Abstract
Treatment of diseases of the posterior segment of the eye, such as age-related macular degeneration, cytomegalovirus retinitis, diabetic retinopathy, posterior uveitis and retinitis pigmentosa, requires novel drug delivery systems that can overcome the many barriers for efficacious delivery of therapeutic drug concentrations. This challenge has prompted the development of biodegradable and nonbiodegradable sustained-release systems for injection or transplantation into the vitreous as well as drug-loaded nanoparticles, microspheres and liposomes. These drug delivery systems utilize topical, systemic, subconjunctival, intravitreal, transscleral and iontophoretic routes of administration. The focus of research has been the development of methods that will increase the efficacy of spatiotemporal drug application, resulting in more successful therapy for patients with posterior segment diseases. This article summarizes recent advances in the research and development of drug delivery methods of the posterior chamber of the eye, with an emphasis on the use of implantable devices as well as micro- and nanoparticles.
Keywords: biodegradable, drug delivery, intravitreal, iontophoresis, microbubbles, microspheres, nonbiodegradable, subconjunctival, sustained release, transscleral
Drug delivery, in its simplest, most common form, is comprised of fast-acting chemical compounds that are dispensed either topically or systemically. Depending on the method of application, the drugs are distributed locally, regionally or systemically, and may lead to various undesired side effects, such as drug accumulation and toxicity. While the topical and systemic forms of drug delivery are useful in certain disease processes, they pose many limitations to the treatment of diseases of the posterior eye (Figure 1), including age-related macular degeneration (AMD), diabetic macular edema (DME), endophthalmitis and retinitis pigmentosa. While many review articles in recent years have focused on drug delivery to the eye as shown in Table 1 [1–17], this article emphasizes recent advances in ocular drug delivery techniques most suitable for drug delivery to the posterior segment (Figure 2).
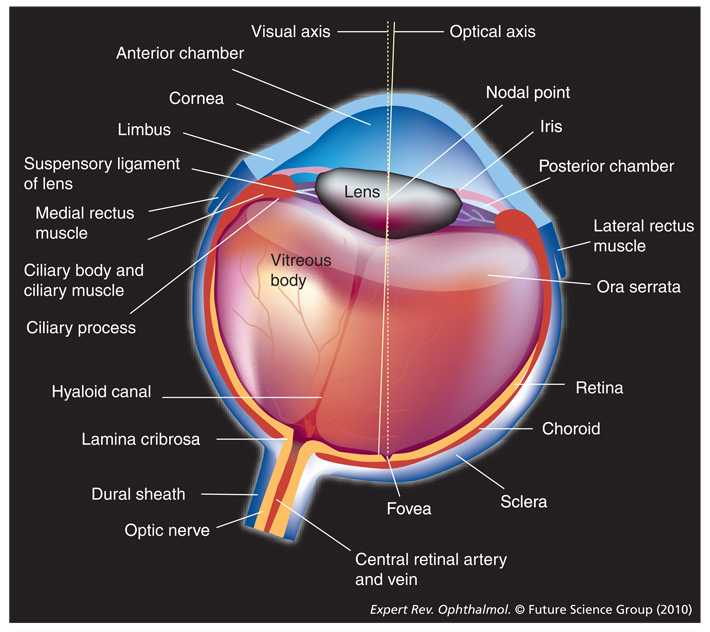
Figure 1.
The eye.
Table 1.
Recent reviews in drug delivery.
| Subject | Article title | Year | Ref. |
|---|---|---|---|
| General drug delivery reviews |
Novel drug delivery systems for retinal diseases. A review. | 2009 | [1] |
| Review of ocular drug delivery. | 2006 | [2] | |
| Sustained-release drug implants for the treatment of intraocular disease. | 2004 | [3] | |
| Sustained transscleral drug delivery. | 2008 | [4] | |
| Current and future ophthalmic drug delivery systems. A shift to the posterior segment. | 2008 | [5] | |
| Recent perspectives in ocular drug delivery. | 2009 | [6] | |
| Drug delivery methods for posterior segment disease. | 2007 | [7] | |
| The origins and evolution of ‘controlled’ drug delivery systems. | 2008 | [8] | |
| Ophthalmic drug delivery: development and regulatory considerations. | 2009 | [9] | |
| Membranes and barriers | Ocular novel drug delivery: impacts of membranes and barriers. | 2008 | [10] |
| Macular disorders | A review of treatments for macular degeneration: a synopsis of currently approved treatments and ongoing clinical trials. |
2004 | [11] |
| Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: present and future applications. |
2007 | [12] | |
| Microbubbles | Therapeutic use of ultrasound targeted microbubble destruction: a review of non-cardiac applications. |
2006 | [13] |
| Lipid-based systems | A review of the application of lipid-based systems in systemic, dermal/transdermal and ocular drug delivery. |
2008 | [14] |
| Endophthalmitis | Endophthalmitis: a review of current evaluation and management. | 2007 | [15] |
| Neovascular disease | Anti-vascular endothelial growth factor (VEGF) therapy for ocular neovascular disease. | 2007 | [16] |
| Transporters | Role of transporters in ocular drug delivery system. | 2009 | [17] |
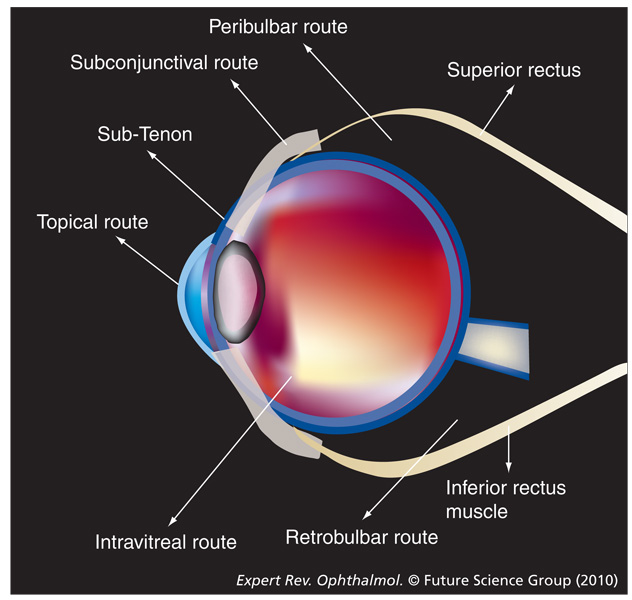
Figure 2.
Alternate methods of drug delivery.
Topical drug application
Topical application to the anterior eye has been proven successful in the treatment of diseases owing to easy access to the target site. However, the adoption of mechanisms in ensuring topical drug penetration to the posterior eye presents numerous challenges. While being the least invasive method of drug application, topically applied drugs are hindered by many components of the anterior eye, including the corneal epithelium, corneal endothelium, conjunctiva and sclera [18]. In addition, the longer diffusion distance to the posterior eye and the acellular nature of the vitreous negatively impact the pharmacokinetics and distribution of the topical drugs [18]. Simple physiologic processes such as tear production, blinking, drug metabolism and drug binding also impact topical applications, hindering the access of topical drugs to the target locations. All of these factors and limitations lead to increased dosing and a higher frequency of drug application in order to attain therapeutic concentrations, making the use of topical drugs relatively inefficient for patients and leading to decreased patient compliance.
Recent research has focused on small-molecule penetration into the vitreous, with evidence that molecules with lower molecular weight have increased permeability into the posterior chamber. Molecules with higher molecular weights and superior water solubility (highly charged) may have longer half-lives than those with lower molecular weights [19]. Thus, lower molecular-weight compounds have increased access to the posterior eye and may minimize the risk of toxicity compared with higher molecular-weight compounds, which degrade at slower rates. These characteristics are generalizations. Therefore, each drug must be individually assessed and its uptake, efficiency and safety must be determined.
Systemic drug delivery
The systemic application of drugs is another method of access to the posterior segment. The drugs are administered orally or intravenously, enabling distribution throughout the body via the blood-stream. From the blood, the drugs can easily enter the choroidal extravascular space as the choroid has an extensive vascular network and leaky walls. However, the entry of the drug into the posterior segment is often limited by the outer and inner blood–retinal barriers that are made up of retinal pigment epithelium (RPE) (Figure 3) and endothelial cells of the retinal blood vessels, respectively. The RPE contains several efflux pumps including P-glycoprotein and multidrug resistance-associated protein, which reduce the permeability of various endogenous compounds into the vitreous [10]. The systemic application of drugs not only increases the quantity of a drug necessary to achieve therapeutic concentrations, but it also increases the risk of adverse effects due to the accumulation of a drug in other tissues throughout the body. Another limitation of systemic application includes potential reduced time of therapeutic effects and potency due to the dilution and degradation of the drug before reaching the target site [20]. Moreover, drug–drug interactions in patients being treated for coexisting medical conditions also influence the administration of systemic drugs for the treatment of retinal disease. A summary of the major limiting factors with topical and systemic drug administration can be seen in Table 2 [18].
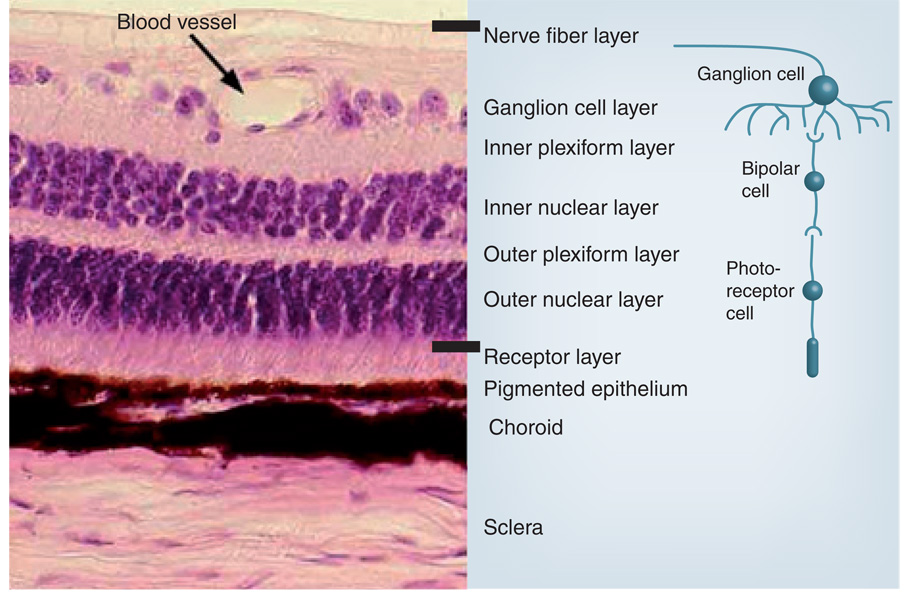
Figure 3.
Layers of the retina.
Redrawn with permission from the Southern Illinois University School of Medicine, IL, USA [201].
Table 2.
Limitations of topical and systemic drug administration.
| Topical | Systemic |
|---|---|
| Lacrimation | Blood–retinal barrier |
| Impermeability of corneal epithelium and endothelium |
RPE efflux pumps: P-glycoprotein and multidrug resistance-associated protein |
| Aqueous production | Higher therapeutic index |
| Blood flow | Increased drug accumulation throughout other body tissues |
| Stromal tissues of cornea and sclera |
RPE: Retinal pigment epithelium.
Data from [18].
Despite these limitations, there have been advances in the use of systemic medications for the treatment of ophthalmic diseases. Generally, there is increasing focus on creating drug delivery methods that control the rate and delivery time to the posterior eye, thus increasing the spatiotemporal efficacy and reducing drug accumulation. One major advance has been the efficacious use of the prodrug of ganciclovir, valganciclovir, for the treatment of cytomegalovirus (CMV) retinitis. Valganciclovir, which metabolizes to ganciclovir, provides an oral route of treatment for CMV retinitis as an alternative to the previously used intravenous (iv.) ganciclovir treatment. A study of 52 patients with CMV retinitis has shown that valganciclovir has a similar tolerability profile to that of ganciclovir, and that it also reduces the side effects associated with iv. injections [21].
The use of newer, more potent and more soluble antibiotics might result in an increased efficacy in treating endophthalmitis. Increased penetration of some fluoroquinolones yields better treatment outcomes when compared with the aminoglycoside amikacin and cephalosporin ceftazidime [22]. Endophthalmitis treatment with fluoroquinolones is superior at targeting several strains of bacteria including Klebsiella pneumoniae and Pseudomonas spp., which are showing increasing resistance to other antibiotics, including amikacin and β-lactams [22–24].
Intravitreal injection
Intravitreal injection is one means of drug delivery that is becoming more popular in the clinical setting. Anti-VEGF drugs, such as pegaptanib, ranibizumab and bevacizumab are new intravitreal treatments for AMD and macular edema; intravitreal injection is currently the most acceptable and effective method to treat vitreoretinal disease. This method allows a direct application of the drug into the posterior eye, thus eliminating the barriers common with topical and systemic administration. As a result, a much higher dose of drug can reach the target site and yield a more efficacious treatment of posterior eye diseases. Intravitreal triamcinolone acetonide is being used to treat AMD and macular edema. However, multiple injections may be necessary as a result of the limited half-life of many compounds in the vitreous, potentially causing trauma and increasing the risk of cataract, retinal detachment, hemorrhage and endophthalmitis [20].
Transscleral diffusion
A relatively newer method of drug delivery is transscleral delivery, a less invasive method in which the drug permeates through ocular tissues to reach the neuroretina. Transscleral delivery includes such avenues as subconjunctival, retrobulbar, peribulbar, sub-Tenon’s and intrascleral delivery [20]. An overview of these avenues of application is seen in Table 3 [20,25].
Table 3.
Overview of existing transscleral drug delivery techniques.
| Avenue of delivery |
Mechanism of application | Risks and limitations | Common uses |
|---|---|---|---|
| Retrobulbar | Drug is injected between the inferior and lateral rectus muscles, and the needle is directed posteriorly until resistance from the orbital septum is met. The needle is then directed towards the apex until resistance from the intermuscular septum is met [20,25] |
Blood vessel laceration, globe perforation, orbital hemorrhage, diplopia, artery occlusion, ptosis and brainstem anesthesia [20] |
Preoperative analgesia, postoperative analgesia, akinesia and control of intraocular pressure |
| Peribulbar | This method can be further classified as circumocular, periocular, periconal and apical based on depth of needle penetration [20] |
Similar to retrobulbar delivery, but the risk of injury to intraorbital structures is milder [20] |
Preoperative analgesia, postoperative analgesia, akinesia and control of intraocular pressure |
| Sub-Tenon’s | Injection of drug into a fascial sheath of connective tissue between the conjunctiva and episcleral plexus [20] |
Difficult penetration of drugs through the sclera and choroid. Rapid drug removal by the choroidal circulation [20] |
Analgesia, local anesthesia, triamcinolone acetonide and antibiotics |
| Subconjunctival | Injection of drug beneath the conjunctiva, providing a localized and minimally invasive means of delivery to the posterior eye [20] |
Dependent on pharmacodynamics of drug and diffusion through sclera and choroid [20] |
Bioactive proteins, prostaglandins and dexamethasone |
Although transscleral methods eliminate some of the side effects of intravitreal delivery, they in turn have their own limitations. Because the drug molecules must cross through several layers of tissue, the bioavailability of the drug at the target site can sometimes be drastically reduced and, thus, require very high doses to be effective. These barriers are categorized into three major groups: static, dynamic and metabolic [25]. Table 4 provides a quick overview of these three barrier types [25]. Static barriers include the tissues that must be penetrated (e.g., sclera, Bruch’s membrane-choroid and RPE). Dynamic barriers include blood flow, lymphatic drainage, transport proteins of the RPE, drug efflux pumps, organic ion transporters and bulk fluid flow from intraocular drainage systems. Metabolic barriers include enzyme systems such as cytochrome P450 and lysosomal enzymes, which have the ability to degrade or detoxify drugs. Mathematical models of posterior segment drug delivery by subconjunctival injection reveal that the dominant pathway for entry into the vitreous is direct penetration, while recirculation or movement from the aqueous to vitreous chambers is not significant [26–29].
Table 4.
Barriers to transscleral drug delivery.
| Static | Dynamic | Metabolic |
|---|---|---|
| Sclera: permeability decreases with increasing molecular radius and lipophilicity. Permeability increases with negatively charged solutes |
Blood and lymphatic flow: high flow rates in conjunctiva and choroid may lead to faster drug elimination and minimal drug penetration |
Cytochrome P450 |
| Choroid and Bruch’s membrane: permeability decreases with increasing molecular weight and lipophilicity. Permeability increases with negatively charged solute |
Bulk fluid flow: convective flow may lead to decreased penetration of drugs |
Lysosomal enzymes |
| RPE: permeability decreases with increasing molecular radius and increases with increasing lipophilicity |
Transport proteins, drug efflux pumps and ion transporters |
RPE: Retinal pigment epithelium.
Data from [25].
In addition to the static, dynamic and metabolic barriers, other factors that must be considered in transscleral delivery include the individual pharmacokinetic properties of the drug. The pharmacokinetics of drug diffusion across these barriers is dependent on the molecular dimensions, molecular weight, atomic charge and chemical components of the drug. In vitro studies demonstrated that the human sclera is permeable to 70 kDa dextran [30]. The radius of the molecule is considered to be a more important predictor of permeability than the weight and charge [31]. Finally, the solubility of the drug compounds is impacted by the water and lipid interactions. Hydrophilic compounds tend to permeate through the sclera more rapidly than lipophilic (hydrophobic) molecules, making the delivery of lipid-dominant molecules such as corticosteroids via transscleral routes more challenging [31]. However, a balance may be critical since many lipophilic compounds can easily penetrate the RPE; a problem can arise owing to toxicity caused by a lack of drug elimination. The delivery of drugs via the transscleral route continues to undergo investigation owing to the potential benefits over systemic and intravitreal delivery; however, this method provides barrier and permeability limitations.
Transscleral iontophoresis
Another transscleral method involves an electrodynamic process of drug delivery termed iontophoresis. In this technique, charged molecules are delivered across the sclera and into the posterior chamber of the eye via a direct electric current. In most cases, an iontophoretic probe is placed over the pars plana, enabling a bypass of the lens–iris barrier. This arrangement permits the precise delivery of high quantities of drugs through changes in the intensity of the applied current, yielding improved control of constant, uniform drug delivery. Animal and human studies have shown that iontophoresis eliminates many of the unwanted side effects of intravitreal injections and may improve the efficacy of periocular injections by decreasing the risk of retinal detachment, endophthalmitis, globe perforation and ptosis [32]. In effect, iontophoresis reduces the risk of injections and surgical procedures. Iontophoresis has been shown to be successful in delivering biologically relevant concentrations of numerous ophthalmic drugs including corticosteroids, antibiotics, anti-inflammatory agents and immunosuppressants [32].
In the utilization of an iontophoretic device, the main elements impacting the amount of drug delivered are the amount of current, concentration of drug, the duration of treatment, pH of the drug and the permeability of the tissue [33]. Another major concern is that the resistance in the tissue may change over time after repetitive applications of current and heat, altering the electrical field and leading to changes in drug permeation, thus altering drug peaks and troughs.
Several types of iontophoretic devices are available and each provides its own set of benefits. For example, the coulomb-controlled iontophoresis unit allows an automatic adjustment in electrical current based on the changes in resistance across the conjunctival epithelium [32,34]. This unit also provides self-calibration and acts as an indicator for proper electrical probe contact [32,34]. Other iontophoretic units include the mini-ion unit and the EyeGate® II iontophoresis device (EyeGate Pharma, MA, USA) [32]. The portable mini-ion unit provides a variable electrical current for a preset amount of time. It uses a hydrogel probe to deliver charged drugs to the posterior eye [35,36]. The EyeGate II device is a new, updated version of the original EyeGate iontophoresis device. The EyeGate II uses an electrical current to hydrolyze water and, thus, increase ion mobility, allowing greater concentrations of drug to reach the posterior eye. As the current induces like-charged ions to repel each other, more drugs are delivered through tissues. Preliminary results of Phase II studies of the EyeGate II Delivery System have shown it not only to be effective in delivering siRNAs, but also in increasing the cellular uptake of oligonucleotides and drug delivery to the target site as a result of the applied electrical current.
Research and development of new intravitreal, transscleral and iontophoretic drug delivery methods continue with the goal of optimizing therapeutic drug concentrations while minimizing risks and side effects. Table 5 provides an overview of the limiting factors of these delivery methods [20,25,31–34]. As a response to these limitations, there is a recent increase in the research of other drug delivery methods, including sustained-release devices, injectable colloids, microparticles, nanoparticles and hydrogels, which also show promise in the treatment of posterior eye diseases.
Table 5.
Limiting factors of several drug delivery methods.
| Intravitreal injection | Transscleral diffusion | Iontophoresis |
|---|---|---|
| Retinal detachment [32] | Tissue permeability [20,31] | Tissue permeability [33] |
| Endophthalmitis [32] | Static, dynamic and metabolic barriers [25] | Resistance of tissue to current and heat [33] |
| Globe perforation and hemorrhage [32] | Decreased bioavailability [20] | Changes in electrical field leading to alterations in drug peaks and troughs [32,34] |
| Decreased patient compliance | Exposure to direct electrical current [32,33] |
Nonbiodegradable implants
Intravitreal, transscleral and iontophoretic routes of drug administration tend to achieve high drug levels in the posterior eye, and are typically more efficacious than systemic and topical methods. However, these drugs are susceptible to rapid clearance and require frequent administration. The half-life of most drugs in the vitreous is limited to a few hours [37]. This has led to the development of sustained-release drug delivery systems that minimize the frequency of drug application and decrease the importance of patient compliance. In addition, these controlled systems deliver drug without an initial burst. Many types of controlled-release drug delivery systems have been developed, including nanoparticles, microcapsules, liposomes and implants [4].
Much research has been focused on implantable sustained-release vehicles of drug delivery. These implants provide the opportunity to remove the drug should toxicity occur. Specifically, an implantable device can be explanted as required; however, injectable liposomes, microparticles and nanoparticles would be difficult to retract once toxicity is obvious. There are three approved implantable devices available; one is biodegradable and two are nonbiodegradable polymer implants, which may achieve diffusive zero-order kinetics over a period of time. These implants are not metabolized in vivo, and may require replacement or removal once the drug is depleted. As a result, there has been increasing research into biodegradable vehicles that would allow the implants to be slowly converted to soluble forms via enzymatic and nonenzymatic reactions in the eye [4]. This, in turn, eliminates the need for the implants to be removed and replaced in different locations and, thus, decreases side effects from multiple invasive procedures.
A ganciclovir implant was the first nonbiodegradable, sustained-release vehicle to be approved by the US FDA [12]. This device, which became widely available in March 1996, was developed to deliver concentrated drug directly into the eye for the treatment of CMV retinitis in patients with AIDS. This device, surgically inserted through the pars plana into the posterior eye, is made of a central pellet of ganciclovir (4.5 mg) that is placed in a coating of polyvinyl alcohol (PVA), which is a permeable, water-soluble polymer that serves as the framework of the device and provides regulation of drug release. The PVA is then surrounded by ethylene vinyl acetate (EVA), which is impermeable and restricts the surface area available for drug diffusion. The EVA is surrounded by yet another level of PVA to increase regulation of the rate of drug release. This system delivers the drug at a rate of 1 µg/h and lasts for 6–8 months [38]. This drug system has been shown to produce a statistically significant improvement over iv. administration in delivering ganciclovir to the posterior eye for the treatment of CMV retinitis [38]. One study randomly assigned CMV retinitis patients with no history of prior treatment to one of three treatment groups: 1-µg/h implant, 2-µg/h implant and iv. ganciclovir [38]. Retinitis was categorized as an extension of a lesion border by at least 750 µm, the development of a new area of retinitis of at least 750 µm, a decrease in visual acuity (VA) to less than 20/200, or the presence of retinal detachment in conjunction with an area of retinitis. The results demonstrated that the median time to progression of CMV retinitis was 221 days in the 1-µg group, 191 days in the 2-µg group and 71 days in the iv. ganciclovir group. The risk of progression was nearly three-times as high in the iv. ganciclovir group compared with the 1-µg implant group (p < 0.001) [38].
Another study, conducted by the National Eye Institute, randomly assigned patients to two separate groups: treatment with a ganciclovir implant and a no treatment group. The results of this study showed a progression time of only 15 days for the deferred treatment group, and a time of 226 days for the implant group (p < 0.0001) [39]. While being effective, the implant presents the risk of side effects and injury to the eye. These side effects, including endophthalmitis, cystoid macular edema (CME), rhegmatogenous retinal detachment and vitreous hemorrhage were shown to occur in approximately 13 out of 110 eyes [40].
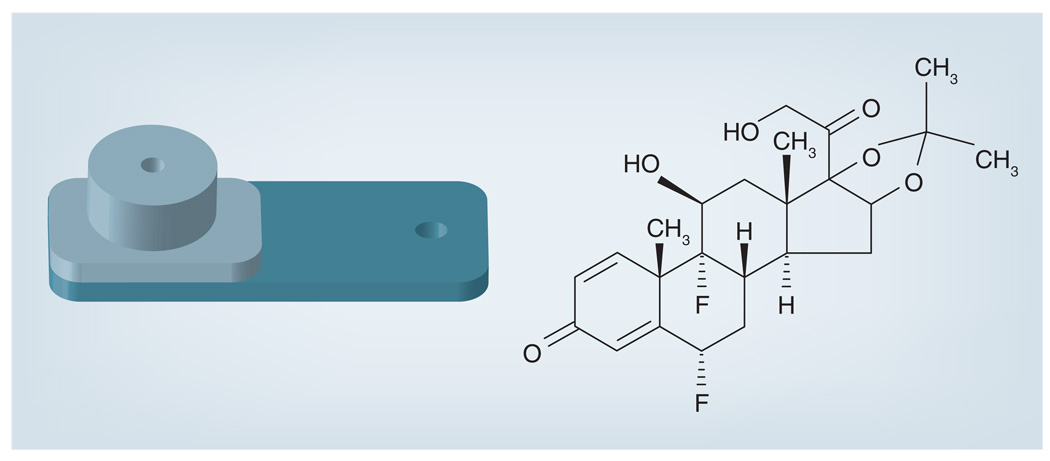
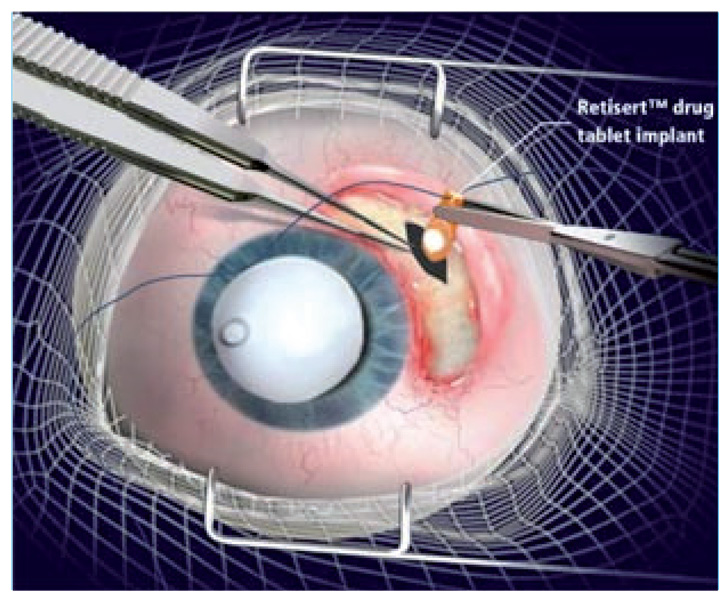
The efficacy of nonbiodegradable ganciclovir implants has enabled its expansion into the research and development of other drug-releasing devices for the treatment of diseases of the posterior eye. Several other implants have been designed to treat severe uveitis with drugs including fluocinolone acetonide (FA), dexamethasone and cyclosporine. The FDA approved a FA-containing sustained-release drug delivery device in 2005. This sterile implant, Retisert® (Bausch & Lomb Surgical, Inc., CA, USA) (Figure 4 & Figure 5), is used for the treatment of chronic noninfectious uveitis of the posterior segment. It contains FA 0.59 mg, which is released initially at a rate of 0.6 µg/day, decreasing over the first month to a steady state between 0.3–0.4 µg/day for approximately 30 months. A reformatted version of Retisert contains FA 2.1 mg, which is released at an initial rate of 2 µg/day, decreasing to a steady state of 1 µg/day over a 3-year period. The implant itself measures 3 × 2 × 5 mm with a 1.5-mm FA drug core encased in a silicone elastomer cup, which holds a single- (0.59 mg) or double- (2.1 mg) release orifice [41]. The orifice has a PVA membrane that surrounds it to permit diffusion of the drug. The entire device is attached using silicone adhesive to a head-cured PVA suture tab with a suture hold that anchors the implant inside the eye. Many studies have been performed to test the efficacy of this implant system. The first pilot study involved seven eyes with severe posterior uveitis, and demonstrated stabilization or improvement of VA along with control of inflammation in all patients over a 10-month period [41].
Figure 4.
Retisert®.
Figure 5.
Implantation of Retisert®.
One of the largest trials of FA implants is a 3-year, multicenter, controlled trial using the 0.59- and the 2.1-mg implants. Results of this study demonstrated that the uveitis recurrence in the 110 patients of the 0.59-mg group was reduced from 62% during the 1-year preimplantation period to 4, 10 and 20% during the 1-, 2- and 3-year postimplantation periods, respectively (p < 0.01) [42]. In the 168 patients of the 2.1-mg dose group, the recurrence was decreased from 58% to 7, 17 and 41%, respectively (p < 0.01) [42]. In addition to decreasing the recurrence of uveitis, this study also demonstrated that the implantation significantly reduced the need for adjunctive systemic therapy or periocular injections by nearly 80%. VA in the 0.59- and 2.1-mg implant groups improved by 23 and 18%, respectively, compared with 6 and 4% in nonimplanted eyes [42]. Finally, the implanted eyes demonstrated a greater reduction in the area of CME. At the 3-year visit, there was a reduction of CME by 73% in the 0.59-mg implanted group and 45% in the 2.1-mg implanted group, compared with 22% in the nonimplanted group [42]. However, the implanted eyes had higher rates of adverse events including increased intraocular pressure (IOP) and cataract formation. During the 3-year period, 67% of the 0.59-mg group and 79% of the 2.1-mg group had an IOP increase of 10 mmHg or more. A total of 78% of total implanted eyes required IOP-lowering eye drops compared with 36% of nonimplanted eyes. In addition, 40% of implanted eyes required IOP-lowering surgery compared with only 2% of nonimplanted eyes (p < 0.01) [42]. During the 3-year period, 93% of phakic-implanted eyes underwent cataract surgery compared with only 20% of phakic-nonimplanted eyes (p < 0.01) [42]. This study demonstrates both the efficacy of the implant and the heightened risk of increased IOP and cataracts in the implanted eyes, thus requiring increased medical or surgical IOP control and cataract extraction [42].
The FA implant is also currently being tested for treating macular edema from diabetic retinopathy. A multicentered, randomized clinical trial enrolled 197 patients who were placed in a FA 0.59 mg implant group or in a nonimplant group. Initial 12-month results of this 2-year study demonstrated that 57% of the implant group had no evidence of macular edema versus only 20.3% of the control group (p < 0.001) [43]. Improvement in retinal thickness at the center of the macula was shown in 55.6% of eyes receiving the implant as compared with only 17.2% in the control group (p > 0.001). As with the FA implants for posterior uveitis, the risk of cataracts and IOP rise was significantly higher in the implant groups [43].
A new FA sustained-delivery device has been developed for the treatment of DME. Iluvien™ (Alimera Sciences, Inc., GA, USA) is a new injectable, nonbiodegradable, intravitreal insert, which uses the Medidur™ FA insert (Alimera Sciences, Inc.) to deliver a low dose of corticosteroid to the retina for the treatment of DME [44]. Iluvien comes in two doses: the high dose (0.5 µg/day) is set to release the drug over an 18–24-month period and the low dose (0.2 µg/day) releases the drug over a 24–30-month period [44]. Iluvien is inserted via a 25-gauge injector system into the vitreous, through a sutureless procedure. Iluvien (3.5 × 0.37-mm cylinder) provides a smaller size than Retisert, eliminating the need for surgical implantation in the operating room and allowing the unit to be placed directly in the posterior eye. In vitro studies have shown that Iluvien’s release rate of 0.5 µg/day drops to 0.2 µg/day after 6 months [44]. Like Retisert, Iluvien has a PVA cap that regulates the rate of drug release. However, unlike Retisert, it is not composed of microcrystalline cellulose or magnesium stearate. The device is currently under Phase III trials in the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study.
In addition to treating uveitis and DME, FA implants have recently been used in clinical studies for the treatment of chronic central retinal vein occlusion (CRVO) [45]. In a recent study, 13 eyes with chronically persistent macular edema associated with CRVO received 0.59-mg intraocular FA implantation. Initial results of this study show that the median VA improved to 20/60 (p = 0.013) within 2 months after implantation and 20/80 (p = 0.06) within 12 months, from a baseline of 20/126 [45]. From the 13 eyes, nine gained two or more lines of VA from baseline, two eyes remained stable and the other two eyes lost two lines from the baseline within 12 months after FA implantation. Moreover, at 12 months, the mean and median central foveal thickness decreased from 622 ± 111 and 600 µm, respectively, before surgery to 294 ± 162 and 219 µm at 2 months, respectively. At 12 months, the mean and median foveal thicknesses measured 307 and 199 µm, respectively (p = 0.0078) [45]. As with other implantation studies, cataracts had developed in all phakic patients, and increased IOP was seen in all patients. This pilot study demonstrates the promise of the FA implant as an alternative in the treatment of macular edema from CRVO.
In the past few years, there has been an increasing focus on the application of intravitreal triamcinolone acetonide (TA) as a treatment option for various neovascular and edematous proliferative disorders of the eye. Intravitreal TA may be useful as an antiangiogenesis therapy in eyes with neovascularization and proliferative ischemic retinopathies. In addition, it may be useful in exudative AMD. TA is a water-insoluble drug, which remains in the vitreous for longer periods of time, thus making it superior to many other steroids that have a shorter duration of action [46]. As a result, many studies have been performed to demonstrate the efficacy of a TA sustained-release drug delivery implant. The polymers used for TA implants consist of PVA and EVA [46]. PVA, a permeable polymer, regulates the rate of drug permeation. EVA, an impermeable polymer, limits the surface area through which the drug can diffuse. In an in vivo study, TA pellets measuring 1 × 1 × 2 mm were implanted into the vitreous of rat eyes and measurements of the thickness of fibrovascular membranes were made at various intervals [47]. The study showed a statistically significant inhibition of fibrovascular proliferation disease for at least 35 days. This study tested for the inhibition of fibrovascular proliferation, but did not test for regression of pre-existing neovascular tissue [48]. In vitro studies demonstrated steady-state zero-order release of the TA, with the pellets delivering drug for a total of up to 35 days [48].
Beeley et al. performed a study of a sustained-release, subretinal TA implant in rabbits [49]. A rod-shaped implant consisting of a 3.5-mm long filament with an outer drug-loaded polymer coating was used in this study. The implants were fabricated by coating nitinol, poly(methyl methacrylate) or chromic gut core filaments with a drug-eluting polymer matrix made of a mixture of poly(butyl methacrylate) and poly(ethylene-co-vinyl acetate), a design similar to that of many sirolimus-eluting cardiovascular stents used in human patients. In this in vivo study to evaluate clinical tolerability and levels of in vivo drug elution, implants were inserted into the subretinal space of 20 rabbits. The implants were well tolerated and were able to elute TA for a period of 4 weeks. Although there was a positive retinal response, six out of the 20 eyes developed cataract and corneal edema [50].
SurModics, Inc. (MN, USA) is currently studying the I-vation™ TA intravitreal implant in a Phase I human clinical trial, Sustained Triamcinolone Release for Inhibition of Diabetic Macular Edema (STRIDE). The I-vation TA is a nonbiodegradable implantable device, which is available as slow-release (1 µg/ day) and fast release (3 µg/day) [46]. It consists of a helical coil coated with TA, poly(butyl methacrylate) and poly(ethylene-vinyl acetate) polymers. The implant’s helical shape maximizes the surface area available for drug coating. This shape also ensures secure anchoring of the implant at the scleral wall, and enables its easy retrieval. The device permits customization of drug delivery rates through variation of the polymer ratios.
Biodegradable implants
While the safety and efficacy of several nonbiodegradable implants have been demonstrated, the process of surgical implantation and removal has many potential deleterious side effects, including vitreous hemorrhage, retinal detachment and endophthalmitis. In addition, once the drug is depleted, the device may need to be removed and a new device implanted. To eliminate this, more research is focusing on the development of biodegradable implants, which are soluble and, thus, do not need to be removed or reimplanted when the drug is depleted. These implants have been manufactured in a variety of forms including rods, discs, pellets, plugs and sheets, and allow implantation through smaller incisions than the nonbiodegradable counterparts [7]. The drugs in the biodegradable implants are conjugated to a variety of polymers including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(caprolactone) and poly(methylene malonate).
One such implant incorporates a combination of drugs for the treatment of proliferative vitreoretinopathy (PVR) using a PLGA matrix polymer for the delivery of 5-fluorouridine, TA and tissue plasminogen activator (t-PA) [7,26]. The implant (7 mm long and 0.8 mm in diameter) consists of three cylindrical segments, each containing one of the drugs [7]. TA and 5-fluorouridine are released over 4 weeks, and t-PA over a period of 2 weeks. The device utilizes the PLGA coating over the t-PA segment to supply a lag-time for the delivery of t-PA. The system demonstrates the benefits of allowing a multidrug release from a single implant. While promising results were obtained in a preliminary in vitro controlled-release study for PVR treatment, there have been no recent in vivo trials of the multisegment implant.
The efficacy and safety of a TA biodegradable intrascleral implant were evaluated. The implant (1 mm thick × 3 mm diameter) was made of PLA (poly[d,l-lactide]) with TA 6.4 mg [51]. The design was unique in that it utilized the high molecular weight of PLA as a one-side coating, permitting a unidirectional drug absorption in the sclera. A 12-week in vivo study in 20 rabbit eyes was performed to measure the efficacy of the drug reaching the vitreous. The preliminary study demonstrated statistically significant levels of TA in the aqueous humor until 4 weeks postimplantation and in the retina–choroid until 8 weeks. TA was detected constantly over the full 12-week period in the vitreous. The preliminary study shows promise for a biodegradable TA implant, and further studies are underway to determine the efficacy of such an implant in humans [51].
Another biodegradable implant integrates dexamethasone for the treatment of uveitis and DME. This implant (Ozurdex™; Allergan, Inc., CA, USA), a sustained-release formulation made of a PLGA matrix, is currently in Phase III clinical trials. The results from a controlled Phase II, prospective, multicentered study were recently published. In this study, patients were assigned to three different treatment groups: Ozurdex implant containing dexamethasone 350 µg, Ozurdex implant with dexamethasone 700 µg and a control group with no treatment. The study enrolled 306 patients who were diagnosed with macular edema from a variety of conditions including DME in 54.3% of the patients, retinal vein occlusion in 32.7%, uveitis in 4.4% and Irvine–Gass syndrome (postcataract surgery macular edema) in 8.6%. All patients had macular edema that persisted for more than 90 days after laser or medical treatment and a best corrected VA of 20/40 or worse, but no worse than 20/200. Results of this study demonstrated improvements in VA and retinal thickness in the Ozurdex treatment groups. At 90 days, 24% of eyes in the 350-µg group, 35% in the 700-µg group and 13% in the control group showed a ten-letter or greater improvement in VA. Furthermore, the percentage of patients showing an improvement of VA of greater than 15 letters was 10% in the 350-µg group, 18% in the 700-µg group and 6% in the control. At day 180, 18% of eyes in the 700-µg group showed at least a 15-letter improvement in VA, while 13% of eyes in the 350-µg group and 8.0% of eyes in the control group showed similar improvements in VA at 180 days (p = 0.02). Mean retinal thickness decreased by more than 160 µm in the 700-µg group while it increased by more than 20 µm in the control group (p < 0.001). In addition, in the 700-µg group, fluorescein leakage decreased by two or more levels in a third of the patients and by three or more levels in a quarter of the patients compared with the control groups (p < 0.001) [52,53].
In addition to these benefits, there were minimal side effects of the implantation. No cases of endophthalmitis were reported, and there was no significant difference in the number of reports of cataract among the different study groups. Most cases of vitreous hemorrhage were seen within the first week after surgery and were mild in severity. The number of patients with an increase in IOP of 10 mmHg or more anytime during the study was 12 out of 100 in the 350-µg group, 17 out of 101 in the 700-µg group and three out of 100 in the observation group. Most of these only had one occurrence of elevated IOP, and all patients were successfully managed with observation or a topical medication. The only other adverse effect to occur significantly more frequently in the treatment group was anterior chamber flare. A total of 5% of patients in the 700-µg group were observed to have anterior chamber flare, while 0% was seen in the control group. These adverse effects seem minimal compared with trials of FA implants; however, it must be noted that the follow-up periods in this study were also shorter. A Phase III study of the Ozurdex device in patients with DME has recently completed enrollment and is in the follow-up phase, while a Phase III study of patients with macular edema due to retinal vein occlusion is complete with results expected in the near future.
Apart from this controlled study, an additional Phase IIb clinical trial evaluated the safety and performance of an extruded form of the Ozurdex implant, which is implanted using a 22-gauge applicator through a self-sealing wound in the pars plana. This sutureless implantable method was compared with incisional implant. In this prospective, multicentered trial, all patients received Ozurdex 700 µg. A total of 29 patients participated, of which 19 received the extruded Ozurdex and ten received the surgically implanted version. The 6-month results demonstrated VA improvements similar to those in the Phase II trial of Ozurdex previously discussed. VA improvements of three or more lines were seen in 20% of patients in both treatment groups. Only two patients in the incision group experienced vitreous hemorrhage, while none of the applicator group patients had vitreous hemorrhage. At 6 months, the IOP did not rise in any of the applicator group patients. Further studies of this applicator system may elucidate future promise for sutureless delivery of the biodegradable Ozurdex implant, with the hopes of minimizing side effects from incisional implantation [7,52].
Novel drug delivery: microparticles & nanoparticles
Many sustained intraocular drug delivery methods have been developed as alternatives to implantation. Ocular drug delivery systems using particulates have been developed, which provide sustained release with high target specificity in the form of microspheres and microcapsules with diameters of 1–1000 µm, as well as nanospheres and nanocapsules with diameters of less than 1 µm [54–57]. Drugs can be incorporated into biodegradable polymers to form either a matrix system or a reservoir system [58]. In a matrix system, the drugs and polymer are combined and the drug is released through diffusion from the polymer matrix with simultaneous polymer degradation. This system is used for micro-and nanospheres. The reservoir-type system involves encapsulating drugs within polymeric shells and is the system used for biodegradable micro- and nanocapsules [58]. Some of the commonly used synthetic biodegradable polymers are the aliphatic polyesters such as PLA, PGA, PLGA and poly(caprolactone) [56,57,59]. These polymers are appropriate for controlled-release applications because they are nontoxic, nonimmunogenic and degrade through enzymatic reactions and hydrolysis to natural metabolic products over a period of months to years [60,61]. Drug-release profiles can be modified through variations in polymer molecular weights and copolymer formulations [57]. An emulsion–diffusion process is used to encapsulate drugs in micro- and nanocapsules [62,63], while solvent evaporation is used to prepare micro- and nanospheres [64] using an oil-in-water emulsion for hydrophobic drugs or an oil-in-oil emulsion for improved encapsulation efficiency for hydrophilic drugs [58]. The particulates are suspended in a carrier solution to enable ocular injection. Intravitreal injections can potentially impair vision due to clouding. However, microspheres larger than 2 µm tend to settle out owing to gravity and nanoparticulates diffuse quickly and localize within ocular tissues [7].
Polymeric microspheres have been used to target the RPE. Moritera et al. studied the use of surface-modified polymeric microspheres to localize drugs to the RPE [65]. Phagocytosis by RPE was tracked by incorporating fluorescent dye into PLA microspheres with the rate of phagocytosis enhanced with gelatin-precoating as compared with bare microspheres. Kimura et al. used polymeric microspheres formulated using l-lactic acid and dl-lactic acid and their copolymers loaded with a fluorescent dye, rhodamine 6GX, to evaluate drug targeting to the RPE [66]. Intracellular dye release occurred following phagocytosis and could be controlled by varying the polymer formulation of the microspheres. Tuovinen et al. studied targeting drugs to the RPE using microparticles (11 µm in diameter) produced with starch acetate, which degrades more slowly than native starch [67]. These microparticles could be phagocytosed and degraded within the RPE.
Nanospheres have also been used to target the RPE for sustained drug delivery. Sakurai et al. studied the intraocular kinetics of nanospheres and found that polystyrene nanospheres containing fluorescein (2 µm in diameter) were detectable in the retina, vitreous and trabecular meshwork more than 1 month following an intravitreal injection in vivo in rabbits [68]. Bourges et al. showed the feasibility of targeting the retina and the RPE using a single intravitreal injection of polylactide nanoparticles loaded with the dye, rhodamine 6G and Nile Red, which quickly accessed the retina and were observed for 4 months postinjection [54]. Kim et al. used human serum albumin nanoparticles to track the movement of intravitreally injected nanoparticles as a function of surface charge and retinal injury [69]. Anionic nanoparticles traversed the collagen fibrils of the vitreous more readily than the cationic nanoparticles, showing potential as drug delivery vehicles for the subretinal space and the RPE. Müller cells take up the nanoparticles, possibly playing a key role in retinal penetration. Gaudana et al. reported that ligands, such as folate and biotin, attached to the surface of steroidal nanoparticles, can increase uptake by the RPE [6].
Steroids such as budesonide and dexamethasone have been tested in polymeric nano- and microparticles for sustained drug delivery. Kompella et al. determined that nano- and microparticles containing budesonide, a corticosteroid, could inhibit VEGF expression in vitro in a RPE cell line (ARPE-19) [70]. PLA nano-(345 nm) and microparticles (3.6 µm) containing budesonide were subconjunctivally injected in rats and were able to sustain retinal levels of budesonide compared with the steroid solution alone. Loftsson et al. evaluated delivering steroids to the retina in rabbits by topical application of a low viscosity aqueous suspension, which contained dexamethasone/γ-cyclodextrin microparticles with a mean diameter of 20.4 µm, and achieved vitreal and retinal steroid concentrations comparable to levels observed 1 month after an intravitreal injection [71]. Gomez-Gaete et al. prepared dexamethasone-loaded PLGA nanoparticles (230 nm) and optimized the solvent evaporation process to produce particles with the highest drug entrapment [72]. Gomez-Gaete et al. then devised a new drug vehicle for the intravitreal delivery of dexamethasone, called Trojan particles, which were formed by spray drying 1,2-dipalmitoyl-SN-glycero-3-phosphocholine, hyaluronic acid and different concentrations of dexamethasone-loaded PLGA nanoparticle suspensions [73]. The Trojan particles, which are spherical, hollow and have surface irregularities due to encapsulated nanoparticles, permit slower in vitro drug release owing to the protection provided by the excipient matrix.
The efficacy of encapsulating antiviral drugs such as ganciclovir and acyclovir into polymeric micro- and nanospheres has been presented. Veloso et al. used ganciclovir-loaded PLGA microspheres (300–500 µm in diameter) in rabbits inoculated with human CMV and were able to control the progression of fundus disease using an intravitreal injection of 10 mg of microspheres (89.77 µg ganciclovir/mg) suspended in 0.1 ml of 2% hydroxypropylmethylcellulose [74]. Herrero-Vanrell et al. prepared ganciclovir-loaded PLGA microspheres (300–500 µm) by dispersing ganciclovir in fluorosilicone oil, which enabled a high ganciclovir loading (95%) [75]. Microsphere sterilization using γ-radiation at a dose of 2.5 megarads did not affect the drug-release kinetics. Merodio et al. studied the ocular toxicity of an intravitreal injection in rats of ganciclovir-loaded bovine serum albumin nanoparticles, which were well tolerated in vivo and did not result in any inflammatory reactions based on histological evaluation [76]. Albumin nanoparticles contain a significant number of charged amino acids, which enable the adsorption of ganciclovir or particles that carry a negative charge such as oligonucleotides, as shown in a study by Irache et al. [77]. Duvvuri et al. investigated drug entrapment, surface morphology, particle size analysis and drug release from ganciclovir-loaded PLGA microspheres using various polymer blends, showing how blends can modify drug release for controlled delivery systems [78]. Duvvuri et al. presented empirical equations to describe drug release from ganciclovir-loaded PLGA microspheres and developed a thermogelling polymer solution to carry the dispersed microspheres for sustained delivery [79]. Duvvuri et al. developed a sustained-release formulation of ganciclovir-loaded PLGA microspheres in a thermogelling PLGA–poly(ethylene glycol) (PEG)–PLGA solution that was tested in vivo by intravitreal administration in a microdialysis rabbit model [80,81]. The microspheres in the thermogel polymer solution could maintain mean ganciclovir levels of 0.8 µg/ml for 14 days as compared with 54 h with direct injections. Martinez-Sancho et al. prepared PLGA microspheres loaded with vitamin A palmitate (10–80 mg) and acyclovir (40–80 mg) with in vitro drug release sustained for 49 days using an optimal formulation of acyclovir 40 mg, vitamin A palminate 80 mg and polymer 400 mg [82]. Cortesi et al. used spray drying to encapsulate acyclovir in polyacrylic microparticles that exhibited a controlled drug-release profile [83].
Pharmaceutical agents for the treatment of PVR have been incorporated into injectable particulates and tested for sustained delivery [56]. Moritera et al. studied the drug-release kinetics of microspheres (50 µm in diameter) prepared with polymers of PLA or copolymers of PGA and PLA containing 5-fluorouracil (5-FU), a potent inhibitor of fibroblast proliferation [84]. Copolymers increased the rate of drug release over homopolymers. Drug-release rates were accelerated with lower molecular-weight polymers or a copolymer matrix. In vivo studies in rabbits found increased microsphere clearance in vitrectomized eyes and electroretinograms, and histological study demonstrated no retinal toxicity. Moritera et al. investigated PLA microspheres loaded with adriamycin for the treatment of PVR [85]. In an experimental rabbit model, a single injection of microspheres containing adriamycin 10 µg decreased traction retinal detachment from 50% in controls to 10%, and was shown to be nontoxic to the retina by electroretinography and histological studies. However, retinal necrosis and detachment were observed with an injection of the same amount of free adriamycin, showing the potential for reduced toxicity through drug incorporation into biodegradable polymers. Giordano et al. showed that PLGA (50:50) microspheres loaded with retinoic acid could provide sustained release for 40 days in vitro and reduced the incidence of traction retinal detachment in a rabbit model of PVR after 2 months following a single intravitreal injection [86]. Peyman et al. tested the drug kinetics of microspheres formulated from copolymers of PLA and PGA (85:15), which incorporated cytosine arabinoside or 5-FU [87]. The drug-loaded microspheres were injected intravitreally in primates with both drugs detectable at 11 days postinjection and exhibiting similar rates of drug clearance. Yeh et al. prepared 3-µm microparticles using PLGA loaded with 5-FU and optimized the formulation to achieve an in vitro sustained release of 5-FU for 21 days with a delivery rate of 0.4 µg 5-FU/mg particles/day [88].
De Kozak et al. investigated the efficacy of incorporating tamoxifen, a nonsteroidal estrogen-receptor modulator, into PEG-coated nanoparticles for the treatment of experimental autoimmune uveoretinitis. Intravitreal injection in a rat model performed 1–2 days before expected disease onset in controls significantly inhibited the disease owing to a shift in the immune response from a Th1 to a Th2-type response [89]. He et al. evaluated cyclosporine-loaded PLGA microspheres, 50 µm in diameter, for the treatment of uveitis [90]. Drug release was monitored following intravitreal injections in healthy rabbits, maintaining therapeutic concentrations for at least 65 days in the choroid–retina and iris–ciliary body. Sakai et al. investigated the iv. administration of PLA nanoparticles loaded with β-methasone phosphate and tagged with rhodamine to target experimental autoimmune uveoretinitis induced with S-antigen peptide in a rat model [91]. The nanoparticles accumulated in the retina and choroid within 3 h and remained for 7 days postinjection, resulting in a reduction in the ocular infiltration of activated T cells and macrophages, as well as reduced hypertrophy of Müller cells. Barcia et al. tested the short- and long-term efficacy of dexamethasone-loaded PLGA microspheres (20–53 µm in diameter) to reduce ocular inflammation in a rabbit model of uveitis elicited by intravitreal lipopolysaccharide injection [92]. Both the short-term (15 days in length) and the long-term study (33 days in length) demonstrated reduced inflammation by clinical evaluation, electroretinography and histopathologic evaluation.
A novel approach for scavenging reactive oxygen species prominent in retinal degenerative diseases was presented by Chen et al. [93,94] and reviewed by Edelhauser et al. [95]. Cerium oxide nanoparticles (CeO2, nanoceria particles), which are nontoxic, nonimmunogenic and protective at a very low dosage, provided protection in vivo using a light-damage animal model. In this case, these rare earth particulates are not the carrier of a specific drug, but the therapeutic agent itself.
Micro- and nanoparticles have potential in the field of gene therapy by functioning as nonviral vectors to enable cellular penetration, guard against degradation and maintain sustained delivery. Panyam et al. demonstrated that PLGA nanoparticles could escape the endo–lysosomal compartment and prevent degradation by lysosomal nucleases, a quality necessary for a drug delivery vehicle [96]. The method of escape involves a reversal of the nanoparticle’s surface charge from anionic to cationic owing to the acidic environment of the endo–lysosomal compartment. This enables the nanoparticle to exit into the cytosol by interacting with the endo–lysosomal membrane. Endo–lysosomal escape makes PLGA nanoparticles an attractive delivery vehicle for macromolecules, such as DNA and low-molecular-weight drugs such as dexamethasone. Bejjani et al. explored the use of PLA and PLGA nanoparticles as vectors for gene transfer to a bovine and a human ARPE-19 cell line [97]. The plasmids employed were green fluorescent protein for expression within the cytoplasm or red nuclear fluorescent protein for expression within the nucleus. Intravitreal injections in vivo in a rat model concluded that PLGA could successfully sequester and internalize plasmids, resulting in gene expression in RPE detectable 48 h postinjection and maintained for 8 days. Mo et al. used human serum albumin nanoparticles loaded with the CuZn superoxide dismutase (SOD1) gene for in vitro transfection studies using human ARPE-19 cells [98]. The gene-loaded nanoparticles had a transfection efficiency of 80%, a fivefold increase in SOD1 expression over untreated cells and no cytotoxicity. In vivo studies employing an intravitreal injection in a mouse model resulted in detectable fusion protein at 48 h, while levels were undetectable in the control group.
Another therapeutic approach in the treatment of ocular disease is the inhibition of gene expression using antisense oligonucleotides, aptamers and siRNA [99–101]. Aukunuru et al. showed that nanoparticles formulated using a PLGA (50:50) copolymer could deliver VEGF antisense oligonucleotide to the human ARPE-19 cell line, and inhibit VEGF secretion and mRNA expression [102]. In a study performed by Carrasquillo et al. [103] and summarized by Moshfeghi and Peyman [104], the anti-VEGF RNA aptamer (EYE001, Macugen®, OSI Pharmaceuticals, NY, USA) was incorporated into PLGA microspheres to develop a sustained-release inhibition of VEGF for the treatment of neovascular AMD. The aptamer-loaded PLGA microspheres could deliver 2 µg/day over a 20-day period, effectively inhibiting VEGF-induced cell proliferation in human umbilical vein endothelial cells. The potential for using the PLGA microspheres for transscleral drug delivery to the choroid and retina was assessed in vitro using a device in which harvested rabbit sclera was mounted and drug permeation could be measured. Spectrophotometry was used to verify aptamer transfer across the sclera for the 6-day test period. Singh et al. presented a novel application using an iv. injection of surface-functionalized PLGA nanoparticles to target neovascular tissue for gene delivery of anti-VEGF intraceptor, an intracellular VEGF inhibitor, in a laser-induced, rodent model of choroidal neovascularization [105]. Anti-VEGF intraceptor expression was increased in retinal vascular endothelial cells, photoreceptor outer segments and RPE cells using iv. administration of nanoparticles functionalized with either transferrin, arginine–glycine–aspartic acid peptide or both, thereby inhibiting the progression of neovascularization.
Transport and drug efficacy studies of micro- and nanoparticles administered via periocular injection have been published. Amrite and Kompella determined that subconjunctivally administered nanoparticles and microparticles, of 200 nm and larger, could be retained at the injection site in rats for at least 2 months [106]. Amrite et al. showed that periocular blood and lymphatic circulation affected the clearance rate of 20-nm particles administered through periocular injection in dead and living rats, observing only minor transport across the sclera and insignificant transport across the sclera–choroid–RPE [107]. Chiang et al. observed in vivo sustained-release kinetics for more than 1 week using a subconjunctival injection of PLGA microspheres loaded with 5-FU injected in rabbits [108]. In an effort to develop a sustained delivery treatment for choroidal neovascularization, Saishin et al. prepared PLGA glucose microspheres incorporating PKC412, a kinase inhibitor that blocks receptors for VEGF, thereby inhibiting ocular neovascularization [109]. The PKC412-loaded microspheres were administered by periocular injection in young pigs after rupture of Bruch’s membrane via laser photocoagulation. Drug levels were detectable in the choroid, vitreous and retina 20 days postinjection. Ayalasomayajula and Kompella showed that celecoxib, a selective COX2 inhibitor, given orally could inhibit VEGF in a streptozotocin-induced diabetic rat model [110] and retinal celecoxib delivery improved via a subconjunctival administration [111]. In an effort to sustain retinal celecoxib delivery, Ayalasomayajula and Kompella then incorporated celecoxib into PLGA (85:15) microparticles and administered them subconjunctivally in rats [112]. Retinal drug levels were maintained for a 2-week period and inhibited diabetes-induced retinal oxidative stress. Amrite et al. were able to inhibit diabetes-induced elevations in prostaglandin E2, VEGF and blood–retinal barrier leakage using a posterior subconjunctival (periocular) injection of celecoxib-loaded PLGA microparticles in a streptozotocin diabetic rat model [113]. Therapeutic concentrations of celecoxib were maintained in the retina in vivo for 60 days and resulted in no damage to the retina or periocular tissues.
In summary, nano- and microparticles have shown great potential for expanding the arsenal of drug-delivery systems available for treating posterior segment disease due to their ability to provide sustained delivery and reduce complications that result from treatments requiring multiple injections. iv. administration of nanoparticles with surface modifications that can target the retina was a novel approach demonstrated in studies by Sakai et al. [91] and Singh et al. [105]. Transscleral delivery of anti-VEGF drugs loaded in PLA or PLGA nano- and microparticles is gaining much attention as a feasible and effective method of administration for the treatment of posterior segment disease [12]. Herrero-Vanrell et al. [75] and Barcia et al. [92] proved the feasibility of particle sterilization using γ-radiation at an effective USP sterilizing dose of 25 KGy (2.5 Mrad) [114], which will advance efforts toward clinical trials.
Ultrasound-mediated microbubble drug delivery
Ultrasound-mediated microbubble drug delivery stems from the field of ultrasonic contrast imaging. Commercially available microbubble contrast agents are gas-filled bubbles (1–8-µm diameter) made with a shell coating that is stabilized with phospholipids, surfactant, denatured serum albumin or synthetic polymer [115]. Since they are efficient reflectors of acoustic ultrasound energy, liquid suspensions of microbubbles are being used in medical applications as contrast agents for the enhancement of ultrasound images and visualization of perfusion. Contrast agents used in imaging and drug delivery studies are shown in Table 6 [115–117].
Table 6.
Ultrasound contrast agents used in imaging and drug delivery.
| Agent | Shell | Core | Mean diameter (µm) |
|---|---|---|---|
| Optison™ (GE Healthcare Systems, NJ, USA) |
Albumin | C3F8 | 3.0–4.5 |
| Definity® (Lantheus Medical Imaging, MA, USA) |
Lipid | C3F8 | 1.1–3.3 |
| PESDA (Porter, University of Nebraska, NE, USA [116]) |
Dextrose Albumin |
C4F10 | 2.5–4.9 |
Ultrasound has long been used for diagnostic imaging, but recently there has been a focus on its therapeutic applications such as drug delivery and gene transfection. Based on in vivo and in vitro experiments, exposure to ultrasound increased drug release from the matrix of biodegradable polymeric implants up to 20-fold, and tenfold for nonbiodegradable implants [118]. The release rates were proportional to the ultrasound intensity and reversible when the ultrasonic stimulus was removed. Studies show that ultrasound improves DNA transfection [119–123] and protein delivery [124] into tissues. The ultrasound-mediated drug delivery of sodium fluorescein, a hydrophilic compound, through the rabbit cornea was enhanced by tenfold with only minor changes in the corneal epithelium [125].
Drug delivery with ultrasound and microbubbles can be performed via two methods:
The drug carrier can be coinjected with the microbubbles
The drug can be incorporated on or within the microbubble
Application of ultrasound to the targeted tissue destroys the microbubble, releasing its contents and enhancing membrane permeability [126]. Encapsulating drugs/genes on or within the microbubble shell can be accomplished by:
Attaching to the microbubble shell
Embedding within the microbubble shell
Noncovalently binding to the microbubble surface
Encapsulating with gas within the microbubble
Incorporating hydrophobic drugs in an oily layer within the microbubble [127–129]
However, in order to maximize this drug delivery system, the optimal ultrasound parameters need to be determined as well as a molecular model explaining the varying degrees of ultrasound-induced membrane permeability [130].
Ultrasound combined with microbubbles affords a promising drug/gene delivery system owing to the ability of ultrasound to destroy the bubbles, which aids in the tissue-targeted release of bound or encapsulated drugs. Microbubbles respond to ultrasound based on the local output power, which depends on the strength of the ultrasound signal, the transmit frequency and the depth attenuation of the ultrasound beam. The gas in the microbubble lowers the cavitation threshold and the degree of cavitation is controlled by the output power. Stable cavitation is the induction of low-amplitude oscillations from pre-existing bubbles resulting from low-intensity ultrasound. It can generate local shearing forces and cause acoustic microstreaming in the adjacent fluid [131]. Inertial cavitation occurs when an ultrasound signal with a high acoustic power induces a series of strong oscillations of the microbubble shell, causing it to become unstable and collapse owing to the inertia of the inrushing fluid, thereby releasing its contents into the surrounding medium [131]. Shockwaves with their associated shear forces can be generated in the fluid, directing high-velocity fluid jets, which can disrupt or penetrate a nearby membrane or solid surface [131,132]. Drug delivery and gene transfection are also improved via the enhanced membrane permeability attributable to the ultrasound cavitation energy sometimes referred to as sonoporation [132,133]. Ultrasound-mediated drug/gene delivery has been tested in small animals, successfully delivering drugs, proteins, genes and gene-silencing therapies to various organ systems [134].
Ocular drug delivery using ultrasound and microbubbles is advantageous because the eye is easily accessible, it can be evaluated in vivo with ophthalmoscopy and it contains a variety of target tissues that can be treated with concentrated vectors while minimizing systemic immune response owing to the blood–retinal and blood–aqueous barriers. A recent study demonstrated that microbubble-enhanced ultrasound improved luciferase transfection in the adult rat brain by tenfold, indicating the potential for the ultrasound-mediated transfection of ocular neural tissue [135]. Hirokawa et al. observed transient changes in vascular permeability in the vessels of the rabbit eye due to insonation (228 W/cm2 at 2 MHz) for 5 min following iv. administration of lipid microbubble contrast agent (Definity®, Lantheus Medical Imaging, MA, USA) [136]. Sonoda et al. were able to strongly increase gene transfection efficiency for green fluorescent protein to the cornea using ultrasound (0.5–2 W/cm2 at 1 MHz) with an albumin-shelled contrast agent (Optison™, GE Healthcare, NJ, USA) [137]. Peeters et al. demonstrated that low-intensity ultrasound (i.e., 0.5 W/cm2 at 1 MHz) could improve retinal permeation of polystyrene nanoparticles 53 and 131 nm in diameter [138].
Our laboratories are developing a sustained, controlled-release delivery system for the posterior segment comprised of biodegradable, polymeric nano- and microbubbles that use ultrasound to selectively trigger the release of drugs or genes encapsulated within an oily core. The delivery system has been mathematically modeled to determine the frequency required for drug release given the size and material properties of the microbubble shell [139,140]. A minimum threshold pressure is required to induce microbubble cavitation, which is detectable by a subharmonic backscatter signal [141] and can be correlated with drug release. The microbubble suspension can be administered subconjunctivally, eliminating or reducing the need for multiple-injection treatments or surgical procedures to insert or remove ocular implants. The applied acoustic signal induces drug release by fracturing the polymeric shell and transiently increasing scleral permeability for drug transfer into the vitreous. This delivery method could assist in posterior segment treatments for age-related eye diseases, such as AMD, diabetic retinopathy and DME, which are becoming more prevalent as the percentage of the population 65 years of age and older increases.
Novel drug delivery: thermoresponsive gels
A recent study examined the use of thermoresponsive gels for the delivery of anti-VEGF for the regulation of angiogenesis. VEGF acts as an endothelial cell mitogen and increases vascular permeability. VEGF is known to be increased in diabetic retinopathy and AMD. Many current clinical trials have already demonstrated the efficacy of several anti-VEGF drugs. For example, the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) and Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Nevoascular AMD (MARINA) trials have shown the success of ranibizumab for the treatment of neovascularization and angiogenesis. While these drugs are currently very common in the clinical setting and have shown abundant positive results, they have the drawback of requiring repeated injections every 4–6 weeks, increasing the risk of side effects and decreasing patient compliance [142].
Hydrogels have been proposed as an alternative method of delivering anti-VEGF drugs to the posterior eye. Anti-VEGF therapies may not be effective in eyes after vitrectomy owing to a shortened half-life time in the vitreous cavity. Hydrogels may prolong the drug residence in the vitreous even after vitrectomy; this could provide potential treatment benefits. Hydrogels are polymeric networks that absorb large amounts of water while remaining insoluble in aqueous solutions. Hydrogels permit the manipulation of permeation and diffusion characteristics, allowing the optimization of drug delivery. As these hydrogels do not have hydrophobic interactions that normally denature biomolecules, they are excellent for encapsulating biomacromolecules including proteins and DNA. In addition, compared with hydrophobic polymers such as PLA or PLGA, the formation of hydrogels usually occurs at ambient temperatures and organic solvents are rarely needed. Hydrogels can be made from natural or synthetic polymers, each with its own benefits and drawbacks. Natural polymers provide increased biocompatibility, biodegradability and biologically recognizable moieties that support cellular activities; however, at the same time they do not provide the mechanical properties that synthetic polymers possess and they may evoke inflammatory responses within the body [143].
The main factor that determines the degree of hydrophilicity of hydrogels is the nanostructure of cross-linked hydrogel networks. This nanostructure consists of three major factors: polymer volume fraction in the swollen state, number of average molecular weight between cross-links and network mesh size. The manipulation of these factors allows scientists to develop hydrogels for various drug delivery targets. The hydrophilicity of hydrogels provides an increased in vivo circulation time as a result of evading host immune responses and decreasing phagocytic activities [143]. In addition, hydrogels can be made to be bioadhesive, thus facilitating drug targeting through mucus membranes for noninvasive drug administration. Finally, hydrogels permit various mechanisms of drug release, including diffusion-controlled, swelling-controlled and chemically controlled drug release.
Recent studies have examined the use of temperature-responsive hydrogels for drug delivery. Poly(N-isopropylacrylamide) hydrogel is a thermosensitive material that has a lower critical solution temperature (LCST), which defines the physical state of the hydrogel. The gel is swollen below the LCST, and collapses above the LCST. This rapid, reversible change in physical state prompted further research into thermoresponsive gels for the application of anti-VEGF drugs. Zhang et al. showed that the combination cross-linked poly(N-isopropylacrylamide) hydrogels with crosslinked PEG-diacrylate yielded a sustained-release version of a thermoresponsive hydrogel with homogenous pores [144].
Current scientific evidence supports the potential of hydrogels as a drug delivery vehicle for molecules to reach a target site through an external stimulus such as temperature (thermosensitive), pH, glucose or light. These hydrogels are biocompatible and biodegradable in nature and are considered a useful tool in nano-drug delivery, and have applications in the field of sustained-release drug delivery.
In a recent study, the PNIPAAm–PEG-diacrylate thermosenstive hydrogel was encapsulated with various proteins including bovine serum albumin, IgG, bevacizumab and ranibizumab. The goal of the study was to assess the rate of release of drug at various time points, and to analyze the bioactivity of the released anti-VEGF drugs. The rate of release was measured as a function of cross-link density, which was manipulated for increased efficacy. Cross-linked PNIPAAm hydrogel showed a fast and reversible phase change. The hydrogel can be injected as a liquid to the juxtascleral region or the vitreous cavity via a small-gauge needle, and once it is exposed to the body temperature it rapidly becomes a solid gel that releases the encapsulated anti-VEGF. Release profiles of the drugs showed an initial burst of release within 48 h of hydrogel application. This was followed by a steady-state level of drug, which was maintained for the length of the study (3 weeks). Manipulations of cross-linking showed that less cross-linking yielded a faster release and a more pliable gel. This system shows promise in potentially replacing the need for repetitive injections of anti-VEGF.
Expert commentary
Over the past 5 years, basic research and open clinical trials have provided breakthrough therapies for treatment of diseases of the posterior segment of the eye, such as the use of anti-VEGF agents for the treatment of AMD. Accelerated diagnosis, followed by prompt treatment, has also improved patient clinical outcomes for certain acute conditions in the posterior segment. However, despite the optimism, no standard of drug delivery to the posterior segment has emerged, and therapy for some widespread diseases such as diabetic retinopathy has not kept pace with the treatment for AMD. More effective drugs and drug-delivery systems are needed to decrease the frequency of drug administration. Multiple drugs and drug delivery systems may be required to safely and successfully treat some conditions. With the continued development of more potent drugs combined with research into novel delivery methods, there is a realistic hope that optimal therapeutic drug delivery for diseases of the posterior segment will be available in the near future.
Five-year view
Over the next 5 years, we predict several therapeutic and drug-delivery breakthroughs for the treatment of diseases of the posterior segment. We envision one paradigm utilizing combinations of drugs and drug delivery devices; for example, an anti-neovascularization drug plus an anti-inflammatory agent delivered utilizing two or more devices or systems. Although this paradigm is attractive, to date no studies have utilized all these components. Ultimately, this model must be specifically focused and tested in well-controlled animal studies and proceed to successful open clinical trials. The ultimate validation will be provided by successful randomized clinical trials. We predict that such an achievement will be accomplished during the next 5 years for therapy to the posterior segment.
Key issues
Although drug delivery to the posterior segment has significantly improved in the last 10 years, new systems, devices or unique combinations are required to treat blinding eye diseases, such as age-related macular degeneration and diabetic retinopathy.
Many diseases and/or clinical conditions have some degree of pharmacological treatment success, but better and more potent drugs are required for age-related macular degeneration and diabetic retinopathy.
There is no ‘gold standard’ or ‘standard of care’ for drug delivery to the posterior segment.
All methods and devices for drug delivery to the posterior segment have advantages and disadvantages.
Although the current methods and devices used in patients and in animal models have some efficacy, a major limitation is the lack of a longer sustained period of drug release.
Since many diseases or clinical conditions of the posterior segment are of a chronic nature, long sustained-release (months to years) devices and/or systems are of critical need.
This article provides the concepts for various types of drug delivery systems, such as biodegradable and nonbiodegradable sustained-release systems. None of these systems are ideal.
Since some types of treatment employ combination pharmacological therapy, perhaps a future development will combine two or more types of drug delivery systems.
Footnotes
Financial & competing interests disclosure
The authors are supported in part by NIH grants EY006311 (James M Hill), EY019144 (Partha S Bhattacharjee) and EY02377 (LSU Eye Center Core Grant for Vision Research), by a Research to Prevent Blindness Senior Scientific Investigator Award (James M Hill), by the Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents (James M Hill), an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (NY, USA) and funding from the Louisiana Lions Eye Foundation, New Orleans and Lions International. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Shalin S Shah, Department of Ophthalmology, Louisiana State University Health Sciences Center (LSUHSC), 2020 Gravier St. Suite B, Room 3E6, New Orleans, LA 70112-2234, USA, Tel.: +1 678 296 2334, Fax: +1 504 568 2385, sshah3@lsuhsc.edu.
Lori Vidal Denham, Department of Biomedical Engineering, Tulane University, Lindy Boggs Bldg. Suite 500, New Orleans, LA 70118, USA, Tel.: +1 504 865 5897, Fax: +1 504 862 8779, vidal@tulane.edu.
Jasmine R Elison, Department of Ophthalmology, Louisiana State University Health Sciences Center (LSUHSC), 2020 Gravier St. Suite B, Room 3E6, New Orleans, LA 70112-2234, USA, Tel.: +1 504 568 2081, Fax: +1 504 568 2385, jeliso@lsuhsc.edu.
Partha S Bhattacharjee, Department of Biology, Xavier University of Louisiana, 1 Drexel Drive, New Orleans, LA 70125, USA, Tel.: +1 504 520 5269, Fax: +1 504 520 7918, pbhattac@xula.edu.
Christian Clement, Department of Ophthalmology, Louisiana State University Health Sciences Center (LSUHSC), 2020 Gravier St. Suite B, Room 3E6, New Orleans, LA 70112-2234, USA, Tel.: +1 504 568 2552, Fax: +1 504 568 2385, cclem1@lsuhsc.edu.
Tashfin Huq, Department of Biology, Xavier University of Louisiana, 1 Drexel Drive, New Orleans, LA 70125, USA, Tel.: +1 504 452 5913, Fax: +1 504 520 7918, tashfinshuq@gmail.com.
James M Hill, Departments of Pharmacology, Microbiology and Neuroscience, LSU Health Sciences Center (LSUHSC) School of Medicine, 2020 Gravier St. Suite B, Room 3E6, New Orleans, LA 70112-2234, USA, Tel.: +1 504 568 2275, Fax: +1 504 568 2385, jhill@lsuhsc.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1. Lee SS, Robinson MR. Novel drug delivery systems for retinal diseases. A review. Ophthalmic Res. 2009;41:124–135. doi: 10.1159/000209665. •• Excellent review of current and novel drug delivery to the posterior segment.
- 2.Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Curr. Drug Deliv. 2006;3:207–217. doi: 10.2174/156720106776359186. [DOI] [PubMed] [Google Scholar]
- 3.Acharya N, Young L. Sustained-release drug implants for the treatment of intraocular disease. Int. Ophthalmol. Clin. 2004;44:33–39. doi: 10.1097/00004397-200404430-00006. [DOI] [PubMed] [Google Scholar]
- 4.Barocas VH, Balachandran RK. Sustained transscleral drug delivery. Expert Opin. Drug Deliv. 2008;5:1–10. doi: 10.1517/17425247.5.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior egment. Drug Discov. Today. 2008;13:135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 6. Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm. Res. 2009;26:1197–1216. doi: 10.1007/s11095-008-9694-0. • Good review of progress in ocular drug delivery.
- 7. Hsu J. Drug delivery methods for posterior segment disease. Curr. Opin. Ophthalmol. 2007;18:235–239. doi: 10.1097/ICU.0b013e3281108000. • Good discussion of drug delivery to the posterior segment.
- 8.Hoffman AS. The origins and evolution of ‘controlled’ drug delivery systems. J. Control. Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Novack GD. Ophthalmic drug delivery: development and regulatory considerations. Clin. Pharmacol. Ther. 2009;85:539–543. doi: 10.1038/clpt.2008.297. [DOI] [PubMed] [Google Scholar]
- 10.Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008;5:567–581. doi: 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Regillo CD. A review of treatments for macular degeneration: a synopsis of currently approved treatments and ongoing clinical trials. Curr. Opin. Ophthalmol. 2004;15:221–226. doi: 10.1097/01.icu.0000122122.24016.f1. [DOI] [PubMed] [Google Scholar]
- 12. Booth BA, Denham LV, Bouhanik S, Jacob JT, Hill JM. Sustained-release ophthalmic drug delivery systems for treatment of macular disorders: present and future applications. Drugs Aging. 2007;24:581–602. doi: 10.2165/00002512-200724070-00006. •• Excellent review of sustained-release delivery systems with an emphasis on biodegradable and nonbiodegradable implants for macular disorders.
- 13.Bekeredjian R, Katus HA, Kuecherer HF. Therapeutic use of ultrasound targeted microbubble destruction: a review of non-cardiac applications. Ultraschall. Med. 2006;27:134–140. doi: 10.1055/s-2005-858993. [DOI] [PubMed] [Google Scholar]
- 14.Gershkovich P, Wasan KM, Barta CA. A review of the application of lipid-based systems in systemic, dermal/transdermal, and ocular drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:545–584. doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.20. [DOI] [PubMed] [Google Scholar]
- 15.Lemley CA, Han DP. Endophthalmitis: a review of current evaluation and management. Retina. 2007;27:662–680. doi: 10.1097/IAE.0b013e3180323f96. [DOI] [PubMed] [Google Scholar]
- 16.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr. Opin. Ophthalmol. 2007;18:502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 17.Mitra AK. Role of transporters in ocular drug delivery system. Pharm. Res. 2009;26:1192–1196. doi: 10.1007/s11095-009-9862-x. [DOI] [PubMed] [Google Scholar]
- 18.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin. Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 19.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20. Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. •• Extensive review of existing and new developments in the field of retinal drug delivery.
- 21.Cvetkovic RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005;65:859–878. doi: 10.2165/00003495-200565060-00012. [DOI] [PubMed] [Google Scholar]
- 22.Chen KJ, Hwang YS, Chen YP, Lai CC, Chen TL, Wang NK. Endogenous Klebsiella endophthalmitis associated with Klebsiella pneumoniae pneumonia. Ocul. Immunol. Inflamm. 2009;17:153–159. doi: 10.1080/09273940902752250. [DOI] [PubMed] [Google Scholar]
- 23.Hori Y, Nakazawa T, Maeda N, et al. Susceptibility comparisons of normal preoperative conjunctival bacteria to fluoroquinolones. J. Cataract Refract. Surg. 2009;35:475–479. doi: 10.1016/j.jcrs.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Keating GM. Levofloxacin 0.5% ophthalmic solution: a review of its use in the treatment of external ocular infections and in intraocular surgery. Drugs. 2009;69:1267–1286. doi: 10.2165/00003495-200969090-00009. [DOI] [PubMed] [Google Scholar]
- 25. Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39:244–254. doi: 10.1159/000108117. •• Excellent article discussing the barriers and limitations of transscleral drug delivery to the vitreous.
- 26.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye II: development and validation of a simple pharmacokinetic model for subconjunctival injection. J. Ocul. Pharmacol. Ther. 2004;20:43–53. doi: 10.1089/108076804772745455. [DOI] [PubMed] [Google Scholar]
- 27.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye III: the effect of parallel elimination pathway on the vitreous drug level after subconjunctival injection. J. Ocul. Pharmacol. Ther. 2004;20:55–64. doi: 10.1089/108076804772745464. [DOI] [PubMed] [Google Scholar]
- 28.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J. Ocul. Pharmacol. Ther. 2001;17:565–572. doi: 10.1089/10807680152729257. [DOI] [PubMed] [Google Scholar]
- 29.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye IV: theoretical formulation of a drug delivery system for subconjunctival injection. J. Ocul. Pharmacol. Ther. 2009;25:29–37. doi: 10.1089/jop.2008.0010. [DOI] [PubMed] [Google Scholar]
- 30.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability: effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest. Ophthalmol. Vis. Sci. 1995;36:1893–1903. [PubMed] [Google Scholar]
- 31.Ambati J, Canakis CS, Miller JW, et al. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 2000;41:1181–1185. [PubMed] [Google Scholar]
- 32. Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev. 2005;57:2063–2079. doi: 10.1016/j.addr.2005.08.006. • Important review focusing on transscleral iontophoresis.
- 33.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Hayden B, Jockovich ME, Murray TG, et al. Iontophoretic delivery of carboplatin in a murine model of retinoblastoma. Invest. Ophthalmol. Vis. Sci. 2006;47:3713–3721. doi: 10.1167/iovs.06-0365. [DOI] [PubMed] [Google Scholar]
- 35.Frucht-Pery J, Mechoulam H, Siganos CS, Ever-Hadani P, Shapiro MN, Domb A. Iontophoresis–gentamicin delivery into the rabbit cornea, using a hydrogel delivery probe. Exp. Eye Res. 2004;78:745–749. doi: 10.1016/s0014-4835(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 36.Eljarrat-Binstock E, Raiskup F, Stepensky D, Domb AJ, Frucht-Pery J. Delivery of gentamicin to the rabbit eye by drug-loaded hydrogel iontophoresis. Invest. Ophthalmol. Vis. Sci. 2004;45:2543–2548. doi: 10.1167/iovs.03-1294. [DOI] [PubMed] [Google Scholar]
- 37.Bochot A, Fattal E, Boutet V, et al. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Invest. Ophthalmol. Vis. Sci. 2002;43:253–259. [PubMed] [Google Scholar]
- 38. Musch DC, Martin DF, Gordon JF, Davis MD, Kuppermann BD The Ganciclovir Implant Study Group. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. N. Engl. J. Med. 1997;337:83–90. doi: 10.1056/NEJM199707103370203. •• Well known, important study on the sustained-release ganciclovir implant.
- 39.Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch. Ophthalmol. 1994;112:1531–1539. doi: 10.1001/archopht.1994.01090240037023. [DOI] [PubMed] [Google Scholar]
- 40.Lim JI, Wolitz RA, Dowling AH, Bloom HR, Irvine AR, Schwartz DM. Visual and anatomic outcomes associated with posterior segment complications after ganciclovir implant procedures in patients with AIDS and cytomegalovirus retinitis. Am. J. Ophthalmol. 1999;127:288–293. doi: 10.1016/s0002-9394(98)00443-7. [DOI] [PubMed] [Google Scholar]
- 41.Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch. Ophthalmol. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 43.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 44. Kane FE, Burdan J, Cutino A, Green KE. Iluvien: a new sustained delivery technology for posterior eye disease. Expert Opin. Drug Deliv. 2008;5:1039–1046. doi: 10.1517/17425247.5.9.1039. •• Discussion on the mechanism and advances of thermoresponsive hydrogels.
- 45.Ramchandran RS, Fekrat S, Stinnett SS, Jaffe GJ. Fluocinolone acetonide sustained drug delivery device for chronic central retinal vein occlusion: 12-month results. Am. J. Ophthalmol. 2008;146:285–291. doi: 10.1016/j.ajo.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Mansoor S, Kuppermann BD, Kenney MC. Intraocular sustained-release delivery systems for triamcinolone acetonide. Pharm. Res. 2009;26:770–784. doi: 10.1007/s11095-008-9812-z. [DOI] [PubMed] [Google Scholar]
- 47.Ciulla TA, Criswell MH, Danis RP, et al. Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants. Br. J. Ophthalmol. 2003;87:1032–1037. doi: 10.1136/bjo.87.8.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr. Opin. Ophthalmol. 2004;15:211–220. doi: 10.1097/01.icu.0000120711.35941.76. [DOI] [PubMed] [Google Scholar]
- 49.Beeley NR, Stewart JM, Tano R, et al. Development, implantation, in vivo elution, and retrieval of a biocompatible, sustained release subretinal drug delivery system. J. Biomed. Mater. Res. A. 2006;76:690–698. doi: 10.1002/jbm.a.30567. [DOI] [PubMed] [Google Scholar]
- 50.Beeley NR, Rossi JV, Mello-Filho PA, et al. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J. Biomed. Mater. Res. A. 2005;73:437–444. doi: 10.1002/jbm.a.30294. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, Kim JH, Jeon O, Kwon IC, Park K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009;71:420–430. doi: 10.1016/j.ejpb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irving–Gass syndrome. Am. J. Ophthalmol. 2009;147:1048–1054. e2. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 53.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007;125:309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 54.Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest. Ophthalmol. Vis. Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 55.Bu HZ, Gukasyan HJ, Goulet L, et al. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr. Drug Metab. 2007;8:91–107. doi: 10.2174/138920007779815977. [DOI] [PubMed] [Google Scholar]
- 56.Guidetti B, Azema J, Malet-Martino M, Martino R. Delivery systems for the treatment of proliferative vitreoretinopathy: materials, devices and colloidal carriers. Curr. Drug Deliv. 2008;5:7–19. doi: 10.2174/156720108783331050. [DOI] [PubMed] [Google Scholar]
- 57.Kearns VR, Williams RL. Drug delivery systems for the eye. Expert Rev. Med. Devices. 2009;6(3):277–290. doi: 10.1586/erd.09.4. [DOI] [PubMed] [Google Scholar]
- 58.Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 2004;23:253–281. doi: 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 2001;52:37–48. doi: 10.1016/s0169-409x(01)00193-4. [DOI] [PubMed] [Google Scholar]
- 60.Kashyap N, Modi S, Jain JP, et al. Polymers for advanced drug delivery. CRIPS (Current Research & Information on Pharmaceutical Science) 2004;5:7–12. [Google Scholar]
- 61.Kranz H, Ubrich N, Maincent P, Bodmeier R. Physicomechanical properties of biodegradable poly(d,l-lactide) and poly(d,l-lactide-co-glycolide) films in the dry and wet states. J. Pharm. Sci. 2000;89:1558–1566. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Moinard-Checot D, Chevalier Y, Briancon S, Beney L, Fessi H. Mechanism of nanocapsules formation by the emulsion–diffusion process. J. Colloid Interface Sci. 2008;317:458–468. doi: 10.1016/j.jcis.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 63.Moinard-Checot D, Chevalier Y, Briancon S, Fessi H, Guinebretiere S. Nanoparticles for drug delivery: review of the formulation and process difficulties illustrated by the emulsion–diffusion process. J. Nanosci. Nanotechnol. 2006;6:2664–2681. doi: 10.1166/jnn.2006.479. [DOI] [PubMed] [Google Scholar]
- 64.Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 65.Moritera T, Ogura Y, Yoshimura N, et al. Feasibility of drug targeting to the retinal pigment epithelium with biodegradable microspheres. Curr. Eye Res. 1994;13:171–176. doi: 10.3109/02713689408995774. [DOI] [PubMed] [Google Scholar]
- 66.Kimura H, Ogura Y, Moritera T, Honda Y, Tabata Y, Ikada Y. In vitro phagocytosis of polylactide microspheres by retinal pigment epithelial cells and intracellular drug release. Curr. Eye Res. 1994;13:353–360. doi: 10.3109/02713689409167299. [DOI] [PubMed] [Google Scholar]
- 67.Tuovinen L, Ruhanen E, Kinnarinen T, et al. Starch acetate microparticles for drug delivery into retinal pigment epithelium–in vitro study. J. Control. Release. 2004;98:407–413. doi: 10.1016/j.jconrel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;3:31–36. doi: 10.1159/000055638. [DOI] [PubMed] [Google Scholar]
- 69.Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm. Res. 2009;26:329–337. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 70.Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano-and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 71.Loftsson T, Hreinsdottir D, Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007;59:629–635. doi: 10.1211/jpp.59.5.0002. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int. J. Pharm. 2007;331:153–159. doi: 10.1016/j.ijpharm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-Gaete C, Fattal E, Silva L, Besnard M, Tsapis N. Dexamethasone acetate encapsulation into Trojan particles. J. Control. Release. 2008;128:41–49. doi: 10.1016/j.jconrel.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Veloso AA, Jr, Zhu Q, Herrero-Vanrell R, Refojo MF. Ganciclovir-loaded polymer microspheres in rabbit eyes inoculated with human cytomegalovirus. Invest. Ophthalmol. Vis. Sci. 1997;38:665–675. [PubMed] [Google Scholar]
- 75.Herrero-Vanrell R, Ramirez L, Fernandez-Carballido A, Refojo MF. Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Encapsulation technique, in vitro release profiles, and sterilization process. Pharm. Res. 2000;17:1323–1328. doi: 10.1023/a:1026464124412. [DOI] [PubMed] [Google Scholar]
- 76.Merodio M, Irache JM, Valamanesh F, Mirshahi M. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials. 2002;23:1587–1594. doi: 10.1016/s0142-9612(01)00284-8. [DOI] [PubMed] [Google Scholar]
- 77.Irache JM, Merodio M, Arnedo A, Camapanero A, Mirshahi M, Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini. Rev. Med. Chem. 2005;53(2):293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- 78.Duvvuri S, Gaurav Janoria K, Mitra AK. Effect of polymer blending on the release of ganciclovir from PLGA microspheres. Pharm. Res. 2006;23:215–223. doi: 10.1007/s11095-005-9042-6. [DOI] [PubMed] [Google Scholar]
- 79.Duvvuri S, Janoria KG, Mitra AK. Development of a novel formulation containing poly(d,l-lactide-co-glycolide) microspheres dispersed in PLGA–PEG–PLGA gel for sustained delivery of ganciclovir. J. Control. Release. 2005;108:282–293. doi: 10.1016/j.jconrel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Duvvuri S, Janoria KG, Pal D, Mitra AK. Controlled delivery of ganciclovir to the retina with drug-loaded poly(d,l-lactide-co-glycolide) (PLGA) microspheres dispersed in PLGA–PEG–PLGA gel: a novel intravitreal delivery system for the treatment of cytomegalovirus retinitis. J. Ocul. Pharmacol. Ther. 2007;23:264–274. doi: 10.1089/jop.2006.132. [DOI] [PubMed] [Google Scholar]
- 81.Duvvuri S, Rittenhouse KD, Mitra AK. Microdialysis assessment of drug delivery systems for vitreoretinal targets. Adv. Drug Deliv. Rev. 2005;57:2080–2091. doi: 10.1016/j.addr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Sancho C, Herrero-Vanrell R, Negro S. Vitamin A palmitate and aciclovir biodegradable microspheres for intraocular sustained release. Int. J. Pharm. 2006;326:100–106. doi: 10.1016/j.ijpharm.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Cortesi R, Ajanji SC, Sivieri E, et al. Eudragit microparticles as a possible tool for ophthalmic administration of acyclovir. J. Microencapsul. 2007;24:445–456. doi: 10.1080/02652040701374889. [DOI] [PubMed] [Google Scholar]
- 84.Moritera T, Ogura Y, Honda Y, Wada R, Hyon SH, Ikada Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest. Ophthalmol. Vis. Sci. 1991;32:1785–1790. [PubMed] [Google Scholar]
- 85.Moritera T, Ogura Y, Yoshimura N, et al. Biodegradable microspheres containing adriamycin in the treatment of proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 1992;33:3125–3130. [PubMed] [Google Scholar]
- 86.Giordano GG, Refojo MF, Arroyo MH. Sustained delivery of retinoic acid from microspheres of biodegradable polymer in PVR. Invest. Ophthalmol. Vis. Sci. 1993;34:2743–2751. [PubMed] [Google Scholar]
- 87.Peyman GA, Conway M, Khoobehi B, Soike K. Clearance of microsphere-entrapped 5-fluorouracil and cytosine arabinoside from the vitreous of primates. Int. Ophthalmol. 1992;16:109–113. doi: 10.1007/BF00918942. [DOI] [PubMed] [Google Scholar]
- 88.Yeh MK, Tung SM, Lu DW, Chen JL, Chiang CH. Formulation factors for preparing ocular biodegradable delivery system of 5-fluorouracil microparticles. J. Microencapsul. 2001;18:507–519. doi: 10.1080/02652040010018100. [DOI] [PubMed] [Google Scholar]
- 89.de Kozak Y, Andrieux K, Villarroya H, et al. Intraocular injection of tamoxifen-loaded nanoparticles: a new treatment of experimental autoimmune uveoretinitis. Eur. J. Immunol. 2004;34:3702–3712. doi: 10.1002/eji.200425022. [DOI] [PubMed] [Google Scholar]
- 90.He Y, Liu Y, Liu Y, et al. Cyclosporine-loaded microspheres for treatment of uveitis: in vitro characterization and in vivo pharmacokinetic study. Invest. Ophthalmol. Vis. Sci. 2006;47:3983–3988. doi: 10.1167/iovs.05-1373. [DOI] [PubMed] [Google Scholar]
- 91.Sakai T, Kohno H, Ishihara T, et al. Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating β-methasone phosphate. Exp. Eye Res. 2006;82:657–663. doi: 10.1016/j.exer.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Barcia E, Herrero-Vanrell R, Diez A, Alvarez-Santiago C, Lopez I, Calonge M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp. Eye Res. 2009;89:238–245. doi: 10.1016/j.exer.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Chen J, Patil S, Seal S, McGinnis JF. Nanoceria particles prevent ROI-induced blindness. Adv. Exp. Med. Biol. 2008;613:53–59. doi: 10.1007/978-0-387-74904-4_5. [DOI] [PubMed] [Google Scholar]
- 94.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 95. Edelhauser HF, Boatright JH, Nickerson JM. Drug delivery to posterior intraocular tissues: third Annual ARVO/Pfizer Ophthalmics Research Institute Conference. Invest. Ophthalmol. Vis. Sci. 2008;49:4712–4720. doi: 10.1167/iovs.08-1904. •• Excellent up-to-date summary of drug delivery to the posterior segment.
- 96.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB. J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 97.Bejjani RA, BenEzra D, Cohen H, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol. Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- 98.Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol. Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- 99.Fattal E, Bochot A. Antisense oligonucleotides, aptamers and SiRNA: promises for the treatment of ocular diseases. Arch. Soc. Esp. Oftalmol. 2006;81:3–6. [PubMed] [Google Scholar]
- 100.Tanito M, Li F, Elliott MH, Dittmar M, Anderson RE. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Invest. Ophthalmol. Vis. Sci. 2007;48:1900–1905. doi: 10.1167/iovs.06-1057. [DOI] [PubMed] [Google Scholar]
- 101.Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int. J. Pharm. 2008;364:237–248. doi: 10.1016/j.ijpharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 102.Aukunuru JV, Ayalasomayajula SP, Kompella UB. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2003;55:1199–1206. doi: 10.1211/0022357021701. [DOI] [PubMed] [Google Scholar]
- 103.Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic) acid microspheres. Invest. Ophthalmol. Vis. Sci. 2003;44:290–299. doi: 10.1167/iovs.01-1156. [DOI] [PubMed] [Google Scholar]
- 104. Moshfeghi AA, Peyman GA. Micro- and nanoparticulates. Adv. Drug Deliv. Rev. 2005;57:2047–2052. doi: 10.1016/j.addr.2005.09.006. • Good review of drug delivery in micro- and nanoparticulates.
- 105.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J. Pharm. Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 107.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol. Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang CH, Tung SM, Lu DW, Yeh MK. In vitro and in vivo evaluation of an ocular delivery system of 5-fluorouracil microspheres. J. Ocul. Pharmacol. Ther. 2001;17:545–553. doi: 10.1089/10807680152729239. [DOI] [PubMed] [Google Scholar]
- 109.Saishin Y, Silva RL, Saishin Y, et al. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest. Ophthalmol. Vis. Sci. 2003;44:4989–4993. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- 110.Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- 111.Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm. Res. 2004;21:1797–1804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- 112.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib–PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005;511:191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 113.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib–PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv. Drug Deliv. Rev. 2001;52:5–16. doi: 10.1016/s0169-409x(01)00200-9. [DOI] [PubMed] [Google Scholar]
- 115.Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers. J. Pharm. Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- 116.Porter TR, Cwajg JM. Contrast echocardiography. In: Prohost GM, editor. Imaging in Cardiovascular Disease. PA, USA: Lippincott Williams & Wilkins; 2000. pp. 85–96. [Google Scholar]
- 117.Qin L, Pahud DR, Ding Y, et al. Efficient transfer of genes into murine cardiac grafts by Starburst polyamidoamine dendrimers. Hum. Gene Ther. 1998;9:553–560. doi: 10.1089/hum.1998.9.4-553. [DOI] [PubMed] [Google Scholar]
- 118.Kost J, Leong K, Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl Acad. Sci. USA. 1989;86:7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med. Biol. 1997;23:953–959. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 120.Huber PE, Pfisterer P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 2000;7:1516–1525. doi: 10.1038/sj.gt.3301242. [DOI] [PubMed] [Google Scholar]
- 121.Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum. Gene Ther. 1996;7:1339–1346. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- 122.Miller DL, Bao S, Gies RA, Thrall BD. Ultrasonic enhancement of gene transfection in murine melanoma tumors. Ultrasound Med. Biol. 1999;25:1425–1430. doi: 10.1016/s0301-5629(99)00105-2. [DOI] [PubMed] [Google Scholar]
- 123.Tata DB, Dunn F, Tindall DJ. Selective clinical ultrasound signals mediate differential gene transfer and expression in two human prostate cancer cell lines: LnCap and PC-3. Biochem. Biophys. Res. Commun. 1997;234:64–67. doi: 10.1006/bbrc.1997.6578. [DOI] [PubMed] [Google Scholar]
- 124.Mukherjee D, Wong J, Griffin B, et al. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J. Am. Coll. Cardiol. 2000;35:1678–1686. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- 125.Zderic V, Clark JI, Vaezy S. Drug delivery into the eye with the use of ultrasound. J. Ultrasound Med. 2004;23:1349–1359. doi: 10.7863/jum.2004.23.10.1349. [DOI] [PubMed] [Google Scholar]
- 126.Price RJ, Kaul S. Contrast ultrasound targeted drug and gene delivery: an update on a new therapeutic modality. J. Cardiovasc. Pharmacol. Ther. 2002;7:171–180. doi: 10.1177/107424840200700307. [DOI] [PubMed] [Google Scholar]
- 127.Kooiman K, Bohmer MR, Emmer M, et al. Oil-filled polymer microcapsules for ultrasound-mediated delivery of lipophilic drugs. J. Control. Release. 2009;133:109–118. doi: 10.1016/j.jconrel.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 128.Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog. Cardiovasc. Dis. 2001;44:45–54. doi: 10.1053/pcad.2001.26443. [DOI] [PubMed] [Google Scholar]
- 129.Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R. Therapeutic applications of microbubbles. Eur. J. Radiol. 2002;42:160–168. doi: 10.1016/s0720-048x(01)00455-7. [DOI] [PubMed] [Google Scholar]
- 130.Tsutsui JM, Grayburn PA, Xie F, Porter TR. Drug and gene delivery and enhancement of thrombolysis using ultrasound and microbubbles. Cardiol. Clin. 2004;22:299–312. doi: 10.1016/j.ccl.2004.02.009. vii. [DOI] [PubMed] [Google Scholar]
- 131.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 132.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 133.Postema M, Gilja OH. Ultrasound-directed drug delivery. Curr. Pharm. Biotechnol. 2007;8:355–361. doi: 10.2174/138920107783018453. [DOI] [PubMed] [Google Scholar]
- 134.Mayer CR, Geis NA, Katus HA, Bekeredijan R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin. Drug Deliv. 2008;5:1121–1138. doi: 10.1517/17425247.5.10.1121. [DOI] [PubMed] [Google Scholar]
- 135.Shimamura M, Sato N, Taniyama Y, et al. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther. 2004;11:1532–1539. doi: 10.1038/sj.gt.3302323. [DOI] [PubMed] [Google Scholar]
- 136.Hirokawa T, Karshafian R, Pavlin CJ, Burns PN. Insonation of the eye in the presence of microbubbles: preliminary study of the duration and degree of vascular bioeffects – work in progress. J. Ultrasound Med. 2007;26:731–738. doi: 10.7863/jum.2007.26.6.731. [DOI] [PubMed] [Google Scholar]
- 137.Sonoda S, Tachibana K, Uchino E, et al. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest. Ophthalmol. Vis. Sci. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- 138.Peeters L, Lentacker I, Vandenbroucke RE, et al. Can ultrasound solve the transport barrier of the neural retina? Pharm. Res. 2008;25:2657–2665. doi: 10.1007/s11095-008-9684-2. [DOI] [PubMed] [Google Scholar]
- 139.Church CC. The effects of an elastic solid surface layer on the radial pulsations of gas bubbles. J. Acoust. Soc. Am. 1995;97:1510–1521. [Google Scholar]
- 140.Hoff L, Sontum PC, Hovem JM. Oscillations of polymeric microbubbles: effect of the encapsulating shell. J. Acoust. Soc. Am. 2000;107:2272–2280. doi: 10.1121/1.428557. [DOI] [PubMed] [Google Scholar]
- 141.Shankar PM, Krishna PD, Newhouse VL. Subharmonic backscattering from ultrasound contrast agents. J. Acoust. Soc. Am. 1999;106:2104–2110. doi: 10.1121/1.428142. [DOI] [PubMed] [Google Scholar]
- 142. Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008;106:206–213. • Good discussion on the mechanism and advances of thermoresponsive hydrogels.
- 143.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug Del. Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Zhang XZ, Yang YY, Chung TS, Ma KX. Preparation and characterization of fast response macroporous poly(N-isopropylacrylamide) hydrogels. Langmuir. 2001;17:6094–6099. [Google Scholar]
Website
- 201.Southern Illinois University School of Medicine. www.siumed.edu/yoc/