Abstract
Rexin-G, a nonreplicative pathology-targeted retroviral vector bearing a cytocidal cyclin G1 construct, was tested in a phase I/II study for gemcitabine-resistant pancreatic cancer. The patients received escalating doses of Rexin-G intravenously from 1 × 1011 colony-forming units (cfu) 2–3× a week (dose 0–1) to 2 × 1011 cfu 3× a week (dose 2) for 4 weeks. Treatment was continued if there was less than or equal to grade 1 toxicity. No dose-limiting toxicity (DLT) was observed, and no vector DNA integration, replication-competent retrovirus (RCR), or vector-neutralizing antibodies were noted. In nine evaluable patients, 3/3 patients had stable disease (SD) at dose 0–1. At dose 2, 1/6 patients had a partial response (PR) and 5/6 patients had SD. Median progression-free survival (PFS) was 3 months at dose 0–1, and >7.65 months at dose 2. Median overall survival (OS) was 4.3 months at dose 0–1, and 9.2 months at dose 2. One-year survival was 0% at dose 0–1 compared to 28.6% at dose 2, suggesting a dose–response relationship between OS and Rexin-G dosage. Taken together, these data indicate that (i) Rexin-G is safe and well tolerated, and (ii) Rexin-G may help control tumor growth, and may possibly prolong survival in gemcitabine-resistant pancreatic cancer, thus, earning US Food and Drug Administration's (FDA) fast-track designation as second-line treatment for pancreatic cancer.
Introduction
Pancreatic cancer is one of the most lethal of cancers that affects ~37,000 and kills ~33,000 persons in the United States each year.1,2 Currently available treatments for pancreatic cancer have minimal impact on disease outcome. The median survival time for patients with locally advanced pancreatic cancer is 6–10 months, and for metastatic pancreatic cancer, this figure drops to 3–6 months.3,4 Gemcitabine, a deoxycytidine analogue, has been shown to improve the quality of life of patients with advanced pancreatic cancer,4,5 although the median survival was extended merely 5 weeks, as compared to 5-fluorouracil (median survival 5.65 months for gemcitabine versus 4.41 months for 5-fluorouracil).6 Recently, the molecular-targeted therapies, cetuximab (Erbitux) and bevacizumab (Avastin), which target the HER-1/EGF and VEGF receptor-mediated pathways, respectively, failed to show significant survival benefits in phase III clinical trials for pancreatic cancer.7 Further, the tyrosine kinase inhibitor, erlotinib (Tarceva), given first-line in combination with gemcitabine, was reported to improve survival by only 2 weeks compared to gemcitabine alone: median survival 6.3 months versus 5.9 months, respectively.8,9 Currently, all existing therapies offer meager patient benefits, and there is no US Food and Drug Administration (FDA)–approved second-line therapy for pancreatic cancer that has failed a gemcitabine-containing regimen.10 Therefore, innovative therapies are urgently needed.
Among the leading alternatives to traditional chemotherapeutics, both cancer gene therapy and cancer immunotherapy strategies are currently under active clinical investigation.11,12,13 Rexin-G, a nonreplicative-targeted retroviral vector, also described as a pathotropic nanoparticle (~100 nm in diameter) bearing a cytocidal dominant negative cyclin G1 construct, represents the first and, so far only, targeted gene therapy vector that has been tested in the clinic.14 Its “pathotropic” guidance system incorporates a physiological surveillance function (a collagen-binding peptide) derived from von Willebrand clotting factor, which is responsible for guiding platelets to injured tissues where collagen is exposed.15 When injected intravenously, this surveillance function guides the Rexin-G nanoparticles into cancerous lesions, where collagenous matrix proteins are either exposed via protease activity and/or are newly deposited as a result of tumor invasion, tumor-associated angiogenesis and stroma formation,16,17 thus enhancing effective vector concentration within the tumor microenvironment. The genetic payload is a dominant negative mutant of the human cyclin-G1 gene,18 an essential and early part of the cell cycle control pathway.19 Targeting and aborting the early regulatory components of the cancer cell's universal replication mechanism arrests the cell cycle in G1 phase, causing cell death via apoptosis mediated pathways.
In the United States, Rexin-G is currently being tested simultaneously in three phase I/II clinical trials for chemotherapy-resistant metastatic sarcoma, pancreatic cancer, and breast cancer, respectively, and in one phase II study for chemotherapy-resistant metastatic osteosarcoma. In this article, we report on the results of the phase I/II study of Rexin-G for metastatic pancreatic cancer that is considered refractory to a gemcitabine-containing regimen (standard therapy).
Results
Patient population
The current phase I/II study, which incorporates a phase II component by adaptive design, enrolled 13 patients at higher doses than a previous phase I study conducted at the Mayo Clinic, Rochester, MN,20 with repeated treatment cycles to evaluate the efficacy and cumulative toxicity of Rexin-G. Escalating doses starting from 1 × 1011 colony-forming units (cfu) intravenously 2–3× a week (or 8–12 × 1011 cfu over 4 weeks; dose level 0–1) to 2 × 1011 cfu 3× a week (or 24 × 1011 cfu over 4 weeks; dose level 2) were given. Patients who had grade 1 or less toxicity received additional treatment cycles. Further, across-the-board dose escalation was allowed by the FDA from dose level 0 to dose level 2 as part of an adaptive study design, when the safety of Rexin-G was documented in concurrent clinical studies for sarcoma.21 In the dose level 0–1 cohort, the median total cumulative dose received was 7 × 1011 cfu, and one of six patients received chemotherapy after Rexin-G was discontinued. In the dose level 2 cohort, the median total cumulative dose was 60 × 1011 cfu (an 8.5-fold higher dose than that received at dose level 0–1 cohort). Two of seven patients received chemotherapy after Rexin-G was discontinued, and one patient received 10 cycles and is continuing treatment with Rexin-G with a sustained partial response (PR) for >1 year.
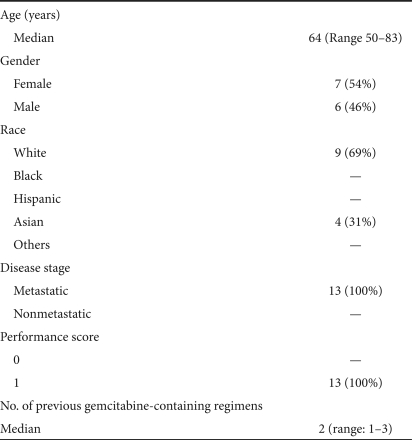
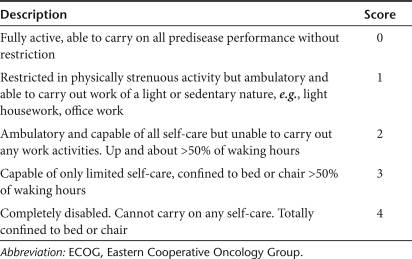
Table 1 shows the demographics of the patient population. The median age was 64 years. There were equal numbers of females and males. There were nine white Caucasians and four Asians. All patients had metastatic disease, thus, bearing a poorer prognosis than those with locally advanced or regional disease, and had failed a median of two gemcitabine-containing regimens. All patients had a performance score of 1 (based on Eastern Cooperative Oncology Group scoring system; Table 2).
Table 1.
Demographics of the patient population (N = 13)
Table 2.
ECOG performance scoring system
Analysis of safety
Treatment-related adverse events occurred in 2/13 patients and are listed in Table 3. Two patients experienced grade 1 fatigue, and one patient had grade 1 chills and grade 1 headache. There was no dose-limiting toxicity (DLT) or organ-related toxicity. There were no antibodies detected in the patients' serum samples tested. Further, all DNA samples from patients' peripheral blood lymphocytes were found to be negative for replication-competent retrovirus (RCR) or vector DNA integration.
Table 3.
Treatment-related adverse events (by NIH CTCAE version 3)
Analysis of efficacy
In the first cohort, three patients were taken off study before completion of one treatment cycle due to disease-related complications (n = 1) or due to a personal decision to discontinue therapy (n = 2). In the second cohort, one patient was taken off study before completion of one treatment cycle. Patients who completed at least one treatment cycle and who had at least one follow-up positron emission tomography–computed tomography (PET-CT) scan were considered evaluable.
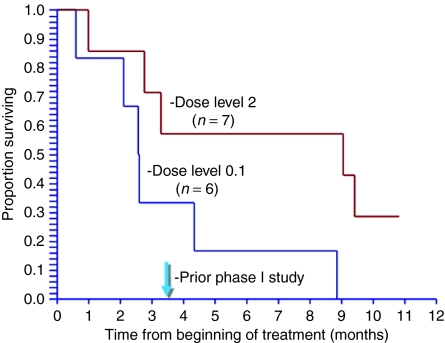
Table 4 gives the tumor responses using response evaluation criteria in solid tumors (RECIST), International PET and CHOI criteria for each dose level. Among the nine evaluable patients, three of three patients had stable disease (SD) at dose level 0–1, whereas one of six patients had a PR and five of six patients had SD at dose level 2 by RECIST. Further, the median progression-free survival (PFS) time at dose level 0–1 was 3 months, and median overall survival (OS) time was 4.3 months (2.6 months for all six patients at dose level 0–1). In contrast, the median PFS time at dose level 2 was ≥7.65 months and median OS time was 9.2 months (9.3 months for all seven patients at dose level 2). The Kaplan–Meier analysis of OS in the “intent-to-treat” groups are shown in Figure 1.
Table 4.
The impact of Rexin-G treatment on tumor response, progression-free survival, and overall survival in patients with pancreatic cancer refractory to gemcitabine (treated analysis)
Figure 1.
Kaplan–Meier analysis for the two cohorts of patients with gemcitabine-resistant pancreatic cancer (intent-to-treat analysis) showing the relationship between overall survival (OS) and Rexin-G dosage.
Correlative analysis showed that FDG uptake was significantly reduced (>25%) in one of three patients at dose level 0–1 and in one of six patients at dose level 2 with the rest having SD in both cohorts (Table 4). Similarly, tumor density was significantly reduced (>15%) in 1/3 patients at dose level 0–1 and in 2/6 patients at dose level 2. These corroborative data indicate that the International PET and CHOI criteria may be sensitive indicators of early tumor response to Rexin-G therapy in pancreatic cancer.
Correlative analysis of changes in CA19.9 level with response and tumor progression by PET-CT scan showed various patterns in three patients who completed at least three treatment cycles of Rexin-G: (i) an early fall in CA19.9 level after the first treatment cycle in a patient with SD for 6 months, followed by a rise in CA19.9 level simultaneous with disease progression by CT, (ii) an initial rise in CA19.9 level followed by a delayed fall after two treatment cycles (12-week follow-up) in one patient with sustained SD, and (iii) a gradual rise in CA19.9 level to moderately high levels over 13 months in a patient with sustained PR by CT.
Table 5 shows the percent of patients surviving 6 months and 12 months according to dose level. At dose level 0–1, only 17% of patients survived ≥6 months and none survived 12 months. In contrast, at dose level 2, 57% of patients survived ≥6 months and 28.6% survived ≥12 months.
Table 5.
The impact of Rexin-G dosage on overall survival in patients with pancreatic cancer refractory to gemcitabine (intent-to-treat analysis)
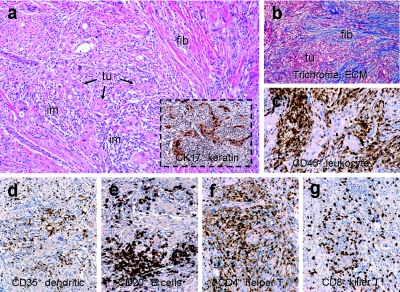
One patient underwent a biopsy of a tumor nodule after three treatment cycles of Rexin-G. Tissue sections of the biopsied tumor showed that the predominant cell population in the residual tumor nodule consisted of host mononuclear cells expressing CD45+ leukocyte common antigen (Figure 2c) and a small proportion (15%) of CK17+ pancreatic cancer cells (Figure 2a, boxed). Immunocytochemical phenotyping of the tumor-infiltrating lymphocytes revealed a complete cadre of T and B cells as well as antigen-presenting cells including CD35+ dendritic cells (Figure 2d), CD20+ B cells (Figure 2e), CD4+ helper T cells (Figure 2f), and CD8+ killer T cells (Figure 2g) surrounding the cancer cells and focal apoptotic nuclei, and significant stroma formation and/or reparative fibrosis (seen as intense blue-staining material by trichrome staining for stromal collagen; Figure 2b). These findings are consistent with those observed in preclinical and clinical studies with Rexin-G treatment.16,17,22,23,24,25 The preponderance of tumor-infiltrating lymphocytes (CD35+ dendritic cells, CD20+ B cells, CD4+ helper T cells, and CD8+ killer T cells) within the tumor nodule affirm the potential of using pathotropic nanoparticles bearing cytokine genes (e.g., GM-CSF) for in situ cancer vaccination strategies.26
Figure 2.
Immunohistochemical characterization of tumor-infiltrating lymphocytes in tumor. (a) Hematoxylin and eosin stain of tumor nodule with CK17+ cancer cells (boxed), (b) trichrome stain for collagen (blue staining material), (c) cells expressing the leukocyte common antigen (reddish brown staining material), (d) CD35+ dendritic cells, (e) CD20+ B cells, (f) CD4+ helper T cells, (g) CD8+ killer T cells (reddish brown staining material). ECM, extracellular matrix; fib, fibrosis; im, immune cells; tu, tumor.
Discussion
Rexin-G is the first targeted genetic medicine to gain orphan drug designation for pancreatic cancer in the United States (http://www.fda.gov/orphan/index.htm). There are no alternative therapies for chemotherapy-resistant pancreatic cancer that impact survival. Therefore, attempts to develop salvage therapy for this rapidly fatal disease could be considered a futile effort. However, the results of pioneering studies conducted in the Philippines,24,27 in Japan (T. Imamura, Chiba Port Medical Clinic, personal communication, April 2008), and now, in the United States, indicate that Rexin-G would have clinical utility as a second-line treatment for pancreatic cancer. The promising results of this phase I/II study suggest clinical benefits of Rexin-G (given as second to fourth-line treatment) that approach those reported in a phase III study wherein gemcitabine and erlotinib were given as first-line treatment.8,9 In that phase III study, the median survival was 5.9 months with gemcitabine given first-line, and 6.3 months with gemcitabine + erlotinib (but with increased toxicity), whereas the median survival with Rexin-G given as second- to fourth-line treatment is 9.2 months with no organ-related toxicity. Therefore, it would be appropriate to expedite the development of this potentially safe and effective drug as a treatment for pancreatic cancer.28
Using higher doses than those used in an early phase I safety study at the Mayo Clinic,20 we report improved tumor responses [PR or SD, and no progression of disease (PD)] using all available measures including RECIST, International PET criteria and CHOI criteria, and extended PFS (median ≥7.65 months) and OS (median 9.2 months) at dose level 2 compared to those reported in the Mayo Clinic study (median PFS of 32 days and median OS of 3.5 months).20 In the current phase I/II study, a dose–response relationship between OS and Rexin-G dosage is apparent (Figure 1). This is consistent with the results of a simultaneous phase I/II study in sarcoma wherein the dose–response relationship between OS time and Rexin-G dosage was statistically significant (P < 0.005) (ref. 21). Taken together, these data confirm the results of previous preclinical and clinical studies conducted in the Philippines that demonstrated that Rexin-G has antitumor activity as a single therapeutic agent in pancreatic cancer.24,27
In summary, we report that the primary objective of this phase I/II study has been met, that is, the safety of Rexin-G has been demonstrated. There was no DLT or organ-related toxicity with repeated infusions of Rexin-G given intravenously at the specified doses. The first of the two secondary objectives has also been met. The results of these studies show no evidence of vector specific or neutralizing antibodies in the sera of patients even after seven treatment cycles, indicating the low immunogenicity of Rexin-G. In contrast, the frequent development of antibodies directed against targeted monoclonal antibodies used as treatment for many types of cancers have precluded their subsequent use. There was no evidence of any vector DNA integration or recombination (RCR) events in nontarget organs (peripheral blood lymphocytes), thereby confirming the exceptional safety record of Rexin-G.
Finally, the second of the secondary objectives has also been met with SD achieved after one treatment cycle of Rexin-G at all dose levels used. Improved tumor responses (one PR and five SDs) as well as longer PFS and OS were achieved in the dose level 2 cohort.
Taken together, the clinical data suggest that (i) Rexin-G is safe and well tolerated, (ii) Rexin-G may help control tumor growth, and (iii) Rexin-G may improve survival in patients with chemotherapy-resistant metastatic pancreatic cancer. In June 2009, the FDA granted Rexin-G fast-track designation as second-line treatment of pancreatic cancer, based on the recognized potential of Rexin-G to address this unmet medical need. Therefore, a phase II/III pivotal two-arm randomized study is planned to confirm the OS benefit of Rexin-G as monotherapy versus physician's choice of therapy in gemcitabine-refractory pancreatic cancer. Overall response rates, PFS, CA19.9 levels, quality of life measures, and histopathology of surgically excised tumors will be further correlated with Rexin-G treatment in a larger number of patients.
Materials and Methods
Study design. The study employed a modification of the standard cohort of three design.29 Three patients were treated at each dose level with expansion to six patients per cohort if DLT was observed in any one of the three first patients at each dose level. Maximum tolerated dose was defined as the highest safely tolerated dose, where ≤1 patient experienced DLT, with the next higher dose level having at least two patients who experienced DLT. DLT was defined as any grade 3, 4, or 5 adverse event considered possibly, probably, or definitely related to the study drug, excluding grade 3 absolute neutrophil count lasting <72 hours, grade 3 alopecia, or any grade 3 or worse nausea, vomiting, or diarrhea where the patient did not receive maximal supportive care.29 Each cohort of three was also expanded to six or seven patients if significant biologic activity was noted.
Adaptive trial design. A phase II efficacy component was incorporated in the phase I/II study by allowing additional treatment cycles to be given if the patient had less than or equal to grade 1 toxicity. Further, across the board, dose escalations were allowed up to dose level 2 for patients with less than or equal to grade 1 toxicity when safety at the specified dose level was documented. The principal investigator was also allowed to recommend surgical resection/debulking, and Rexin-G was continued if residual disease was found by histological examination or PET-CT scan.
Clinical objectives/end points. The primary objective of this study is to determine the clinical toxicity of escalating doses of Rexin-G as defined by patient performance status, toxicity assessment score, and hematologic and metabolic profiles. Secondary objectives include (i) evaluation of the potential of Rexin-G for evoking an immune response, recombination events and/or unwanted vector integration in nontarget organs, and (ii) identification of an antitumor response to Rexin-G.
Patient population. The present phase I/II trial enrolled 13 patients with a pathologic diagnosis of pancreatic adenocarcinoma that was locally advanced or metastatic that had failed gemcitabine or a gemcitabine-containing regimen. Histologic or cytologic confirmation at diagnosis or recurrence was required. Inclusion criteria consisted of an Eastern Cooperative Oncology Group performance score of 0–1 and adequate hematologic, hepatic, and kidney function. Exclusion criteria included human immunodeficiency virus, hepatitis B virus, or hepatitis C virus positivity, clinically significant ascites, medical or psychiatric conditions that could compromise successful adherence to the protocol, and unwillingness to employ effective contraception during treatment with Rexin-G and for 6 weeks following treatment completion. An amendment to the New Investigational Drug application was approved by the FDA (BB-IND#11586) in July 2007, and the clinical protocol (C07-105) was reviewed and approved by the Western Institutional Review Board, Olympia, WA.
Patient recruitment and assignment. The phase I/II clinical trial using Rexin-G for pancreatic cancer was registered on http://www.clinicaltrials.gov (NCT00504998) within 1 week of study initiation, and patients were recruited on a first-come first-serve basis after appropriate screening procedures were conducted. Written informed consent was obtained from each patient at the time of enrollment. This is an open label study using escalating doses of Rexin-G. Six patients were enrolled at dose level 0–1 followed by a dose escalation in six patients at dose level 2 when safety/toxicity data in at least three patients at dose level 1 had been recorded, and no DLT was encountered. One patient was included in the dose level 2 cohort because he received an intrapatient dose escalation from dose level 0 to dose level 2.
Treatment. Rexin-G is a nonreplicative “pathotropic” or disease-seeking nanoparticle bearing a functional collagen-binding motif on its envelope protein18 and encoding an N-terminal deletion mutant construct of human cyclin G1 (ref. 18) under the control of a hybrid Moloney murine leukemia virus long-terminal repeat promoter. The gene expression vector also contains the neomycin phosphotransferase gene driven by the SV40 early promoter, which was used for precise vector titer assays. The Rexin-G vector is produced by transient co-transfection of three separate plasmids in 293T cells (human kidney 293 cells transformed with the SV40 large T antigen) maintained as a fully validated master cell bank.21,24,27 The final product exhibits a vector titer of 5 × 109 cfu/ml, a biologic potency of 50–70% growth inhibitory activity in target cancer cells, <550 base-pair residual DNA, no detectable E1A or SV40 large T antigen, and no detectable RCR.30 The clinical vector is stored in volumes of 23 ml in 30 ml vials or 40 ml in 150 ml cryobags at −80 °C. Preparation of the Rexin-G vector for patient administration consisted of rapid thawing of the vector in the vial in a 34 °C water bath. The vector was thawed 15–30 minutes prior to infusion into the patient, and given intravenously over 5–10 minutes. All personnel who handled and disposed of the vector observed Biosafety Level 2 compliance in accordance with the National Institutes of Health Guidelines for Research Involving Recombinant DNA molecules.
Thirteen patients were treated with escalating doses of Rexin-G. Briefly, each treatment cycle was 6 weeks, consisting of 4 weeks treatment and 2 weeks rest period. The following two dose levels were employed: dose level 0 = 1 × 1011 cfu two times a week for 4 weeks (dose per 4-week cycle: 8 × 1011 cfu); dose level 1 = 1 × 1011 cfu three times a week for 4 weeks (dose per 4-week cycle: 12 × 1011 cfu); dose level 2 = 2 × 1011 cfu three times a week for 4 weeks (dose per 4-week cycle: 24 × 1011 cfu). Treatment was continued if there was less than or equal to grade 1 toxicity.
Safety and efficacy evaluation. Pretreatment evaluation included history, physical exam, complete blood count with differential and platelet count, a serum chemistry panel including aspartate transaminase, alanine transaminase, alkaline phosphatase, creatinine, and total bilirubin, assessment of coagulation status including prothrombin time, international normalized ratio, and activated partial thromboplastin time, testing for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus, imaging evaluation to include a whole body FDG/PET-CT scan, electrocardiography, and chest X-ray. All patients had a complete blood count and serum chemistry panel performed weekly during treatment.
Safety analysis. Toxicity was assessed before each vector infusion, and before beginning an additional treatment cycle. Toxicity was graded using NCI CT-CAE version 3 (ref. 31). Patients had serum collected for vector-specific antibody detection and peripheral blood mononuclear cells collected for assessment of vector DNA integration and RCR at the end of 4 weeks, at 6 weeks, or before the start of a treatment cycle.
Vector-related studies were performed in the Epeius Biotechnologies Quality Control Unit, San Marino, CA using standard operating procedures in compliance with good laboratory practices. Detection of antivector antibodies in serum, testing for presence of RCR and vector DNA integration studies in patient's peripheral blood lymphocytes, were performed as previously described.20,21
Efficacy analysis. Efficacy assessment with FDG PET-CT scan was performed at the end of 4 weeks, at the end of 6 weeks, or before starting an additional treatment cycle up to 12 weeks, and every 12 weeks thereafter. All PET-CT images were performed and reviewed by independent radiologists of the Medical Imaging Center of Southern California, Santa Monica, CA, who are experts at nuclear and PET imaging, and who were blinded to the Rexin-G dose levels. Tumor responses [complete response (CR); PR; or SD] were evaluated using the NCI RECIST criteria.32
Correlative analysis. Tumor responses were also assessed using modifications of the International PET criteria33 and the CHOI criteria.34 The modified International PET Criteria defines a CR as disappearance of FDG avid uptake in target and nontarget lesions with no new lesions; PR as a decrease in maximum standard uptake value of >25% from baseline with no new lesions and no obvious progression of nontarget lesions; SD as not meeting the criteria for CR, PR, or PD, and no symptomatic deterioration attributed to tumor progression; and PD as an increase in maximum standard uptake value of >25% from baseline, any new lesions, and obvious progression of nontarget lesions.
The modified CHOI criteria defines CR as the disappearance of all disease and no new lesions; PR as a decrease in size of ≥10% or a decrease in CT density (Hounsfeld units) ≥15% with no new lesions and no obvious progression of nonmeasurable disease; SD as not meeting the criteria for CR, PR, or PD, and no symptomatic deterioration attributed to tumor progression; and PD as an increase in unidimensional tumor size of >10% and did not meet criteria for PR by CT density, any new lesions, including new tumor nodules in a previously cystic tumor.
Overall evaluation of toxicity/tumor responses was conducted by the principal investigator and associate, Sarcoma Oncology Center, Santa Monica, CA (S.P.C. and V.S.C., respectively).
Statistical analysis. Frequency tables, graphs, and summary statistics were used to describe patient characteristics and outcome data. Follow-up data from October 2007 to 13 January 2009 were analyzed. Kaplan–Meier methodology35 was used to describe graphically the distribution of OS. OS time was calculated in days and divided by 30.4 to convert to months. PFS time was approximated, using the times of patient evaluations. OS and PFS times were compared in groups of patients treated at different dose levels, using permutation tests on the logrank statistic with at least 10,000 replications. Tumor-response data by different specific criteria (RECIST, PET, and CHOI criteria) were reported. Reported P values are two-sided, and P < 0.05 was considered statistically significant. Analysis was done using NCSS software (Number Cruncher Statistical Systems, Kaysville, UT). Statistical analysis was performed by a biostatistician not otherwise involved in the study (W.C.B.).
Acknowledgments
We are grateful to John P. Levy, Rebecca A. Reed, W. Nina Petchpud, and Liqiong Liu (Epeius Quality Control Unit) for quality control testing of product and patient samples, to Antonette C. Balais, registered nurse, and Jun de Guzman (Epeius Clinical Research Unit) for data management. S.P.C., principal investigator; V.S.C.; L.F.; Andreh Saralou; and D.Q.; who participated in the design, patient recruitment, conduct, and evaluation of the clinical safety and efficacy of Rexin-G, and W.C.B., who performed the statistical analysis, have no conflict of interest. F.L.H. and E.M.G., who designed the studies and conducted the correlative analysis are compensated employees of Epeius Biotechnologies Corporation. The study was supported in part by an R01 grant from the FDA-Office of Orphan Product Development (1 R01 FD003071), in part by a grant from the Lazarex Cancer Foundation, Danville, CA, and in part by Epeius Biotechnologies, San Marino, CA. We confirm that the work is original and that the contents have not been published anywhere in its entirety, nor is the work being considered for publication in any other journal. Preliminary results were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in June 2008 in Chicago, IL and at the ASCO Gastrointestinal Symposium in January 2009 in San Francisco, CA.
REFERENCES
- American Cancer Society (2007) Cancer Facts and Figures 2007. American Cancer Society: Atlanta, GA, pp. 16–17; [Google Scholar]
- Nieto J, Grossbard ML., and , Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty. Oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- van Riel JM, Giaccone G., and , Pinedo HM.1999Pancreaticobiliary cancer: the future aspects of medical oncology Ann Oncol 10suppl. 4): 296–299. [PubMed] [Google Scholar]
- el-Kamar FG, Grossbard ML., and , Kozuch PS. Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncologist. 2003;8:18–34. doi: 10.1634/theoncologist.8-1-18. [DOI] [PubMed] [Google Scholar]
- Rosemurgy AS., and , Serafini FM. New directions in systemic therapy of pancreatic cancer. Cancer Control. 2000;7:437–451. doi: 10.1177/107327480000700506. [DOI] [PubMed] [Google Scholar]
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Burris H., 3rd, and , Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist. 2008;13:289–298. doi: 10.1634/theoncologist.2007-0134. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, National Cancer Institute of Canada Clinical Trials Group et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Senderowicz AM, Johnson JR, Sridhara R, Zimmerman P, Justice R., and , Pazdur R.2007Erlotinib/gemcitabine for first-line treatment of locally advanced or metastatic adenocarcinoma of the pancreas Oncology (Williston Park) 211696–1706.discussion 1706–1709, 1712, 1715 [PubMed] [Google Scholar]
- Almhanna K., and , Kim R.2008Second-line therapy for gemcitabine-refractory pancreatic cancer: is there a standard Oncology (Williston Park, NY) 221176–83.discussion 1190, 1192, 1196 [PubMed] [Google Scholar]
- Lieberman SM, Hörig H., and , Kaufman HL. Innovative treatments for pancreatic cancer. Surg Clin North Am. 2001;81:715–739. doi: 10.1016/s0039-6109(05)70157-2. [DOI] [PubMed] [Google Scholar]
- Zwiebel JA. Cancer gene and oncolytic virus therapy. Semin Oncol. 2001;28:336–343. doi: 10.1016/s0093-7754(01)90128-9. [DOI] [PubMed] [Google Scholar]
- Wong HH., and , Lemoine NR. Biological approaches to therapy of pancreatic cancer. Pancreatology. 2008;8:431–461. doi: 10.1159/000151536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R, Russell SJ., and , Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FL, Liu L, Zhu NL, Stapfer M, Anderson WF, Beart RW, et al. Molecular engineering of matrix-targeted retroviral vectors incorporating a surveillance function inherent in von Willebrand factor. Hum Gene Ther. 2000;11:983–993. doi: 10.1089/10430340050015293. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Liu PX, Chen ZH, Liu L, Whitley MD, Gee C, et al. Inhibition of metastatic tumor growth in nude mice by portal vein infusions of matrix-targeted retroviral vectors bearing a cytocidal cyclin G1 construct. Cancer Res. 2000;60:3343–3347. [PubMed] [Google Scholar]
- Gordon EM, Chen ZH, Liu L, Whitley M, Liu L, Wei D, et al. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- Xu F, Prescott MF, Liu PX, Chen ZH, Liau G, Gordon EM, et al. Long term inhibition of neointima formation in balloon-injured rat arteries by intraluminal instillation of a matrix-targeted retroviral vector bearing a cytocidal mutant cyclin G1 construct. Int J Mol Med. 2001;8:19–30. doi: 10.3892/ijmm.8.1.19. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu L, Yee A, Carbonaro-Hall D, Tolo V., and , Hall F. Molecular cloning of the human CYCG1 gene encoding a G-type cyclin: overexpression in osteosarcoma cells. Oncol Rep. 1994;1:705–711. doi: 10.3892/or.1.4.705. [DOI] [PubMed] [Google Scholar]
- Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams RR, et al. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16:979–984. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla SP, Chua VS, Fernandez L, Quon D, Saralou A, Blackwelder WC, et al. 2009Phase I/II and Phase II studies of targeted gene delivery in vivo using pathotropic nanoparticles bearing a dominant negative Cyclin G1 construct (Rexin-G) for chemotherapy-resistant osteosarcoma and other sarcomas Mol Therepub ahead of print). [DOI] [PMC free article] [PubMed]
- Skotzko M, Wu L, Anderson WF, Gordon EM., and , Hall FL. Retroviral vector-mediated gene transfer of antisense cyclin G1 (CYCG1) inhibits proliferation of human osteogenic sarcoma cells. Cancer Res. 1995;55:5493–5498. [PubMed] [Google Scholar]
- Chen DS, Zhu NL, Hung G, Skotzko MJ, Hinton DR, Tolo V, et al. Retroviral vector-mediated transfer of an antisense cyclin G1 construct inhibits osteosarcoma tumor growth in nude mice. Hum Gene Ther. 1997;8:1667–1674. doi: 10.1089/hum.1997.8.14-1667. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Lopez FF, Cornelio GH, Lorenzo CC 3rd, Levy JP, Reed RA, et al. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: three-year clinical experience. Int J Oncol. 2006;29:1053–1064. [PubMed] [Google Scholar]
- Gordon EM, Chan MT, Geraldino N, Lopez FF, Cornelio GH, Lorenzo CC, 3rd, et al. Le morte du tumour: histological features of tumor destruction in chemo-resistant cancers following intravenous infusions of pathotropic nanoparticles bearing therapeutic genes. Int J Oncol. 2007;30:1297–1307. [PubMed] [Google Scholar]
- Gordon EM, Levy JP, Reed RA, Petchpud WN, Liu L, Wendler CB, et al. Targeting metastatic cancer from the inside: a new generation of targeted gene delivery vectors enables personalized cancer vaccination in situ. Int J Oncol. 2008;33:665–675. [PubMed] [Google Scholar]
- Gordon EM, Cornelio GH, Lorenzo CC 3rd, Levy JP, Reed RA, Liu L, et al. First clinical experience using a ‘pathotropic' injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol. 2004;24:177–185. [PubMed] [Google Scholar]
- FDA Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics (2007) DHHS, FDA, CDER, CBER. pp. 1–19.
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- FDA Guidance for Industry: Supplemental Guidance on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors. (2006) DHHS, FDA, CBER: 1–15, October 6, 2006 < http://www.fda.gov/cber/guidelines.htm >. [DOI] [PubMed]
- The NCI Common Terminology Criteria for Adverse Events Version 3 (2006) Cancer Therapy Evaluation Program DCTD, NCI, NIH, DHHS, March, 2003 1–72. < http://ctep.cancer.gov >.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Young H, Baum R, Cremerius U, Herhloz K, Hoekstra O, Lammertsma AA, et al. Position paper. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- Kaplan E., and , Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]