Abstract
To induce a tumor-specific immune response by delivering tumor-associated antigens in tumor cells to antigen-presenting cells (APCs), we designed a fusion protein which consists of heat-shock protein 70 (Hsp70) and the C-terminal 34 amino acids of herpes simplex virus VP22 protein (VP22268–301), the former having a peptide binding domain and an ability to be recognized by APCs, and the latter able to achieve cell penetration. Hsp70-VP22268–301, the fusion protein, was efficiently taken up by mouse dendritic cell (DC) line DC2.4. Major histocompatibility complex (MHC) class I–restricted presentation of an epitope peptide of ovalbumin (OVA) was examined in DC2.4, and Hsp70-VP22268–301 significantly increased the presentation of the peptide compared with Hsp70. Electroporation-assisted injection of naked plasmid vector expressing Hsp70-VP22268–301 (pHsp70-VP22268–301) into subcutaneous tumors of EG7-OVA, a mouse lymphoma–expressing OVA, significantly increased the survival of mice compared with the same treatment with pHSp70, a plasmid expressing Hsp70. Splenocytes from the pHsp70-VP22268–301-treated mice exhibited cytolytic activity against both EG7-OVA and the parent EL4, but not against mouse melanoma B16-F10, suggesting that not only OVA-derived antigens but those common to EG7-OVA and EL4 are delivered to APCs. These results provide a new therapeutic method to induce tumor-specific antitumor immunity without identifying nor isolating tumor-associated antigens.
Introduction
Cancer immunotherapy, which requires the stimulation of the immune system, is expected to be a safe and effective strategy that shows promise in the treatment of cancer patients.1,2,3 Effective induction of a tumor-specific immune response requires efficient delivery of antigens to antigen-presenting cells (APCs) followed by presentation of antigen-derived peptides on major histocompatibility complex (MHC) class I molecules to naive CD8+ T cells.4 Therefore, controlling the in vivo distribution of antigens will increase antigen-specific immune responses, including cytotoxic T lymphocyte (CTL) response.
Heat-shock protein 70 (Hsp70), a molecular chaperon induced under stress conditions, can present a variety of tumor antigens to APCs and elicit innate immunity.5,6,7,8,9,10 A previous study has reported that tumor-derived Hsps initiate protective and tumor-specific CTL responses.11 The mechanisms involved in these processes have been partially identified as follows. Hsps noncovalently form complexes with tumor antigen-derived peptides12,13,14 and bind to dendritic cells (DCs) and macrophages through CD91 and other Hsp receptors,15,16,17 followed by colocalization with the MHC class I molecule in endosomes. In addition, Hsps also activate the innate immunity through interaction with CD40 and Toll-like receptor–2 on DCs, which eventually leads to cytokine release.12,18,19,20,21 These events result in the migration of mature DCs to draining lymph nodes where they present antigens to T cells and initiate the T-cell response.
Because Hsps can deliver antigen peptides to APCs and efficiently activate the immune response to tumor-associated antigens, their application to cancer immunotherapy has been extensively investigated. One of the most common strategies is the use of purified tumor-derived Hsp-peptide complexes, including gp96-peptide complex.11,22,23,24,25 Recently, an Hsp-peptide vaccine (Oncophage; Antigenics, Lexington, MA) has been approved in Russia for the treatment of kidney cancer patients. Such complexes can be reconstituted using several types of Hsps and synthetic peptides.26 All challenges using synthetic or tissue-isolated peptides require the purification and identification of antigen peptides. However, such processes are very expensive, time consuming, and labor intensive. Because tumor tissues are a depot for tumor antigens, any approach to delivering these antigens outside tumor cells to APCs would induce a tumor-specific immune response without exogenous administration of antigens.
In recent years, several peptides and proteins have been reported to translocate across the membranes of mammalian cells.27 These molecules, collectively called cell-penetrating peptides (CPPs) or protein transduction domains, have been applied for the intracellular delivery of a large variety of compounds, including proteins, liposomes, and plasmid DNA complex. Although the precise mechanism of their cellular uptake has not yet been fully identified, cytosolic distribution of CPP-containing compounds has been reported after their addition to cultured cells. VP22, a structural protein of human simple herpes virus-1, has been reported to possess a cell-penetrating activity and to be secreted from cells in which it is produced, thereafter entering surrounding cells.28 Recently, Lemken et al.29 have demonstrated intercellular trafficking of VP22 in living cells. The C-terminal peptide consisting of 34 amino acids (VP22268–301) has been shown to be responsible for this activity. Therefore, conjugation of this peptide with Hsp70 may facilitate the transmembrane transport of Hsp70 and its cargos, such as antigen peptides. Once taken up by APCs, VP22268–301 may accelerate the cytosolic delivery of the complex through the activity of VP22268–301, an important process for inducing Hsp70/peptide-mediated CTL responses.30
These lines of evidence led us to hypothesize that gene delivery of a fusion protein consisting of Hsp70 and VP22268–301 to tumor cells would induce a tumor-specific immune response through the delivery of tumor-associated antigens existing within tumor cells to APCs. To prove this, plasmid vectors encoding mouse Hsp70 or Hsp70-VP22268–301 fusion protein were constructed, and the distribution and antitumor activity of these proteins were examined after gene transfer to cultured cells and to subcutaneous tumors in mice.
Results
Physicochemical properties of Hsp70-VP22268–301
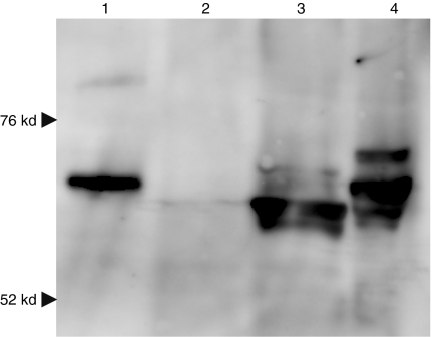
Samples from B16-F10 melanoma cells transfected with pcDNA3.1 (mock), pHsp70, or pHsp70-VP22268–301 were evaluated by western blot analysis (Figure 1, lanes 2–4). All samples showed a weak band for endogenous Hsp70, and cells transfected with pHsp70 (lane 3) or pHsp70-VP22268–301 (lane 4) showed a strong band with a molecular weight representative of Hsp70 and Hsp70-VP22268–301, respectively. There were a few bands in the lanes of lysates from cells expressing pHsp70 (lane 3) or pHsp70-VP22268–301 (lane 4), suggesting that their interaction with cellular proteins. The purified Hsp70-VP22268–301 from bacteria (lane 1) showed a single band with a molecular weight slightly greater than Hsp70 (lane 2), so that the increase in molecular weight of Hsp70 by fusion with the VP22 peptide with a molecular weight of about 3,000 was confirmed. Hsp70-VP22268–301 had a slightly lower electrophoretic mobility (−1.29 ± 0.10 × 10−4 cm2/V·s) compared with Hsp70 (−1.60 ± 0.05), indicating that the surface charge of the fusion protein was slightly less negative due to the presence of many basic amino acids in the VP22 peptide.
Figure 1.
Western blot analysis of Hsp70 and Hsp70-VP22268–301 expressed in B16-F10 cells. B16-F10 cells were transfected with pcDNA3.1 (2, mock), pHsp70 (3) or pHsp70-VP22268–301 (4) using Lipofectamine 2000, and cell lysates were subjected to 10% SDS-PAGE. After transfer to a membrane, Hsp70 and Hsp70-VP22268–301 were detected using mouse anti-Hsp70 monoclonal antibody. Lane 1 shows Hsp70-VP22268–301 expressed and purified from bacteria.
Intracellular distribution of Hsp70 and Hsp70-VP22268–301 in tumor cells
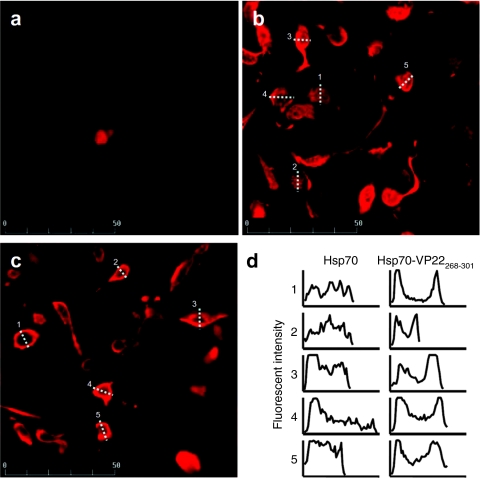
The intracellular distribution of Hsp70 and Hsp70-VP22268–301 was observed in B16-F10 cells after transfection with plasmids expressing each protein. Figure 2 shows the confocal images of B16-F10 cells, in which Hsp70 and Hsp70-VP22268–301 were detected using anti-Hsp70 antibody. Mock (pcDNA3.1) transfected cells showed no significant signals of Hsp70 (Figure 2a), indicating that the level of endogenous Hsp70 was too low to be detected under the conditions used. Cells transfected with pHsp70 exhibited a uniform distribution of Hsp70 within cells (Figure 2b). On the other hand, cells transfected with pHsp70-VP22268–301 showed localized fluorescent signals close to cell membranes (Figure 2c). To compare the intracellular distribution of Hsp70 and Hsp70-VP22268–301, the fluorescent images of cells expressing these proteins were quantitated by fluorescent microscopy. The intensity of cells indicated dotted lines in Figure 2b,c was summarized in Figure 2d. The distribution in cells expressing Hsp70 was rather uniform over the cells. On the other hand, the signal of Hsp70-VP22268–301 showed high distribution to the edge of cells, suggesting their high affinity for the membranes.
Figure 2.
Confocal microscopic images of Hsp70 and Hsp70-VP22268-301 transiently expressed in B16-F10 cells. B16-F10 cells were transfected with (a) pcDNA3.1 (mock), (b) pHsp70 or (c) pHsp70-VP22268–301 using Lipofectamine 2000. After being permeabilized with Triton X-100, cells were stained with mouse anti-Hsp70 monoclonal antibody, followed by Alexa 594-conjugated secondary antibody. Images were obtained by confocal laser scanning microscopy (MRC-1024, Bio-Rad, Hercules, CA). (d) The fluorescent intensity of cells indicated by dotted lines in b and c was quantitated by fluorescent microscopy.
Uptake of Hsp70 and Hsp70-VP22268–301 in DCs
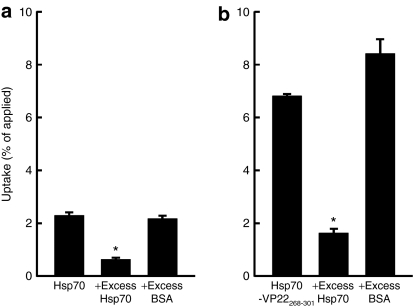
Figure 3 shows the level of radioactivity in DC2.4 cells 1 hour after addition of 111In-Hsp70 or 111In-Hsp70-VP22268–301. As reported in our previous study,30 111In-Hsp70 was efficiently taken up by DC2.4 cells (Figure 3a). This uptake was significantly inhibited by an excess of unlabeled Hsp70, but not by the same amount of bovine serum albumin, suggesting that the uptake of Hsp70 by DC2.4 cells is mediated by some mechanisms specific to Hsp70, such as LOX-1 and other Hsp receptors. Compared with 111In-Hsp70, 111In-Hsp70-VP22268–301 was more effectively taken up by DC2.4 cells (Figure 3b). Again, the uptake was significantly inhibited by an excess of Hsp70, indicating that Hsp70-VP22268 301 is also recognized by the same uptake mechanism as Hsp70. Even when the uptake was inhibited by an excess of Hsp70, the amount of 111In-Hsp70-VP22268–301 taken up by DC2.4 cells was significantly greater than that of 111In-Hsp70. Therefore, it can be speculated that the cellular uptake of Hsp70-VP22268–301 through mechanisms other than the Hsp70-specific ones is more efficient compared with that of Hsp70.
Figure 3.
Uptake of radioactivity in DC2.4 cells after addition of 111In Hsp70 or 111In-Hsp70-VP22268–301. DC2.4 cells were incubated with (a) 2.5 µg 111In-Hsp70 or (b) 111In-Hsp70-VP22268–301 for 1 hour at 37 °C, with or without excess (125 µg) Hsp70 or bovine serum albumin (BSA). Results are expressed as mean ± SD of three determinations. *P < 0.05 compared with the group without any competitor.
MHC class I–restricted presentation of OVA-derived peptide in DC2.4 cells with added Hsp70 or Hsp70-VP22268–301
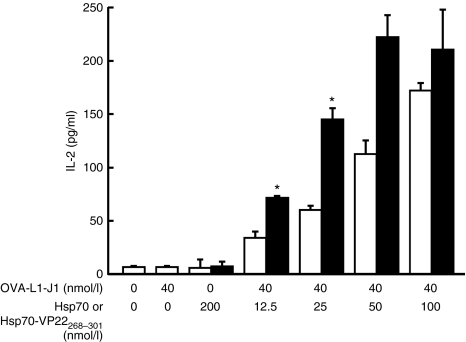
Figure 4 shows the interleukin-2 (IL-2) levels in the supernatant of CD8OVA1.3 T hybridoma cells mixed with DC2.4 cells. The addition of OVA-L1-J1 peptide to the cells resulted in a very low induction of IL-2, indicating that this peptide is hardly presented by itself under the conditions used. The Hsp70/OVA-J1-L1 complex prepared using the same amount of peptide was more effective than the free peptide alone for IL-2 production. These results suggest that the MHC class I–restricted presentation of the peptide was significantly increased by the complex formation with Hsp70, which was in good agreement with the previous results.31 The addition of Hsp70-VP22268–301/OVA-J1-L1 complex resulted in a significantly greater production of IL-2 than that of Hsp70/OVA-J1-L1 complex.
Figure 4.
MHC class I–restricted presentation of OVA-derived peptide in DC2.4 cells. DC2.4 cells were added with OVA-L1-J1 (40 nmol/l), Hsp70 (200 nmol/l), Hsp70-VP22268–301 (200 nmol/l), or OVA-L1-J1 (40 nmol/l) mixed with a varying amount of Hsp70 (open bars) or Hsp70-VP22268–301 (closed bars). At 24 hours after incubation, CD8OVA1.3 T hybridoma cells were added and the IL-2 concentration in supernatants was measured by ELISA. Results are expressed as mean ± SD of three determinations. *P <0.05 compared with the OVA-L1-J1/Hsp70 group.
Tumor-specific immune response after injection of pHsp70 or pHsp70-VP22268–301 into EG7-OVA tumors
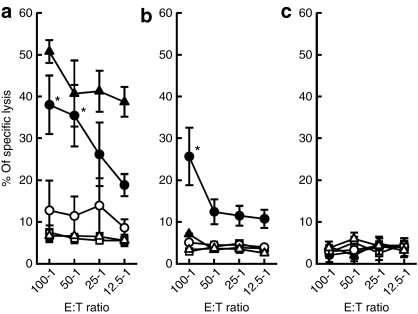
The CTL response was measured to evaluate whether the antigen-specific CTLs were elicited by intratumoral gene transfer of Hsp70 or Hsp70-VP22268–301 (Figure 5). Three cell lines were selected as target cells in the assay to examine whether the CTL response is specific to the type of tumor cells: EG7-OVA, the cell line used to inoculate mice; EL4, the parent cell line of EG7-OVA that shares antigen peptides except for those derived from OVA; and B16-F10, a melanoma cell line that has no relationship to the former two lymphoma cell lines. Splenocytes from mice treated with pHsp70 VP22268–301 showed a higher level of CTL activity against EG7-OVA cells compared with those treated with pHsp70 (Figure 5a). The activity was close to that obtained with OVA in Freund's complete adjuvant, which is a highly effective but very toxic formulation. Splenocytes from the pHsp70-VP22268–301-injected mice were also cytotoxic to EL4 cells (Figure 5b), but not to B16-F10 cells (Figure 5c). These results suggest that not only highly antigenic OVA-derived peptides but antigens common to EG7-OVA and EL4 cells are present on the MHC class I molecules of APCs in mice receiving intratumoral injections of pHsp70-VP22268–301. The absence of CTL activity against B16-F10 cells indicated that the cytotoxic activity produced is specific to EG7-OVA and EL4 cells.
Figure 5.
OVA-specific CTL response after injection of pHsp70 or pHsp70-VP22268–301 into EG7-OVA tumors in mice. EG7-OVA-bearing mice were left untreated (open triangles), or received a subcutaneous injection of 100 µg OVA in Freund's complete adjuvant (closed triangles), or three injections (4 days apart) of pcDNA3.1 (mock, open squares), pHsp70 (open circles), or pHsp70-VP22268–301 (closed circles) into the subcutaneous tumor followed by electroporation. Four days after the last gene transfer, spleen cells were isolated and a standard 51Cr release assay was carried out against (a) EG7-OVA, (b) EL4, and (c) B16-F10 cells. Results are expressed as mean ± SD of at least three determinations. *P < 0.05 compared with the pcDNA3.1 treated and pHsp70-treated groups.
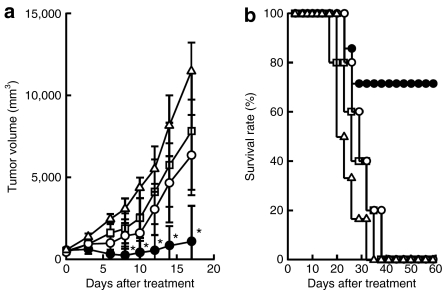
Antitumor effect of intratumoral injection of pHsp70 or pHsp70-VP22268–301
Intratumoral injections of a control pcDNA3.1 plasmid followed by electroporation slightly retarded the tumor growth (Figure 6a). pHsp70 had little additional effects on the tumor growth compared with pcDNA3.1. On the other hand, pHsp70-VP22268–301 significantly inhibited tumor growth. In accordance with the profile of tumor growth, intratumoral injections of pHsp70-VP22268–301 significantly increased the survival of EG7-OVA-bearing mice (Figure 6b).
Figure 6.
Tumor growth and survival of EG7-OVA-bearing mice after injection of pHsp70 or pHsp70-VP22268–301 into EG7-OVA tumors in mice. EG7-OVA-bearing mice were left untreated (open triangles), or injected three times (5 days apart) with pcDNA3.1 (mock, open squares), pHsp70 (open circles), or pHsp70-VP22268–301 (closed circles) into the subcutaneous tumor followed by electroporation. (a) The tumor size was measured periodically and (b) the survival of mice was monitored until 60 days after the start of the treatment. *P < 0.05 compared with the pcDNA3.1-treated and pHsp70-treated groups.
Discussion
Theoretically, the induction of an antigen-specific immune response is achieved by delivering tumor-related antigens to APCs. In addition to the delivery of antigens, many studies have emphasized the importance of the activation of APCs for an efficient induction of such responses. Of the various candidates used as antigen delivery systems, Hsp proteins are considered to be highly effective because they exhibit both delivery and immune stimulation functions.5,6,7,8,9,10 The present study proposes a new approach for delivering endogenous antigen peptides expressed in tumor cells by using Hsp70-VP22268–301 fusion protein after being expressed in tumor cells by in vivo gene transfer. The VP22 peptide, one of the CPPs that has been reported to be translocated through biological membranes, was selected and fused to the C-terminal of Hsp70 to increase the release of Hsp70-antigen peptide complex from tumor cells.
Although the precise mechanism of transmembrane transport of CPPs has not been fully identified, such peptides have been widely used in the delivery of drugs with a variety of physicochemical properties.29,32 However, few challenges have involved the delivery of any compounds present inside cells to their outside. This is, at least partly, due to the fact that many CPPs also have a nuclear transport ability. HIV Tat peptides are frequently used as CPPs but, after entering cells, they localize in the nuclei. On the other hand, HSV-1 VP22 and its derivatives have been reported to be able to deliver transgene products to untransfected cells present around the transfected cells.28,33,34,35 In preliminary experiments, we compared the whole VP22 protein and the VP22268–301 fragment in terms of the induction of CTL responses in tumor-bearing mice, and found that the plasmid expressing Hsp70-VP22268–301 showed better results than that expressing Hsp70-VP22 (data not shown). These findings may be explained by the difference in the size of the fusion proteins, although further studies are needed to prove this.
Hsp70, as well as other Hsps, is believed to possess properties suitable for applications as cancer vaccines. We and others have tried to use Hsps as delivery vehicles for tumor antigens to APCs because they can be recognized by Hsp receptors expressed on APCs.9,10,12,13,14,15,16,17,36 In these approaches, antigens are bound to Hsps through their peptide binding domains, or covalently conjugated. In clinical trials, Hsps binding endogenous tumor antigens have been isolated and administered to cancer patients as vaccines.11,22,23,24,25 Antigens bound to Hsps were reported to be internalized then, through unknown mechanisms, enter the cytosol where antigens can be processed to be presented to MHC class I molecules. In this study, it was suggested that Hsp70 and Hsp70-VP22268–301 interact with some cellular proteins when expressed in melanoma cells (Figure 1). Although the details of such interaction need further study, these results as well as preclinical and clinical observations of Hsp-peptide complexes11,22,23,24,25 would support the hypothesis that Hsp70 and Hsp70-VP22268–301 can be used to deliver endogenous tumor antigens. In addition to these properties of tissue distribution and intracellular trafficking, Hsps can activate APCs through receptors, such as toll-like receptors. These lines of evidence strongly support the usefulness of Hsps as inducers of tumor-specific immune responses.
Fusion of the VP22268–301 peptide to Hsp70 will alter various processes in the distribution of the fusion protein after in vivo gene transfer. Intracellular localization of Hsp70-VP22268–301 is different from that of Hsp70, and the fusion protein showed a facilitated distribution to plasma membranes (Figure 2). The change in the hydrophilic/hydrophobic balance and apparent surface charge of the Hsp70-VP22268–301 could explain the localized distribution of the Hsp70-VP22268–301 to the membranes. Because neither Hsp70 nor Hsp70-VP22268–301 was secreted from cells transduced, the first step in the release from transduced cells is the association with the plasma membranes. Therefore, the localized distribution of Hsp70-VP22268–301 would be beneficial for the release from the cells transduced, the next step in the induction of a tumor-specific immune response.
Once released, the Hsp70 and Hsp70-VP22268–301, especially those complexed with antigen peptides, should be taken up by APCs. Hsp70-VP22268–301 showed a greater uptake in DC2.4 cells than Hsp70 (Figure 3). The uptake of 111In-Hsp70 and 111In-Hsp70-VP22268–301 was specifically inhibited by an excess of Hsp70. Therefore, both would be recognized by cells through receptors specific to Hsp70. It seems that the fusion of VP22268–301 to Hsp70 increases the interaction with those receptors, because the uptake of 111In-Hsp70-VP22268–301 to DC2.4 cells was much higher than that of 111In-Hsp70 and the uptake was efficiently inhibited by an excess of Hsp70. Under conditions where an excess of Hsp70 was added, the amount of 111In-Hsp70-VP22268–301 associated with cells was two- to threefold greater than that of 111In-Hsp70, suggesting the cellular interaction of Hsp70 through mechanisms other than the Hsp70-specific ones is also increased by the fusion of VP22268 301. Furthermore, the fusion protein induced a significantly greater amount of IL-2 than Hsp70 in the antigen presentation assay (Figure 4). Similar to the results of a previous study,31 OVA-L1-J1 alone induced very little IL-2 production and this confirmed that the production of IL-2 is a result of the intracellular delivery of this peptide by delivery systems, i.e., Hsp70 or Hsp70-VP22268 301. Both were found effective in inducing the production of IL-2 from T-cell hybridoma (Figure 4), but the level was significantly higher when Hsp70-VP22268–301-peptide complex was added to the cells. Although the binding of the peptide to Hsp70 proteins was not directly examined in this study, the results of the IL-2 production strongly support the notion that OVA-L1-J1 binds to Hsp70 and Hsp70-VP22268–301 and is delivered efficiently into the cytosol of the cells, then presented on the MHC class I molecules, as demonstrated in the previous study using peptide and Hsp70 (ref. 31). These results suggest that, once released from tumor cells, Hsp70-VP22268–301 more efficiently deliver antigens than Hsp70.
In a previous study, we confirmed that electroporation-assisted in vivo gene transfer is an effective approach for achieving high levels of transgene expression irrespective of the tissues or organs involved.37 In addition, we compared several plasmid-based gene transfer methods to solid tumor tissues in terms of the level of transgene expression.38,39 Based on these studies, we concluded that electroporation-assisted gene transfer is also highly effective for transgene expression in tumor tissues over other methods, including naked plasmid DNA injection without electroporation and injection of plasmid DNA/cationic liposome complex. Therefore, we first examined whether the parameters of electroporation that had been optimized in the previous studies were effective for gene transfer to EG7-OVA tumors. Preliminary experiments demonstrated that EG7-OVA tumor tissues were not an exception and a significant level of transgene expression was obtained after direct tissue injection of naked plasmid DNA followed by electroporation (data not shown). The treatment using control plasmid DNA produced some inhibitory effects on tumor growth. This is probably due to the tissue damage created by both injections and electric pulses. Some cells would be destroyed due to the heat created by the pulses. In addition, the injection of plasmid DNA may induce inflammatory responses against unmethylated CpG dinucleotides, or CpG motifs.40,41,42 Such responses would activate nonspecific antitumor responses. Taken together, the control treatment in which control plasmid DNA was injected in the naked form followed by electroporation has some effect in inhibiting tumor growth. However, such effects are marginal and make hardly any difference to the survival of tumor-bearing mice. In addition, Hsp70 gene transfer was not very effective in inhibiting tumor growth compared with the injection of control plasmid DNA, indicating that a simple supplementation of Hsp70 was not effective in inducing antitumor effects. It has been reported that Hsp70 localizes in the cytosol,8,43,44 and we also confirmed that the Hsp70 expressed in B16-F10 cells evenly distributes within the cytosol (Figure 2). Thus, even although Hsp70-antigen peptide complexes are generated within tumor cells transduced, they have little effect on inhibiting tumor growth probably because they are not significantly released from the cells. However, once released, they should be effective in inducing an antigen-specific immune response as observed in the presentation assay (Figure 4).
Hsp70-VP22268–301 gene transfer to EG7-OVA tumor tissue was effective in inducing CTL responses against EG7-OVA cells. These cells highly express an antigenic protein, OVA, and have been used in vaccine studies in which OVA or its peptides were administered in protein/peptide or DNA form. The specificity of the CTL response induced by Hsp70-VP22268–301 was validated using B16-F10 cells; no significant cell lysis was observed when B16-F10 cells were used as target cells in the CTL assay. Interestingly, a detectable level of CTL response was observed when splenocytes of mice treated with Hsp70-VP22268–301 were mixed with EL4 cells, the parent cells of EG7-OVA. Although the shared antigens remain to be identified, these results indicate that not only OVA-derived peptides but also peptides common to EL4 and EG7-OVA cells are delivered to APCs by gene transfer of Hsp70-VP22268–301 to EG7-OVA-tumor tissues. The difference in the CTL activity between EG7-OVA and EL4 cells may be explained by a fact that the model antigen, OVA, is highly immunogenic compared with other endogenous antigen peptides derived from EG7-OVA and EL4 cells. The importance of Hsp70 in inducing antitumor immune responses has been repeatedly reported in previous publications, but it was not directly examined in the present study. The VP22268–301 part of the fusion protein was 34 amino acids in length and about 3,000 in molecular weight, so that it could be less efficient in forming complexes with antigen-derived peptides than Hsp70, a full-length protein with a molecular weight of about 70,000. Further studies are needed to confirm the roles of each part of Hsp70-VP22268–301 in the induction of antitumor immune responses.
Another approach to inducing CTL responses could be the complex formation of Hsp70 or Hsp70-VP22268–301 with tumor-associated antigens outside cells, not inside cells, which was used in the present study. This possibility needs to be investigated, but we think that gene transfer of Hsp70-VP22268–301 to tumor cells would greatly increase the opportunity for the fusion protein to interact with tumor-associated antigens, which could lead to high CTL responses as observed in this study. Immune reaction to the VP22 peptide could also be elicited after gene transfer of Hsp70-VP22268–301 to tumor cells, because the fusion protein was effectively taken up by immune cells, such as DCs. The effects of such reaction need additional studies to guarantee the safety of this approach.
In conclusion, gene transfer of Hsp70-VP22268–301 to tumor cells has been shown to be a novel approach to inducing a tumor-specific immune response. The fusion of VP22268–301 to Hsp70 was found to be important to obtain the immune response, suggesting that the peptide helps the fusion protein complexed with intracellular antigens to be released from the cells transduced. The increase in the positive charge of the fusion protein increased its uptake by immune cells, which would also be involved in the effective induction of the response. This system does not require identification or purification of tumor antigens, so that it can be applied to a variety of tumor immune therapies without identification or purification of tumor antigens.
Materials and Methods
Plasmid DNA constructs. The pTrc99A expression vector containing genomic mouse clone Hsp70.1 cDNA was kindly supplied by Paul Slusarewicz (Mojave Therapeutics, Hawthorne, NY). The Hsp70.1 cDNA amplified by Ex Taq polymerase (Takara, Tokyo, Japan) was cloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA) at the BamHI and HindIII restriction sites. To construct plasmid encoding Hsp70-VP22268–301, the PCR product of Hsp70 and double strand oligonucleotides for VP22268–301 (5′-aat tct gac gcg gcc acg gcg act cga ggg cgt tct gcg gcg tcg cgc ccc acc gag cga cct cga gcc cca gcc cgc tcc gct tct cgc ccc aga cgg ccc gtc gag tga ggt-3′ and 5′-cta gac ctc act cga cgg gcc gtc tgg ggc gag aag cgg agc ggg ctg ggg ctc gag gtc gct cgg tgg ggc gcg acg ccg cag aac gcc ctc gag tcg ccg tgg ccg cgt cag-3′) were cloned in pcDNA3.1(+). Both plasmid constructs were confirmed by DNA sequencing.
Cell culture. EL4 (C57BL/6, H-2b, T lymphoma), EG7-OVA (EL4 cells transfected with OVA cDNA),45 and B16-F10 (C57BL/6, melanoma) were purchased from American Type Culture Collection (Manassas, VA). EL4 and B16-F10 were cultured in Dulbecco's modified Eagle's medium (Nissui, Tokyo, Japan) supplemented with 10% heat-inactive fetal bovine serum. EG7-OVA was cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 50 µmol/l 2-mercaptoethanol, 2 mmol/l L-glutamine, glucose, sodium pyruvate, HEPES, and G418. The murine DC line, DC2.4 (ref. 46), was kindly supplied by Kenneth Rock (Department of Pathology, University of Massachusetts Medical School, MA). DC2.4 cells were cultured with RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mmol/l L-glutamine, 100 µmol/l nonessential amino acids, 50 µmol/l 2-mercaptoethanol, and antibiotics.
Purification of Hsp70 and Hsp70-VP22268–301. Hsp70 and Hsp70 VP22268 301 proteins were isolated by a method reported previously.36 In brief, the expression of Hsp70 and Hsp70-VP22268–301 in Escherichia coli DH5α cells was induced by 1 mmol/l isopropyl-β-D-thiogalactopyranoside, and the harvested cells were disrupted. The clarified supernatants of Hsp70 and Hsp70-VP22268–301 were loaded onto a glutathion sepharose column (Amersham Pharmacia Biotech, Uppsala, Sweden), and these proteins were eluted by applying pre-scission protease (Amersham Pharmacia Biotech) in 50 mmol/l Tris-HCl solution (pH 7.5). Each eluate was subjected to 8% SDS-PAGE. Then, proteins were transferred to a polyvinyldiene difluoride membrane (Immobilon PTM, Millipore, Bedford, MA), blotted with antibody, and detected by enhanced chemiluminescence (Amersham Pharmacia Biotech). Mouse anti-Hsp70 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used for detection. After washing with phosphate-buffered saline (PBS) containing 0.1% Tween-20, horseradish peroxidase–conjugated mouse anti-IgG antibody was incubated for 1 hour. After washing with PBS containing 0.1% Tween-20, the labeled spots were visualized using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech). The electrophoretic mobility of Hsp70 and Hsp70-VP22268–301 was measured with a laser electrophoresis-ζ potential analyzer (Zetasizer 3000HS, Malvern Instruments, Worcestershire, UK), as an indicator of the net charge of these proteins.47
Western blot analysis of Hsp70 and Hsp70-VP22268–301 expressed in B16-F10 cells. B16-F10 cells were seeded at 2 × 105 per well of six-well plates. After a 24-hour incubation in 5% CO2/95% air, transfection of cells with pHsp70 or pHsp70-VP22268–301 was performed using Lipofectamine2000 (Invitrogen) according to the manufacturer's instructions. At 2 hours after transfection, the medium was replaced with fresh culture medium. The cells were collected at 24 hours after transfection and were lysed, and the lysates were subjected to 10% SDS-PAGE. Then, Hsp70 proteins were assayed by western blot analysis as described above.
Immunofluorescence of Hsp70, Hsp70-VP22268–301 and Hsp70-VP22 in B16-F10 cells. B16-F10 cells were plated on coverplates into 12-well plates at a density of 5 × 105 cells/well, and transfected with pHsp70 or pHsp70-VP22268–301 as described above. Then, cells were washed with PBS and fixed for 15 minutes at room temperature with 4% paraformaldehyde in PBS. After washing with PBS, cells were treated with 0.2% Triton X-100 for permeabilization, and stained with mouse anti-Hsp70 monoclonal antibody, followed by an Alexa 594-conjugated secondary antibody. Images were obtained by confocal laser scanning microscopy (MRC-1024, Bio-Rad, Hercules, CA). The fluorescent intensity of cells expressing Hsp70 or Hsp70-VP22268–301 was quantitated by fluorescent microscopy (Biozero BZ-8000, KEYENCE, Osaka, Japan).
Uptake of Hsp70 and Hsp70-VP22268–301 in DC2.4 cells. Hsp70 and Hsp70-VP22268–301 were radiolabeled with 111In using the bifunctional chelating agent, diethylenetriaminepentaacetic dianhydride, as reported previously.36 DC2.4 cells (1 × 106 cells/well) cultured on 24-well plates were incubated with 2.5 µg 111In-labeled proteins in Hanks' balanced salt solution for 1 hour at 37 °C. Then, the protein solution was removed, and cells were washed and solubilized with 0.3 mol/l NaOH with 0.1% Triton X-100. Radioactivity in cell lysate was measured using a well-type NaI-scintillation counter (ARC-500, Aloka, Tokyo, Japan). To evaluate the involvement of the Hsp70-specific uptake mechanism in the cellular uptake of 111In-Hsp70 and 111In-Hsp70-VP22268–301, a 50-fold excess (125 µg/well) of Hsp70 or bovine serum albumin (Sigma Chemical, St Louis, MO) was added to the 111In-labeled samples.
Antigen presentation assay. The efficacy of the MHC class I presentation activity of Hsp70 and Hsp70-VP22268–301 was examined using DC2.4 cells and T hybridoma cells that specifically recognize the SIINFEKL complex with mouse MHC class I molecule Kb (SIINFEKL-Kb) and release IL-2, a simple method for the evaluation of OVA vaccination systems.48 To avoid direct binding of the SIINFEKL peptide to MHC class I molecules on DC2.4 cells, OVA-L1-J1, a hybrid peptide,31 was selected and obtained from Hokkaido System Science (Sapporo, Japan). This peptide was mixed with Hsp70 or Hsp70-VP22268–301 in PBS, and the mixture was incubated for 1 hour at 25 °C. Then, an amount of 17 ng OVA-L1-J1 peptide with or without Hsp70 derivatives was added to DC2.4 cells (5 × 104 cells/well) cultured on 96-well plates. At 24 hours after incubation, CD8OVA1.3 T hybridoma cells (1 × 105/well) were added to each well and further incubated for 20 hours at 37 °C. Then, supernatants were collected and freeze-thawed. The response of CD8OVA1.3 T cells was determined by measuring IL-2 levels in supernatants with an enzyme-linked immunosorbent assay (ELISA; AN'ALYZA mouse IL-2, Genzyme-techne, Minneapolis, MN).
Animals. Female C57BL/6 mice were purchased from the Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). Animals were maintained under conventional housing conditions and received humane care according to the criteria outlined in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. The protocols for animal experiments were approved by the Animal Experimentation Committee of the Graduate School of Pharmaceutical Sciences of Kyoto University.
CTL assay. Mice were inoculated with EG7-OVA (1 × 106 cells/mouse) in the dorsal skin. When the tumor volume approached 40 mm3, 100 µg plasmid/50 µl was injected into the subcutaneous tumor, and electric pulses (250 V/cm, 5 ms, 4 Hz, 12 pulses) were delivered through forceps-type electrodes (CUY21, Nepagene, Chiba, Japan). The conditions for electroporation were optimized using a plasmid vector expressing firefly luciferase (data not shown). The intratumoral injection and following electroporation were performed three times at intervals of 4 days. A positive control group of mice received a subcutaneous injection of 100 µg OVA protein (Sigma, St Louis, MO) emulsified in Freund's complete adjuvant (ICN Biomedicals, Aurora, OH). At 4 days after the last gene transfer, splenocytes were isolated and restimulated in vitro for 4 days with mitomycin C–treated EG7-OVA. Separately, EG7-OVA, EL4 and B16-F10 cells were labeled with 51Cr by incubation with Na251CrO4 in culture medium for 45 minutes at 37 °C. After washing, 2 × 104 of 51Cr-labeled cells and serially diluted splenocytes were coincubated for 4 hours at 37 °C. Then, cells were centrifuged for 5 min, and 100 µl supernatant was collected for radioactivity measurement. The maximal and spontaneous release of 51Cr from 51Cr-labeled cells was measured in the presence or absence of 1% Nonidet-P40. The cytolytic activity of CTL was calculated as: % of killing = (observed release-spontaneous release)/(maximal release-spontaneous release) × 100.
Antitumor effect of intratumoral gene transfer. EG7-OVA tumor-bearing mice were treated with each plasmid as described above. The intratumoral injection and following electroporation was performed three times at intervals of 5 days. The tumor size was measured with a slide caliper and expressed as a tumor index, determined as the square root of (major axis × minor axis). The survival of tumor-bearing mice was also recorded.
Statistical analysis. Differences were statistically evaluated by one-way ANOVA followed by the Student–Newmann–Keuls multiple comparison test and Kaplan–Meier analysis with a log-rank test to determine survival, and the level of statistical significance was P < 0.05.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (B) and a Grant-in-Aid for Exploratory Research from the Ministry of Education, Science, Sports, and Culture of Japan (to Y.T.), by a grant from Mochida Memorial Foundation (to M.N.), and by a grant from Terumo Life Science Foundation (to M.N.). This work was performed in Kyoto, Japan.
REFERENCES
- Boon T, Coulie PG., and , Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- Hauser H, Shen L, Gu QL, Krueger S., and , Chen SY. Secretory heat-shock protein as a dendritic cell-targeting molecule: a new strategy to enhance the potency of genetic vaccines. Gene Ther. 2004;11:924–932. doi: 10.1038/sj.gt.3302160. [DOI] [PubMed] [Google Scholar]
- Banchereau J., and , Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Campbell FA, Redmond HP., and , Bouchier-Hayes D. The role of tumor rejection antigens in host antitumor defense mechanisms. Cancer. 1995;75:2649–2655. doi: 10.1002/1097-0142(19950601)75:11<2649::aid-cncr2820751102>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Beere HM., and , Green DR. Stress management – heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- Ren W, Strube R, Zhang X, Chen SY., and , Huang XF. Potent tumor-specific immunity induced by an in vivo heat shock protein-suicide gene-based tumor vaccine. Cancer Res. 2004;64:6645–6651. doi: 10.1158/0008-5472.CAN-04-1084. [DOI] [PubMed] [Google Scholar]
- Huang C, Yu H, Wang Q, Ma W, Xia D, Yi P, et al. Potent antitumor effect elicited by superantigen-linked tumor cells transduced with heat shock protein 70 gene. Cancer Sci. 2004;95:160–167. doi: 10.1111/j.1349-7006.2004.tb03198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura Y, Takemoto S., and , Nishikawa M. Hsp-based tumor vaccines: state-of-the-art and future directions. Curr Opin Mol Ther. 2007;9:385–391. [PubMed] [Google Scholar]
- Nishikawa M, Takemoto S., and , Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354:23–27. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M., and , Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- Udono H., and , Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- Suto R., and , Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Wassenberg JJ, Dezfulian C., and , Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112 (Pt 13):2167–2175. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM., and , Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P., and , Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–4852. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Gross C, Hansch D, Gastpar R., and , Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ., and , McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795. [DOI] [PubMed] [Google Scholar]
- Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ., and , Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Testori A, Maio M, Castelli C, Rivoltini L, Pilla L, et al. Heat shock proteins and their use as anticancer vaccines. Clin Cancer Res. 2004;10:8142–8146. doi: 10.1158/1078-0432.CCR-04-1194. [DOI] [PubMed] [Google Scholar]
- Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M, Hällbrink M, Prochiantz A., and , Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- Elliott G., and , O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Lemken ML, Wolf C, Wybranietz WA, Schmidt U, Smirnow I, Bühring HJ, et al. Evidence for intercellular trafficking of VP22 in living cells. Mol Ther. 2007;15:310–319. doi: 10.1038/sj.mt.6300013. [DOI] [PubMed] [Google Scholar]
- Takemoto S, Nishikawa M, Otsuki T, Yamaoka A, Maeda K, Ota A, et al. Enhanced generation of cytotoxic T lymphocytes by increased cytosolic delivery of MHC class I epitope fused to mouse heat shock protein 70 via polyhistidine conjugation. J Control Release. 2009;135:11–18. doi: 10.1016/j.jconrel.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Flechtner JB, Cohane KP, Mehta S, Slusarewicz P, Leonard AK, Barber BH, et al. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J Immunol. 2006;177:1017–1027. doi: 10.4049/jimmunol.177.2.1017. [DOI] [PubMed] [Google Scholar]
- Gupta B, Levchenko TS., and , Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Phelan A, Elliott G., and , O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- Dilber MS, Phelan A, Aints A, Mohamed AJ, Elliott G, Smith CI, et al. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- Zender L, Köck R, Eckhard M, Frericks B, Gösling T, Gebhardt T, et al. Gene therapy by intrahepatic and intratumoral trafficking of p53-VP22 induces regression of liver tumors. Gastroenterology. 2002;123:608–618. doi: 10.1053/gast.2002.34756. [DOI] [PubMed] [Google Scholar]
- Takemoto S, Nishikawa M., and , Takakura Y. Pharmacokinetic and tissue distribution mechanism of mouse recombinant heat shock protein 70 in mice. Pharm Res. 2005;22:419–426. doi: 10.1007/s11095-004-1880-0. [DOI] [PubMed] [Google Scholar]
- Thanaketpaisarn O, Nishikawa M, Yamashita F., and , Hashida M. Tissue-specific characteristics of in vivo electric gene: transfer by tissue and intravenous injection of plasmid DNA. Pharm Res. 2005;22:883–891. doi: 10.1007/s11095-005-4583-2. [DOI] [PubMed] [Google Scholar]
- Nomura T, Nakajima S, Kawabata K, Yamashita F, Takakura Y., and , Hashida M. Intratumoral pharmacokinetics and in vivo gene expression of naked plasmid DNA and its cationic liposome complexes after direct gene transfer. Cancer Res. 1997;57:2681–2686. [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Kobayashi N., and , Takakura Y. Gene silencing in primary and metastatic tumors by small interfering RNA delivery in mice: quantitative analysis using melanoma cells expressing firefly and sea pansy luciferases. J Control Release. 2005;105:332–343. doi: 10.1016/j.jconrel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- McMahon JM, Wells KE, Bamfo JE, Cartwright MA., and , Wells DJ. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998;5:1283–1290. doi: 10.1038/sj.gt.3300718. [DOI] [PubMed] [Google Scholar]
- Yew NS, Zhao H, Wu IH, Song A, Tousignant JD, Przybylska M, et al. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol Ther. 2000;1:255–262. doi: 10.1006/mthe.2000.0036. [DOI] [PubMed] [Google Scholar]
- Kako K, Nishikawa M, Yoshida H., and , Takakura Y. Effects of inflammatory response on in vivo transgene expression by plasmid DNA in mice. J Pharm Sci. 2008;97:3074–3083. doi: 10.1002/jps.21254. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Bukau B., and , Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Moore MW, Carbone FR., and , Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Shen Z, Reznikoff G, Dranoff G., and , Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- Nishikawa M, Hasegawa S, Yamashita F, Takakura Y., and , Hashida M. Electrical charge on protein regulates its absorption from the rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G711–G719. doi: 10.1152/ajpgi.00358.2001. [DOI] [PubMed] [Google Scholar]
- Chefalo PJ, Grandea AG, 3rd, Van Kaer L., and , Harding CV. Tapasin-/- and TAP1-/- macrophages are deficient in vacuolar alternate class I MHC (MHC-I) processing due to decreased MHC-I stability at phagolysosomal pH. J Immunol. 2003;170:5825–5833. doi: 10.4049/jimmunol.170.12.5825. [DOI] [PubMed] [Google Scholar]