Abstract
A phase I clinical trial was conducted to determine the clinical safety of Telomelysin, a human telomerase reverse transcriptase (hTERT) promoter driven modified oncolytic adenovirus, in patients with advanced solid tumors. A single intratumoral injection (IT) of Telomelysin was administered to three cohorts of patients (1 × 1010, 1 × 1011, 1 × 1012 viral particles). Safety, response and pharmacodynamics were evaluated. Sixteen patients with a variety of solid tumors were enrolled. IT of Telomelysin was well tolerated at all dose levels. Common grade 1 and 2 toxicities included injection site reactions (pain, induration) and systemic reactions (fever, chills). hTERT expression was demonstrated at biopsy in 9 of 12 patients. Viral DNA was transiently detected in plasma in 13 of 16 patients. Viral DNA was detectable in four patients in plasma or sputum at day 7 and 14 post-treatment despite below detectable levels at 24 h, suggesting viral replication. One patient had a partial response of the injected malignant lesion. Seven patients fulfilled Response Evaluation Criteria in Solid Tumors (RECIST) definition for stable disease at day 56 after treatment. Telomelysin was well tolerated. Evidence of antitumor activity was suggested.
Introduction
Conditionally replicative oncolytic viruses are engineered to replicate selectively in cancer cells with specified oncogenic phenotypes. Multiple viral backbones have been employed, although the most commonly utilized is derived from the adenovirus serotype 5.
Two different approaches have been used to limit adenoviral replication to cancer cells. One approach is to delete components of viral genes (E1A, E1B) that function in part to neutralize normal cell defense (p53, Rb) mechanisms. Loss of function of the cell defense genes in cancer cells renders the virus cytotoxic to tumor cells but incapable of replication in normal cells, as exemplified by ONYX-015 or Δ24.1 Alternatively, native viral promoters that govern the initiation of viral replication can be replaced with a promoter region for genes that are active and/or overexpressed in cancer cells.2,3 The resulting constructs display viral cytolytic activity that is confined to cancer cells but at a level that approaches that of wild-type adenovirus.2 Numerous studies have confirmed that administration of live, wild-type adenovirus to healthy, adult humans is safe.3
Telomelysin is a novel, replication-competent adenovirus serotype 5-based adenoviral construct that incorporates a human telomerase reverse transcriptase gene (hTERT) promoter. hTERT encodes for the catalytic protein subunit of telomerase, a polymerase that acts to stabilize telomere lengths and is highly expressed in tumors but not in normal, differentiated adult cells.4,5
Additional modifications of Telomelysin include the replacement of the normal transcriptional element of viral E1B gene by an IRES (Internal Ribosomal Entry Site) sequence to minimize “leakiness” further enhancing specificity. Furthermore, Telomelysin is the first replication-competent adenovirus that retains a fully functional viral E3 region.6
In vitro studies have validated the selective infectivity and direct cytolysis of Telomelysin in cancer cells but not nonmalignant cells.5 In animal experiments, intratumoral injection (IT) of Telomelysin demonstrated antitumor activity without significant toxicity to normal organs. Additionally, distant viral uptake was observed following IT evidenced by the presence of adenoviral protein identified in noninjected tumor following intratumoral treatment of the contralateral tumor.5
These encouraging preclinical findings of safety and directed antitumor activity form the basis of our phase I study, which is designed to validate safety, response and pharmacodynamics of Telomelysin in advanced cancer patients.
Results
Patient profile
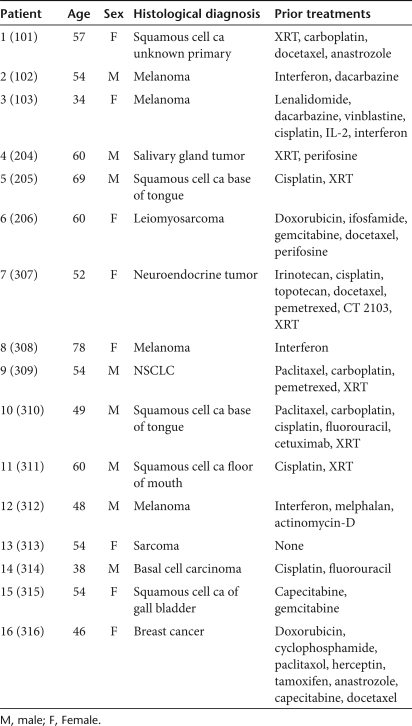
Sixteen patients were entered into trial: three each into cohorts 1 and 2 and 10 into cohort 3. The age, sex, histological diagnosis, and prior treatments of the evaluated patients are shown in Table 1.
Table 1.
Patient demographics
Adverse events
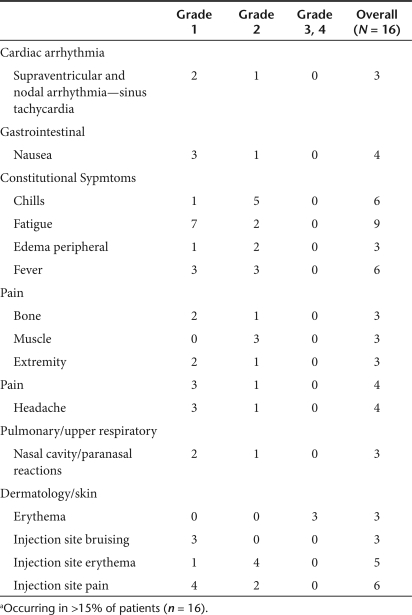
No clinically significant grade 3 or 4 treatment related toxic events were experienced by any patients. There were multiple grade 1 and 2 adverse events, with the most common being fever, chills, fatigue, and injection site pain (Table 2). Thirteen patients developed asymptomatic transient lymphocyte decreases, seven grade 2, five grade 3 and one grade 4, 24 hours after Telomelysin injection with complete recovery by day 7 following injection.
Table 2.
List of commona adverse events
Clinical response
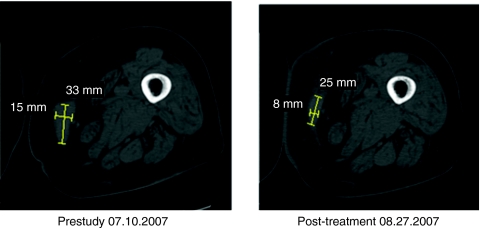
Eleven patients satisfied Response Evaluation Criteria in Solid Tumors (RECIST) criteria for stable disease response to the injected lesion at Day 28, three had progressive disease and two more unevaluable. Seven of the day 28 stable disease patients had stable disease at day 56, two had progressive disease and two were unevaluable. One patient (pt 308) had 33% reduction of injected lesion at day 28 and 56.7% reduction of injected lesion at day 56 (see Figure 1).
Figure 1.
Patient 308: Initial response of the largest of three metastatic melanoma lesions involving the right thigh.
Postinjection biopsies performed at day 28 on four of the patients with stable disease revealed necrosis that may or may not be treatment induced. Three of these patients had melanoma. Survival of all patients ranged from 1 to 21 months (median 10).
Viral pharmacokinetics analysis
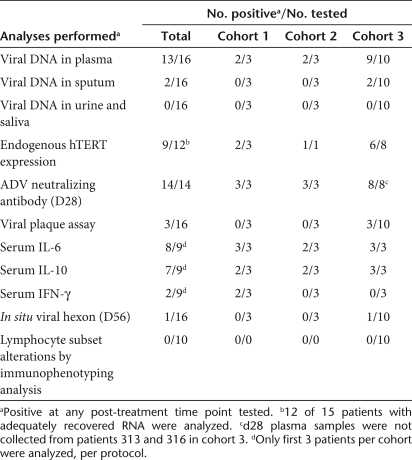
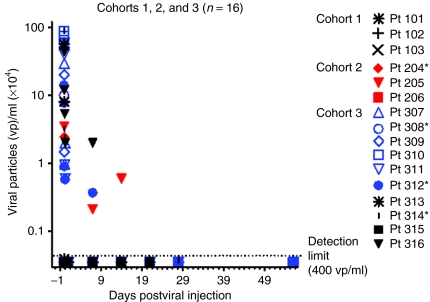
Systemic dissemination of Telomelysin was evaluated by collection of patient plasma, urine, sputum, and saliva at time points before and after IT. Quantitative real-time PCR analysis was carried out with primers that were specific for the Telomelysin E1A and IRES regions. We detected the presence of viral DNA in 13 of 16 patient plasma samples tested, including 9 of 10 patients in cohort 3 (Table 3). Plasma viral DNA was detected between 30 minutes and 6 hours in most patients, at concentrations that ranged from 2.1 × 103 to 1.5 × 107 viral copies/ml. We detected the presence of plasma viral particles in two cohort 3 patients. Viral DNA copies detected on day 7 (pt 312: 3.7 × 103; pt 316: 2 × 104 viral copies/ml, respectively) were ~10–50-fold higher than detection threshold (400 vp/ml). Viral DNA was also detected in one cohort 2 patient on days 7 and 14 [pt 205: 3.7 × 103 (day 7), 6.0 × 103 (day 14)] but not at (Figure 2). No viral DNA was detected at 24 hours post-treatment for these patients, suggesting that detectable levels of viral DNA at days 7 and 14 may constitute a second wave of viremia from replication. Viral DNA was detected in two cohort 3 sputum specimens on day 1 (pt 310: 8.2 × 104 viral copies/ml) and day 7 (pt 307, 5 × 103 viral copies/ml) but not at earlier time points post-treatment. Viral DNA was not detected in any other body fluid compartments examined. The systemic detection of viral DNA at these extended time points is suggestive of viral replicative activity.
Table 3.
Pharmacokinetics and immune response assessments
Figure 2.
Detection of Telomelysin viral DNA in patient plasma samples on various days post-treatment. Data represented at day 1 constituted peak values determined at up to 6 hours post-treatment. All patients exhibited below detection levels of plasma viral particles (≤400 vp/ml) at day 1 post-treatment.
Viral E1A and hexon expression in treated tumors
Immunohistochemical evaluation of adenoviral hexon protein expression in treated tumor biopsies was carried out as a surrogate indicator of viral replicative activity at days 28 and 56 postinjection. Viral hexon protein expression was not detected in Telomelysin treated tumor biopsies collected at days 28 and 56 from 15 of 16 patients (Table 3), whereas one patient displayed an equivocal reaction at day 56 but not day 28. Viral E1A expression was uniformly negative from all 16 patients. The negative findings indicate that viral replicative activity did not extend to these time points, despite suggestion of viral dissemination for up to day 7–14 after the single viral injection.
Neutralizing antibody response
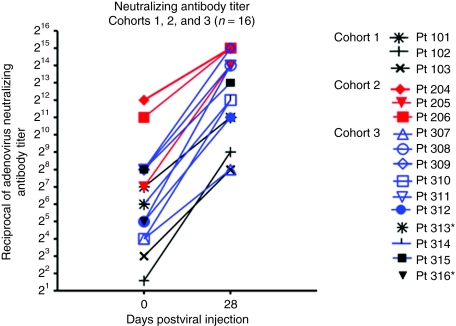
To identify systemic immune-activating events from intratumoral Telomelysin treatment, a functional assay with Telomelysin-infected HEK 293 cells was used to determine the neutralizing antibody (NAb) titer of patients entered into trial. Blocking activity of graded concentrations of the patient's pre- and post-treatment plasma was determined by light microscopy. An elevated NAb titer was observed in 14 of 14 plasma samples collected at day 28 (Table 3). Two patients (pt 313 and pt 316) did not have samples collected. The increase in titer ranged from 8- to 512-fold (Figure 3). However, the magnitude of titer increase did not correlate either with dose or with the presence or absence of a pre-existing NAb titer (Figure 3).
Figure 3.
Neutralizing antibody titer. Change in neutralizing antibody titer on day 28 after injection compared to baseline. *Patients 313 and 316 did not have day 28 plasma samples to determine post-treatment neutralizing antibody titer.
Serum cytokines
Non specific systemic immune activation from intratumoral Telomelysin treatment was observed as evidenced by an elevated increase in serum cytokine levels, in particular, interleukin-6 (IL-6) and IL-10 in all cohorts (Table 3). An elevated IL-6 level (>50%) was observed in 8 of 9 patients tested, as early as 30 minutes after treatment. Increased IL-10 level was also observed in 7 of 9 patients, whereas two patients had elevated interferon-γ.
Peripheral blood lymphocyte immunophenotyping
There were no demonstrable trends of altered post-treatment changes in the frequency distribution of CD4+ T, CD8+ T, B, and NK cells that correlated with viral treatment (Table 3) in 10 tested patients.
hTERT mRNA
To validate viral replication permissiveness of injected tumor specimens, real time, quantitative real-time-PCR assays were carried out retrospectively using tumor biopsy specimens collected before treatment, using total RNA from frozen patient tumor biopsy and primers and a TaqMan probe specific to hTERT or the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Tumor hTERT expression was carried out in 12 of 15 tumor biopsies that yielded adequate RNA. Tumor biopsy was not available from pt 316. Endogenous hTERT expression was detected in 9 of the 12 tumor biopsy specimens (Table 3). These included two with high hTERT (>104 copies/µg) mRNA expression (one in cohort 2 and one in cohort 3), four with moderate expression (103–104 copies/µg) (one in cohort 1, three in cohort 3), and three with low expression (≤103 copies/µg) (one in cohort 1, two in cohort 3). hTERT was below detection limit in three other patients tested (one in cohort 1, two in cohort 3). Of the three patients with prolonged, detectable plasma viral DNA at day 7 post-treatment, pt 205 displayed a high level of endogenous hTERT mRNA, whereas tumor samples were either unavailable (pt 316) or inadequate (pt 312) for assessment. These limited findings confirm hTERT expression in the majority of human tumors.
Discussion
Telomelysin administration in this Phase I safety trial demonstrated safety with no treatment related grade 3/4 adverse effects. Further, we observed the encouraging findings of one patient with partial response at day 56 after a single IT. The transient presence of systemic Telomelysin dissemination following IT was documented early after IT injection. Immune activation was observed, with cytokine upregulation of IL-6 and IL-10 and the induction of viral neutralizing antibodies. Limited suggestive evidence of vial replication was observed at day 7 post-treatment in three patients, for whom plasma viremia was not detected on day 1. One of these three patients had elevated malignant tissue hTERT expression with a significant clinical response. However, these limited findings require additional confirmation as we cannot completely exclude the unlikely possibility of delayed viral clearance. Immunohistochemical analysis of viral E1A and hexon was negative 28 days after injection suggesting rapid clearance. In Galanis' Phase II osteosarcoma trial with ONYX-015, 5 of 6 patients had detectable viral DNA on Day 5 of the first cycle.7 In Makower's hepatobiliary tumor trial with ONYX-015, no viral DNA was detected in plasma following intralesional injection.8 In our previous work with ONYX-015 we showed that 41% of patients had detectable viral DNA at days 5 and 6, and 9% had circulating DNA at day 10.
Adenoviral immunogenicity can be affected by viral structural modification (E3 region function), physical properties (temperature), other agents (enbrel, steroids), serotype status, removal of neutralizing antiviral antibodies (plasmapheresis) or presence of antibody producing cells (B-cell inhibition secondary to Ribavirin, Rituxan), use of physical shields (liposome, polymer, cellular delivery), and/or alteration of neutralizing surface epitopes (hexon, knob, fiber).9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32
Evidence of clinical efficacy has previously been demonstrated with a E1B-55 kd deleted oncolytic adenoviral therapeutic (ONYX-015); however, the opportunity to move towards systemic administration was hampered by efficacy results and the limitations imposed by rapid viral clearance and low replication capacity. These data were insufficient for advancement of phase III development with ONYX-015. Telomelysin was designed with a structure to enhance tumor selective viral gene expression (hTERT promoter) thereby allowing the opportunity to consider systemic administration in tandem with masked delivery approaches.33,34 The adenovirus early transcription unit (E3) encodes for polypeptides (14.7 k, 10.4, 14.5),35,36,37,38,39 which function to directly block tumor necrosis factor–α activation as well as apoptotic pathways shared by tumor necrosis factor–α and fas.38,40,41 The E3 gp19k protein functions to bind and retain MHC class I molecules within the endoplasmic reticulum, thus preventing surface presentation of viral antigens, thereby limiting class I-restricted CTL clearance of virally infected cells.35,36,42,43,44,45 The expression of the E3 gene region products may, therefore, decrease viral clearance, increase the expression of those viral genes that suppress immune recognition and enhance viral replication.40,46
In conclusion, both activity and safety of a single injection approach for Telomelysin has been demonstrated. However, despite activity in a subset of patients, limited clinical relevant responses were observed in others. This may be attributed to the single viral treatment administered to each patient. An increase in viral NAb titer in all patients tested is indicative of systemic immune sensitization following IT. We and others have shown previously that systemic viremia can be maintained at 3–6 days after second intravenous or intra-arterial treatments in spite of the presence of high levels of NAb titers and antiviral cytokines.47,48 Thus repeat intratumoral or intranvenous injection of Telomelysin is a viable treatment option to achieve an improved clinical response. Alternatively, artificial envelopment of Telomelysin with bilamellar cationic liposomes for “stealth” systemic delivery may be applicable for improving systemic pharmacokinetics and coxsackie and adenovirus receptor-independent tropism.33,34 With these considerations, data support further clinical assessment of a multi treatment schedule.
Materials and Methods
Test article. Telomelysin is manufactured at Introgen Therapeutics, Houston, TX. Telomelysin was reconstituted using aseptic technique in a Biocontainment Level 2 ISO Class 5 Biosafety Cabinet.
Study design. This was a dose escalation study in patients with advanced solid tumors. A single IT of virus particles (vp) was administered through a single injection site using a radial method of distribution in order to evenly distribute material to both peripheral and central sites of growing tumor without removing the needle completely from the tumor. Most of the viral dose was administered at the tumor periphery and at the interface between normal tissue and tumor; prior studies have indicated improved efficacy with this administration approach.49 Attempts were made to distribute the virus uniformly along the needle tracks by gradually depressing the syringe plunger during withdrawal of the needle. Each patient was enrolled into one of the following cohorts: Cohort 1: 1 × 1010 vp/tumor (n = 3); Cohort 2: 1 × 1011 vp/tumor (n = 3); Cohort 3: 1 × 1012 vp/tumor (n = 10). Patients in cohorts 1 and 2 remained on study for 28 days after injection. Cohort 3 patients were followed until day 56 post-treatment.
Viral DNA was monitored using quantitative PCR (Q-PCR) technique. After the first patient was enrolled into Cohort 1, each of the remaining patients (i.e., pt 2 and pt 3) was enrolled. In Cohort 1, clearance of viral DNA in all body fluid specimens including blood, saliva, sputum, and urine of the preceding patients by two consecutive negative Q-PCR results at least 3 days apart was required. Enrollment of the first patient in Cohort 2 began when viral DNA results on the last patient in Cohort 1 were negative on two consecutive tests at least 3 days apart.
If a dose-limiting toxicity was observed in one of three patients related to Telomelysin, an additional three patients were enrolled. If only one of the six total patients experienced a dose-limiting toxicity, then the dose escalation would be continued to the next cohort. If two or more of the six patients experienced a dose-limiting toxicity, the maximum tolerated dose would be defined as exceeded and an additional three patients would be treated at the dose level below. Toxicities were graded and reported according to the National Cancer Institute common terminology criteria for adverse events, version 3.0. Response was evaluated in this study using the international criteria proposed by the RECIST Committee.
Study population. Patients with superficial accessible cancer who had failed at least one prior therapeutic regimen and for whom effective conventional therapy was not available were eligible for the study. All patients were required to be at least 18 years old, have histologically confirmed carcinoma and a Karnofsky performance status of at least 70%. Inclusion was also predicated on normal laboratory assessment. All patients were required to provide written consent according to local institutional review board–approved guidelines. Women and men of reproductive potential were required to use contraception.
Baseline assessments included: concomitant medications, interval history, physical examination, performance status, tumor assessment, medical laboratory studies, adenoviral NAb, urinalysis, tumor biopsy, viral DNA in blood, saliva, sputum, and urine. Viral plaque forming titer in serum, cytokine levels (IL-6, IL-10, INF-γ). Peripheral blood immunephenotype analyses were performed for Cohort 3 patients.
Assessments were performed using samples collected as follows: plasma viral DNA: pretreatment and at 30 minutes, 1 hour, 3 hours, 6 hours and on days 1, 7, 14, 21, 28, and 56 post-treatment; viral DNA in sputum, urine, and saliva: pretreatment and days 1, 7, 14, 21, 28, and 56 postinjection; endogenous hTERT expression: assessed with pretreatment tumor biopsy; adenovirus NAb: pretreatment and day 28 post-treatment; cytokine: pretreatment and 30 minutes, 1 hour, 6 hours, and on days 1, 14, and 28 post-treatment for first three patients per cohort only; immunohistochemistry for viral hexon: tumor biopsies collected pretreatment and on days 28 and day 56 post-treatment; immunephenotyping analysis: pretreatment and days 7, 14, and 28 post-treatment. Viral plaque assay was performed only on patient plasma samples that yielded ≥1 × 105 vp/ml by Q-PCR analysis.
Detection of viral DNA. Patient samples were collected previral infusion and on day 0 (1 hour, 3 hours, 6 hours post-treatment), day 1, 7, 14, 21, 28, and 56 post-IT. DNA extraction was carried out from patient's archived, frozen tumor biopsy specimen, plasma, sputum, saliva, and urine specimens. Viral DNA was quantified by real-time Q-PCRs. Briefly, DNA was extracted with the Qiagen QIAmp DNA Mini Kit (plasma and saliva samples) or QIAamp Viral RNA Mini Kit (urine and sputum samples). Plasma, saliva, sputum, and urine samples from normal donors were used for protocol validation, with or without “spiking” with known amounts of Telomelysin immediately prior to DNA extraction. Q-PCRs were carried out on the iQ5 Q-thermal cycler (BioRad, Hercules, CA), using Telomelysin-specific primers for the E1A and IRES region and the 2 × Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The amounts of detectable viral particles were quantified by extrapolation with a standard curve, generated with serially diluted (1:10) DNA templates with predetermined copy numbers (10 to 1 × 106 copies) of pure Telomelysin viral DNA. A positive response is based on the detection of both IRES- and E1A amplification products with an assay threshold of 4 × 102 vp/ml for plasma and saliva; 1 × 103 vp/ml for urine; 2 × 103 vp/ml for sputum samples for both reactions.
Primer sequences
IRES-Forward 5′-GAT TTT CCA CCA TAT TGC CG
IRES-Reverse 5′-TTC ACG ACA TTC AAC AGA CC
E1A-Forward 5′-CCT GTG TCT AGA GAA TGC AA
E1A-Reverse 5′-ACA GCT CAA GTC CAA AGG TT.
Endogenous hTERT expression in patient tumor. To validate viral replication, real time, quantitative real-time-PCR assays were carried out with total RNA from patient tumor biopsy. Briefly, Q-PCR assays were carried out on iQ5 Q-PCR machine (BioRad), using primers and a TaqMan probe specific to hTERT or GAPDH (Sigma/Proligo, St Louis, MO), and TaqMan Core PCR reagents (Applied Biosystems). Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNAs were generated according to manufacturer's instructions (RETROscript kit; Ambion, Foster City, CA). PCR standard curves for determination of gene copy number in the reaction template were generated with triplicate reactions, using 1:10, serially diluted samples of either the hTERT or GAPDH PCR amplification products.
Primer sequences
hTERT Forward primer: 5′-GCACTGGCTGATGAGTGTGT-3′
hTERT Reverse primer: 5′-CTCGGCCCTCTTTTCTCTG-3′
hTERT TaqMan probe: 5′-(FAM) TTGCAAAGCATTGGAATCAGACAGCACT-(TAMRA)-3′
GAPDH Forward primer: 5′-GAAGGTGAAGGTCGTAGTC-3′
GAPDH Reverse primer: 5′-GAAGATGGTGATGGGATTTC-3′
GAPDH TaqMan probe: 5′-(FAM) CAAGCTTCCCGTTCTCAGCC (TAMRA)-3′.
Immunohistochemical analysis. A previously described automated immunoperoxidase staining technique was used to characterize viral protein expression.50 Briefly, viral E1A and hexon expression was determined with the avidin-biotin-complexed immunoperoxidase reaction (iVIEW DAB Detection kit; Ventana Medical Systems, Tucson, AZ) following initial incubation with antibodies specific to viral E1A (prediluted mouse monoclonal adenovirus type 5 E1A antibody, GeneTex, Irvine, CA), or hexon (goat antiadenovirus polyclonal antibody (Millipore, Billerica, MA), using the Ventana 320ES System (Ventana Medical Systems, Tucson, AZ).
Flow cytometric immunophenotype analysis. Peripheral blood immunophenotype analysis was carried by a two color immunofluorescence reaction and flow cytometric analysis as described previously.50 The frequency distribution of T, B, and NK cell subsets: CD45-FITC/CD14-PE, CD3-FITC/CD19-PE, CD4-FITC/CD8-PE, CD13-FITC [CD16 CD56]-PE (all from BD Biosciences, San Jose, CA) were determined.
Serum cytokine analysis. ELISA assays (R&D Quantikine kits, Minneapolis, MN) were used to quantify patient serum cytokine levels.50 Serial serum samples were analyzed simultaneously, using cytokine-specific immunoassay reagents. The colorimetric reaction was quantified as a function of optical density absorbance at 450 nm with the correction wavelength set at 540 nm (SpectraMax 340; Molecular Devices, Sunnyvale, CA). The minimal detectable concentration was as follows: interferon-γ: <16 pg/ml; IL-10: <8 pg/ml; IL-6: <1 pg/ml. The percent increase in cytokine level at any time point post-treatment was determined through comparison with serum harvested before Telomelysin injection. Based on inter- and intra-sample variations, increases in cytokine level of ≥50% over baseline were considered significant.
Antiadenovirus antibodies. Adenovirus-NAb titer in patient plasma samples was measured as a function of blocking human adenovirus infection of 293 cells. Briefly, twofold serially diluted patient plasma samples were added to 293 cells that were infected with Telomelysin virus. The plates were evaluated microscopically for the percentage of cells that lysed in presence of patient plasma samples at 72 and 96 hours postinfection. The adenovirus-NAb titer for a given sample was the highest dilution of the plasma that showed a blocking effect (>60% 293 cells intact and attached as monolayer).
Acknowledgments
We acknowledge Susan Mill and Brenda Marr for their competent and knowledgeable assistance in the preparation of this manuscript.
REFERENCES
- Tong AW. Oncolytic Viral Therapy for Human Cancer: Challenges Revisited (review) Drug Development Research. 2006;66:260–277. [Google Scholar]
- Taki M, Kagawa S, Nishizaki M, Mizuguchi H, Hayakawa T, Kyo S, et al. Enhanced oncolysis by a tropism-modified telomerase-specific replication-selective adenoviral agent OBP-405 (‘Telomelysin-RGD') Oncogene. 2005;24:3130–3140. doi: 10.1038/sj.onc.1208460. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL., and , Wold WS. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 2004;11:819–829. doi: 10.1038/sj.cgt.7700765. [DOI] [PubMed] [Google Scholar]
- Shay JW, Zou Y, Hiyama E., and , Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10:677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10 1 Pt 1:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Toth K, Doronin K, Tollefson AE., and , Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693–702. [PubMed] [Google Scholar]
- Pan Q, Liu B, Liu J, Cai R, Wang Y., and , Qian C. Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell Biochem. 2007;304:315–323. doi: 10.1007/s11010-007-9514-6. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–893. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- Lin E., and , Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther. 2004;11:643–664. doi: 10.1038/sj.cgt.7700733. [DOI] [PubMed] [Google Scholar]
- Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG., and , Crystal RG. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KD, Green NK, Hale A, Subr V, Ulbrich K., and , Seymour LW. Passive tumour targeting of polymer-coated adenovirus for cancer gene therapy. J Drug Target. 2007;15:546–551. doi: 10.1080/10611860701501014. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y., and , Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y., and , Tanaka N. Diagnostic and therapeutic application of telomerase-specific oncolytic adenoviral agents. Front Biosci. 2008;13:1881–1886. doi: 10.2741/2807. [DOI] [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE., and , Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- Delgado-Enciso I, Cervantes-García D, Martínez-Dávila IA, Ortiz-López R, Alemany-Bonastre R, Silva-Platas CI, et al. A potent replicative delta-24 adenoviral vector driven by the promoter of human papillomavirus 16 that is highly selective for associated neoplasms. J Gene Med. 2007;9:852–861. doi: 10.1002/jgm.1071. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, et al. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther. 2003;14:1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- Vile RG., and , Hart IR. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 1993;53:962–967. [PubMed] [Google Scholar]
- Savontaus MJ, Sauter BV, Huang TG., and , Woo SL. Transcriptional targeting of conditionally replicating adenovirus to dividing endothelial cells. Gene Ther. 2002;9:972–979. doi: 10.1038/sj.gt.3301747. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Hamada K, Zhang Z, Okada H, Tagawa M, Kamidono S, et al. A cox-2 promoter-based replication-selective adenoviral vector to target the cox-2-expressing human bladder cancer cells. Clin Cancer Res. 2004;10:4342–4348. doi: 10.1158/1078-0432.CCR-03-0267. [DOI] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M., and , Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Haisma HJ, Pinedo HM, Rijswijk A, der Meulen-Muileman I, Sosnowski BA, Ying W, et al. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 1999;6:1469–1474. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Rogers BE, Douglas JT, Sosnowski BA, Ying W, Siegal GP, et al. Targeted gene delivery to Kaposi's sarcoma cells via the fibroblast growth factor receptor. Cancer Res. 1997;57:1447–1451. [PubMed] [Google Scholar]
- Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- Grill J, Van Beusechem VW, Van Der Valk P, Dirven CM, Leonhart A, Pherai DS, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7:641–650. [PubMed] [Google Scholar]
- Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- Zhang YA, Nemunaitis J, Samuel SK, Chen P, Shen Y., and , Tong AW. Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Res. 2006;66:9736–9743. doi: 10.1158/0008-5472.CAN-06-1617. [DOI] [PubMed] [Google Scholar]
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK., and , Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67:11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- Lamfers ML, Idema S, Bosscher L, Heukelom S, Moeniralm S, van der Meulen-Muileman IH, et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin Cancer Res. 2007;13:7451–7458. doi: 10.1158/1078-0432.CCR-07-1265. [DOI] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Kawakami K, Takeshita F., and , Puri RK. Identification of distinct roles for a dileucine and a tyrosine internalization motif in the interleukin (IL)-13 binding component IL-13 receptor alpha 2 chain. J Biol Chem. 2001;276:25114–25120. doi: 10.1074/jbc.M100936200. [DOI] [PubMed] [Google Scholar]
- Yotnda P, Chen DH, Chiu W, Piedra PA, Davis A, Templeton NS, et al. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5:233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- Thompson DH. Adenovirus in a synthetic membrane wrapper: an example of hybrid vigor. ACS Nano. 2008;2:821–826. doi: 10.1021/nn800279s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Droguett G, Chowdhury NR, Li Y, Sengupta K, Thummala NR, et al. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Abina MA, Haddada H., and , Perricaudet M. The constitutive expression of the immunomodulatory gp19k protein in E1-, E3- adenoviral vectors strongly reduces the host cytotoxic T cell response against the vector. Gene Ther. 1995;2:256–262. [PubMed] [Google Scholar]
- Wold WS, Hermiston TW., and , Tollefson AE. Adenovirus proteins that subvert host defenses. Trends Microbiol. 1994;2:437–443. doi: 10.1016/0966-842x(94)90801-x. [DOI] [PubMed] [Google Scholar]
- Wold WS, Tollefson AE., and , Hermiston TW. E3 transcription unit of adenovirus. Curr Top Microbiol Immunol. 1995;199 (Pt 1):237–274. doi: 10.1007/978-3-642-79496-4_13. [DOI] [PubMed] [Google Scholar]
- Bett AJ, Haddara W, Prevec L., and , Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Fabricius EM, Schneeweiss U, Schaepe C, Benedix A, Weissbrich C, et al. Application of microbiological cancer test to cattle infected with bovine leucosis virus. Arch Exp Veterinarmed. 1990;44:205–212. [PubMed] [Google Scholar]
- Horwitz MS, Tufariello J, Grunhaus A., and , Fejer G. Model systems for studying the effects of adenovirus E3 genes on virulence in vivo. Curr Top Microbiol Immunol. 1995;199 (Pt 3):195–211. doi: 10.1007/978-3-642-79586-2_10. [DOI] [PubMed] [Google Scholar]
- Wold WS., and , Gooding LR. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991;184:1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Rawle FC, Tollefson AE, Wold WS., and , Gooding LR. Mouse anti-adenovirus cytotoxic T lymphocytes. Inhibition of lysis by E3 gp19K but not E3 14.7K. J Immunol. 1989;143:2031–2037. [PubMed] [Google Scholar]
- Feuerbach D., and , Burgert HG. Novel proteins associated with MHC class I antigens in cells expressing the adenovirus protein E3/19K. EMBO J. 1993;12:3153–3161. doi: 10.1002/j.1460-2075.1993.tb05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier DC, Cox JH, Vining DR, Cresswell P., and , Engelhard VH. Association of human class I MHC alleles with the adenovirus E3/19K protein. J Immunol. 1994;152:3862–3872. [PubMed] [Google Scholar]
- Kaplan JM, Armentano D, Sparer TE, Wynn SG, Peterson PA, Wadsworth SC, et al. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- Nemunaitis J, Cunningham C, Tong AW, Post L, Netto G, Paulson AS, et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther. 2003;10:341–352. doi: 10.1038/sj.cgt.7700585. [DOI] [PubMed] [Google Scholar]
- Heise CC, Williams A, Olesch J., and , Kirn DH. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread and distribution effects. Cancer Gene Ther. 1999;6:499–504. doi: 10.1038/sj.cgt.7700071. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]