene expression in pancytopenic mice was still moderate to high compared to the eGFP expression at time of final evaluation (experiment 1, Supplementary Figure S5).
Figure 3.
Molecular analysis of chronic myeloproliferative disease (CMPD) and myelodysplastic-like syndrome (MDS) samples. (a) Kaplan–Meyer survival chart of mice transplanted with Mpl-transduced bone marrow (BM) cells (black, n = 22, experiments 1 and 2) compared to an enhanced green fluorescent protein (eGFP) control group (light grey, n = 7, experiment 3). The mice that developed CMPD were killed 20 weeks post-transplantation before pancytopenia and are not included in the survival chart (n = 5, experiment 2). (b) Thrombopoietin (Thpo) concentration in the plasma of peripheral blood (PB) from mice of experiment 1 where plasma samples have been collected. Mice were transplanted with SF91.Mpl.pre-transduced BM cells and were killed at pancytopenic state for final analysis (n = 6). Mpl expressing group is compared to a control group transplanted with SF91.eGFP.pre-transduced BM cells (n = 5) and untransduced Mpl−/− mice with high plasma Thpo levels (n = 9). Normal Thpo levels in untreated healthy C57Bl6/J mice are indicated as shaded box (150–1,300 pg/ml). (c) Measurement of the LSK cell fraction from nucleated total BM in CMPD animals (n = 4) and pancytopenic animals (n = 3, *P ≤ 0.03) at final analysis that received SF91.Mpl.pre-transduced BM cells compared to an eGFP control group (n = 7) or healthy C57Bl6/J mice (n = 10). (d) Colony assay of BM cells from animals in CMPD state. Colony-forming unit-mix colonies were counted in methylcellulose assays (duplicates of four individual mice n = 4, 2.5 × 105 total BM cells were plated into each assay, P ≤ 0.03).
The retroviral expression leads to high Mpl levels on almost all leukocytes and red cells in addition to megakaryocytes and platelets. Binding of Thpo to Mpl results in internalization and destruction of the ligand/receptor complex. Physiological Thpo levels are regulated by Mpl expression on platelets and megakaryocytes.1,22,23 Reduced numbers of platelets result in increased Thpo levels. As a consequence, Mpl knockout (Mpl−/−) mice, and CAMT patients, have low platelet counts with elevated Thpo levels throughout life. However, Mpl-expressing mice with low platelet counts had Thpo levels similar to eGFP-expressing mice with regular platelet counts or normal untreated C57Bl6/J mice (150–1,300 pg/ml in the plasma) (Figure 3b). This observation demonstrates that ectopic expression of Mpl indeed disturbed the Thpo/Mpl balance.
Because Thpo/Mpl imbalance was recognized in our experiments and the lack of Mpl signaling in HSCs may trigger proliferative exhaustion,5,6 we asked whether the Lin−, Sca1+, ckit+ cell (LSK) fraction in the BM of pancytopenic mice was altered. Flow cytometry data showed a significant reduction of the lineage negative LSK cell fraction of the nucleated BM in pancytopenic animals (0.01 ± 0.01% of the nucleated BM, *P ≤ 0.04) compared to mice that received eGFP-transduced cells (0.05 ± 0.015%) or healthy nontransplanted C57Bl6/J mice (0.05 ± 0.02, Figure 3c). Four months after BMT, when hematopoiesis was still high in the recipients, no significant reduction of LSK cells in the BM (0.05 ± 0.02%) was observed but complete BM cells already had a lower clonogenic potential in methylcellulose assays than cells from eGFP-expressing mice (four individual mice tested each, Figure 3d, P ≤ 0.03). Ectopic Mpl expression thus influences hematopoiesis by cell-intrinsic mechanisms and also results in an imbalance of the ligand/receptor ratio. This eventually led to progressive pancytopenia that even involved co-transplanted eGFP-transduced and untransduced competitor cells.
Leukemias developing from Mpl-expressing cells required insertional mutagenesis
Due to the known function of MPL in human MPD the development of leukemias in Mpl-overexpressing mice was anticipated. However, only 3 of 27 mice transplanted with SF91.Mpl.pre-transduced cells developed leukemia (latency 2, 3.5, and 7.5 months). Moribund mice presented with massively enlarged spleens (680, 720, and 980 mg), cherry red livers, and leukocytosis (blood counts 18.8, 36.3, and 60.2 × 103/µl). Nucleated erythroid precursors were found in the PB, compatible with erythroid leukemias (Figure 4d, arrows). The leukemic cells were CD71+ and TER119+ (Figure 4a). In the spleens, all three animals showed diffuse infiltrations by blasts, which amounted for 80–90% of cells (Figure 4b–d). The BM was infiltrated by a significantly lower amount of blasts (>20% in two mice, <20% with marked increase of mature giant megakaryocytes, and signs of a chronic MPD in the third) (Figure 4c, arrows). Southern blot and ligation mediated-PCR showed the same band pattern in two different tissues (spleen and BM in mice #2 and #3, spleen and PB in mouse #1), indicating that each mouse developed one leukemic clone containing several retroviral insertions (Figure 4e, Supplementary Table S1). In leukemias #1 and #3, one of the insertions was located in the proto-oncogenes Sfpi1 and Fli1, respectively. Quantitative PCR revealed an upregulation of the mRNA for Sfpi1 (1.7-fold versus mouse #3; 2.5-fold versus mouse #2). Fli1 was also increased (4.9-fold versus mouse #2; 1.8-fold versus mouse #1). Sequencing of the Mpl cDNA gave no hints for activating mutations.
Figure 4.
Phenotypic and molecular analysis of erythroid leukemias. (a) Three (#1, 2, 3) out of 27 animals transplanted with SF91.Mpl.pre-transduced BM cells developed erythroid leukemias. Splenocytes analyzed by flow cytometry were negative for CD11b, Gr1, CD3, and CD19 but highly expressed CD71, TER119, and Mpl transgene (experiment 2, case #2). (b–d) Histopathology of leukemias. Diffuse infiltration of (b) blasts and (c, arrows) atypical megakaryocytes in the spleen, and (d, arrow) infiltration of the liver by erythroid precursors and detection of nucleated erythroid precursors within the lumen of the blood vessels. Magnification and staining: (b,c) ×400, hematoxylin–eosin (HE); (d) ×200, HE. (e) Ligation mediated (LM)-PCR and Southern blot analysis showed monoclonality of leukemias. The number of insertion sites ranged from 4 to 6. Insertion sites are listed in Supplementary Table S1. Arrow in LM-PCR indicates internal control bands.
Mpl expression by improved γ-retroviral vectors containing cellular promoters reduce adverse reactions
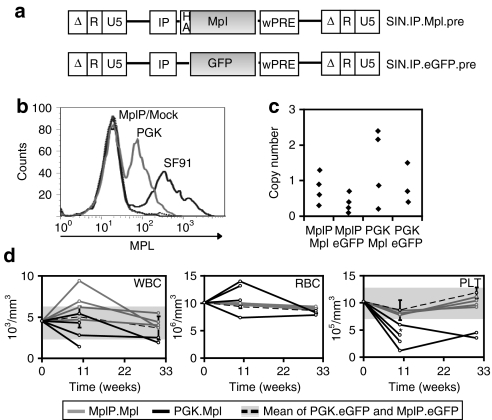
The above experiments showed that ectopic Mpl expression causes severe adverse reactions (like chronic MPD and MDS) due to the high expression on unnatural target cells. Insertional genotoxicity may exacerbate the chronic MPD to erythroid leukemia. Gene therapy for CAMT therefore requires the development of safer vectors. We constructed self-inactivating (SIN) γ-retroviral vectors expressing Mpl from the PGK promoter (SIN.PGK.Mpl.pre) or an endogenous Mpl promoter fragment (SIN.MplP.Mpl.pre) (Figure 5a). The MplP fragment spanned a 2-kb region upstream of the Mpl's ATG as described by Ziegler et al.21 The MplP vectors should restrict Mpl expression to megakaryopoiesis and the early HSC compartment.21 As compared to LTR-driven vectors (SF91), the PGK promoter mediated greatly decreased transgene expression levels in Lin− BM cells (Figure 5b, PGK: mean fluorescence intensity: 144 relative units, SF91: mean fluorescence intensity 520 relative units). Probably due to the lineage specificity of MplP21 only very few Lin− BM cells transduced by SIN.MplP showed Mpl expression detectable by flow cytometry despite equal multiplicity of infection (SF91.Mpl.pre: 47%, SIN.PGK.Mpl.pre: 43%, and SIN.MplP.Mpl.pre: 4%). After BMT, in vivo the SFFV and PGK promoter mediated robust expression in every hematopoietic lineage analyzed. The MplP, however, conferred only very low expression levels in mature hematopoietic cell lineages. Expression was more prominent in platelets, and also in the LSK cells, which are the sites of endogenous Mpl signaling (Supplementary Figure S6a,b).
Figure 5.
Controlled transgene expression level reduces side effects. (a) Self- inactivating (SIN) vectors harboring different internal promoters (IP: SFFV, PGK, or MplP) and expressing the transgenes Mpl or eGFP. (b) Transgene expression levels detected by flow cytometry in Lin− bone marrow (BM) cells before transplantation, comparing the two SIN vectors to SF91.Mpl.pre. (c) Vector copy number in the peripheral blood (PB) of the animals 6 weeks post-transplantation. (d) Diagram of the development of PB parameters over an observation time of 30 weeks. White blood cell (WBC), red blood cell (RBC), and platelet (PLT) counts are normal for animals transplanted with SIN.MplP.Mpl.pre-transduced BM cells (light grey) compared to the eGFP control groups (mean ± SD, dotted black line, with grey shaded area for SD, n = 8). Mice receiving cells expressing Mpl from the PGK promoter (black lines) show severe alterations especially in PLT counts (zero time points originate from the analysis of wild-type C57BL/6J mock mice (n = 4): WBC 4.5 ± 1.1 103/µl, RBC 10.1 ± 0.5 106/µl, PLT 10.0 ± 2.4 105/µl).
We transplanted WT BM cells transduced with SIN.PGK.Mpl.pre or SIN.MplP.Mpl.pre into WT C57BL/6J mice to analyze the new vectors for their increased safety. Efficient gene marking by LTR and SIN vectors was shown by qPCR-mediated quantification of the vector copy number in PB and BM of transplanted mice (Figure 5c). Similar to the SF91.Mpl.pre-transplanted mice, the mice that received BM cells transduced with SIN.PGK.Mpl.pre developed alterations in hematopoiesis, yet not as strongly as seen in experiments using the LTR-driven (SF91) vector: erythrocyte counts increased (n = 2, P = 0.001) or decreased in one mouse and platelet counts decreased (n = 4) to levels as low as 121 × 103 platelets/µl (~10% of normal counts, P = 0.007, Student's t-test, two sided, unpaired). Two mice died before final analysis because of severe hematopoietic insufficiency (Figure 5d). Only mice that were transplanted with BM cells that expressed Mpl under control of MplP exhibited largely unchanged PB counts. Cell counts after transplantation of SIN.MplP.Mpl.pre-transduced cells showed only moderate increase in white blood cell and no change in red blood cell and platelet counts compared to animals repopulated with eGFP+ cells (Figure 5d). Final histological analysis of mice receiving SIN.MplP.Mpl.pre-transduced BM cells after 7 months gave no hints of dysplastic features, while these were visible in mice that received BM cells transduced with SIN.PGK.Mpl.pre including an atypical mast cell proliferation (Supplementary Figure S7). Thus, Mpl expression from the MplP promoter, which directs expression into the physiological target cells, avoids potentially lethal adverse reactions of Mpl overexpression.
Mpl expression in Mpl−/− cells can correct deficient megakaryocyte differentiation in vitro and transduced Mpl−/− cells engraft long term in the BM
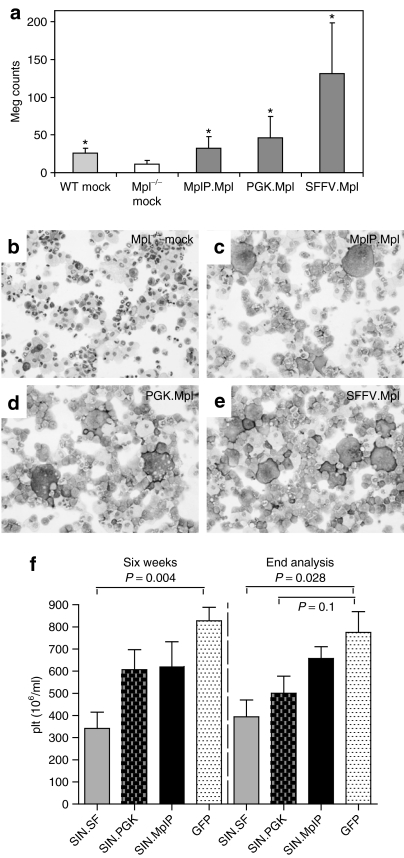
The reduction of Mpl expression levels by the use of the MplP promoter avoided toxic effects. We next wanted to test whether this vector expressing Mpl from the MplP would have potential to correct Mpl deficiency. Because defects in megakaryopoiesis as well as defects in long-term engraftment of stem cells are the prominent features in CAMT we tested for the potential to correct those phenotypes in two assays: (i) correction of megakaryocytic development in vitro and (ii) engraftment of gene corrected Mpl−/− cells in vivo in WT mice. We transduced Lin− BM cells of Mpl−/− mice with the different SIN vectors expressing Mpl by the SFFV, PGK, or MplP promoters (multiplicity of infection 3–5) and differentiated them toward megakaryocytes using the cytokines interleukin-6, stem cell factor, and Thpo in liquid culture or transplanted cells into conditioned WT CD45.1 C57BL/6J mice (Table 1). Mpl−/− cells are CD45.2+. Untransduced Mpl−/− BM cells produced less than half of the numbers of megakaryocytes that were counted in WT BM cultured (Figure 6a,b, megakaryocytes from a defined amount of cells on cytospins were counted, *P ≤ 0.04). Mpl−/− BM cells that were transduced by the SIN vectors expressing Mpl from the PGK or the MplP promoter restored megakaryocytic differentiation similar to the numbers by WT cells (Figure 6a,c,d, *P ≤ 0.04). When using the SIN vector that expressed Mpl from the SFFV promoter the megakaryocyte number increased far above physiological levels (approximately fourfold increase, Figure 6a,e, *P ≤ 0.04).
Figure 6.
In vitro megakaryocyte differentiation assay. (a) Megakaryocyte counts in 20,000 cultured cells on cytospins. Cells were cultured in liquid media for 14 days post-transduction containing thrombopoietin (Thpo), stem cell factor (SCF), and interleukin(IL)-6 (n = 5, *P ≤ 0.04). (b–e) Pictures of cytospin-containing megakaryocytes after transduction with (c) SIN.MplP.Mpl.pre, (d) SIN.PGK.Mpl.pre, (e) SIN.SF.Mpl.pre vectors, or (b) control Mpl−/− mock bone marrow (BM). Magnification and staining: (b–e) ×100, May-Grünwald/Giemsa. (f) Platelet counts in mice that received SIN.SF.Mpl.pre (n = 8), SIN.PGK.Mpl.pre (n = 7), SIN.MplP.Mpl.pre (n = 7), or SIN.SF.eGFP.pre (n = 4) transduced BM cells. The cell counts 6 weeks after BMT and at the day the mice were killed are shown. The platelet counts were significantly reduced in the mice that expressed Mpl by the SFFV promoter.
Mice transplanted with transduced Mpl−/− BM cells showed a chimerism of 2–20% in the blood. However, Mpl vector transduced Mpl−/− cells increased over time as detected by fluorescence activated cell sorting when using the SIN.SF.Mpl.pre vector, while the eGFP expression by the SIN.SF.eGFP.pre was lost (Supplementary Figure S8). In mice that received SIN.MplP.Mpl.pre, SIN.PGK.Mpl.pre, and SIN.SF.Mpl.pre-transduced Mpl−/− cells showed a higher average copy number in the donor cell fraction in the BM 5 months after BMT compared to the SIN.SF.eGFP.pre [SIN.MplP.Mpl.pre (n = 5): 0.33 ± 0.30, P = 0.1, SIN.PGK.Mpl.pre (n = 5): 0.47 ± 0.49, P = 0.06, SIN.SF.Mpl.pre (n = 4): 0.94 ± 0.71, P = 0.03, and SIN.SF.eGFP.pre (n = 4): 0.10 ± 0.10, P values versus GFP]. Transduced donor Mpl−/− cells repopulated the LSK compartment, while mice that received unmodified BM cells eventually reconstituted hematopoiesis by recipient cells (Supplementary Figure S9).
In agreement with the toxic effects observed in the WT BMT model, mice that received SIN.SF.Mpl.pre-transduced Mpl−/− BM cells showed lower PB cell counts that were significantly reduced in the platelet number [SIN.SF.Mpl.pre (n = 8): 341 ± 208 × 103/µl, versus SIN.SF.eGFP.pre (n = 4): 827 ± 122 × 103/µl, P ≤ 0.004 6 weeks after BMT and at the day of killing). Importantly, mice that received Mpl−/− BM cells transduced with the SIN.MplP.Mpl.pre vector stayed healthy without changes in their hematologic parameters (platelet counts of 621 ± 296 103/µl and Figure 6f and Supplementary Figure S10). Histopathological analysis of mice that had long-term engraftment of SIN.SF.Mpl.pre and SIN.PGK.Mpl.pre-transduced Mpl−/− BM cells showed MDS-like features similar to mice that received WT BM transduced with the LTR-driven retroviral vector, however, the phenotype was not as severe. The donor chimerism in these mice was low and therefore the alterations in hematopoiesis more subtle yet distinctly visible. Only mice receiving SIN.MplP.Mpl.pre-transduced BM cells had no pathologic alterations in hematopoiesis underscoring the necessity of controlled Mpl expression.
Discussion
The development of safe approaches for correction of monogenetic diseases by semirandomly integrating vectors requires special attention to potential adverse reactions caused by ectopic transgene expression (phenotoxicity) and insertional mutagenesis of cellular genes (genotoxicity). Although great efforts have recently been invested to develop vectors with reduced genotoxicity,24,25,26,27 the issue of phenotoxicity is less carefully investigated. We reasoned that CAMT, a lethal aplastic anemia caused by deficiency of the Mpl gene, may serve as a paradigm to address whether improved vector design may prevent both phenotoxicity and genotoxicity.
In this study, we demonstrate two potentially lethal adverse reactions caused by ectopic Mpl expression: (i) exhaustion of hematopoiesis with profound loss of primitive cell populations in the BM, skewed differentiation and peripheral pancytopenia, and (ii) induction of a MPD with potential progression to erythroleukemia. Although the latter were clonal events associated with insertional upregulation of proto-oncogenes, the former appeared oligoclonal and even involved co-transplanted cells that were untransduced or transduced with neutral marking vectors. Accordingly, the hematopoietic organs showed histopathological features of an MDS-like disorder with dysmegakaryopoiesis, incomplete maturation of erythro- and granulopoiesis, and increased mast cell production. Such profound systemic effects induced by the transplantation of gene-modified hematopoietic cells overexpressing Mpl likely reflect the disturbance of a major homeostatic function of the Mpl/Thpo balance. Indeed, endogenous Mpl is tightly controlled and expressed only during megakaryocytic differentiation and in HSC.1,6 Uncontrolled ectopic Mpl expression may result in abundant binding of Thpo with subsequent uptake and destruction.22,23 This hypothesis is supported by our finding that reduced platelet counts observed in the pancytopenic phase did not trigger increased Thpo levels. In a study by Yan et al.,19 similar to our work, BMT experiments were performed using BM cells that were transduced with a retroviral Mpl vector. All mice that overexpressed Mpl in the BM developed thrombocytopenia and dysplastic megakaryocytes were found in the BM. However, no effects on HSC numbers were noticed nor a decrease in the other PB cell lineages. Yan et al. postulated a dominant negative effect induced by ectopic Mpl overexpression and indeed, injection of Thpo increased platelet counts. In addition to dysmyelopoiesis and loss of LSK cells half of the mice that overexpressed Mpl in our experiments had increased numbers of mast cells in the BM. Mast cell differentiation is controlled by an interplay of c-Kit and Mpl signaling and Mpl−/− mice present with reduced numbers of mast cell progenitors but an increase of mature mast cells in the BM.28,29 Thus all symptoms found in the pancytopenic phase argue for a severe systemic crisis of hematopoiesis induced by ectopic Mpl expression, leading to exhaustion of HSC and altered differentiation of progeny cells.
In two further studies, Mpl was overexpressed in the mouse BM. While one of the studies was hampered by the use of the human rather than murine Mpl,30 the other study utilized replication competent viruses and thus demonstrated the oncogenic potential of Mpl.18 Mice developed a profound MPD that especially affected the erythroid lineage. The contribution of retroviral insertions was not addressed. When we expressed Mpl from replication-deficient vectors with constitutive promoters (SFFV or PGK), leukemias were observed in a minority of recipients (10% of mice transplanted with the LTR-driven γ-retroviral vector), while the majority succumbed to pancytopenia. The leukemic clones contained activating retroviral integrations in known proto-oncogenes, namely Sfpi1 and Fli1. Strikingly, exactly these proto-oncogenes are frequent targets of insertional mutagenesis in erythroid leukemias induced by Friend murine leukemia virus (F-MLV) or the complex of F-MLV with the acutely transforming form of SFFV, which encodes a viral oncogene that activates the Mpl-related erythropoietin receptor.31,32 In our study conducted with replication-deficient vectors encoding Mpl under control of the SFFV LTR, the erythroid leukemias had a rather fast onset (2–7 months after transplantation in primary recipients) and are as such different to insertional leukemias that were induced by γ-retroviral vectors expressing fluorescent marker genes, which typically develop after prolonged observation in secondary recipients and present with myeloid or lymphoid phenotypes.33,34,35 In our previously reported experiments, only three cases of a total of 115 primary recipients (2.6%) of BM cells transduced with retroviral gene marking vectors and no pretransplant expansion had developed insertional leukemias. These observations suggest that the induction of leukemia by retroviral vectors expressing Mpl reflects a combination of phenotoxic and genotoxic effects.
We thus show that gene therapy of Mpl deficiency has to overcome potentially lethal phenotoxic and genotoxic effects, and that the phenotoxic effects even involve systemic mechanisms that act on bystander cells that have not been transduced by Mpl vectors. With the intention to obtain more physiological expression, we constructed vectors expressing Mpl from the internal cellular promoters PGK or a fragment of the endogenous murine Mpl gene (MplP). Although MplP vectors did not alter hematopoiesis, PGK vectors still induced thrombocytopenia and dysmyelopoiesis, even though not as severely as the SFFV vectors. This underscores the great importance to identify physiological promoters for transgene expression 36,37,38,39,40.
Interestingly, none of the mice transplanted with cells expressing Mpl or eGFP from the cellular promoters PGK and MplP succumbed to insertional leukemias (PGK n = 15, MplP n = 15). This is in agreement with the low transforming activity of cellullar internal promoters in the in vitro immortalization assay as well as the Cdkn2a−/− mouse tumor model.24,27 However, insertional mutagenesis was not the major cause of side effects induced by Mpl vectors and the PGK promoter was not sufficient to prevent the phenotoxic effects.
In the absence of suitable transplantation conditions for Mpl−/− mice, recently two studies reported their partial correction by a transgenic approach.41,42 Both studies expressed Mpl from the 2-kb fragment of MplP that was also used in our vectors. In one study the genome of the transgenic mice contained 38 gene copies.42 Nonetheless, the correction of Mpl deficiency was incomplete and transgenic mice developed an unexpected thrombocytosis in association with reduced megakaryocytic expression of Mpl from the MplP fragment.41 The authors postulated that low Mpl levels on platelets disturbed Thpo regulation, which in turn increased platelet production. In one of the studies, transgenic mice present with elevated Thpo levels,42 while in the other study no changes in Thpo levels were observed.41 In our experiments retroviral vectors expressing Mpl from the 2-kb MplP fragment corrected megakaryocyte formation of Mpl−/− cells in vitro. Furthermore we obtained evidence that gene corrected Mpl−/−cells engrafted in the LSK compartment of lethally irradiated WT recipient mice and that the percentage of Mpl-transduced cells within the Mpl−/− compartment increased over time. However, we cannot rule out that the promoter fragment may lack some important regulatory regions of the endogenous promoter.43 Suitable transplantation conditions for Mpl−/− mice are urgently required to address whether MplP or other promoters within a semirandomly integrating construct are able to mediate physiologic Mpl expression with full correction of the hematopoietic phenotype.
Materials and Methods
Retroviral vectors and vector production. For transgene expression we constructed LTR-driven γ-retroviral vectors containing the SFFV enhancer–promoter elements.44,45 expressing the murine Mpl receptor (SF91.Mpl.pre) or the eGFP (SF91.eGFP.pre). For the detection by flow cytometry, a hemagglutinin tag was added to Mpl at position bp 78 between the signal peptide and the extracellular domain. Further retroviral vectors were constructed based on γ-retroviral SIN vectors (kindly provided by Axel Schambach, Hannover), which have a deletion in the U3 region to remove enhancer–promoter elements46 and express Mpl or eGFP by the SFFV, PGK, or the murine endogenous Mpl promoter (SIN.SF.Mpl.pre, SIN.SF.eGFP.pre, SIN.PGK.Mpl.pre, SIN.PGK.eGFP.pre, SIN.MplP.Mpl.pre, SIN.MplP.eGFP.pre). The 2-kb Mpl promoter fragment was kindly provided by Radek Skoda, Basel.21 Cell-free vector supernatants were generated by transient transfection of Phoenix gp packaging cells by using a packaging construct coding for the ecotropic envelope protein, as described previously.47 Virus titrations were performed on SC1 fibroblasts.
Animals. C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). Mpl knockout (Mpl−/−) mice were obtained from W. Alexander.7 All mice were bred and kept in the pathogen-free animal facilities of the Hannover Medical School, Germany. Animal experiments were approved by the local ethical committee and performed according to their guidelines.
Murine BMT model. Lin− BM cells of C57BL/6J mice were transduced as previously described34,48 (Supplementary Materials and Methods).
Western blot analysis. Protein lysates of transduced 32D cells were separated by electrophoresis as described previously49 (Supplementary Materials and Methods).
Electromobility shift assay. Electromobility shift assays were performed to detect STAT3 and STAT5 activation. Same protein lysates as for western blots were used following the protocol previously described in ref. 49. Double stranded oligonucleotides with either STAT3 or STAT5 binding sites where radioactively labeled and incubated with the protein lysates. The DNA/protein mixture was separated by electrophoresis and the gel was analyzed by autoradiography.
Southern blot and ligation mediated-PCR anaylsis. DNA of PB leukocytes, BM or spleen was purified using QIAmp Blood DNA Preparation Kit (Qiagen, Hilden, Germany) following manufacturer's instructions. Genomic DNA (10 µg) was digested with BglII as internal control and Southern blot was performed according to standard protocols. DNA of wPRE (woodchuck hepatitis virus posttranscriptional regulatory element) transgenic cells was hybridized with the 700-bp probe. For ligation mediated-PCR analysis see Supplementary Materials and Methods.
Vector copy number and mRNA expression levels. For quantification of vector copy numbers from BM or PB genomic DNA was used detecting a 94-bp wPRE specific sequence. The wPRE specific signal was normalized by the signal of a housekeeping gene, flk1. Results were quantified using the comparative CT method (primer sequences and cycling parameters see Supplementary Materials and Methods).
Thpo enzyme-linked immunosorbent assay. Plasma was collected from PB samples by centrifugation 20 minutes at ~300 g. Supernatant was collected and frozen at −20 °C. Thpo enzyme-linked immunosorbent assay on 96-well plates was performed following manufacturer's protocols (Quantikine M; R&D Systems, Wiesbaden, Germany).
Statistical analysis. As not indicated otherwise all measurements result from triplicates and show the mean of all measurements with standard deviation. Statistical analysis was performed using standard calculations like Student's t-test, two tailed with unequal variations or Wilcoxon signed rank test as a nonparametric test for two continuous variables.
SUPPLEMENTARY MATERIALFigure S1. Mpl signaling can support growth of 32D cells in vitro.Figure S2. Competitive growth of Mpl and eGFP expressing cells in vivo.Figure S3. FACS analysis of the lineage distribution of hematopoietic cells in the spleen.Figure S4. MDS-like disorder clonality.Figure S5. Transgene expresssion in mice that developed MDS-like symptoms.Figure S6. Expression profile by different retroviral vectors.Figure S7. Atypical mast cell proliferation in the BM of a mouse that received BM cell expressing Mpl by the PGK promoter.Figure S8. Analysis of chimerism and transgene expression after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.Figure S9. Analysis of chimerism and transgene expression in the LSK cell fraction after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.Figure S10. Analysis of peripheral blood cell counts in mice transplanted with retroviral SIN vector transduced Mpl−/− BM cells.Table S1. Retroviral insertion sites (RIS).Supplementary Materials and Methods.
Supplementary Material
Mpl signaling can support growth of 32D cells in vitro.
Competitive growth of Mpl and eGFP expressing cells in vivo.
FACS analysis of the lineage distribution of hematopoietic cells in the spleen.
MDS-like disorder clonality.
Transgene expresssion in mice that developed MDS-like symptoms.
Expression profile by different retroviral vectors.
Atypical mast cell proliferation in the BM of a mouse that received BM cell expressing Mpl by the PGK promoter.
Analysis of chimerism and transgene expression after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.
Analysis of chimerism and transgene expression in the LSK cell fraction after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.
Analysis of peripheral blood cell counts in mice transplanted with retroviral SIN vector transduced Mpl−/− BM cells.
Retroviral insertion sites (RIS).
Acknowledgments
We are indebted to Sabine Knoess, Cindy Elfers and Martin Hapke for their excellent technical assistance, to Martijn Brugman for statistical analysis, to Bernhard Schiedlmeier for help with flow cytometry, to Axel Schambach for providing the vector backbones and to Joerg Fruehauf from the irradiation facility (all Hannover Medical School, Hannover, Germany). Mpl promoter fragment was kindly provided by Radek Skoda, Basel. The authors have no conflicting financial interest. The research was financed by the grants of Deutsche Forschungsgemeinschaft (KFO 110, SFB 566, and Excellence Cluster REBIRTH).
REFERENCES
- Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG, Griffin JD., and , Kaushansky K. The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-Mpl. J Biol Chem. 1995;270:4979–4982. doi: 10.1074/jbc.270.10.4979. [DOI] [PubMed] [Google Scholar]
- Deutsch VR., and , Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134:453–466. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- Kaushansky K, Broudy VC, Grossmann A, Humes J, Lin N, Ren HP, et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. J Clin Invest. 1995;96:1683–1687. doi: 10.1172/JCI118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland C, Jensen C, Antonchuk J, Mansson R, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:1–14. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Roberts AW, Nicola NA, Li R., and , Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- Kimura S, Roberts AW, Metcalf D., and , Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff T, Horn PA, Valli VE, Gown AM, Wardwell S, Wood BL, et al. Pharmacologically regulated in vivo selection in a large animal. Blood. 2002;100:2026–2031. doi: 10.1182/blood-2002-03-0792. [DOI] [PubMed] [Google Scholar]
- Jin L, Siritanaratkul N, Emery DW, Richard RE, Kaushansky K, Papayannopoulou T, et al. Targeted expansion of genetically modified bone marrow cells. Proc Natl Acad Sci USA. 1998;95:8093–8097. doi: 10.1073/pnas.95.14.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Germeshausen M, Schulze H, Cherkaoui K, Lang S, Gaudig A, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139–146. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- Freedman MH., and , Estrov Z. Congenital amegakaryocytic thrombocytopenia: an intrinsic hematopoietic stem cell defect. Am J Pediatr Hematol Oncol. 1990;12:225–230. [PubMed] [Google Scholar]
- King S, Germeshausen M, Strauss G, Welte K., and , Ballmaier M. Congenital amegakaryocytic thrombocytopenia: a retrospective clinical analysis of 20 patients. Br J Haematol. 2005;131:636–644. doi: 10.1111/j.1365-2141.2005.05819.x. [DOI] [PubMed] [Google Scholar]
- Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigon I, Mornon JP, Cocault L, Mitjavila MT, Tambourin P, Gisselbrecht S, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci USA. 1992;89:5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Varlet P, Charon M., and , Tambourin P. MPLV: a retrovirus complex inducing an acute myeloproliferative leukemic disorder in adult mice. Virology. 1986;149:242–246. doi: 10.1016/0042-6822(86)90125-x. [DOI] [PubMed] [Google Scholar]
- Souyri M, Vigon I, Penciolelli JF, Heard JM, Tambourin P., and , Wendling F. A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell. 1990;63:1137–1147. doi: 10.1016/0092-8674(90)90410-g. [DOI] [PubMed] [Google Scholar]
- Cocault L, Bouscary D, Le Bousse Kerdiles C, Clay D, Picard F, Gisselbrecht S, et al. Ectopic expression of murine TPO receptor (c-mpl) in mice is pathogenic and induces erythroblastic proliferation. Blood. 1996;88:1656–1665. [PubMed] [Google Scholar]
- Yan XQ, Lacey DL, Saris C, Mu S, Hill D, Hawley RG, et al. Ectopic overexpression of c-mpl by retroviral-mediated gene transfer suppressed megakaryopoiesis but enhanced erythropoiesis in mice. Exp Hematol. 1999;27:1409–1417. doi: 10.1016/s0301-472x(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Abkowitz JL., and , Chen J. Studies of c-Mpl function distinguish the replication of hematopoietic stem cells from the expansion of differentiating clones. Blood. 2007;109:5186–5190. doi: 10.1182/blood-2006-08-044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S, Bürki K., and , Skoda RC. A 2-kb c-mpl promoter fragment is sufficient to direct expression to the megakaryocytic lineage and sites of embryonic hematopoiesis in transgenic mice. Blood. 2002;100:1072–1074. doi: 10.1182/blood-2002-01-0281. [DOI] [PubMed] [Google Scholar]
- Fielder PJ, Gurney AL, Stefanich E, Marian M, Moore MW, Carver-Moore K, et al. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood. 1996;87:2154–2161. [PubMed] [Google Scholar]
- Stoffel R, Wiestner A., and , Skoda RC. Thrombopoietin in thrombocytopenic mice: evidence against regulation at the mRNA level and for a direct regulatory role of platelets. Blood. 1996;87:567–573. [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Xiong D, Stamatoyannopoulos G., and , Emery DW. Genomic and functional assays demonstrate reduced gammaretroviral vector genotoxicity associated with use of the cHS4 chromatin insulator. Mol Ther. 2009;17:716–724. doi: 10.1038/mt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove PW, Kepes S, Hanawa H, Obenauer JC, Pei D, Cheng C, et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in β-thalassemic hematopoietic cells. Mol Ther. 2008;16:525–533. doi: 10.1038/sj.mt.6300394. [DOI] [PubMed] [Google Scholar]
- Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- Migliaccio AR, Rana RA, Vannucchi AM., and , Manzoli FA. Role of thrombopoietin in mast cell differentiation. Ann N Y Acad Sci. 2007;1106:152–174. doi: 10.1196/annals.1392.024. [DOI] [PubMed] [Google Scholar]
- Ghinassi B, Zingariello M, Martelli F, Lorenzini R, Vannucchi AM, Rana RA, et al. Increased differentiation of dermal mast cells in mice lacking the Mpl gene. Stem Cells Dev. 2009;18:1081–1092. doi: 10.1089/scd.2008.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves F, Lacout C, Villeval JL, Wendling F, Vainchenker W., and , Dumenil D. Thrombopoietin does not induce lineage-restricted commitment of Mpl-R expressing pluripotent progenitors but permits their complete erythroid and megakaryocytic differentiation. Blood. 1997;89:3544–3553. [PubMed] [Google Scholar]
- Ben-David Y, Giddens EB., and , Bernstein A. Identification and mapping of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci USA. 1990;87:1332–1336. doi: 10.1073/pnas.87.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Schuetze S, Kozak SL, Kozak CA., and , Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991;65:464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Düllmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- Modlich U, Kustikova OS, Schmidt M, Rudolph C, Meyer J, Li Z, et al. Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood. 2005;105:4235–4246. doi: 10.1182/blood-2004-11-4535. [DOI] [PubMed] [Google Scholar]
- Modlich U, Schambach A, Brugman MH, Wicke DC, Knoess S, Li Z, et al. Leukemia induction after a single retroviral vector insertion in Evi1 or Prdm16. Leukemia. 2008;22:1519–1528. doi: 10.1038/leu.2008.118. [DOI] [PubMed] [Google Scholar]
- Charrier S, Dupré L, Scaramuzza S, Jeanson-Leh L, Blundell MP, Danos O, et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther. 2007;14:415–428. doi: 10.1038/sj.gt.3302863. [DOI] [PubMed] [Google Scholar]
- Moreau-Gaudry F, Xia P, Jiang G, Perelman NP, Bauer G, Ellis J, et al. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood. 2001;98:2664–2672. doi: 10.1182/blood.v98.9.2664. [DOI] [PubMed] [Google Scholar]
- Chang AH., and , Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the ltr, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Hargrove PW, Kepes S, Srivastava DK, Nienhuis AW., and , Persons DA. Extended β-globin locus control region elements promote consistent therapeutic expression of a γ-globin lentiviral vector in murine β-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- Marangoni F, Bosticardo M, Charrier S, Draghici E, Locci M, Scaramuzza S, et al. Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models. Mol Ther. 2009;17:1073–1082. doi: 10.1038/mt.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt R, Coers J, Ziegler S, Wiestner A, Hao-Shen H, Bornmann C, et al. Pronounced thrombocytosis in transgenic mice expressing reduced levels of Mpl in platelets and terminally differentiated megakaryocytes. Blood. 2009;113:1768–1777. doi: 10.1182/blood-2008-03-146084. [DOI] [PubMed] [Google Scholar]
- Lannutti BJ, Epp A, Roy J, Chen J., and , Josephson NC. Incomplete restoration of Mpl expression in the mpl−/− mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis. Blood. 2009;113:1778–1785. doi: 10.1182/blood-2007-11-124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu SN. Mpl and thrombocytosis: levels matter. Blood. 2009;113:1617–1618. doi: 10.1182/blood-2008-11-186981. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Abel KL, Ostertag W., and , Baum C. Design of 5′ untranslated sequences in retroviral vectors developed for medical use. J Virol. 1999;73:4083–4089. doi: 10.1128/jvi.73.5.4083-4089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambach A, Wodrich H, Hildinger M, Bohne J, Kräusslich HG., and , Baum C. Context dependence of different modules for posttranscriptional enhancement of gene expression from retroviral vectors. Mol Ther. 2000;2:435–445. doi: 10.1006/mthe.2000.0191. [DOI] [PubMed] [Google Scholar]
- Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA, et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol Ther. 2006;13:391–400. doi: 10.1016/j.ymthe.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Schambach A, Mueller D, Galla M, Verstegen MM, Wagemaker G, Loew R, et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther. 2006;13:1524–1533. doi: 10.1038/sj.gt.3302807. [DOI] [PubMed] [Google Scholar]
- Li Z, Schwieger M, Lange C, Kraunus J, Sun H, van den Akker E, et al. Predictable and efficient retroviral gene transfer into murine bone marrow repopulating cells using a defined vector dose. Exp Hematol. 2003;31:1206–1214. doi: 10.1016/j.exphem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer J, Jucker M, Ostertag W., and , Stocking C. Carboxyl-truncated STAT5β is generated by a nucleus-associated serine protease in early hematopoietic progenitors. Blood. 1998;91:1901–1908. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mpl signaling can support growth of 32D cells in vitro.
Competitive growth of Mpl and eGFP expressing cells in vivo.
FACS analysis of the lineage distribution of hematopoietic cells in the spleen.
MDS-like disorder clonality.
Transgene expresssion in mice that developed MDS-like symptoms.
Expression profile by different retroviral vectors.
Atypical mast cell proliferation in the BM of a mouse that received BM cell expressing Mpl by the PGK promoter.
Analysis of chimerism and transgene expression after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.
Analysis of chimerism and transgene expression in the LSK cell fraction after transplantation of Mpl−/− BM cells in WT CD45.1 C57Bl/6 mice.
Analysis of peripheral blood cell counts in mice transplanted with retroviral SIN vector transduced Mpl−/− BM cells.
Retroviral insertion sites (RIS).