Abstract
An otherwise healthy 6-week-old girl who presented with an isolated left third cranial nerve palsy underwent MRI that revealed an enhancing mass intrinsic to the left third cranial nerve. Rapid enlargement of the lesion over 1 month led to subtotal neurosurgical resection of an atypical teratoid/rhabdoid tumor (AT/RT), a rare, highly aggressive malignancy of infancy closely related histologically to medulloblastoma and primitive neuroectodermal tumor. Despite aggressive chemotherapy, the patient died within 6 months of presentation. This is the first report of an AT/RT presenting as an isolated third cranial nerve palsy caused by tumor arising from within the nerve.
A typical teratoid/rhabdoid tumor (AT/RT) is a rare, highly aggressive, central nervous system (CNS) malignancy of infancy that was definitively distinguished from its closest histologic relatives, medulloblastoma and primitive neuroectodermal tumor (PNET), in a 32-patient case series in 1995 (1). Since then, approximately 200 cases have been reported, 94% of which have been seen in patients younger than 5 years (2). Symptoms of AT/RT are either nonspecific, including lethargy, vomiting, or failure to thrive, or related to the location of the tumor, including cranial nerve palsies, ataxia, head tilt, or extremity paresis (3). We report an infant presenting with an isolated third cranial nerve palsy caused by AT/RT intrinsic to the third cranial nerve, a previously undescribed phenomenon.
CASE REPORT
An otherwise healthy 6-week-old girl was seen with 3 days of left eye outward deviation. The patient was the product of an unremarkable pregnancy and was delivered without complications by cesarean section, weighing 9 pounds 8 ounces, at full term. The patient had no history of illness, travel, or trauma. There was no pertinent family history.
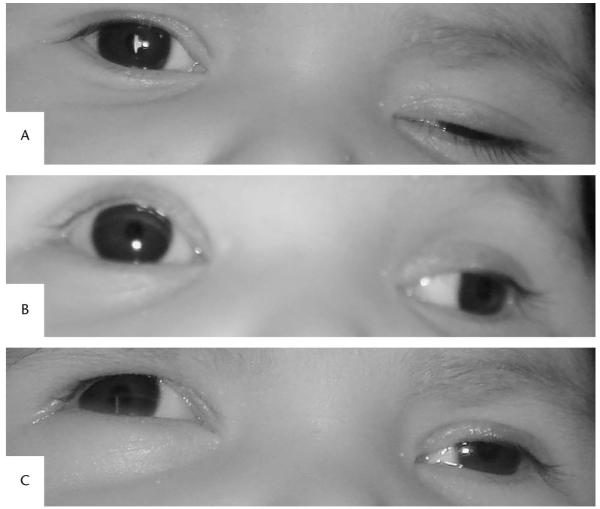
Examination revealed that each eye was capable of fixing and following intermittently with equal aversion to occlusion. In primary gaze, the right eye was centered with normal eyelid position, and the left eye was exotropic and hypotropic with ptosis (Fig. 1A). Ocular ductions of the right eye were full. The left eye displayed reduced supraduction (Fig. 1B) and adduction (Fig. 1C). There was variable upper lid ptosis. A review of photographs of the patient taken during the first 4 weeks of life revealed that these ophthalmic abnormalities were not present at that time. The right pupil measured 4 mm in dark and 2 mm in light, the left pupil measured 6 mm in dark and 4 mm in light. There was no afferent pupillary defect. Anterior segment and dilated ophthalmoscopic examinations were normal in both eyes.
FIG. 1.
External photographs of the infant showing features of a left third cranial nerve palsy. A. Primary gaze position: right eye centered and left upper lid ptosis. B. Partial upgaze: reduced elevation of the left eye. C. Partial right gaze: reduced abduction of the left eye.
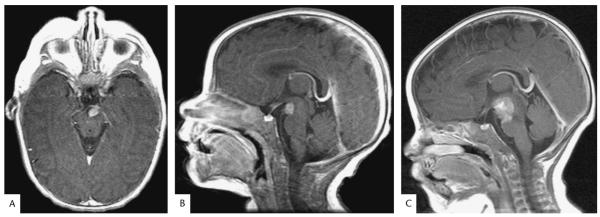
Brain MRI revealed an 8-mm homogenously enhancing mass within the interpeduncular cistern inseparable from the left third cranial nerve (Fig. 2A,B). Results of CT angiography, MRA, and lumbar puncture with cytology and flow cytometry were unremarkable. Results of complete blood count, basic chemistry panel, C-reactive protein, erythrocyte sedimentation rate, liver function tests, thyroid function tests, and Coombs test were normal. Repeat MRI of the brain and spine after 1 month revealed a significant increase in the size of the mass to 18 mm with extension along the left third cranial nerve and invasion of the midbrain and pons (Fig. 1C). There was no evidence of leptomeningeal dissemination.
FIG. 2.
Magnetic resonance images of the infant with an atypical teratoid/rhabdoid tumor. A, B. Postcontrast T1 axial and sagittal MRIs at presentation shows a 6 × 8 × 8 mm mass with homogenous enhancement within the interpeduncular cistern inseparable from the left third cranial nerve. C. Postcontrast T1 sagittal MRI performed 1 month later shows an increase in the size of the mass to 22 × 18 × 18 mm with extension along the third cranial nerve and invasion of the midbrain and pons.
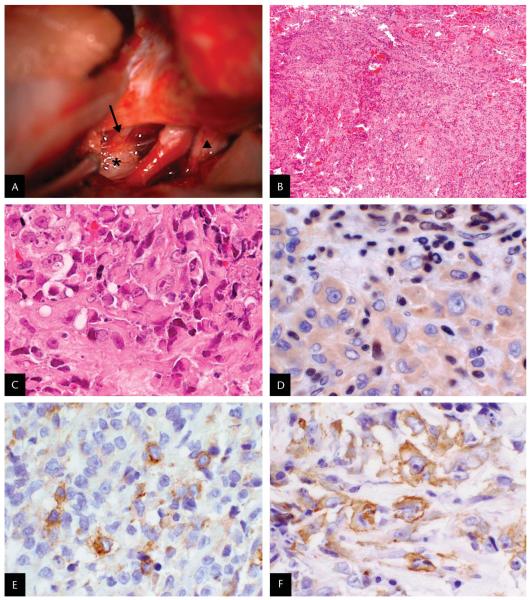
A left frontotemporal craniotomy with orbitozygomatic extension disclosed that the left third cranial nerve appeared normal anteriorly but was expanded by tumor posteriorly (Fig. 3A). With no clear boundary between tumor and brainstem parenchyma, a subtotal resection was performed.
FIG. 3.
A. Intraoperative photograph: superior view of the anterior brainstem. The third cranial nerve (arrow) is lateral to the internal carotid artery, and the optic nerve (triangle) is located medially. A white, gelatinous tumor (star) is seen arising from the posterior aspect of the third cranial nerve. B–F. Histopathology of the surgical specimen. B. Small and large blue cells arranged in cords and nodules separated by fibrous septae with hemorrhage, necrosis, and mitotic figures (hematoxylin and eosin: original magnification ×40). C. Anaplastic, large pink cells with cytoplasmic inclusions and vesicular nuclei with prominent nucleoli (hematoxylin and eosin; original magnification: ×400). D. Large tumor cells exhibit no nuclear labeling for integrase interactor-1 (INI1), whereas infiltrating lymphocytes and endothelial cells show the expected nuclear reactivity of normal cell types (BAF47 anti-INI1 immunohistochemical staining; original magnification ×400) (4). E. Tumor cells exhibit positive labeling for epithelial membrane antigen (EMA) (anti-EMA immunohistochemical staining; original magnification: ×400). F. Tumor cells exhibit positive labeling for smooth muscle actin (SMA) (anti-SMA immunohistochemical staining; original magnification ×400).
Light microscopy revealed hemorrhage, necrosis, mitotic figures, and large pink, anaplastic cells with vesicular nuclei and prominent nucleoli with cytoplasmic inclusions, cells characteristic of rhabdoid tumors (Fig. 3B–C). Immunohistochemistry demonstrated tumor cells to be positive for epithelial membrane antigen (EMA) (Fig. 3E), smooth muscle actin (SMA) (Fig. 3F), and glial fibrillary acidic protein (GFAP) and negative for desmin, synaptophysin, and human melanoma black-45 (HMB-45). There was loss of nuclear expression of integrase interactor-1 (INI1) within tumor cells, supporting a diagnosis of AT/RT (Fig. 3D). Analysis of DNA isolated from formalin-fixed tissue did not reveal a coding sequence mutation in the INI1 gene.
A five-cycle chemotherapeutic regimen was started according to the Head Start II Protocol, including vincristine, etoposide, cyclophosphamide, methotrexate, and cisplatinum, with the eventual goal of hematopoietic stem cell transplantation. Five months after the initial presentation, the patient developed vomiting. Repeat MRI revealed hydrocephalus with widespread leptomeningeal disease extending around the brainstem and spine. A palliative ventriculoperitoneal shunt was placed. The patient died several weeks later, 6 months after the initial presentation.
DISCUSSION
Among acquired, pupil-involving third cranial nerve palsies in a child, 10% are due to neoplasm (5,6). Although the sixth and seventh cranial nerves are most commonly involved with AT/RT (3), the current patient demonstrates that AT/RT may occur intrinsic to the third cranial nerve and present as an isolated third cranial nerve palsy.
AT/RTs represent approximately 1.3% of pediatric brain tumors (2). MRI typically reveals isointense or hypointense signal on both T1 and T2 sequences and enhancement with gadolinium. The signal is heterogenous due to cysts, calcification, hemorrhage, and necrosis (7). However, there are no specific imaging features for AT/RTs. PNETs have similar imaging characteristics. Compared with PNETs, AT/RTs may have an increased rate of leptomeningeal metastasis at presentation and may be more likely to have associated hemorrhage (7). Both types of tumors require assessment of cerebrospinal fluid cytology and MRI scanning of the entire neuroaxis to rule out subarachnoid dissemination.
Definitive diagnosis of AT/RT is made by correlating histologic and immunohistochemical findings, as in the present patient. Histologically, rhabdoid cells can vary in size from small to large. The larger forms typically have homogeneous bright pink cytoplasm that may appear to contain an inclusion. Cell margins are usually well-defined and nuclei are round, often with a prominent nucleolus (1). The immunohistochemical profile of these tumors is complex, as there is often variation of expression within a given tumor, but it is essential to distinguish them from other primary nervous system tumors, most commonly germ cell tumors and PNETs (3). Rhabdoid cells are most commonly positive for EMA, vimentin, and SMA. They may also express GFAP, neurofilament protein, keratin, and synaptophysin. In contrast, they are consistently negative for desmin, which is confined to mesenchymal tumors and PNETs. Similarly, germ cell markers, such as placental alkaline phosphatase and α-fetoprotein, are consistently negative in rhabdoid tumor cells (1,3,8,9). HMB-45, a marker for melanocytic tumors, is also negative.
Supplementing these immunohistochemical characteristics, the absence of nuclear expression of INI1 has emerged as a critical tool for accurate AT/RT diagnosis (10). The INI1 protein is a component of the chromatin-remodeling complex SW1/SNF/BAF that functions in the transcriptional activation and repression of a variety of genes. Although its precise role in tumorigenesis is not defined, INI1 is the predominant rhabdoid tumor suppressor gene. Mutation or deletion of both copies of the INI1 gene is observed in approximately 70% of rhabdoid tumors, with an additional 20%–25% having reduced expression at the RNA or protein level. In addition, approximately 18% of patients with nervous system AT/RTs have germline mutations (4). As all normal cells at all stages of development exhibit nuclear INI1 expression, blood vessels, fibroblasts, and infiltrating lymphocytes serve as internal positive controls for INI1 immunohistochemistry, as in the present patient. Although loss of nuclear expression of INI1 is believed to be highly specific for AT/RT and retention of nuclear expression of INI1 strongly suggests an alternate diagnosis, loss of INI1 staining has been reported rarely in other tumors (4,10).
Compared with an 85% 5-year survival for standardrisk pediatric medulloblastoma, the prognosis of AT/RT is poor, with death occurring in 85% of patients within 2 years (4,11). Therefore, correct diagnosis using histologic evaluation and immunohistochemistry is important to allow appropriate prognostication and treatment.
This patient demonstrates that AT/RT may present as an isolated third cranial nerve palsy and highlights the importance of neuroimaging in instances of acquired cranial nerve palsy in children, as well as the histologic and genetic features that differentiate AR/RT from other primary nervous system malignancies of infancy.
Acknowledgments
The authors acknowledge the technical assistance of Laura Tooke.
This work was supported by a National Institutes of Health Center Grant P30-EY014801 and CA 46274 (to J.A.B.) and an unrestricted grant to the University of Miami from Research to Prevent Blindness.
REFERENCES
- 1.Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24:21–8. doi: 10.1007/BF01052653. [DOI] [PubMed] [Google Scholar]
- 2.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–11. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 3.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 4.Biegel JA. Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus. 2006;20:E11. doi: 10.3171/foc.2006.20.1.12. [DOI] [PubMed] [Google Scholar]
- 5.Miller NR. Solitary oculomotor nerve palsy in childhood. Am J Ophthalmol. 1977;83:106–11. doi: 10.1016/0002-9394(77)90197-0. [DOI] [PubMed] [Google Scholar]
- 6.Ng YS, Lyons CJ. Oculomotor nerve palsy in childhood. Can J Ophthalmol. 2005;40:645–53. doi: 10.1016/S0008-4182(05)80062-6. [DOI] [PubMed] [Google Scholar]
- 7.Parmar H, Hawkins C, Bouffet E, et al. Imaging findings in primary intracranial atypical teratoid/rhabdoid tumors. Pediatr Radiol. 2006;36:126–32. doi: 10.1007/s00247-005-0037-6. [DOI] [PubMed] [Google Scholar]
- 8.Burger PC, Yu IT, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a pediatric oncology group study. Am J Surg Pathol. 1998;22:1083–92. doi: 10.1097/00000478-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Parwani AV, Stelow EB, Pambuccian SE, et al. Atypical teratoid/rhabdoid tumor of the brain: cytopathologic characteristics and differential diagnosis. Cancer. 2005;105:65–70. doi: 10.1002/cncr.20872. [DOI] [PubMed] [Google Scholar]
- 10.Haberler C, Laggner U, Slavc I, et al. Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol. 2006;30:1462–8. doi: 10.1097/01.pas.0000213329.71745.ef. [DOI] [PubMed] [Google Scholar]
- 11.Hilden JM, Meerbaum S, Burger P, et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22:2877–84. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]