Abstract
OBJECTIVE
We examined whether mild traumatic brain injuries in children and adolescents, especially when associated with acute clinical features reflecting more severe injury, result in different postinjury trajectories of postconcussive symptoms compared with mild orthopedic injuries.
PARTICIPANTS AND METHODS
Participants in this prospective and longitudinal cohort study were 8- to 15-year-old children, 186 with mild traumatic brain injuries and 99 with mild orthopedic injuries, who were recruited from consecutive admissions to emergency departments in 2 large children’s hospitals. Parents rated current postconcussive symptoms within 3 weeks of injury and at 1, 3, and 12 months after injury. At the initial assessment, parents also provided retrospective ratings of preinjury symptoms, and children with mild traumatic brain injuries received MRI of the brain. Clinical features examined as predictors of postconcussive symptoms included loss of consciousness, Glasgow Coma Scale score below 15, other injuries, acute symptoms of concussion, and intracranial abnormalities on the MRI.
RESULTS
Finite mixture modeling identified 4 longitudinal trajectories of postconcussive symptoms (ie, no postconcussive symptoms, moderate persistent postconcussive symptoms, high acute/resolved postconcussive symptoms, high acute/persistent postconcussive symptoms). The mild traumatic brain injuries and orthopedic injuries groups demonstrated a different distribution of trajectories. Children with mild traumatic brain injuries were more likely than those with orthopedic injuries to demonstrate high acute/resolved and high acute/persistent trajectories relative to the no postconcussive symptoms group. The 2 trajectories with high acute levels of postconcussive symptoms were especially likely among children with mild traumatic brain injuries whose acute clinical presentation reflected more severe injury.
CONCLUSIONS
Mild traumatic brain injuries, particularly those that are more severe, are more likely than orthopedic injuries to result in transient or persistent increases in postconcussive symptoms in the first year after injury. Additional research is needed to elucidate the range of factors, both injury related and non–injury related, that place some children with mild traumatic brain injuries at risk for postconcussive symptoms.
Keywords: mild traumatic brain injury, postconcussive symptoms, clinical predictors
Traumatic brain injuries (TBIs) are a leading cause of death and disability among children and adolescents.1 Moderate and severe TBIs account for most of the mortality and morbidity in children.2 However, mild injuries represent the vast majority of TBIs.3 Approximately 500 000 children aged 14 and younger sustain TBIs resulting in emergency department visits annually in the United States, and 80% to 90% of TBIs can be classified as mild.1,3 Even if only a small proportion of children with mild TBIs suffer negative outcomes, then mild TBI is a serious public health problem.
Recent reviews have suggested that mild TBIs have little short- or long-term effect on children.4–6 However, most previous studies of mild TBIs have assessed outcomes by using standardized tests of cognitive abilities or broad-based ratings of behavioral adjustment. Few studies have focused specifically on postconcussive symptoms (PCSs). PCSs are subjective somatic, cognitive, and emotional problems that are embodied in the diagnostic criteria for postconcussion syndrome in the International Classification of Diseases, 10th Revision,7 as well as in the research criteria for postconcussional disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.8
Of the limited studies conducted to date, most have suggested that PCSs are more common and severe in children with mild TBIs than in children with injuries not involving the head or in healthy children matched for sociodemographics.9–13 Group differences in PCSs tend to be most pronounced shortly after the injury and to resolve over time, although the likelihood that PCSs will persist and be associated with psychosocial morbidity after mild TBI in children remains uncertain, because only a handful of studies have followed children for more than a few months.11,14–15
The etiology of PCSs after mild TBI remains a topic of debate. The controversy is often framed in terms of “psychogenesis versus physiogenesis.”16–17 Proponents of psychogenesis argue that PCSs reflect children’s premorbid adjustment, postinjury psychological or family factors, or outright malingering in the context of litigation.11,18–19 In contrast, proponents of physiogenesis point to experimental studies of nonhuman animals and clinical research with humans suggesting that mild TBI can result in acute neuropathology and abnormalities in brain function20–22 and that the occurrence of PCSs after mild TBI may be transiently associated with deficits on standardized cognitive testing and abnormalities on neuroimaging.13,23–25
Much of the existing research on mild TBI in children suffers from methodologic shortcomings that preclude a resolution of this controversy.26,27 One of the major limitations of previous research involves vague and inconsistent definitions of mild TBI.28 Most studies have defined mild TBI in part based on Glasgow Coma Scale (GCS)29 scores ranging from 13 to 15, but the studies have been inconsistent in their use of other criteria, such as presence or duration of unconsciousness or posttraumatic amnesia. Children with positive findings on neuroimaging have often been omitted, despite evidence suggesting that so-called “complicated” mild TBI (ie, GCS score of 13–15 but with intracranial abnormalities on neuroimaging) are associated with a higher risk of negative sequelae.30,31
Another shortcoming of the existing literature is that statistical analyses reflect an underlying assumption that children with mild TBI are a homogenous group. Thus, most analyses compare the mean level of PCSs of children with mild TBI to that of a comparison group. However, the assumption of homogeneity may be incorrect, and individual children may display significant variations in PCSs, particularly when studied across time. The advent of techniques such as mixture modeling, which can be used to empirically identify latent classes of individuals based on different developmental trajectories, should enable a more sophisticated examination of individual outcomes of mild TBI and the clinical features that are related to them.32,33
We recently completed a prospective, longitudinal study intended to clarify the outcomes of mild TBI in children.27 The study focused specifically on PCSs and was designed to address the methodologic shortcomings of previous research. Participants were children with mild TBI or with orthopedic injuries (OIs). Mild TBIs were defined based on specific inclusion and exclusion criteria encompassing a range of severity. Parents rated PCSs at multiple times during the first year after injury. Preinjury symptoms were measured retrospectively shortly after the injury. Multiple acute clinical features were documented to assess the severity of mild TBIs. Developmental trajectory analysis was used to classify individual children in terms of their patterns of PCSs across time. In this article, we examine whether mild TBI, as well as specific clinical features indicative of brain injury, are associated with different trajectories of PCSs as compared with OI.
METHODS
Participants
Children were recruited from consecutive admissions to the emergency departments at Nationwide Children’s Hospital and Rainbow Babies & Children’s Hospital. All children from 8 to 15 years of age with blunt head traumas or OIs were screened for participation.
Children were defined as having a mild TBI if they sustained a blunt head trauma resulting in an observed loss of consciousness (LOC) no longer than 30 minutes, a GCS score of 13 or 14, or at least 2 acute symptoms of concussion as documented by emergency department medical personnel. Acute symptoms included posttraumatic amnesia, vomiting, nausea, headache, diplopia, dizziness, disorientation to time, place or person, or any other indications of mental status changes (ie, dazed, foggy, slow to respond, lethargic, confused, asking repetitive questions, sleepy). Exclusion criteria for the mild TBI group included any GCS score below 13, delayed neurologic deterioration, or any medical contraindication to MRI. Children were not excluded if they were hospitalized or demonstrated intracranial lesions or skull fractures on acute computed tomography. Thus, the mild TBI group included injuries often described as complicated (eg, those with intracranial abnormalities) but excluded injuries that would typically be defined as moderate in severity.
Children with OIs were eligible if they sustained upper or lower extremity factures associated with an Abbreviated Injury Scale (AIS)34 score of ≤3. The AIS is a widely used scoring system that assesses the severity of injuries to specific anatomic regions on a scale of 1 to 6. Children were excluded from the OI group if they displayed any evidence of head trauma or symptoms of concussion.
Both groups were subject to multiple exclusion criteria: neurosurgical or surgical intervention; any associated injury with an AIS score of >3; any associated injury that interfered with neuropsychological testing; hypoxia, hypotension, or shock; ethanol or drug ingestion involved with the injury; previous head injury requiring medical treatment; premorbid neurologic disorder or mental retardation; any injury resulting from child abuse or assault; or premorbid severe psychiatric disorder requiring hospitalization. Children were not excluded for premorbid learning difficulties or attention problems. The mild TBI and OI groups did not differ on retrospective parent ratings of premorbid school performance or attention problems.
Among children meeting criteria for mild TBI, the participation rate was 48%; participation among those meeting criteria for OI was 35%. Within the mild TBI and OI groups, participants and nonparticipants did not differ significantly in age, gender, ethnic/racial minority status, or census tract measures of socioeconomic status (ie, median family income, % below poverty line, % minority).
The final sample included 186 children with mild TBI and 99 children with OI. The groups did not differ in age at injury, gender, ethnic/racial minority status, socioeconomic status, or number of premorbid PCSs as assessed by a retrospective parent report (see Table 1). The mild TBI group displayed greater overall injury severity as measured by the Modified Injury Severity Scale, which is an overall index derived from AIS scores, calculated as the sum of the squares of the 3 most severely injured body areas.35 Recreational and sports-related injuries were the most common cause of injury (57% of mild TBIs, 62% of OIs), with falls the second leading cause (20% of mild TBIs, 21% of OIs). Transportation-related injuries accounted for significantly more mild TBIs (17%) than OIs (3%).
TABLE 1.
Demographic and Clinical Characteristics of Participants
| Variable | OI | Mild TBI |
|---|---|---|
| n | 99 | 186 |
| Age at injury, mean (SD), y | 11.76 (2.23) | 11.96 (2.22) |
| Socioeconomic status, mean (SD)a | −0.09 (1.15) | 0.05 (0.91) |
| No. of premorbid PCSs, mean (SD) | 0.96 (1.79) | 1.05 (1.41) |
| Modified Injury Severity Scale, mean (SD)b | 3.25 (1.52) | 4.62 (4.54) |
| Male, n (%) | 64 (65) | 132 (71) |
| White, n (%) | 64 (65) | 132 (71) |
| Loss of consciousness, n (%) | — | 74 (40) |
| Duration of loss of consciousness, median (range), min | — | 1 (<1 to 15) |
| Glasgow Coma Scale score <15, n (%) | — | 24 (13) |
| Persistent posttraumatic amnesia, n (%) | — | 60 (32) |
| Vomiting, n (%) | — | 82 (44) |
| Nausea, n (%) | — | 77 (41) |
| Headache, n (%) | — | 141 (76) |
| Diplopia, n (%) | — | 22 (12) |
| Dizziness, n (%) | — | 48 (26) |
| Disorientation, n (%) | — | 18 (10) |
| Other mental status changes, n (%) | — | 63 (33) |
| Presence of other injuries, n (%) | — | 63 (25) |
| Intracranial abnormality on MRI, n (%) | — | 32 (18) |
Socioeconomic status was assessed by averaging sample z scores for years of maternal education, median family income for census tract, and the Duncan Socioeconomic Index, which is a measure of occupational prestige.47
Groups differ significantly (P <.05).
Study Design and Sample Attrition
The research was approved by the appropriate institutional review boards, and informed parental consent and child assent were obtained in writing before participation. Eligible children whose parents consented completed an initial assessment no later than 3 weeks after injury, with 80% completed between 1 and 2 weeks after injury (mean: 11.35 days [SD: 3.42]). Parents reported current PCSs at the initial assessment and again at 1, 3, and 12 months after injury. At the initial assessment, parents also completed a retrospective report of preinjury symptoms before reporting current symptoms. In addition, children with mild TBI received MRI of the brain.
Of the 285 children who completed the initial assessment, 280 (98%) completed the assessment at 1 month after injury (183 [98%] with mild TBIs, 97 [98%] with OIs), 268 (94%) completed the 3-month assessment (178 [96%] with mild TBIs, 90 [91%] with OIs), and 253 (89%) completed the 12-month assessment (169 [91%] with mild TBIs, 84 [85%] with OIs). Four children from the mild TBI group could not complete the MRI.
Assessment of PCSs
PCSs were assessed by using the Postconcussive Symptom Interview,11 which asks parents to report the presence or absence of 15 symptoms during the preceding week (see Table 2). The symptoms are similar to those listed for postconcussion syndrome in the International Classification of Diseases, 10th Revision and for postconcussional disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.8 The total number of symptoms was used as the measure of PCSs and displayed satisfactory internal consistency at each of the 4 assessments (Cronbach’s α = .78–.82).
TABLE 2.
Postconcussive Symptom Interview
| Has your child been tired a lot in the last week? | Yes | No |
| Has your child had headaches in the last week? | Yes | No |
| Has your child had any trouble remembering things in the past week? | Yes | No |
| Has bright light hurt your child’s eyes in the last week? | Yes | No |
| Has your child’s head been dizzy in the past week? | Yes | No |
| Has your child been cranky or irritable in the last week? | Yes | No |
| Has your child felt nervous or scared in the last week? | Yes | No |
| Has your child had trouble paying attention in the last week? | Yes | No |
| Has your child been sad or depressed in the last week? | Yes | No |
| Has it been hard for your child to think in the last week? | Yes | No |
| Has your child had trouble seeing in the last week? | Yes | No |
| Has loud noise hurt your child’s ears in the last week? | Yes | No |
| Has your child had trouble sleeping in the last week? | Yes | No |
| Has your child been less interested in doing things in the last week? | Yes | No |
| Has your child’s personality seemed different in the last week? | Yes | No |
Neuroimaging
The MRI pulse sequence included sagittal T1-weighted spin echo images, axial T2-weighted and proton density fast spin echo images, coronal 2-dimensional gradient echo images, coronal fluid attenuated inversion recovery images, and axial diffusion-weighted echo planar images. Board-certified radiologists reviewed each MRI by using a standard protocol, unaware of the results of any other assessments. Each MRI was scored dichotomously in terms of whether it contained trauma-related intracranial abnormalities.
Assessment of Acute Clinical Status
Based on previous studies of acute clinical features that predict significant intracranial injury in children with mild TBI,36,37 the following clinical features were considered likely indicators of brain injury: LOC, GCS score of <15, injuries to other body regions, and 8 acute symptoms of concussion (ie, posttraumatic amnesia, vomiting, nausea, headache, diplopia, dizziness, disorientation to time, place, or person, or other mental status changes). Trauma-related intracranial abnormalities on MRI were also considered to be an indicator of more severe brain injury.
Data Analysis
The number of preinjury symptoms was subtracted from the number of PCSs reported at each follow-up assessment to adjust for premorbid status. Developmental trajectory analysis, a semi-parametric group-based method that relies on finite mixture modeling, was used to empirically identify groups of individuals displaying distinctive longitudinal trajectories of PCSs relative to preinjury symptom levels.32–33 The Bayesian information criterion was used to select the optimal number of trajectory groups. Children were assigned to the trajectory group for which they had the highest posterior probability of membership. All children with at least 1 assessment of PCSs were included in the analysis, which yields estimates for each individual by using all available data.
Membership in the trajectory groups for children with mild TBI versus OI was examined by using multinomial logistic regression. Gender, race, age at injury, and socioeconomic status were included as additional independent variables, to control for demographic factors. The relationship of acute clinical features to PCS trajectories was assessed by dichotomizing the mild TBI group based on whether a specific clinical feature was present or absent, and comparing membership in the PCS trajectory groups for children with mild TBI who did and did not display that feature versus children with OI. Analyses were conducted separately for each clinical feature.
RESULTS
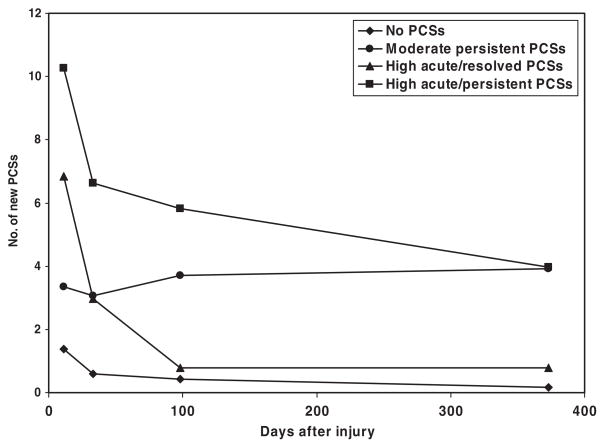
The developmental trajectory analysis yielded the best fit with a solution involving 5 groups. One group contained only 5 children and demonstrated a substantial reduction in PCSs relative to preinjury levels. This group was not included in additional analyses because of its small size. Figure 1 presents the mean number of PCSs relative to preinjury levels for the remaining 4 groups at each assessment.
FIGURE 1.
PCS trajectory groups.
The largest group (no PCSs in Fig 1; n = 193) displayed a small increase in PCSs 2 weeks after injury, followed by few if any PCSs subsequently. The second largest group (moderate persistent PCSs; n = 36) demonstrated a moderate increase in PCSs that persisted over all assessments. The last 2 groups (high acute/resolved PCSs and high acute/persistent PCSs; n = 33 and n = 18, respectively) showed large acute increases in PCSs 2 weeks after injury. The high acute/resolved PCS group showed a gradual resolution of PCSs, whereas the high acute/persistent PCS group showed moderate persistent PCSs even 12 months after injury.
As Table 3 shows, the mild TBI and OI groups differed in their membership in the trajectory groups (χ2 = 19.16; P < .001). Relative to membership in the no PCS group, children with mild TBI were more likely to belong to the high acute/resolved PCS group and the high acute/persistent PCS group than children with OI. The mild TBI and OI groups did not differ significantly in membership in the moderate persistent PCS group.
TABLE 3.
Group Membership in PCS Trajectories
| PCS Trajectory | Group |
|||||
|---|---|---|---|---|---|---|
| OI (Reference) |
Mild TBI |
OR | 95% CI | |||
| n | % | n | % | |||
| No PCSs | 76 | 79 | 117 | 64 | — | — |
| Moderate persistent PCSs | 14 | 15 | 22 | 12 | 1.19 | 0.56–2.53 |
| High acute/resolved PCSs | 5 | 5 | 28 | 15 | 3.82 | 1.40–10.45 |
| High acute/persistent PCSs | 1 | 1 | 17 | 9 | 13.68 | 1.75–106.74 |
OR indicates odds ratio. The multinomial logistic regression analyses examined the odds of belonging to trajectory groups 2, 3, and 4 compared with group 1, for the mild TBI group relative to the OI group.
Specific clinical features were associated with higher odds of belonging to the high acute/resolved and high acute/persistent PCS groups (see Table 4). Persistent posttraumatic amnesia, disorientation, and other mental status changes each predicted substantially higher overall odds of belonging to the high acute/resolved PCS group relative to the OI group than did mild TBI without those features. Similarly, LOC, injuries to other body regions, nausea, dizziness, disorientation, and other mental status changes each predicted higher overall odds of belonging to the high acute/persistent PCS group relative to the OI group than did mild TBI without those features.
TABLE 4.
Relationship of Acute Clinical Features to PCS Trajectories
| PCS Trajectory | Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OI (Reference) |
Mild TBI |
|||||||||
| Feature Not Displayed |
Feature Displayed |
|||||||||
| n | % | n | % | OR | 95% CI | n | % | OR | 95% CI | |
| LOC (overall χ2 = 20.71; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 73 | 65 | — | — | 44 | 61 | — | — |
| Moderate persistent PCSs | 14 | 15 | 14 | 13 | 1.15 | 0.50–2.62 | 8 | 11 | 1.19 | 0.56–2.53 |
| High acute/resolved PCSs | 5 | 5 | 16 | 14 | 3.44 | 1.19–9.95 | 12 | 17 | 3.82 | 1.40–10.45 |
| High acute/persistent PCSs | 1 | 1 | 9 | 8 | 10.81 | 1.32–88.77 | 8 | 11 | 13.68 | 1.75–106.74 |
| MRI abnormal (overall χ2 = 20.34; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 94 | 64 | — | — | 20 | 63 | — | — |
| Moderate persistent PCSs | 14 | 15 | 17 | 12 | 1.14 | 0.52–2.53 | 4 | 13 | 1.47 | 0.41–5.23 |
| High acute/resolved PCSs | 5 | 5 | 23 | 16 | 3.99 | 1.43–11.14 | 5 | 16 | 3.93 | 1.00–15.39 |
| High acute/persistent PCSs | 1 | 1 | 14 | 10 | 14.36 | 1.81–114.28 | 3 | 9 | 15.33 | 1.44–163.18 |
| GCS <15 (overall χ2 = 26.34; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 105 | 66 | — | — | 12 | 50 | — | — |
| Moderate persistent PCSs | 14 | 15 | 19 | 12 | 1.13 | 0.52–2.45 | 3 | 13 | 1.80 | 0.42–7.65 |
| High acute/resolved PCSs | 5 | 5 | 20 | 13 | 3.03 | 1.07–8.53 | 8 | 33 | 12.62 | 3.35–47.47 |
| High acute/persistent PCSs | 1 | 1 | 16 | 10 | 13.85 | 1.77–108.25 | 1 | 4 | 9.56 | 0.53–173.17 |
| Associated injury (overall χ2 = 21.02; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 87 | 63 | — | — | 30 | 65 | — | — |
| Moderate persistent PCSs | 14 | 15 | 18 | 13 | 1.30 | 0.59–2.85 | 4 | 9 | 0.87 | 0.26–2.95 |
| High acute/resolved PCSs | 5 | 5 | 22 | 16 | 3.98 | 1.42–11.13 | 6 | 13 | 3.32 | 0.92–11.95 |
| High acute/persistent PCSs | 1 | 1 | 11 | 8 | 11.60 | 1.44–93.56 | 6 | 13 | 20.54 | 2.29–184.05 |
| PTA (overall χ2 = 24.69; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 84 | 67 | — | — | 33 | 56 | — | — |
| Moderate persistent PCSs | 14 | 15 | 14 | 11 | 1.01 | 0.44–2.30 | 8 | 14 | 1.73 | 0.64–4.67 |
| High acute/resolved PCSs | 5 | 5 | 14 | 11 | 2.68 | 0.91–7.86 | 14 | 24 | 6.82 | 2.23–20.88 |
| High acute/persistent PCSs | 1 | 1 | 13 | 10 | 14.15 | 1.78–112.56 | 4 | 7 | 11.91 | 1.24–114.04 |
| Vomiting (overall χ2 = 32.86; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 57 | 56 | — | — | 60 | 73 | — | — |
| Moderate persistent PCSs | 14 | 15 | 12 | 12 | 1.53 | 0.63–3.71 | 10 | 12 | 0.94 | 0.38–3.27 |
| High acute/resolved PCSs | 5 | 5 | 23 | 23 | 7.36 | 2.54–21.36 | 5 | 6 | 1.28 | 0.35–4.67 |
| High acute/persistent PCSs | 1 | 1 | 10 | 10 | 20.56 | 2.45–172.47 | 7 | 9 | 9.33 | 1.10–79.09 |
| Nausea (overall χ2 = 27.95; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 69 | 64 | — | — | 48 | 63 | — | — |
| Moderate persistent PCSs | 14 | 15 | 9 | 8 | 0.67 | 0.33–2.05 | 13 | 17 | 1.74 | 0.73–4.13 |
| High acute/resolved PCSs | 5 | 5 | 22 | 20 | 5.15 | 1.83–14.54 | 6 | 8 | 1.96 | 0.56–6.87 |
| High acute/persistent PCSs | 1 | 1 | 8 | 7 | 11.09 | 1.33–92.86 | 9 | 12 | 17.24 | 2.08–143.10 |
| Headache (overall χ2 = 19.86; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 30 | 68 | — | — | 87 | 62 | — | — |
| Moderate persistent PCSs | 14 | 15 | 4 | 9 | 0.84 | 0.25–2.82 | 18 | 13 | 1.32 | 0.60–2.89 |
| High acute/resolved PCSs | 5 | 5 | 6 | 14 | 3.24 | 0.91–11.54 | 22 | 16 | 4.02 | 1.44–11.26 |
| High acute/persistent PCSs | 1 | 1 | 13 | 9 | 12.49 | 1.31–119.62 | 13 | 9 | 14.07 | 1.77–112.06 |
| Diplopia (overall χ2 = 198.46; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 103 | 64 | — | — | 14 | 64 | — | — |
| Moderate persistent PCSs | 14 | 15 | 20 | 12 | 1.23 | 0.57–2.65 | 2 | 9 | 0.92 | 0.18–4.64 |
| High acute/resolved PCSs | 5 | 5 | 24 | 15 | 3.72 | 1.34–10.31 | 4 | 18 | 4.58 | 1.08–19.49 |
| High acute/persistent PCSs | 1 | 1 | 15 | 9 | 13.64 | 1.73–107.36 | 2 | 9 | 13.95 | 1.14–170.22 |
| Dizziness (overall χ2 = 20.32; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 89 | 65 | — | — | 28 | 58 | — | — |
| Moderate persistent PCSs | 14 | 15 | 16 | 12 | 1.13 | 0.51–2.52 | 6 | 13 | 1.40 | 0.48–4.13 |
| High acute/resolved PCSs | 5 | 5 | 19 | 14 | 3.40 | 1.20–9.64 | 9 | 19 | 5.22 | 1.58–17.20 |
| High acute/persistent PCSs | 1 | 1 | 12 | 9 | 12.29 | 1.54–98.35 | 5 | 10 | 18.74 | 2.02–173.69 |
| Disorientation (overall χ2 = 29.55; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 111 | 67 | — | — | 6 | 33 | — | — |
| Moderate persistent PCSs | 14 | 15 | 20 | 12 | 1.13 | 0.52–2.42 | 2 | 11 | 2.67 | 0.47–15.30 |
| High acute/resolved PCSs | 5 | 5 | 21 | 13 | 3.06 | 1.10–8.58 | 7 | 39 | 19.84 | 4.62–85.13 |
| High acute/persistent PCSs | 1 | 1 | 14 | 8 | 11.85 | 1.50–93.64 | 3 | 17 | 59.47 | 4.97–711.60 |
| Other mental status changes (overall χ2 = 22.43; P < .001) | ||||||||||
| No PCSs | 76 | 79 | 82 | 67 | — | — | 35 | 57 | — | — |
| Moderate persistent PCSs | 14 | 15 | 16 | 13 | 1.25 | 0.56–2.80 | 6 | 10 | 1.08 | 0.37–3.10 |
| High acute/resolved PCSs | 5 | 5 | 15 | 12 | 2.94 | 1.01–8.55 | 13 | 21 | 5.87 | 1.92–17.94 |
| High acute/persistent PCSs | 1 | 1 | 10 | 8 | 11.64 | 1.43–94.92 | 7 | 12 | 18.06 | 2.10–155.45 |
PTA indicates persistent posttraumatic amnesia. For the analysis of each clinical feature, the mild TBI group was divided into those who did and did not display the feature. The multinomial logistic regression analyses examined the odds of belonging to trajectory groups 2, 3, and 4 compared with group 1, for the TBI groups relative to the OI group.
The individual clinical features were not independent of one another. LOC was positively associated with abnormalities on MRI (χ2 = 3.60; P < .05) posttraumatic amnesia (χ2 = 3.66; P < .05), and other injuries ( ; P < .05). Other injuries also were marginally associated with abnormalities on MRI (χ2 = 3.24; P < .07). A GCS score of <15 was positively associated with posttraumatic amnesia (χ2 = 6.19; P < .05), disorientation (χ2 = 17.35; P < .05), and other mental status changes (χ2 = 10.73; P < .05).
A multivariate multinomial logistic regression that included all individual clinical features was not feasible because of the study sample size. Instead, a cumulative severity index was calculated by summing the total number of clinical features displayed by each child with mild TBI. The mild TBI group was dichotomized into low and high severity groups based on the median number of features. Children who displayed ≤3 acute clinical features were classified as low severity and those who displayed ≥4 features were classified high severity. The distribution of membership in the PCS trajectory groups for children with mild TBI at low and high severity versus children with OI was examined by using multinomial regression analysis.
The mild TBI severity groups and OI group differed in their membership in the PCS trajectory groups (see Table 5). Both mild TBI severity groups were more likely than the OI group to belong to the high acute/resolved or high acute/persistent PCS groups (33% of high severity group, 18% of low severity group, 6% of OI group), but the odds were significantly higher only for the high severity group. When the 2 mild TBI severity groups were compared directly in an analysis that included only children with mild TBI, the high severity group demonstrated higher odds of belonging to the high acute/persistent group than did the low severity group (14% for high severity versus 6% for low severity; odds ratio: 3.27 [95% confidence interval (CI): 1.02–10.43]).
TABLE 5.
Relationship of Mild TBI Severity to PCS Trajectories
| PCS Trajectory | Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OI (Reference) |
Mild TBI, Low Severity |
Mild TBI, High Severity |
||||||||
| n | % | n | % | OR | 95% CI | n | % | OR | 95% CI | |
| No PCSs | 76 | 79 | 64 | 70 | — | — | 50 | 56 | — | — |
| Moderate persistent PCSs | 14 | 15 | 11 | 12 | 1.05 | 0.43–2.54 | 10 | 11 | 1.39 | 0.55–3.49 |
| High acute/resolved PCSs | 5 | 5 | 11 | 12 | 2.83 | 0.92–8.68 | 17 | 19 | 5.45 | 1.86–16.01 |
| High acute/persistent PCSs | 1 | 1 | 5 | 6 | 7.45 | 0.83–66.89 | 12 | 14 | 24.15 | 2.95–197.54 |
Overall χ2 = 25.94; P < .001.
The mild TBI group was divided into those who displayed ≤3 (low severity) and ≥4 (high severity) acute clinical features of brain injury. The multinomial logistic regression analyses examined the odds of belonging to the moderate persistent, high acute/resolved, and high acute/persistent trajectories compared with the no PCSs trajectory, for the TBI groups relative to the OI group.
DISCUSSION
The findings indicate that mild TBI are associated with a greater likelihood of longitudinal trajectories characterized by high levels of acute PCSs, and the likelihood of membership in those trajectories is highest in the presence of certain clinical features. Several of the clinical features examined in this study have served as proxies of more severe brain injury, sometimes referred to as complicated mild TBI.28 Indeed, the clinical features that were most strongly associated with trajectories involving high levels of acute PCSs (ie, loss of consciousness, posttraumatic amnesia, GCS score of <15, disorientation, other mental status changes) have also been shown in previous research to be predictive of important clinical events (eg, significant intracranial injury) in children with mild TBI.36,37
Many previous studies of PCSs have excluded children with more severe injuries, and this has engendered potentially erroneous conclusions about the outcomes of mild TBI. Indeed, the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury reviewed the prognosis of mild TBIs and cited 2 studies to justify their conclusion that PCSs in children “are usually transient in nature”4 (p88) and “appear to be largely resolved within 2 to 3 months of the injury”4 (p85). However, both studies they cited excluded children with complicated mild TBI, as have some more recent studies.15 Studies that have included children with more severe injuries have tended to find more pronounced and persistent differences in PCSs compared with children with OI or healthy children.11,13
Few previous studies have directly compared the outcomes of complicated (ie, more severe) versus uncomplicated (ie, less severe) mild TBI in children, but their results have generally suggested that children with more severe injuries display poorer outcomes.30,31,38,39 However, only 1 of those studies examined the occurrence of PCSs,30 and it suffered from a variety of methodologic problems (ie, small sample size, no control group). As best we can tell, ours is the first study of PCSs in children that involved a definition of mild TBI that encompassed a spectrum of injury severity and also embodied other methodologic strengths, including prospective recruitment of consecutive patients, inclusion of a comparison group of children with OI, and longitudinal follow-up with minimal attrition. We believe the current results provide rigorous support for assertions that acute increases in PCSs and, in some cases, persistent PCSs, are more likely after mild TBI than OI, and especially after complicated mild TBI.
Unlike previous investigations, the current study did not treat children with mild TBI as a homogenous group. The developmental trajectory analysis used in this study highlights the importance of examining individual patterns of outcomes across time. Thus, children in the moderate persistent and high acute/persistent PCS trajectories displayed similar symptom levels 12 months after injury but differed markedly in acute symptom levels, as well as in their proportional representation in the mild TBI and OI groups. The same could be said for the no PCS and high acute/resolved PCS groups. A cross-sectional comparison 1 year after injury would have led to the erroneous conclusion that mild TBI and OI result in similar outcomes, whereas the developmental trajectory analysis showed that the groups differed in the pattern of PCSs across time.
The differences between the mild TBI and OI groups in the likelihood of the moderate persistent and high/acute persistent PCS trajectories suggests that persistent PCSs can result for different reasons for different children. Children with mild TBI were more likely than those with OI to belong to the high acute/persistent PCS trajectory, particularly if their injuries were more severe. Thus, membership in the high acute/persistent PCS trajectory probably was more strongly influenced by brain injury. In contrast, similar proportions of the mild TBI and OI groups belonged to the moderate persistent PCS trajectory, regardless of severity, suggesting that PCSs may increase and persist for some children for reasons other than brain insult. Thus, the long-standing debate regarding physiogenesis versus psychogenesis probably needs to be reframed. The 2 explanations are not mutually exclusive, and the relative importance of brain injury versus noninjury-related factors in accounting for PCSs after mild TBIs likely varies across individual children.27
If high acute and persistently elevated levels of PCSs predict significant functional impairment, then the identification of factors that distinguish the high acute/persistent PCS group from the high acute/resolved PCS group, and both high acute groups from the moderate persistent PCS group, will be an important goal with significant clinical implications. In the interim, the current results are heartening, because most children with mild TBIs do not display significant increases in PCSs. Nearly two thirds of the children with mild TBIs belonged to the no PCS group. On the other hand, the findings also suggest that a substantial minority of children with mild TBIs display significant acute increases in PCSs that in some cases persist over time. Thus, nearly 25% of the children with mild TBIs belonged to the high acute/persistent and high acute/resolved PCS groups compared with only 5% of the OI group, and the proportion was even higher among children with complicated mild TBI. Physicians and other health care providers should consider monitoring children with mild TBI, particularly in the presence of clinical features indicative of more severe brain injury, so that they can identify the minority who display acute and persistent increases in PCSs and intervene appropriately. Anticipatory guidance may help prevent the onset of PCSs40 and brief cognitive-behavioral therapy may help to ameliorate PCSs when they do occur.41,42
The clinical implications of the findings are tempered by several study limitations. The recruitment rates for the mild TBI and OI groups were both below 50%. Participants may have differed from nonparticipants, resulting in recruitment bias.43 However, participants and nonparticipants did not differ demographically. In contrast, attrition is unlikely to have introduced any bias, because the analysis of the longitudinal trajectories of PCSs included all children with at least 1 assessment of PCSs. Another shortcoming was that all clinical features were weighted equally in the cumulative severity index and multiple symptoms were summed in the measure of PCSs. A larger sample would permit an examination of the specific contributions of different clinical indicators to different symptoms.
Importantly, the presence of mild TBIs, as well as clinical features indicative of more severe brain injury, accounted only partly for membership in the developmental trajectory groups. Research with children and adults indicates that both injury characteristics and non-injury-related variables help explain outcomes after mild TBI, offering tentative support for both neurogenic and psychogenic explanations of PCSs.12,44,45 The occurrence of PCSs after mild TBI in children is likely to reflect multiple factors, including premorbid vulnerability, changes in brain function, and postinjury child and family adjustment. The current findings suggest that severity of injury helps to account for persistent PCSs in some children with mild TBI, but not all. Additional research is needed to identify the entire range of factors that place children with mild TBI at risk for increased PCSs and to thereby improve their clinical care.46
What’s Known on This Subject
Mild TBIs are a common occurrence in children. Mild TBIs result in PCSs more often than injuries not involving the head, but whether and why PCSs persist over time remains controversial.
What This Study Adds
Children with mild TBI display different longitudinal trajectories of PCSs than children with OIs. They are more likely to display trajectories that involve acute increases in PCSs, especially when their acute clinical presentation reflects more severe brain injury.
Acknowledgments
This study was supported by National Institutes of Health grants HD44099 and HD39834 (to Dr Yeates).
Abbreviations
- TBI
traumatic brain injury
- PCS
postconcussive symptom
- OI
orthopedic injury
- LOC
loss of consciousness
- GCS
Glasgow Coma Scale
- AIS
Abbreviated Injury Scale
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Kraus JF. Epidemiological features of brain injury in children: occurrence, children at risk, causes and manner of injury, severity, and outcomes. In: Broman SH, Michel ME, editors. Traumatic Head Injury in Children. New York, NY: Oxford University Press; 1995. pp. 22–39. [Google Scholar]
- 2.Yeates KO. Closed-head injury. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric Neuropsychology: Research, Theory, and Practice. New York, NY: Guilford; 2000. pp. 92–116. [Google Scholar]
- 3.Bazarian JJ, McClung J, Shah MN, et al. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005;19(2):85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- 4.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 5.Satz P. Mild head injury in children and adolescents. Curr Dir Psychol Sci. 2001;10(3):106–109. [Google Scholar]
- 6.Satz P, Zaucha K, McCleary C, Light R, Asarnow R. Mild head injury in children and adolescents: a review of studies (1970–1995) Psychol Bull. 1997;122(2):107–131. doi: 10.1037/0033-2909.122.2.107. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.Farmer MY, Singer HS, Mellitis ED, Hall D, Charney E. Neurobehavioral sequelae of minor head injuries in children. Pediatr Neurosci. 1987;13(6):304–308. doi: 10.1159/000120348. [DOI] [PubMed] [Google Scholar]
- 10.Fay GC, Jaffe KM, Polissar NL, et al. Mild pediatric traumatic brain injury: a cohort study. Arch Phys Med Rehabil. 1993;74(9):895–901. [PubMed] [Google Scholar]
- 11.Mittenberg W, Wittner MS, Miller LJ. Postconcussion syndrome occurs in children. Neuropsychology. 1997;11(3):447–452. doi: 10.1037//0894-4105.11.3.447. [DOI] [PubMed] [Google Scholar]
- 12.Ponsford J, Willmott C, Rothwell A, et al. Cognitive and behavioral outcomes following mild traumatic head injury in children. J Head Trauma Rehabil. 1999;14(4):360–372. doi: 10.1097/00001199-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Yeates KO, Luria J, Bartkowski H, et al. Post-concussive symptoms in children with mild closed-head injuries. J Head Trauma Rehabil. 1999;14(4):337–350. doi: 10.1097/00001199-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 14.McKinlay A, Dalrymple-Alford JC, Horwood LJ, Fergusson DM. Long-term psychosocial outcomes after mild head injury in early childhood. J Neurol Neurosurg Psychiatry. 2002;73(3):281–288. doi: 10.1136/jnnp.73.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nacajauskaite O, Endziniene M, Jureniene K, Schrader H. The validity of post-concussion syndrome in children: a controlled historical cohort study. Brain Dev. 2006;28(8):507–514. doi: 10.1016/j.braindev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Alexander MP. Minor traumatic brain injury: a review of physiogenesis and psychogenesis. Semin Clin Neuropsychiatry. 1997;2(3):177–187. doi: 10.1053/SCNP00200177. [DOI] [PubMed] [Google Scholar]
- 17.Lishman WA. Physiogenesis and psychogenesis in the postconcussional syndrome. Br J Psychiatry. 1988;153:460–469. doi: 10.1192/bjp.153.4.460. [DOI] [PubMed] [Google Scholar]
- 18.Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma: part II. Clinical implications. J Clin Exp Neuropsychol. 1997;19(3):432–457. doi: 10.1080/01688639708403871. [DOI] [PubMed] [Google Scholar]
- 19.Gasquoine PG. Postconcussion symptoms. Neuropsychol Rev. 1997;7(2):77–85. doi: 10.1023/b:nerv.0000005945.58251.c0. [DOI] [PubMed] [Google Scholar]
- 20.Elson LM, Ward CC. Mechanisms and pathophysiology of mild head injury. Semin Neurol. 1994;14(1):8–18. doi: 10.1055/s-2008-1041053. [DOI] [PubMed] [Google Scholar]
- 21.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 22.Levin HS, Amparo E, Eisenberg HM, et al. Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg. 1987;66(5):706–713. doi: 10.3171/jns.1987.66.5.0706. [DOI] [PubMed] [Google Scholar]
- 23.Leininger BE, Gramling SE, Farrell AD, Kreutzer JS, Peck E., III Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry. 1990;53(4):293–296. doi: 10.1136/jnnp.53.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruff RM, Crouch JA, Troster AI, et al. Selected cases of poor outcome following a minor brain trauma: comparing neuropsychological and positron emission tomography assessment. Brain Inj. 1994;8(4):297–308. doi: 10.3109/02699059409150981. [DOI] [PubMed] [Google Scholar]
- 25.Varney NR, Bushnell DL, Nathan M, et al. NeuroSPECT correlates of disabling mild head injury: preliminary findings. J Head Trauma Rehabil. 1995;10:18–28. [Google Scholar]
- 26.Dikman SS, Levin HS. Methodological issues in the study of mild head injury. J Head Trauma Rehabil. 1993;8(3):30–37. [Google Scholar]
- 27.Yeates KO, Taylor HG. Neurobehavioral outcomes of mild head injury in children and adolescents. Pediatr Rehabil. 2005;8(1):5–16. doi: 10.1080/13638490400011199. [DOI] [PubMed] [Google Scholar]
- 28.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 30.Falk A-C, Cederfjäll C, Von Wendt L, Klang B. Are the symptoms and severity of head injury predictive of clinical findings three months later? Acta Paediatr. 2006;95(12):1533–1539. doi: 10.1080/08035250600731957. [DOI] [PubMed] [Google Scholar]
- 31.Levin HS, Hanten G, Roberson G, et al. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. J Neurosurg Pediatr. 2008;1(6):416–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- 32.Nagin DS. Analyzing developmental trajectories: a semi-parametric, group-based approach. Psychol Methods. 1999;4(2):129–177. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 34.American Association for Automotive Medicine. The Abbreviated Injury Scale (AIS): 1990 Revision. Des Plaines, IL: American Association for Automotive Medicine; 1990. [Google Scholar]
- 35.Mayer T, Matlak M, Johnson D, Walker M. The modified injury severity scale in pediatric multiple trauma patients. J Pediatr Surg. 1980;15(6):719–726. doi: 10.1016/s0022-3468(80)80271-5. [DOI] [PubMed] [Google Scholar]
- 36.Dunning J, Batchelor J, Stratford-Smith P, et al. A meta-analysis of variables that predict significant intracranial injury in minor head trauma. Arch Dis Child. 2004;89(7):653–659. doi: 10.1136/adc.2003.027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunning J, Daly JP, Lomas J-P, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child. 2006;91(11):885–891. doi: 10.1136/adc.2005.083980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polissar NL, Fay GC, Jaffe KM, et al. Mild pediatric traumatic brain injury: adjusting significance levels for multiple comparisons. Brain Inj. 1994;8(3):249–264. doi: 10.3109/02699059409150977. [DOI] [PubMed] [Google Scholar]
- 39.Hessen E, Nestvold K, Sundet K. Neuropsychological function in a group of patients 25 years after sustaining minor head injuries as children and adolescents. Scand J Psychol. 2006;47(4):245–251. doi: 10.1111/j.1467-9450.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 40.Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- 41.Miller LJ, Mittenberg W. Brief cognitive behavioral interventions in mild traumatic brain injury. Appl Neuropsychol. 1998;5(4):172–183. doi: 10.1207/s15324826an0504_2. [DOI] [PubMed] [Google Scholar]
- 42.Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829–836. doi: 10.1076/jcen.23.6.829.1022. [DOI] [PubMed] [Google Scholar]
- 43.McCullagh S, Feinstein A. Outcome after mild traumatic brain injury: an examination of recruitment bias. J Neurol Neurosurg Psychiatry. 2003;74(1):39–43. doi: 10.1136/jnnp.74.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000;6(5):568–579. doi: 10.1017/s1355617700655066. [DOI] [PubMed] [Google Scholar]
- 45.Luis CA, Vanderploeg RD, Curtiss G. Predictors of postconcussion symptom complex in community dwelling male veterans. J Int Neuropsychol Soc. 2003;9(7):1001–1015. doi: 10.1017/S1355617703970044. [DOI] [PubMed] [Google Scholar]
- 46.Schnadower D, Vazquez H, Lee J, Dayan P, Roskind CG. Controversies in the evaluation and management of minor blunt head trauma in children. Curr Opin Pediatr. 2007;19(3):258–264. doi: 10.1097/MOP.0b013e3281084e85. [DOI] [PubMed] [Google Scholar]
- 47.Stevens G, Cho JH. Socioeconomic indexes and the new 1980 census occupational classification scheme. Soc Sci Res. 1985;14(2):142–168. [Google Scholar]