Duchenne muscular dystrophy (DMD), one of the most prevalent pediatric genetic disorders (1 in 3,500 newborns), is caused by point mutations or deletions in the gene encoding dystrophin, a major component of the cytoskeleton of muscular fibers. Disruption of dystrophin results in structural instability within cardiac and skeletal muscle and accelerates turnover of the myogenic stem cell pool, ultimately leading to death in afflicted individuals.1 Gene transfer approaches to treat DMD are hampered by the very large size of the dystrophin locus (2.4 Mb) and the limited penetration into muscle by therapeutic viral vectors carrying the mini-dystrophin gene or exon-skipping constructs. Furthermore, obtaining autologous myogenic progenitors for use in DMD patients presents a particularly difficult challenge for the development of cell-based therapies.
Since the discovery of the dystrophin gene, several investigators have attempted to exploit adult stem cells and gene transfer as therapeutic approaches to DMD. In an important step toward that end, as described in this issue of Molecular Therapy, Kazuki et al. deftly combined two recent innovations so as to provide an unprecedented opportunity to overcome the obstacles facing DMD therapy2: the use of a human artificial chromosome (HAC) to express full-length dystrophin (DYS) and induced pluripotent stem (iPS) cells derived from a DMD patient's own fibroblasts to provide the autologous cellular resource (Figure 1).
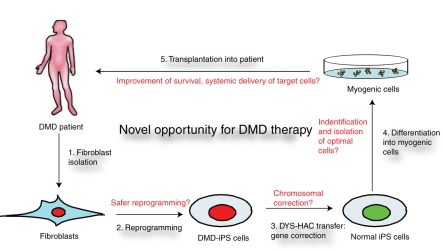
Figure 1.
A novel approach to treating Duchenne muscular dystrophy (DMD) using a combination of gene and iPS cell-based therapy. The sequential completion of combinatorial DMD therapy is represented in the diagram. Fibroblasts are readily cultured from a DMD patient (1) and reprogrammed to generate iPS cells (2). Using a human artificial chromosome (HAC) expressing the whole chromosomal region of dystrophin (DYS), the genetic defect in DMD can be repaired in fibroblasts before reprogramming, or in iPS cells (3) as described by Kazuki et al.2 Myogenic cells differentiated from corrected normal iPS cells (4) can then be transplanted back into the patient to cure the DMD (5). Shown in red are challenges to this therapeutic approach to DMD. Further optimization of reprogramming and genetic correction needs to be achieved. Laborious efforts will be directed at identifying and isolating the most efficient cells for transplantation and toward defining the optimal transplantation method.
Several vector systems have been developed to express defective genes for gene therapy, including retro-, lenti-, adeno-, and adeno-associated viruses.3 The application of adenovirus has been successful, but the duration of gene expression is limited, whereas adeno-associated virus is characterized by a restricted DNA packaging capacity, such that it can carry only a mini-dystrophin gene or small exon-skipping constructs. Retro- and lentiviral vectors have the capacity to deliver a gene of greater length, but their use can lead to adverse events due either to the integration of vector constructs into endogenous chromosomes or to the induction of an immune response stimulated by viral gene expression. To overcome the limitations encountered when using such vectors and to allow expression of the complete dystrophin gene in an endogenous context, the Oshimura laboratory developed an HAC vector that carries the whole genomic locus of dystrophin (DYS-HAC).4 In addition, a gene encoding green fluorescent protein was inserted into the vector to allow the presence of the latter to be monitored within cells, and a suicide gene encoding thymidine kinase was also introduced into the vector so as to allow negative selection against the transduced cells if necessary.
Forced expression of four transcription factors (Oct4, Sox2, Myc, and Klf4) dedifferentiates somatic cells and induces them to become iPS cells that possess the critical features of embryonic stem (ES) cells: self-renewal and pluripotency.5,6 Regaining the potential to differentiate into all three germ layers of the body, these iPS cells provide a powerful resource for cell-based therapy for DMD patients who require cellular regeneration of their muscle.7 Because iPS cells are derived from the patient's own cells, they do not induce immune rejection when transplanted back into patients—a huge obstacle for strategies that make use of allogeneic transplantation.
In their experiments, Kazuki et al. generated iPS cells from fibroblasts harvested from mdx mice—a murine model of DMD—by expressing only three reprogramming factors; they excluded the potentially oncogenic Myc. Using microcell-mediated chromosome transfer, the DYS-HAC vector was transferred to the mdx-iPS cells, where it restored dystrophin expression in the teratomas that grew from the cells, as well as in chimeric mice derived from such cells, demonstrating the potential of the combined therapy for DMD. Transferring DYS-HAC into human DMD-iPS cells proved more challenging because of the intrinsic difficulty of performing single-cell cloning of human ES and iPS cells. DMD-iPS cells were generated only when DYS-HAC was first transferred into the DMD fibroblasts before iPS cell generation. Subsequent in vivo differentiation of a teratoma confirmed the functional expression of dystrophin from the DYS-HAC-iPS cells.
Thus, the laborious collaborative efforts of stem cell and gene therapy scientists have allowed the development of the materials necessary for DMD treatment. However, the cure for DMD or other genetic muscular dystrophic diseases is far from imminent. Figure 1 depicts a popular model of pluripotent stem cell–based DMD cell therapy in a sequential manner. The derivation of a patient's specific iPS cells and the genetic rescue are performed in vitro, and they can currently be routinely achieved despite the need to improve on the reprogramming approach. The current methods used to derive iPS cells—using retro- or lentiviruses to overexpress reprogramming genes—raise the concern of potential tumorigenicity.8 However, new approaches for reprogramming that avoid genetic alterations should be refined within the near future thanks to the pioneering efforts of a fairly large number of stem cell scientists. Indeed, nonintegrating viruses, multiple transient transfections, and protein transduction have all been used to produce iPS cells, albeit at a low efficiency.9,10,11,12 Key issues that must yet be addressed are how to (i) maximize the utility of iPS cells through the identification and isolation of specific cell populations that can efficiently generate myogenic cells and repair damaged tissue and (ii) optimize the cellular delivery method to preserve the function of the transplanted cells.
Candidate myogenic cells can be selected from differentiated ES and iPS cells using methods developed to isolate adult stem cells of a particular muscular lineage,13 such as satellite cells,14 muscle-derived stem cells,15 side-population cells,16 bone marrow–derived stem cells,17 mesoangioblasts,18 pericytes,19 and muscle-derived CD133+ stem cells.20 Established knowledge of cell surface markers used to prospectively isolate the above adult stem cells facilitates the identification of the desired cell types from iPS cells. For example, cells expressing markers of satellite cells (CD56), side-population cells (CD34), myoendothelial cells (CD34, CD114), pericytes (CD146, PDGFRβ), mesoangioblasts (NG2+), and CD133+ cells can be isolated during iPS cell differentiation and then tested for their myogenic potential. However, because most of the cell surface markers are shared by adult stem cells of other lineages, isolating candidate cells with multiple markers will enhance the likelihood of obtaining a pure myogenic cell population. Barberi and colleagues' 2007 report exemplifies how to successfully isolate engraftable skeletal muscle from human ES cells in two steps, using a mesenchymal stem cell marker (CD73) and a myoblast marker (CD56).21 The genetic approach based on the overexpression of known genes (e.g., Pax3) essential for myogenic commitment will be very useful in developing therapeutic approaches for DMD, because this method drives differentiating cells to become myogenic, thereby reducing the portion of uncommitted or nonmyogenic populations of cells.22
In addition to the difficulties associated with isolating the optimal cell types that have myogenic potential for transplantation, the iPS cell–based DMD therapy will confront the same barriers that hinder the successful application of adult stem cell–based approaches23,24: poor survival, limited self-renewal, and limited homing and migration after transplantation. Recent reports of myogenic functional recovery after the intra-arterial injection of mesoangioblasts and pericytes underscore the therapeutic potential of systemic delivery of myogenic cells.18,19 Continuous efforts to optimize the use of adult stem cells in treating muscular dystrophy will be essential to the future application of myogenic cells derived from iPS cells. In addition, a completely new cell population distinct from known adult stem cells may yet be isolated from pluripotent stem cells, providing a population more closely resembling early embryonic multipotent cells with the capacity to be systematically transplanted.25 Meanwhile, we should maintain our search for novel approaches to DMD therapy. Toward this end, Kazuki and colleagues have pioneered a unique therapeutic approach to the treatment of DMD by harnessing gene correction within pluripotent ES or ES-like iPS cells.
REFERENCES
- Wallace GQ., and , McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H, et al. Complete genetic correction of iPS cells from Duchenne muscular dystrophy. Mol Ther. 2010;18:386–393. doi: 10.1038/mt.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom GL, Gregorevic P., and , Chamberlain JS. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim Biophys Acta. 2007;1772:243–262. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiya H, Kazuki Y, Abe S, Takiguchi M, Kajitani N, Watanabe Y.et al. (2009A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene Mol Ther. 17309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA.et al. (2008Reprogramming of human somatic cells to pluripotency with defined factors Nature 451141–146. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Goldstein RA., and , Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T., and , Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II.et al. (2009Human induced pluripotent stem cells free of vector and transgene sequences Science 324797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T., and , Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS.et al. (2009Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins Cell Stem Cell 4472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G., and , Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T.et al. (2007Stem and progenitor cells in skeletal muscle development, maintenance, and therapy Mol Ther 15867–877. [DOI] [PubMed] [Google Scholar]
- Kuang S., and , Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G.et al. (2004Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages Lancet 3641872–1883. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A., and , Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S.et al. (2005Bone marrow stromal cells generate muscle cells and repair muscle degeneration Science 309314–317. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A.et al. (2006Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs Nature 444574–579. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L.et al. (2007Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells Nat Cell Biol 9255–267. [DOI] [PubMed] [Google Scholar]
- Benchaouir R, Meregalli M, Farini A, D'Antona G, Belicchi M, Goyenvalle A.et al. (2007Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice Cell Stem Cell 1646–657. [DOI] [PubMed] [Google Scholar]
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND., and , Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE.et al. (2008Functional skeletal muscle regeneration from differentiating embryonic stem cells Nat Med 14134–143. [DOI] [PubMed] [Google Scholar]
- Skuk D., and , Tremblay JP. Progress in myoblast transplantation: a potential treatment of dystrophies. Microsc Res Tech. 2000;48:213–222. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<213::AID-JEMT9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Skuk D., and , Tremblay JP. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil. 2003;24:285–300. [PubMed] [Google Scholar]
- Sakurai H, Okawa Y, Inami Y, Nishio N., and , Isobe K. Paraxial mesodermal progenitors derived from mouse embryonic stem cells contribute to muscle regeneration via differentiation into muscle satellite cells. Stem Cells. 2008;26:1865–1873. doi: 10.1634/stemcells.2008-0173. [DOI] [PubMed] [Google Scholar]