Abstract
To enhance the strength of activation afforded by tumor antigen-specific receptors, we investigated the effect of adding combined CD28 and 4-1BB costimulatory signaling domains to a chimeric antigen receptor (CAR) specific for prostate-specific membrane antigen (PSMA). Having transferred receptors encompassing the CD28, 4-1BB, and/or CD3ζ cytoplasmic domains in primary human CD8+ T cells, we find that the P28BBz receptor, which includes all three signaling domains, is superior to receptors that only include one or two of these domains in promoting cytokine release, in vivo T-cell survival and tumor elimination following intravenous T-cell administration to tumor-bearing severe combined immunodeficient (SCID)/beige mice. Upon in vitro exposure to PSMA, the P28BBZ receptor-induced the strongest PI3Kinase/Akt activation and Bcl-XL expression, and the least apoptosis in transduced peripheral blood CD8+ T cells. These findings further support the concept of integrating optimized costimulatory properties into recombinant antigen receptors to augment the survival and function of genetically targeted T cells within the tumor microenvironment.
Introduction
Immune-mediated tumor eradication requires adequate survival and intratumoral activation of tumor antigen-specific T cells. To meet these requirements, T cells must be given appropriate activating signals at the time of antigen priming and restimulation. Suboptimal activation exposes T cells to the risks of anergy or apoptosis upon re-exposure to antigen.1,2 Such an outcome is a concern in the context of tumor responses, because tumor cells most often lack activating costimulatory ligands. Thus, the transfection of tumor cells with costimulatory ligands such as B7.1,3 4-1BBL,4 OX40L,5 and CD40L6 enhances tumor rejection. However, it is not yet clear what costimulatory signals or combinations thereof are best suited to initiate and/or sustain tumor eradication, or what T-cell activating mechanisms are redundant, antagonistic, or additive, or how to effectively provide T-cell costimulation in a safe and effective way.
T-cell activation can be initiated by human leukocyte antigen–restricted T-cell receptors or genetically engineered chimeric antigen receptors (CARs). In the context of CARs,7 we and others have shown that the addition of CD28 sequences to CD3ζ chain-based receptors increases antigen-induced secretion of interleukin-2 (IL-2) and in vitro T-cell expansion.8 The immunoglobulin superfamily member CD28 potently enhances T-cell receptor–induced proliferation and differentiation of naive T cells, especially at low T-cell receptor occupancy.9 CD28 enhances the expression of downstream regulators that impact on T-cell proliferation, death, differentiation, and effector functions, for hours or days after the initial T cell–antigen presenting cell (APC) encounter.9 These events are crucial for effector T-cell function and the establishment of long-term memory. In the absence of CD28 costimulation, T cells exposed to antigen become anergic or are eliminated by programmed cell death.10 However, CD28 only postpones activation-induced cell death, and its effect gradually diminishes upon repeated restimulation.2,9,10 Specifically in the context of CARs, receptors bearing both CD28 and CD3ζ signaling domains are more potent than their CD3ζ-based counterparts,8 augmenting the response rates induced by both murine and human targeted T cells.11,12,13,14,15,16,17
Here, we investigate whether CD28 signaling can be enhanced by incorporating in tandem the cytoplasmic domain of 4-1BB receptor (CD137), a member of the tumor necrosis factor receptor family. Cell-surface 4-1BB expression is induced upon T-cell activation and provides late-acting signals that augment cell proliferation, cell survival and the production of interferon-γ and other cytokines.18,19 Engagement of the 4-1BB receptor also inhibits activation-induced cell death in vitro20 and in vivo21,22, particularly in CD8+ T cells.23 Overall, 4-1BB enhances primary CD8+ T-cell responses and the maintenance of memory CD8+ T cells.18,19 Expression of 4-1BBL in murine sarcomas and lymphomas has been shown to enhance the potency of tumor vaccines, more so in tumors that also express B7.1,4,24,25 suggesting some complementarity between CD28 and 4-1BB-mediated costimulation. This complementarity is especially noticeable when coexpressing CD80 and 4-1BBL in primary T cells, which results in potent T-cell auto- and trans-costimulation.26 The molecular basis for the combined effects of CD28 and 4-1BB signaling has not yet been elucidated.
To provide combined CD28 and 4-1BB signals in antigen-dependent fashion, we generated combinatorial antigen receptors and investigated their effect on human primary CD8+ T-cell proliferation, cytokine secretion, T-cell survival, and tumor elimination after intravenous infusion in tumor-bearing immunodeficient mice. We demonstrate that CD28 and 4-1BB signals are functionally additive when combined within a single CAR and that the improved T-cell activation is at least in part dependent on the activation of the Akt pathway, which is independently and additively recruited by CD28 and 4-1BB. The P28BBz receptor, which encompasses both cytoplasmic domains, is thus superior to receptors incorporating either one component in promoting in vitro and in vivo T-cell survival and function.
Results
APC-encoded CD80 and 4-1BBL enhance PSMA-induced CD8+ T-cell expansion
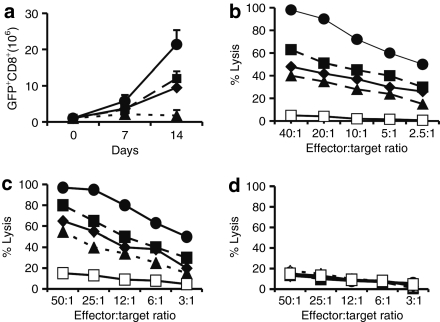
To assess whether combined CD28 and 4-1BB signaling enhances the response of human primary T cells to antigen, we established a cell culture system in which the proliferative and tumoricidal capacities of CD8+ T cells activated in the presence of 4-IBBL (CD137) and/or B7.1 (CD80) could be investigated. To this end, we constructed a series of fibroblast-derived artificial APCs (AAPCs)27,28 expressing prostate-specific membrane antigen (PSMA), PSMA+B7.1, PSMA+4-1BBL, or PSMA+B7.1+4-1BBL. Following transduction with the ζ chain-based Pz1 receptor29 (Figure 1a), highly purified CD8+ cells were cocultured with the different AAPCs and counted over time (Figure 1b). Exposure to PSMA induced proliferation followed by T-cell death within a few days, as previously observed.28,29,30 Both B7.1 and 4-1BBL individually enabled about tenfold greater T-cell accumulation after two consecutive stimulations. Pz1-transduced CD8+ T cells stimulated by B7.1+4-1BBL+ AAPCs expanded further, reaching threefold higher absolute numbers by day 14 than the T cells expanded with AAPCs expressing either costimulatory ligand alone (Figure 1b). No T-cell expansion was obtained with PSMA− AAPCs (data not shown and ref. 28). These T cells also exhibited stronger cytolytic activity, as reflected by greater lysis of PSMA+ RM1.PGLS cells (Figure 1c).
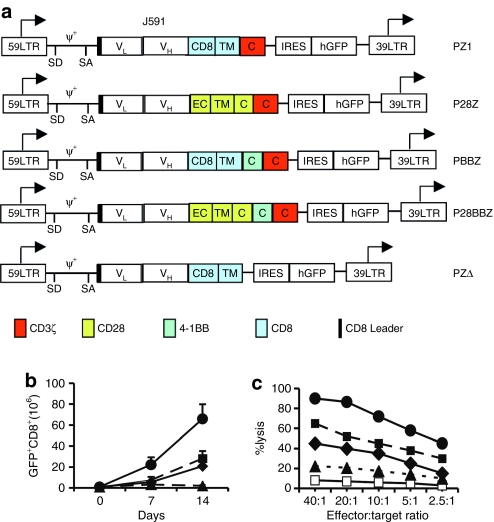
Figure 1.
In vitro proliferation and tumor cytotoxicity of human PSMA-specific primary CD8+ T cells activated with individual or combined CD28 and 4-1BB ligands. (a) Schematic diagram of the Pz1 receptor. The prostate-specific membrane antigen (PSMA)–specific fusion receptors encompass an scFv (white box) derived from the J591 hybridoma, joining the VH and VL fragments through a serine/glycine linker.29 Black: CD8 leader sequence. Red box: CD3ζ chain cytoplasmic domain. Retroviral vector elements: LTR, long terminal repeat; SD, splice donor; SA, splice acceptor; arrows, start of transcription. An EMCV-IRES-humanized renilla green fluorescent protein cassette was cloned downstream of each CAR. (b) Combined CD28 and 4-1BB costimulatory ligands enhance T-cell proliferation and absolute T-cell accumulation following two weekly antigen-specific stimulations. Peripheral blood CD8+ T cells were transduced with Pz1 and activated on AAPCs expressing PSMA and either CD80, CD137, both, or neither. Transduction levels were in the 40–50% range for all receptors (data not shown). T cells were stimulated twice, on day 0 and day 7. Absolute CAR+ T-cell counts are indicated on the y axis. The data represent the mean and SD of nine data points (triplicate cell counts from three separate cultures). (c) Cytolytic activity of antigen-stimulated, CAR-transduced peripheral blood CD8+ T cells. T-cells transduced and activated as described in b were tested on day 7 against RM1.PGLS tumor cells28 and the parental, PSMA− RM1. E:T ratios represent the CD8+CAR+ to target cell ratio. Similar results were obtained using EL4PSMA and EL4 cell lines26,29,48 as targets (data not shown). 19z1+ transduced T cells did not lyse PSMA+ tumor cell lines. Closed circles, AAPC-PSMA/B7.1/4-1BBL; closed squares, AAPC-PSMA/B7.1; closed diamond, AAPC-PSMA/4-1BBL; closed triangle, AAPC PSMA; open squares, 19z1 receptor used as negative control.
Fused 4-1BB and CD28 receptors enhance human CD8+ T-cell proliferation
These results prompted us to generate fusion receptors encompassing both 4-1BB and CD28 signaling domains. Four receptors, termed Pz1, P28z, PBBz, and P28BBz (Figure 2a) were transduced in primary T cells and tested using AAPCs expressing PSMA alone. Transduced T cells were identified on the basis of coexpressed green fluorescent protein, which is independent of the cell surface expression level of the four receptors (see Supplementary Figure S1). Unlike Pz1+ T cells, T cells expressing either P28z or PBBz survived in vitro restimulation and accumulated to comparable numbers by day 14 (Figure 2b), confirming previous studies with CD28/ζ-based fusion receptors.28 Following two antigenic stimulations, CD8+ T cells transduced with the P28BBz receptor reached higher numbers, twofold greater than for P28z+ and PBBz+ T cells and 20-fold more than for the Pz1+ T cells (Figure 2b). Control AAPCs lacking PSMA failed to sustain T-cell survival (data not shown and ref. 9). T cells stimulated by the different PSMA+ AAPCs also showed graded levels of lytic potential when adjusted to the same effector-to-target ratio (Figure 2c). These findings thus established that tandem CD28/4-1BB signaling domains juxtaposed to the CD3ζ chain enhanced antigen-induced clonal expansion and in vitro tumoricidal effector functions in human CD8+ T lymphocytes.
Figure 2.
In vitro proliferation and tumor cytotoxicity of PSMA-targeted human primary CD8+ T cells activated with or without integrated CD28 and 4-1BB costimulation. (a) Integrated CD28 and 4-1BB receptors enhance T-cell proliferation and absolute T-cell accumulation following two weekly antigen-specific stimulations. Peripheral blood CD8+ T cells were transduced with different chimeric antigen receptors (CARs) and subsequently stimulated on the same artificial antigen-presenting cells expressing prostate-specific membrane antigen (PSMA). Transduction levels were in the 40–50% range for all receptors (data not shown). T cells were stimulated twice, on day 0 and day 7. Absolute CAR+ T-cell counts are indicated on the y axis. The data represent the mean and SD of nine data points (triplicate cell counts from three separate cultures). (b) Cytolytic activity of antigen-stimulated, CAR-transduced peripheral blood CD8+ T cells against RM1.PGLS. (c) Cytolytic activity of antigen-stimulated, CAR-transduced peripheral blood CD8+ T cells against the PSMA+ cell line LNCaP. (d) Cytolytic activity of antigen-stimulated, CAR-transduced peripheral blood CD8+ T cells against the PSMA− cell line DU145. T cells were transduced, activated, and tested as described in Figure 1. Closed circles, P28BBz; closed squares, P28z; closed diamonds, PBBz; closed triangles, Pz1; open squares, 19z1 receptor (specific for CD19) used as a negative control.
Combined 4-1BB and CD28 signals enhance CD8+ T cell–mediated prostate cancer elimination in vivo
Our findings on T-cell proliferation and cytotoxicity prompted us to compare the relative potency of human CD8+ T cells transduced with anyone of the Pz1, P28z, PBBz, and P28BBz receptors in tumor bearing immunodeficient mice. To this end, equal numbers of transduced CD8+ T cells were infused intravenously, without further therapy or supportive cytokine administration, in mice inoculated with RM1.PGLS, a virulent prostate adenocarcinoma cell line expressing human PSMA that predominantly seeds in the lung.30 Pz1-transduced T cells resulted in ~15% survival, as previously reported26,30 (P = 0.01 relative to the control 19z1-treated group). Recipients of P28z-transduced T cells fared slightly better, albeit not significantly, with PBBz-treated mice showing intermediate responses. Mice treated with P28BBz+ CD8+ T cells were the only group to show statistically improved survival relative to the Pz1-treated group, P = 0.02, Figure 3). These findings thus demonstrated superior therapeutic efficacy of primary CD8+ T cells transduced with the triple-fusion receptor, establishing that its in vivo signaling properties enhanced intratumoral T-cell activation and subsequent tumor eradication.
Figure 3.
Comparison of the relative potency of prostate-specific membrane antigen-targeted T cells in the RM1.PGLS pulmonary metastases model. Equal numbers of transduced peripheral blood T cells (10 million green fluorescent protein positive CD8+ T cells per mouse) were infused intravenously in tumor-inoculated severe combined immunodeficient/beige mice as previously described.30 Kaplan–Meier survival curves for all treatment groups are represented. Data are pooled from three experiments for a total of 60 mice (Pz1, n = 13; P28z, n = 16; PBBz, n = 13; P28BBz, n = 13 and 19z1, n = 5). Pz1 versus 19z1: P = 0.01; P28BBz versus Pz1: P = 0.025, Log-rank (Mantel–Cox) test.
CD80 and 4-1BB costimulated CD8+ T cells display enhanced effector functions
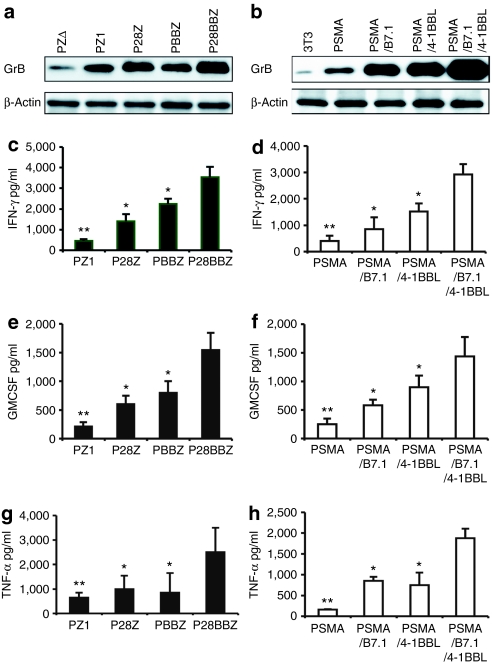
To investigate the mechanism underlying the superior therapeutic efficacy of P28BBz-transduced T cells, we first examined the degree to which effector functions were induced when T cells were activated in the presence of CD80 and/or 4-1BBL. Pz1-transduced CD8+ T cells cocultured with AAPCs expressing PSMA, CD80, and/or 4-1BBL (Figure 4). These were compared to T cells transduced with P28z, PBBz, or P28BBz activated by AAPCs expressing PSMA only (Figure 4). The patterns of expression of Granzyme B, interferon-γ, granulocyte macrophage–colony stimulating factor (GM-CSF), and tumor necrosis factor-α showed the same trends under both conditions, increasing two- to fourfold in the presence of either CD28 or 4-1BB. Combined 4-1BB and CD80 signals increased these levels by a factor of 3.5–6.5, twofold above either stimulus alone on average. IL-10 and IL-4 secretion were either reduced or not increased relative to levels obtained with Pz1 and PSMA alone (data not shown).
Figure 4.
Integrated 4-1BBL and CD28 costimulation upregulates antigen-elicited Granzyme B, TNF-α, IFN-γ), and granulocyte macrophage–colony stimulating factor (GM-CSF) expression. 1 × 106 chimeric antigen receptor-transduced, rested CD8+ T cells were cocultured with 5 × 105 irradiated cells artificial antigen-presenting cells (AAPCs) expressing either prostate-specific membrane antigen (PSMA), PSMA+B7.1, PSMA+4-1BBL, PSMA+B7.1+4-1BBL or none of the above (“mock,” PSMA− AAPC) in the presence or absence of 20 U/ml IL-2. (a,b) Granzyme B expression analyzed by western blot analysis, 24 hours after exposure to antigen. The expression of β-actin was used as a loading control. (a) The Granzyme B expression level in the P28BBz/AAPC-PSMA and (b) Pz1/AAPC-PSMA/B7.1/4-1BBL groups is 3.5- and 6.5-fold greater than the reference Pz1/AAPC-PSMA group, respectively. (c–h) Cytokine expression was analyzed using ELISA or Luminex beads at different time points: IFN-γ (72 hours, c,d), GM-CSF (48 hours, e,f), TNF-α (24 hour, g,h). Error bars indicate standard error (SE) in triplicate samples. *P < 0.05 and **P < 0.001 versus P28BBZ or combination CD28/B7.1 and 4-1BB/4-1BBL signaling.
P28BBz-transduced CD8+ T cells survive repeated antigenic stimulation in vitro and persist longer in vivo
We next examined the apoptotic rate in antigen-stimulated, cultured CD8+ T cells. The rate was elevated in T cells exposed to PSMA+ AAPCs, and reduced in either B7.1 or 4-1BBL, particularly the former. Consistent with the absolute accumulation of expanding T cells shown in Figure 2a, the combination of the two costimulatory ligands, B7.1 and 4-1BBL, additively reduced apoptosis level from 19 and 30% (or with either one alone), respectively, to 8% on day 7 (Figure 5a).
Figure 5.
Integrated 4-1BB and CD28 signals increase in vitro T-cell survival upon antigen restimulation. (a) Inhibition of antigen-induced postactivation cell death. Chimeric antigen receptor (CAR)-transduced CD8+ T cells were restimulated with 3T3-prostate-specific membrane antigen artificial antigen-presenting cells in the presence of 20 U/ml IL-2. After 7 days of coculture, apoptosis was quantified using annexin V and 7-AAD staining. Mean values and SD are from quadruplicate wells from one of three independent experiments performed from different blood donors. *P < 0.05 and **P < 0.001 versus P28BBz. (b) Splenic accumulation of carboxyl fluorescent succinimidyl ester (CFSE)-labeled, CAR-transduced CD8+ T cells 5 days after intravenous adoptive transfer to RM1.PGLS-inoculated severe combined immunodeficient/beige mice (monocistronic vectors encoding CARs only were used in these experiments). (c) Absolute numbers of CD8+ CFSE+ were calculated as the product of percentage transduced cells (determined by flow cytometry) by the total nucleated cell count in the spleen of SCID/beige mice 5 days after adoptive transfer. Each data point represents the average of two or three mice per group. *P < 0.05 and **P < 0.001 versus P28BBz versus CD28/B7.1 and 4-1BB/4-BBL. (d) Cytofluorometric analysis for CFSE-labeled CD8+ T cells 5 days after adoptive transfer, gating on CD3+CD8+ lymphocytes.
This survival advantage carried over in vivo, following adoptive transfer in SCID/beige mice. Five days after intravenous infusion, the number of splenic human T cells was threefold higher for P28BBz relative to P28z and eightfold superior to PBBz (Figure 5a,b). Carboxyl fluorescent succinimidyl ester labeling indicated that, five days after adoptive transfer, many of the surviving P28BBz-transduced, CD8+ T cells had divided two- and fourfold more than T cells stimulated through the P28z and PBBz receptors, respectively (Figure 5d).
Graded levels of PI3kinase/Akt activation by different CARs
To explore the mechanism underlying the decreasing apoptosis in CD80/4-1BB-costimulated T cells, we first measured Bcl-XL induction in the different T-cell groups. We found Bcl-XL levels to be the lowest in Pz1- and PBBz-transduced T cells, higher in P28z+ T cells, and highest in P28BBz+ T cells (Figure 6a). This finding prompted us to examine signaling pathways known to increase Bcl-xL expression, such as the PI3kinase/Akt pathway, which both CD28 and 4-1BB have been reported to activate in human T lymphocytes.20,31 As a first step, Pz1-transduced CD8+ T cells were rested, then treated with anti-CD3, anti-CD28, and/or anti-4-1BB antibodies, and analyzed at multiple time points (Supplementary Figure S2). Akt phosphorylation was slightly increased by CD3/CD28 and CD3/4-1BB stimulation, and further increased when anti-CD28 and 4-1BB antibodies were combined (fourfold higher than anti-CD3 alone, Figure 6b). AKT phosphorylation was also greater in PSMA-stimulated P28BBz-transduced CD8+ T cells, compared to T cells expressing Pz1, P28z, or PBBz (Figure 6c). LY29400 nearly abolished AKT phosphorylation under all of these conditions (Figure 6b,c). To further ascertain the importance of PI3kinase/Akt activation, we tested whether PI3kinase inhibition interfered with the expression of PSMA-induced P28BBz-dependent Bcl-XL expression. Bcl-XL upregulation was completely inhibited by LY294002 in CD8+ T cells (Figure 6a), as well as that induced in Pz1+ CD8+ T cells by PSMA+B7.1+4-1BBL+ AAPCs (data not shown).
Figure 6.
Integrated CD28 and 4-1BB–mediated upregulation of Bcl XL is PI3kinase/Akt-dependent. (a) Upregulation of Bcl-XL in P28BBz-transduced, prostate-specific membrane antigen (PSMA)-stimulated CD8+ T cells. Western blot analysis of Bcl-XL expression was performed 24 hours after exposure to PSMA+ artificial antigen-presenting cells (AAPCs). Bcl-XL expression in P28BBz CD8+ T cells is 1.8–3.9-fold greater than with other vectors, as measured by densitometry. (b) Pz1-transduced, rested CD8+ T cells were stimulated with anti-CD3, anti-CD28 and/or anti-4-1BB antibodies in the absence of IL-2. As a control, an isotype-matched IgG was used. Western blot analysis to detect phospho-Akt (ser437) was performed at multiple time points (see Supplementary Figure S2). The 2-hours data shown here are representative of three independent experiments utilizing T cells from three healthy volunteers. (c) P28BBz-transduced CD8+ T cells sustain higher PKB phosphorylation following antigen stimulation. Rested chimeric antigen receptor-transduced CD8+ T cells were cocultured with PSMA-expressing AAPCs without exogenous IL-2, without or with 10 µmol/l Ly29400 or with 0.05% dimethyl sulfoxide for 2 hours. Western blot analysis for phospho-Akt (ser437) and total Akt are shown. Data representative of three independent experiments.
Discussion
We have generated tumor antigen-specific receptors that signal through serial endodomains derived from CD3ζ, CD28, and/or 4-1BB. Their function was analyzed in comparative fashion in cultured human primary CD8+ T lymphocytes. All chimeric receptors effectively killed PSMA+ tumor cell lines in vitro, underscoring their antigen-specificity and sufficient signaling strength to trigger cytolysis. In proliferative assays, the ζ chain receptor was not able to offset T-cell apoptosis, unless it was complemented with either CD28 (P28z) or 4-1BB (PBBz) cytoplasmic domains. Both dual-fusion receptors increased secretion of interferon-γ, GM-CSF, and tumor necrosis factor-α upon restimulation of rested T cells with cell-bound PSMA. Both decreased T-cell death following exposure to antigen. Serial integration of the two costimulatory domains showed additive effects in all studied functions, including in vitro tumor lysis, cytokine secretion, proliferation, survival, and protection from postactivation apoptosis. Our results corroborate and extend recent findings by Wang et al.32, who found improved in vitro CTL activation, proliferation, and CD20-specific cytotoxicity in polyclonal T cells expressing a CD28/CD137-based CAR, as well as those of Wilkie et al.33, who found similarly increased in vitro effector functions in Muc1-targeted T cells. After submission of our study, C. June et al. reported an in vivo comparison of similarly designed vectors in an intraperitoneal model of mesothelioma and found their 4-1BBz-based receptor to be superior to their CD28z receptor.34 The comparison of our vectors, targeting a different antigen in a different tumor model, shows a different outcome, whereby the CD28z fusion is equal or superior to the 4-1BBz fusion, and where the triple fusion receptor (P28BBz) outperforms either one of the dual fusion receptors (P28z and PBBz). These differences underscore the importance of not generalizing findings from any single series of receptors and the importance of eventually comparing side-by-side the receptors generated by different investigators.8
The therapeutic potential of activating the 4-1BB:4-1BBL axis to enhance antitumor responses using 4-1BBL-transfected tumors or anti-4-1BB antibodies is well established in animal models.35,36 Recent studies have begun to examine combinations of different costimulatory molecules in tumor model systems. Ex vivo T-cell activation with antibodies directed against CD3, 4-1BB, and CD28 increased the activity of adoptively transferred T-cell receptor transgenic murine T cells, resulting in delayed tumor progression.35 Consistent with reports suggesting a cooperative effect between the B7:CD28 and 4-1BB:4-1BBL costimulatory pathways,4,25,32,33,37,38 our results demonstrate superior effector functions and survival of T cells expressing P28BBz as compared to Pz1, P28z, and PBBz. More studies are needed to establish whether these properties extend to all CARs—which likely differ in affinity for antigen, conformation and exact composition of the tandem costimulatory and activating domains.8
Our results further provide some insight into the molecular basis of the cooperativity between these two pathways and their effect on cytokine production, CTL activity, antigen-induced proliferation, and T-cell survival. All receptors that incorporate 4-1BB and/or CD28 signaling domains increased these functions, albeit to varying degrees. PBBz induced at least as much effector cytokine secretion as P28z, albeit less IL-2 (data not shown). These results suggested that integrated CD28 and 4-1BB costimulation of antigen-specific human peripheral CD8+ T cells might increase antitumor reactivity by inducing effector molecules such as Granzyme B and polarizing the T-cell response toward type 1 cytokines including GM-CSF and interferon-γ.
The cooperativity of 4-1BB and CD28 supports the notion that full-fledged 4-1BB activity is achieved in concert with CD28 signaling. Thus, whereas 4-1BB-dependent TRAF2-mediated signaling is preserved in CD28-deficient T cells22 and although it is established that CD28 plays an early role in stimulating clonal expansion and CD137 a later one in sustaining the response,10,18,26,39 our results and others based on antibody-mediated CD28 blockade4 and costimulatory ligand overexpression26 establish the importance of combined, simultaneous signaling. Here, we show that AKT was phosphorylated by both CD28/B7.1 and 4-1BB/4-1BBL at early time points (within 30 minutes) after antigen activation and peaked at 2 hours. 4-1BB signaling synergized with CD28 signaling, prolonging PI3Kinase/Akt activity in the presence of CD28 engagement. AKT phosphorylation upregulated by 4-1BB signaling was not as sustained as with CD28 or combined CD28/4-1BB stimulation. This shows that CD137 is a potentiator of CD28 through the PI3K/AKT signaling pathway in human CD8 T cells.
Our findings place PI3Kinase/Akt activation at the center of the cooperativity between CD28 and 4-1BB. Akt serine/threonine kinases are crucial mediators of proliferation and survival downstream of PI3K,37,40,41 which is activated by cytokines,42 antigen-receptors43 and the costimulatory receptors CD28 (ref. 44) and 4-1BB.20 Although Akt-dependent cell survival may be mediated through a number of effectors, Bcl-XL stands as a critical target in T cells.40,41,42,43,44 Our results show that PI3Kinase/Akt activation was required to achieve CD28/4-1BB–induced upregulation of Bcl-xL. This pathway may be essential to sustain the persistence of adoptively transferred T cells, a critical determinant of the efficacy of adoptively transferred T cells.14,30,45 Our results suggest a quantitative model of CAR-mediated T-cell activation, in which the net activity of human tumor-specific CD8+ T cells is dependent on and proportional to the level and duration of PI3Kinase/Akt activation. This model is illustrated by the hierarchy we find between the Pz1, PBBz, P28z, and P28BBz receptors in terms of their graded strength of PI3kinase/Akt activation and the magnitude of their in vitro response to antigen.
A number of clinical studies have demonstrated the effectiveness of targeting costimulatory molecules in the B7:CD28 pathway to enhance tumor immunity.46 Our data suggest that studies combining 4-1BB and CD28 stimulation will be more powerful. A potential caveat with the use of antibodies or drugs, however, is the risk of autoimmunity secondary to indiscriminate, systemic T-cell activation, as illustrated by the use of high doses of anti-CTLA4 monoclonal antibody.47 An important advantage of the genetic approaches described here and elsewhere30 is the restriction of the combined 4-1BB and CD28 signals to tumor specific T cells, which averts indiscriminate systemic T-cell activation and should thus preempt deleterious autoimmunity.
Methods
Cell lines and vectors. PG13 and H29 retroviral producer cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum. NIH3T3 fibroblasts (American Type Culture Collection, Rockville, MD) were maintained in Dulbecco's modified Eagle's medium with 10% donor calf serum (Hyclone, Logan, UT). RM1 and RM1.PGLS, were grown in high-glucose Dulbecco's modified Eagle's medium supplemented with sodium pyruvate, 10% fetal bovine serum, and 2 mmol/l glutamine (Invitrogen). All media contained penicillin (100 units/ml) and streptomycin (100 µg/ml). NIH3T3-derived AAPCs expressing CD80 and/or PSMA were previously described.26,28 Additional AAPCs encoding 4-1BBL were generated in similar fashion. The coding region of human 4-1BBL was cloned from a human T-cell complementary DNA library and inserted into the NcoI and BamHI restriction sites of SFG, a variant of MFG.S.28,29,30 The oncoretroviral vectors encoding Pz1, P28z, human PSMA, 19z1, and CD80/B7.1 and enhanced green fluorescent protein/Luc are described.26,28,48 In PBBz, the scFv is joined to the hinge and transmembrane of CD8, and much of the intracellular domain of human 4-1BB (Forward primer:5′-CGTTTCTCTGTTAAACGGGGC-3′, Reverse primer:5′CAGTTCACATCCTCCTTCTT-3′. In P28BBz, the intracellular, transmembrane and cytoplasmic domain of CD28 fused to the 4-1BB cytoplasmic domain followed by the CD3ζ cytoplasmic domain. Reverse constructs unexpectedly did not function.49 In Pzdelta, the CD3ζ-chain cytoplasmic domain was truncated from the Pz1 receptor (see Figure 2a).
Peripheral blood lymphocyte collection, transduction, and expansion. All blood samples were obtained from healthy volunteers under an institutional review board-approved protocol. Peripheral blood lymphocytes isolated by low-density centrifugation on Lymphoprep (Accurate Chemical and Scientific Corporation, Westbury, NY) were transduced using gibbon ape leukemia virus envelope-pseudotyped Pzdelta, Pz1, P28z, PBBz, P28BBz, or 19z1 vector in 6-well nontissue culture plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) coated with 15 µg/ml retronectin (Takara Biomedicals, Otsu, Japan) as per the manufacturer's instructions, with fresh viral supernatants daily for 3 days by spinoculation at 80g at 4 °C for 1 hour. For ex vivo expansion, 2 × 106 CD8+ T cells isolated by CD8− selection using magnetic beads (Dynal, Oslo, Norway) were expanded on AAPCs as described28 in the presence of 20 units/ml IL-2 supplemented every 3 days. This cycle was performed once before adoptive transfer or repeated on day 7 for in vitro restimulation studies. 19Z1-transduced T cells were expanded on CD19+CD80+ AAPCs as described.48
Cytotoxicity assays. We determined the cytotoxic activity of transduced T cells by standard 51Cr-release assays using EL4, EL4PSMA, RM1, and RM1.PGLS as targets.30 In selected studies, T cells were incubated with 51Cr-labeled target cells after incubation with 10 µm of Ly294002 for 30 minutes. The effector-to-target cell (E:T) ratio was based on the count of CD8+CAR+ T cells by fluorescence-activated cell sorting analysis. Specific 51Cr release was calculated using the following formula: percentage specific lysis = [(cpm experimental release − cpm spontaneous release)/(cpm maximum release − cpm spontaneous release)] × 100. The SD of triplicates was in all cases <5%.
Flow cytometry. Flow cytometry was performed on a FACScan with Cellquest software (BD Biosciences, San Jose, CA). Expanded Pz1, P28z, PBBz, P28BBz, and 19z1-transduced peripheral blood lymphocytes were stained for the expression of differentiation markers immediately before injection into SCID beige mice or in single-cell spleen suspensions obtained 5 days after infusion. The following antibodies were used for subset identification: anti-CD8-APC, and anti-CD3-PE (eBioscience, San Diego, CA), and, as isotype controls, APC-conjugated mouse IgG2b (BD Biosciences) or mouse IgG1-PE. For carboxyl fluorescent succinimidyl ester labeling, human CD8+ T cells were washed and resuspended in phosphate-buffered saline at a concentration of 2 × 107/ml. An equal volume of 0.25 µmol/l carboxyl fluorescent succinimidyl ester (Molecular Probes, Eugene, OR) was added, and incubated for 5 minutes at room temperature with intermittent mixing. After 5 minutes, an equal volume of heat-inactivated fetal calf serum was added and cells were incubated an additional 1 minute at room temperature, spun down, and washed three times with complete RPMI. Ten million CD8+CAR+ carboxyl fluorescent succinimidyl ester–labeled T cells were injected in 8–12-week-old Fox Chase C.B-17-bg/bg (SCID/beige) mice (Taconic, Germantown, NY). The retrieved spleen cells were resuspended in phosphate-buffered saline containing 2% heat-inactivated fetal calf serum and acquired on a Becton Dickinson FACScan. The data were analyzed using Cellquest. For annexin-V staining, the T cells were washed with phosphate-buffered saline, and labeled with annexin V-FITC, propidium iodide, or both using an Apoptosis Detection Kit (R&D Systems, Minneapolis, MN) according to instructions from the manufacturer. Samples were run on a FACScan, and data were analyzed using Cellquest.
Immunoblotting. Highly purified CD8+ T cells transduced with different CARs were rested overnight in media lacking rhIL-2 and seeded in 24-well plate coated 1 µg/ml of OKT 3 antibody and mixed with 1 ug/ml CD28 antibody and 4-1BB antibody (BD PharMingen,San Diego,CA) or cocultured with AAPCs expressing PSMA, PSMA+B7.1, PSMA+4-1BBL, or PSMA+B7.1+4-1BBL without exogenous IL-2. In some experiments, the T cells were treated with the PI3kinase inhibitor LY294002 or western blotting is same as ref. 50 phospho-Akt (Ser473) and Akt antibodies were from Cellular Signaling (Beverly, MA) or Granzyme B (BD PharMingen), Bcl-xL antibody (Santa Cruz Biotechnology,Santa Cruz, CA), β-actin (Sigma, St Louis, MO).
Cytokine assays. To measure cytokines released by CD8+ T cells in response to PSMA, one million resting 1.0 × 106 CD8+ T cells were cocultured with 0.1 × 106 irradiated different AAPCs (3T3, 3T3-PSMA. 3T3-PSMA/B7.1, 3T3-PSMA/4-1BBL, 3T3-PSMA/B7.1/4-1BBL) in 24-well tissue culture plate for the indicated time at 37 °C. The culture supernatants were then collected and analyzed for cytokine (IFN-γ, GM-CSF and tumor necrosis factor-α production using ELISA (R&D Systems); GM-CSF, IL-4 ad IL-10 production using Luminex beads.
Mouse tumor model. Eight- to twelve-week-old Fox Chase C.B-17-bg/bg (SCID/beige) mice (Taconic, Germantown, NY) were administered 5,000 RM1.PGLS tumor cells by tail vein injection as described.30 Transduced T cells were infused intravenously on the two following days, for a total of 10 million CD8+ CAR+ T cells per recipient. No preconditioning was administered, nor any cytokine or antibody support to support the function of the infused T cells. All mouse studies were carried out under a protocol approved by the Institutional Animal Care and Use Committee.
Statistics. Statistical analyses and curve fittings were done using SAS 9.1 (SAS Institute, Cary, NC).
SUPPLEMENTARY MATERIALFigure S1. CAR cell surface expression.Figure S2. Phospho-AKT time course.
Supplementary Material
CAR cell surface expression.
Phospho-AKT time course.
Acknowledgments
We thank Yan Nikhamin for help with Luminex assays, Jan Hendricks for cell sorting, and Zheng Hu for statistical analyses. This work was supported by NIH grants CA59350, CA23766, and CA08748; Golfers Against Cancer; Alliance for Cancer Gene Therapy; and William H. Goodwin and Alice Goodwin and the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC.
REFERENCES
- Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Townsend SE., and , Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Melero I, Bach N, Hellström KE, Aruffo A, Mittler RS., and , Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- Couderc B, Zitvogel L, Douin-Echinard V, Djennane L, Tahara H, Favre G, et al. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) costimulatory molecules in tumor cells. Cancer Gene Ther. 1998;5:163–175. [PubMed] [Google Scholar]
- Sadelain M, Rivière I., and , Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R., and , Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., and , Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. 2007Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts Clin Cancer Res 1318 Pt 1): 5426–5435. [DOI] [PubMed] [Google Scholar]
- Teng MW, Kershaw MH, Moeller M, Smyth MJ., and , Darcy PK. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum Gene Ther. 2004;15:699–708. doi: 10.1089/1043034041361235. [DOI] [PubMed] [Google Scholar]
- Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G., and , Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- Moeller M, Haynes NM, Trapani JA, Teng MW, Jackson JT, Tanner JE, et al. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. Cancer Gene Ther. 2004;11:371–379. doi: 10.1038/sj.cgt.7700710. [DOI] [PubMed] [Google Scholar]
- Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity. Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- Stärck L, Scholz C, Dörken B., and , Daniel PT. Costimulation by CD137/4-1BB inhibits T cell apoptosis and induces Bcl-xL and c-FLIP(short) via phosphatidylinositol 3-kinase and AKT/protein kinase B. Eur J Immunol. 2005;35:1257–1266. doi: 10.1002/eji.200425686. [DOI] [PubMed] [Google Scholar]
- Hurtado JC, Kim YJ., and , Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Whitmire JK, Ahmed R, Pearson TC., and , Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- Guinn BA, DeBenedette MA, Watts TH., and , Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–5010. [PubMed] [Google Scholar]
- Guinn BA, Bertram EM, DeBenedette MA, Berinstein NL., and , Watts TH. 4-1BBL enhances anti-tumor responses in the presence or absence of CD28 but CD28 is required for protective immunity against parental tumors. Cell Immunol. 2001;210:56–65. doi: 10.1006/cimm.2001.1804. [DOI] [PubMed] [Google Scholar]
- Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Latouche JB., and , Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol. 2000;18:405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- Maher J, Brentjens RJ, Gunset G, Rivière I., and , Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Gong MC, Latouche JB, Krause A, Heston WD, Bander NH., and , Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade TP, Hassen W, Santos E, Gunset G, Saudemont A, Gong MC, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65:9080–9088. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, et al. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Cheuk AT, Mufti GJ., and , Guinn BA. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004;11:215–226. doi: 10.1038/sj.cgt.7700670. [DOI] [PubMed] [Google Scholar]
- Strome SE, Martin B, Flies D, Tamada K, Chapoval AI, Sargent DJ, et al. Enhanced therapeutic potential of adoptive immunotherapy by in vitro CD28/4-1BB costimulation of tumor-reactive T cells against a poorly immunogenic, major histocompatibility complex class I-negative A9P melanoma. J Immunother. 2000;23:430–437. doi: 10.1097/00002371-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr Opin Immunol. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Kane LP., and , Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Reif K, Smith G, Sansom DM, Hemmings BA., and , Ward SG. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL., and , June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- Zhong XS, Matsushita M, Saudemont A, Santos E, Sadelain M. Integrated CD28 and 4-1BB signals strongly potentiate CD8+ T cell mediated eradication of metastatic prostate cancer. Mol Ther. 2006;13:S103. [Google Scholar]
- Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C, et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24:3154–3165. doi: 10.1038/sj.onc.1208525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CAR cell surface expression.
Phospho-AKT time course.