Abstract
There is currently no suitable replacement for damaged temporomandibular joint (TMJ) discs after discectomy. In the present study, we fabricated bilayer biodegradable polylactide (PLA) discs comprising a non-woven mat of poly(L/D)lactide (P(L/D)LA) 96/4 and a P(L/DL)LA 70/30 membrane plate. The PLA disc was examined in combination with adipose stem cells (ASCs) for tissue engineering of the fibrocartilaginous TMJ disc in vitro. ASCs were cultured in parallel in control and chondrogenic medium for a maximum of six weeks. Relative expression of the genes, aggrecan, type I collagen and type II collagen present in the TMJ disc extracellular matrix increased in the ASC-seeded PLA discs in the chondrogenic medium. The hypertrophic marker, type X collagen, was moderately induced. Alcian blue staining showed accumulation of sulphated glycosaminoglycans. ASC differentiation in the PLA discs was close to that observed in pellet cultures. Comparison of the mRNA levels revealed that the degree of ASC differentiation was lower than that in TMJ disc-derived cells and tissue. The pellet format supported the phenotype of the TMJ disc-derived cells under chondrogenic conditions and also enhanced their hyalinization potential, which is considered part of the TMJ disc degeneration process. Accordingly, the combination of ASCs and PLA discs has potential for the development of a tissue-engineered TMJ disc replacement.
Keywords: adipose stem cell, non-woven fabric, polylactide, temporomandibular joint disc, tissue engineering
1. Introduction
Temporomandibular joint (TMJ) disc degeneration owing to displacement or trauma causes considerable pain and dysfunction of the TMJ. In severe cases, the degeneration proceeds until the disc becomes morphologically too altered to be repaired by other therapies, eventually requiring disc removal (Dolwick 1997; Eriksson & Westesson 2001). Subsequently, discectomy leaves the joint surfaces exposed to direct mechanical loading and abrasion, which predispose the joint to arthritic deformation and may ultimately lead to total joint replacement (Mercuri et al. 2007).
The TMJ disc is a biconcave structure positioned between the mandibular condyle and the fossa. The TMJ disc is characterized as specialized fibrocartilaginous tissue distinct from both hyaline and meniscal cartilage (Almarza & Athanasiou 2004a). Approximately two-thirds of the cells in the TMJ disc are described as fibroblasts and one-third have a chondrocyte-like morphology (Landesberg et al. 1996; Detamore et al. 2006). Type I collagen forms the major structural framework of the extracellular matrix (ECM), whereas collagen types II, III, VI, IX and XII are reported in lesser amounts (Mills et al. 1994; Ali & Sharawy 1996b; Landesberg et al. 1996; Minarelli & Liberti 1997). Chondroitin sulphates and dermatan sulphates are the major constituents of the glycosaminoglycans (GAGs) in the TMJ disc (Nakano & Scott 1989, 1996; Mills et al. 1994; Detamore et al. 2005). The amount and distribution of the ECM components in the TMJ disc are heterogeneous and largely dependent on the species and age (Mills et al. 1994).
Like hyaline cartilage, the TMJ disc lacks intrinsic regenerative capacity (Tanaka et al. 2008). Therefore, tissue-engineered TMJ discs may provide another treatment option. Several different materials have been used to replace the removed discs, including autografts taken from different anatomic sites, such as auricular cartilage (Tucker & Spagnoli 1996) and dermis (Dimitroulis 2005), and synthetic materials manufactured from silicone and polytetrafluoroethylene (Estabrooks et al. 1990; Mercuri & Giobbie-Hurder 2004). The disadvantages of autografts, however, include their poor mechanical performance and damage induced at the site of harvesting (Feinberg & McDonnell 1995; Tucker & Spagnoli 1996). The failure of synthetic implants owing to detrimental tissue reactions (Estabrooks et al. 1990; Eriksson & Westesson 1992; Mercuri & Giobbie-Hurder 2004) has directed interest towards biodegradable biomaterials and tissue engineering of the TMJ disc with structurally and functionally analogous properties equivalent to the native TMJ disc. The first attempt to engineer the TMJ disc was performed using type I collagen mesh, which as a natural component of the ECM seemed to be an ideal scaffold material for TMJ disc reconstruction (Thomas et al. 1991). Subsequently, synthetic polyglycolide (PGA) has been the most extensively studied biomaterial used for tissue engineering of the TMJ disc. Both biomaterials have good compatibility with TMJ disc-derived cells, but exhibit poor mechanical durability mainly because of their rapid resorption (Thomas et al. 1991; Puelacher et al. 1994; Springer et al. 2001; Almarza & Athanasiou 2004b). Consequently, polylactide (PLA), which has a longer degradation time, has attracted interest as a promising synthetic material for engineering the TMJ disc (Allen & Athanasiou 2008).
Different cell sources may be considered for TMJ disc engineering. Culturing cells isolated from the TMJ disc during diagnostic arthroscopy is a practical method of obtaining autologous cells for engineering artificial TMJ discs (Thomas et al. 1991; Springer et al. 2001; Johns & Athanasiou 2007). These cells may be cultured and expanded in the laboratory; nonetheless, they are prone to changing their phenotype during culture (Landesberg et al. 1996; Springer et al. 2001; Allen & Athanasiou 2007). Alternatively, there have also been attempts to use articular cartilage-derived chondrocytes or closely related cells in TMJ disc engineering (Puelacher et al. 1994; Girdler 1998; Springer et al. 2001); the use of autologous cartilage is always limited by donor site scarcity and morbidity. These factors provide a perspective for the importance of developing alternative cell sources, such as stem cells, for artificial tissue constructs to replace degenerated TMJ discs (Allen & Athanasiou 2007). Adipose tissue is an expendable and abundant source of autologous multi-potent stem cells capable of undergoing differentiation towards cells of mesenchymal origin, including the fibroblasts and chondrocytes found in the TMJ disc (Zuk et al. 2001; Erickson et al. 2002; Huang et al. 2004). Adipose stem cells (ASCs) differentiate along chondrogenic lineages (Erickson et al. 2002; Awad et al. 2003; Huang et al. 2004; Estes et al. 2006), but, to the best of our knowledge, this is the first time that ASCs have been used in TMJ disc engineering.
The aim of this in vitro study was to develop tissue-engineered constructs to replace fully degenerated TMJ discs by culturing ASCs in biodegradable PLA discs under conditions that support chondrogenic differentiation. The growth and differentiation of ASCs were evaluated in TMJ disc-shaped PLA discs on the basis of gene expression changes and sulphated GAG formation during the in vitro culture period. The differentiation of ASCs in the PLA discs was compared with that of pellet cultures. We also used conventional pellet cultures to maintain TMJ disc-derived cells. The course and level of ASC differentiation were further examined in comparison with TMJ disc-derived cells cultured in pellet cultures and in comparison with TMJ disc tissue.
2. Material and methods
2.1. ASC isolation and expansion
Adipose tissue was harvested from skeletally mature male New Zealand white rabbits (n = 7) immediately after sacrifice. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committees of the University of Tampere and the University of Helsinki. ASC isolation was based on the protocol described previously (Zuk et al. 2001). Briefly, fat pads were finely minced, and ECM was digested with 0.15 per cent type I collagenase (Sigma, St Louis, MO, USA). Subsequently, the ASC-rich stromal vascular fraction was separated from buoyant mature adipocytes by centrifugation. Isolated ASCs were expanded in control medium containing Dulbecco's modified Eagle's medium (Sigma-Aldrich Chemie GmbH, Steinheim, Germany); 10 per cent foetal bovine serum (FBS) (Gibco, Invitrogen Life Technologies, Paisley, UK); 1 per cent antibiotic/antimycotic (100 U ml−1 penicillin, 100 µg ml−1 streptomycin and 250 ng ml−1 amphotericin B; Gibco, Invitrogen) and 1 per cent l-glutamine (Gibco, Invitrogen) for five passages prior to use in subsequent experiments.
In addition to adipose tissue, the TMJ discs from one of the rabbits were harvested for positive controls. Cells were isolated by dissecting the TMJ discs into smaller fragments and allowing the cells to migrate onto the cell culture plastic in control medium. TMJ disc-isolated cells at passage 3 were used for further experiments.
2.2. Scaffold preparation
The scaffold designed for the rabbit TMJ disc was composed of two layers: a non-woven mat of poly(L/D)lactide (P(L/D)LA) 96/4 that was closed from the other side with a P(L/DL)LA 70/30 membrane plate to prevent cell migration out from the scaffold. Two compositions of P(L/D)LA 96/4 non-woven mats were compared with regard to cell attachment, growth and differentiation.
2.2.1. Non-woven mat 1
Medical grade P(L/D)LA 96/4 (intrinsic viscosity of approx. 1.7 dl g−1, Purac Biochem b.v., Gorinchem, The Netherlands) polymer was melt-spun using a monofilament nozzle diameter 0.5 mm and a laboratory-sized extruder (Gimac, Gastronno, Italy). The filament was drawn to a thickness of 0.02–0.03 mm using a Fourné high-speed spinning machine (Fourné Polymertechnik GmbH, Alfter-Impekoven, Germany).
2.2.2. Non-woven mat 2
Medical grade P(L/D)LA 96/4 (intrinsic viscosity of approx. 3.5 dl g−1, Purac Biochem b.v.) polymer was melt-spun using a multi-filament nozzle (12 filaments) with a single orifice (diameter 0.4 mm) and a laboratory-sized extruder. The filaments were drawn to a thickness of 0.06–0.07 mm using a laboratory-scale drawing line that consisted of ovens and caterpillars. Both fibre types were cut to staple fibres, carded and needle-punched to form non-woven mats. The average porosity of both non-woven mats was estimated to be 80–95%, using water as a penetrative liquid.
2.2.3. The membrane plate
Medical grade P(L/DL)LA 70/30 (LR 708, Boehringer Ingelheim, Ingelheim, Germany) polymer was extruded through a 30 × 0.5 mm die and drawn using caterpillars to a continuous strip with dimensions of 10 × 0.16 mm.
2.2.4. PLA disc
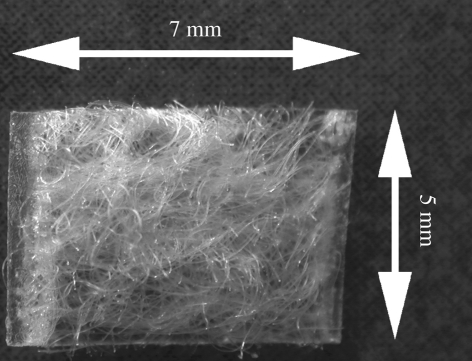
The membrane plate and non-woven mat were cut into 10 × 60 mm pieces. The mat was placed on the strip, and the edges of the two materials were welded together using an ultrasonic-welding machine. From this preform, approximately 5 × 7 × 1.8 mm samples were prepared. Non-woven mats 1 and 2 were used for manufacturing of PLA discs 1 and 2, respectively. The PLA discs were washed twice in ethanol for 20 min using an ultrasonic cleaner. Samples were dried for 24 h in a laminar hood and 24 h under vacuum, after which the samples were packed and gamma-irradiated (25 kGy). The design of the disc is presented in figure 1.
Figure 1.
A structure of the PLA disc. A non-woven mat (P(L/D)LA 96/4) closed from one side with a (P(L/DL)LA 70/30) membrane plate. Thickness of the disc is approximately 1.8 mm.
2.3. Chondrogenic differentiation of ASCs
ASCs were seeded onto the PLA discs 1 and 2 by using the micromass technique (Denker et al. 1995). Briefly, the cells were harvested, counted and resuspended in control medium at a concentration of 107 cells ml−1. Two droplets (10 µl) of the suspension were placed onto the non-woven side of a dry PLA disc in 24-well cell culture plates. Micromasses were allowed to adhere on the disc for 3 h at 37°C under 5 per cent CO2 in a cell incubator in a humidified atmosphere before adding the media. Pellet cultures of ASCs and TMJ disc-isolated cells were performed according to a modification from the previously described methods (Ballock & Reddi 1994). In brief, pellet formation of a suspension of 500 000 ASCs was induced by centrifugation at 180g for 10 min in 15 ml conical polypropylene tubes (Sarstedt, Nümbrecht, Germany).
One half of the PLA disc and pellet cultures was maintained in control medium and the other half in chondrogenic medium comprising DMEM/F12, supplemented with 1 per cent FBS, 1 per cent antibiotic/antimycotic, 1 per cent l-glutamine, 6.25 µg ml−1 insulin (Sigma, Steinheim, Germany), 50 nM ascorbic acid (Sigma) and 10 ng ml−1 transforming growth factor (TGF)-β1 (Sigma). The experimental groups with the time points of the analyses are presented in table 1. The same nomenclature will be used in the following sections. Chondrogenic medium was supplemented with TGF-β1 in the first medium exchange after 24 h of culture. Media were changed at 3 day intervals.
Table 1.
Nomenclature of sample series and number of observations (n) in the analyses. (C, control; D, differentiation; qRT–PCR, real-time quantitative reverse transcription–polymerase chain reaction; nd, not determined.)
| PLA disc |
pellet |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ASC disc 1 |
ASC disc 2 |
ASC pellet |
TMJ disc cell pellet |
||||||
| week | analysis | C | D | C | D | C | D | C | D |
| 1 | Alcian blue | 1 | 1 | 1 | 1 | nd | nd | nd | nd |
| qRT–PCR | 4 | 4 | 4 | 4 | 3 | 3 | 1 | 1 | |
| 1.5 | Alcian blue | nd | nd | nd | nd | 3 | 3 | nd | nd |
| qRT–PCR | nd | nd | nd | nd | nd | nd | nd | nd | |
| 2 | Alcian blue | 1 | 1 | 1 | 1 | nd | nd | nd | nd |
| qRT–PCR | 3 | 3 | 4 | 4 | nd | nd | nd | nd | |
| 3 | Alcian blue | 3 | 3 | 2 | 2 | 3 | 3 | nd | nd |
| qRT–PCR | 4 | 4 | 4 | 4 | 3 | 3 | 1 | 1 | |
| 6 | Alcian blue | 2 | 2 | 2 | 2 | 3 | 3 | nd | nd |
| qRT–PCR | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | |
2.4. Histology
For histological analyses, ASC pellets and the ASC discs were fixed in 4 per cent paraformaldehyde, embedded in paraffin and sectioned at 5 µm thickness. The sections were stained with Alcian blue (pH 1.0) to detect sulphated GAGs, which are abundant in cartilaginous matrices using Nuclear Fast Red solution (Biocare Medical, Concord, MA, USA) as a counterstain.
2.5. Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis
Total RNA was isolated from ASC discs, ASC pellets, TMJ disc cell pellets and a TMJ disc tissue sample using Trizol reagent (Invitrogen) according to the manufacturer's instructions. First-strand cDNA was reverse transcribed from total RNA using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). The mRNA level of cartilage ECM components including aggrecan and types I, II and X collagen were analysed by the qRT–PCR method. The sequences and accession numbers of the primers (Oligomer Oy, Helsinki, Finland) are presented in table 2. The qRT–PCR mixture contained 25 ng cDNA, 330 nM forward and reverse primers and SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). The reactions were conducted and monitored with ABI Prism 7000 Sequence Detection System (Applied Biosystems) beginning with enzyme activation at 95°C for 10 min, and then 45 cycles of denaturation at 95°C for 15 s and anneal and extend at 60°C for 1 min. The relative expression level for each gene of interest was calculated according to a previously described mathematical model (Pfaffl 2001). The housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was used as an internal control.
Table 2.
Primer sequences for qRT–PCR.
| gene | accession number | primer sequence | product size (bp) |
|---|---|---|---|
| GAPDH | L23961 | forward 5′-GGG TGG TGG ACC TCA TGG T-3′ | 57 |
| reverse 5′-CGG TGG TTT GAG GGC TCT TA-3′ | |||
| aggrecan | L38480 | forward 5′-GGG ACG TGT GCG CAT CA-3′ | 54 |
| reverse 5′-GTA GTT GGG CAG CGA GAC CTT-3′ | |||
| type I collagen | D49399 | forward 5′-GGG ACA CAA CGG ATT GCA A -3′ | 59 |
| reverse 5′-GCA CCT TGA TCA CCA TGT TGA C-3′ | |||
| type II collagen | D83229 | forward 5′-CCC CGT CTG CCC TAC TGA-3′ | 67 |
| reverse 5′-GTT CTC CTT TCT GCC CCT TTG-3′ | |||
| type X collagen | AY598937 | forward 5′-GCA CGC CTG TAA TGT ACA CCT ATG-3′ | 65 |
| reverse 5′-CGC TCC CTG AAG CCT GAT C-3′ |
2.6. Statistical analyses
The qRT–PCR results of ASC discs 1 and 2 were analysed with the Kruskal–Wallis test followed by Dunn's post-test, and the Wilcoxon test was used for ASC pellets. Individual observations were used to calculate statistically significant differences between the control and chondrogenic conditions without separating different time points and between ASC discs 1 and 2. Differences were regarded as statistically significant with p < 0.05. Statistical analyses were performed using GraphPad Prism 3.03 software.
3. Results
3.1. Gross appearance of the ASC disc, ASC pellet and TMJ disc cell pellet cultures
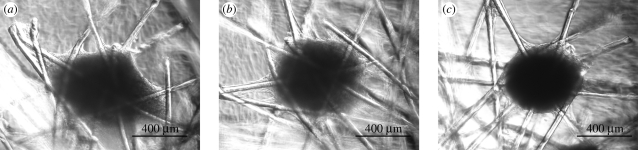
The PLA discs with the two different non-woven mats 1 and 2 of P(L/D)LA 96/4 were prepared for ASC cultures to examine their potential and possible differences as scaffolds in vitro aimed at TMJ disc reconstruction. Although the ASC activity was distinguishable depending on the culture conditions used, no differences between ASC discs 1 and 2 were detected according to histological examination and qRT–PCR, and, accordingly, the results of the PLA disc types 1 and 2 are viewed collectively in the following sections. Differentiated ASCs formed aggregates that favoured the fibre crossing points in the PLA discs. These cell aggregates were detectable as early as 3 days after beginning culture (figure 2a). Subsequently, the size of the aggregates increased and became more condensed by week 2 (figure 2c). Cell aggregates were not observed in ASC discs under control conditions. In ASC pellet cultures, the difference between culture conditions was observed in the pellet size. The size of the control ASC pellets decreased, whereas under chondrogenic conditions, the size of the ASC pellets increased for up to six weeks of culturing. The size of the TMJ disc cell pellets was generally smaller than that of the ASC pellets.
Figure 2.
A micromass attached on the fibre crossing point in a PLA disc under chondrogenic conditions. The condensation of the micromass after 3 days (a), 4 days (b) and two weeks (c) of culturing.
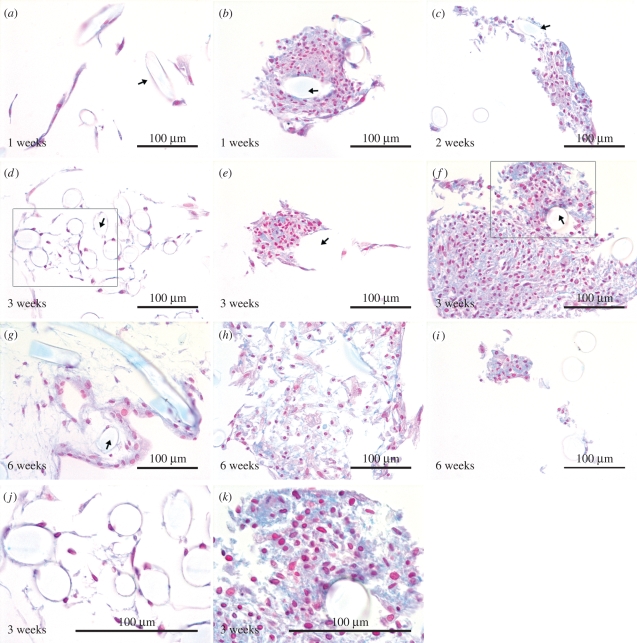
3.2. Accumulation of sulphated GAGs in high-density cell aggregates
ASC cell attachment and growth on the fibres of the PLA discs were also confirmed in histological sections. Under control conditions, ASCs grew as a monolayer following the contour of fibres (figure 3a,d) as well as the membrane plate at the bottom. Under chondrogenic conditions, the PLA fibres were surrounded by ASC aggregates. The largest aggregates were detected at week 3, supporting the observations made microscopically. The formation of sulphated GAGs was demonstrated with Alcian blue staining, which emerged visibly in the aggregates at weeks 1 (figure 3b) and 2 (figure 3c), but was most extensive at week 3 (figure 3e, f). At week 6, the partly degraded, largest aggregates showed only mildly bluish areas (figure 3h), but strong blue staining was still detected in the smaller aggregates under chondrogenic conditions (figure 3i). ASCs were also present under control conditions after six weeks of culture, but showed neither aggregate formation nor staining (figure 3g).
Figure 3.
The ASC discs stained with Alcian blue. The control ASC discs (a,d,g) are represented on the left column and the ASC discs under differentiation conditions (b,c,e,f,h,i) in two columns on the right. Boxes in (d) and (f) represent areas shown at higher magnifications in (j) and (k), respectively. Arrows indicate locations of the P(L/D)LA 96/4 fibres. The ASC discs are chosen from the different experiments, and the ASC discs 1 and 2 are not separated in the panel owing to general similarity in their behaviour.
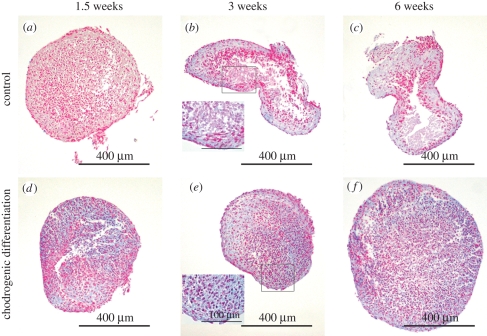
In ASC pellets, sulphated GAG accumulation was observed under both culture conditions. Alcian blue-stained areas were detected in some of the control ASC pellets, but staining seemed more intense and extensive in the differentiated ones (figure 4d–f). In the course of culture, some areas lacking cells emerged in the cores of the pellets, in both control and chondrogenic cultures; however, this was more discernible under control conditions (figure 4a–c). Chondrogenic medium visibly enhanced the viability of ASCs, both in ASC pellets and in aggregates inside the ASC discs.
Figure 4.
Alcian blue-stained ASC pellets from control (a–c) and differentiation (d–f) at the time points of 1.5 (a,d), 3 (b,e) and 6 (c,f) weeks of culture. The higher magnification insets are represented in (b) and (e). Pictures represent samples from different experiments.
3.3. Temporal pattern of gene expression changes in differentiated ASCs
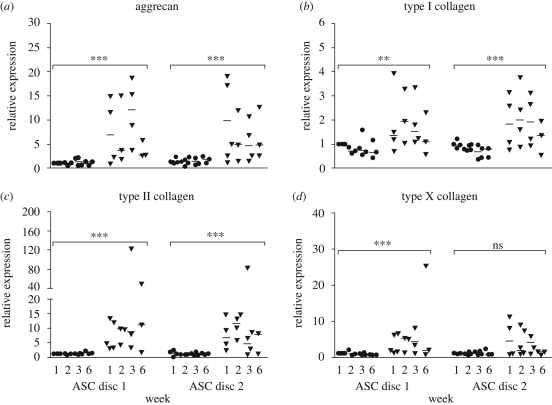
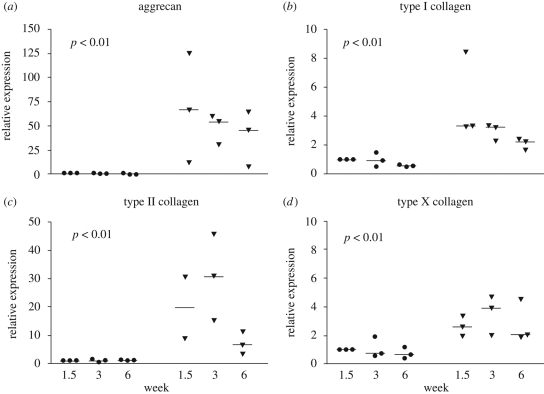
Quantitative RT–PCR was used to detect relative changes in the expression of aggrecan and collagen type I, II and X genes observed in the ECM of cartilaginous tissues. The relative expression of all detected genes was induced in ASCs under chondrogenic conditions. The increases were statistically significant between the control and chondrogenic conditions and established in both the ASC discs (figure 5) and ASC pellets (figure 6). Time points were not tested individually. The overall scale of the relative expression changes was larger for ASC pellet cultures. There were no statistically significant differences between ASC discs 1 and 2. Under control conditions, the gene expression changes were not detected at any time point.
Figure 5.
The relative mRNA levels of the ASC discs 1 and 2 for aggrecan (a), type I (b), type II (c) and type X (d) collagen in the scatter charts. The results of the ASC discs 1 and 2 are compared side by side in the same chart. The controls are presented on the left side and the differentiations on the right side. The significantly changed mRNA levels were noticed between controls, and differentiation conditions for the disc are marked with ** (p < 0.01), *** (p < 0.001) and ns (not significant). Differences between the ASC discs 1 and 2 were not found to be statistically significant. (Filled circle, control; filled upside down triangle, differentiation; line, median of the results at a time point.)
Figure 6.
The scatter charts of the relative mRNA levels of aggrecan (a), type I (b), type II (c) and type X (d) collagen in ASC pellets. An expression point for type II collagen at 1.5 weeks was defined as an outlier and thus discarded. The relative changes in gene expression were statistically significant (p < 0.01) for all the detected genes. (Filled circle, control; filled upside down triangle, differentiation; line, median of the results at a time point.)
The relative expression of aggrecan, the core protein of sulphated proteoglycans present in the ECM of the TMJ disc, seemed highest at the beginning of culture both in the ASC discs and in the ASC pellets, although aggrecan was still strongly expressed in ACS disc 1 at week 3. There was a gradual decrease in the general expression of aggrecan by week 6 (figures 5a and 6a).
Type I collagen is the major structural component in the fibrocartilage of the TMJ disc. Type II collagen is the most prominent collagen in hyaline cartilage, but is also found in the TMJ disc ECM (Mills et al. 1994; Landesberg et al. 1996; Detamore et al. 2005). The relative expression of type I collagen was approximately two- and threefold higher in the differentiated ASC discs and ASC pellets, respectively (figures 5b and 6b). The expression of type I collagen seemed highest by week 2 and decreased thereafter through week 6 in both ASC disc types and ASC pellets. The differences in type II collagen relative expression were higher than those for type I collagen. In the ASC discs, the expression of type II collagen was still high at week 6, whereas its expression in ASC pellets was maintained at the increased level only for the first three weeks and then suddenly decreased by the end of the culture period (figures 5c and 6c). When the expression of type I and II collagen is viewed collectively, chondrogenic differentiation at the mRNA level in rabbit ASCs seemed to peak at week 2.
The expression of type X collagen, regarded as a component of hypertrophic cartilage, was moderately increased under chondrogenic conditions. The level of increase remained mainly less than 5- and 10-fold in the ASC pellets and ASC discs, respectively (figures 5d and 6d). The decrease in type X collagen expression was detected by week 6 in both culture systems. The difference in the expression of type X collagen was not found to be significant between the control and differentiated ASC disc 2.
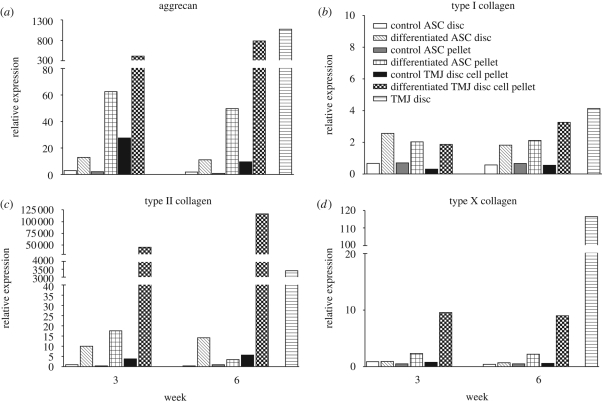
3.4. Comparison of chondrogenesis between ASCs, TMJ disc cells and TMJ disc tissue derived from the same rabbit
In addition to the differentiation series, samples of ASC pellets and ASC discs 1 and 2 at weeks 3 and 6 of one rabbit were re-run in a comparative qRT–PCR analysis with TMJ disc cell pellets at weeks 3 and 6 and original TMJ disc tissue derived from the same rabbit. The overall examination revealed that, in ASCs cultured under chondrogenic conditions, gene expression increases remained noticeably lower than those in TMJ disc cell pellets in chondrogenic medium or tissue sample from the TMJ disc (figure 7). The expression of all detected genes remained mainly unchanged under control conditions. An exception was provided by TMJ disc cell pellets, in which the expression of aggrecan was higher than that in the ASC discs and pellets under control conditions at week 3 but was decreased at close to the same level with the other control samples by week 6. In contrast, in chondrogenic medium, aggrecan expression in the TMJ disc cell pellets was reaching the level of native TMJ disc tissue by week 6 (figure 7a).
Figure 7.
The bar charts of qRT–PCR results comparing control and differentiated samples between ASC pellets and ASC discs, and TMJ disc cell pellets at weeks 3 and 6; in addition, an mRNA sample of the TMJ disc served as a positive control. The results of one PLA disc type were included because of the general similarity between ASC discs 1 and 2. All material was derived from the same rabbit.
In the differentiated TMJ disc cell pellets, the trend of gene expression changes was increasing, whereas in ASC cultures, the expression first increased and then decreased by week 6. Similar to the preceding findings, the mRNA level of type II collagen was an exception, being higher in the ASC discs than in ASC pellets at week 6 (figure 7c). The relative expression of type II collagen in differentiated TMJ disc cell pellets was at extremely high levels: 45 500- and 120 000-fold increases were observed at weeks 3 and 6, respectively. The expression levels of type II collagen in TMJ disc cells exceeded even the level detected in TMJ disc tissue.
The relative expression of type I collagen in differentiated ASC cultures was approximately half of what was observed in the native TMJ disc (figure 7b). The relative expression of type I collagen was uniformly two- to threefold higher than that of controls in all differentiated samples. Importantly, type I collagen was expressed even more strongly in the ASC discs than in the ASC pellets.
The qRT–PCR results revealed strong expression of type X collagen in TMJ disc tissue compared with both undifferentiated and differentiated cell samples (figure 7d). The expression of type X collagen remained relatively similar between cell samples at the different time points. Increased expression was detected only in differentiated TMJ disc cell pellets, and a subtle increase was also observed in differentiated ASC pellets.
4. Discussion
Tissue engineering provides the possibility to construct an original-like TMJ disc. In the present study, we evaluated biodegradable PLA discs as a potential implant for TMJ disc replacement in combination with ASCs seeded in a micromass format. The fibrocartilaginous potential of ASCs in the PLA discs was compared with ASCs and TMJ disc-derived cells cultured and differentiated in conventional pellet cultures.
Recent in vitro studies have demonstrated the advantages of PLA over PGA in terms of scaffold structural stability (Allen & Athanasiou 2008). PGA loses its stability and contracts prior to sufficient ECM formation after only three weeks of culture in vitro (Almarza & Athanasiou 2004b, 2005; Allen & Athanasiou 2008). PLA polymers, which are a more durable material, offer a good combination of mechanical stability and a slower degradation rate, from six months to 2 years for full reformation of the TMJ disc in vivo (Springer et al. 2001; Richardson et al. 2006; Allen & Athanasiou 2008). Here, the fibres of the P(L/D)LA 96/4 mat in the bilayer P(L/D)LA 96/4+P(L/DL)LA 70/30 disc were manufactured in a non-woven form to resemble roughly the structural collagens in the native TMJ disc (Berkovitz 2000; Scapino et al. 2006). The preference for the non-woven mesh-like architecture for TMJ scaffolds was previously demonstrated using porcine TMJ disc cells (Almarza & Athanasiou 2004b, 2005). Additionally, the non-woven mat was closed from the bottom side with the P(L/DL)LA 70/30 membrane, designed to provide a sliding surface over the condyle in the TMJ but also to prohibit ASC migration out of the PLA disc in vitro. PLA has previously been demonstrated as a suitable material for the survival and proliferation of TMJ disc cells (Allen & Athanasiou 2008) and has been shown to support a cartilaginous phenotype and the differentiation of chondrocytes and stem cells in vitro, respectively (Freed et al. 1993; Puelacher et al. 1994; Richardson et al. 2006).
Chondrogenic induction requires specific culture conditions such as a high cell density, appropriate growth factors and a mechanoinductive environment. High cell density allows cells to condense so that cellular contacts, which are necessary for chondrogenesis in vitro, can be established (Tacchetti et al. 1992). The process is analogous to the pre-cartilage condensation that occurs in limb skeletogenesis (DeLise et al. 2000). In the ASC discs, high cell density was provided by seeding ASCs in the micromass format. Subsequent cell condensation was observed in chondrogenic medium but was absent under control conditions. Although cell aggregates are considered to be essential for chondrogenic events (Tacchetti et al. 1992), the high cell density also limits the mass transfer in the centre of aggregates, which becomes further hindered by the cartilaginous ECM accumulation, especially during extended culture periods. Here regions devoid of cells emerged inside ASC pellets in the course of culture, especially under control conditions, suggesting that the chondrogenic medium improved the viability of ASCs in the ASC pellets. On average, ASCs have been shown to survive in a low oxygen environment, making them good candidates for cell-based therapies where the oxygen supply may be limited during laboratory handling and immediately after implantation (Follmar et al. 2006).
In chondrogenic medium, ASC differentiation was demonstrated by the accumulation of sulphated GAGs in high-density cell aggregates and increased the expression of the structural component genes found in cartilaginous ECM, including aggrecan, and types I, II and X collagen. The best ASC differentiation in the ASC discs under these conditions with respect to TMJ disc composition was obtained between 1.5 and 3 weeks. ASC differentiation was not significantly different between the PLA discs 1 and 2, indicating that these cells are probably not sensitive to minor variations in the intrinsic viscosity of PLA. The chondrogenic differentiation of ASC discs was close to that in conventional ASC pellets, although the relative expression differences between control and differentiated ASCs were generally higher in the ASC pellets than in the ASC discs. In previous comparison studies in vitro, chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells was relatively similar between pellets and three-dimensional biomaterial scaffold cultures (Li et al. 2005; Wang et al. 2005).
The combined qRT–PCR analysis provided novel information about ASC differentiation in comparison with mature TMJ disc cell pellets and the TMJ disc tissue sample. The expression of type X collagen in mature TMJ disc tissue was somewhat surprising in the sense that type X collagen is classically considered as a hypertrophy marker expressed by terminally differentiated chondrocytes. Type X collagen is also not found in the collagen of the TMJ disc (Mills et al. 1994; Ali & Sharawy 1996b; Landesberg et al. 1996; Minarelli & Liberti 1997). A modest increase in type X collagen was also detected in some differentiated PLA discs and pellets. Type X collagen may be a component of normal articular cartilage in other synovial joints (Rucklidge et al. 1996). Hence, it is possible that small amounts of type X collagen belong to a normal composition of rabbit TMJ discs.
Generally, the level of chondrogenic differentiation of ASCs remained lower than that in mature cells differentiated in pellets and in TMJ disc tissue samples. In the case of aggrecan, expression was visibly higher in ASC pellets than in the PLA discs, and the expression of type II collagen in ASC cultures did not even reach the same scale of mature TMJ disc samples. Type I collagen, however, was expressed in both discs and pellets at similar levels, close to the expression in TMJ disc tissues. It is the most prominent component of fibrocartilaginous TMJ disc tissue, whereas type II collagen is expressed in lesser amounts and is localized with chondroitin sulphate proteoglycans, mainly in close vicinity to the chondrocyte-like cells (Mills et al. 1994; Ali & Sharawy 1996a; Minarelli & Liberti 1997; Detamore et al. 2005, 2006).
To date, most studies on TMJ disc engineering in which the effects of most suitable culture conditions with different growth factors and their combinations were tested have been concentrated on TMJ disc cells. Many of the tested growth factors, including insulin-like growth factor-1, basic fibroblast growth factor and platelet-derived growth factor, enhance cell proliferation but are not sufficient in restoring TMJ disc-specific matrix production (Detamore & Athanasiou 2004). In addition to its effects on cell proliferation (Landesberg et al. 1996), TGF-β1 also increases the total number of collagen and sulphated GAGs in porcine TMJ disc cell three-dimensional cultures in vitro (Allen & Athanasiou 2008).
In the present study, the chondrogenic medium supplemented with TGF-β1 had a marked effect on the gene expression of rabbit TMJ disc cell pellets. The expression of aggrecan and types I and II collagen was noticeably higher and further increased by week 6 under chondrogenic conditions, whereas the same genes in control TMJ disc cell pellets remained close to the levels of undifferentiated ASCs, suggesting that the control medium alone was not sufficient for the phenotype maintenance of TMJ disc cells and dedifferentiation occurred. These observations together with previous findings suggest that TGF-β1 is a promising factor for supporting the phenotype maintenance or even redifferentiation of passaged TMJ disc cells in vitro.
Traditionally, TGF-β has been considered essential for chondrogenic differentiation of mesenchymal stem cells (Roelen & Dijke 2003), but its effect on ASC differentiation is controversial. ASCs have been suggested to possess reduced capacity for TGF-β-induced chondrogenesis because of their deficient expression of the TGF-β type I receptor (ALK-5) that is required for TGF-β signalling (Hennig et al. 2007). On the other hand, ASCs have been shown to enhance cell proliferation (Awad et al. 2003) and the synthesis of cartilaginous ECM components in TGF-β1-supplemented chondrogenic medium (Erickson et al. 2002; Awad et al. 2003). The positive effect of TGF-β1 on both chondrogenic differentiation of ASCs and phenotype maintenance of TMJ disc cells was also demonstrated in this study. Nevertheless, the state of cell differentiation affects the required culture conditions, and thus the essential factors for the maintenance of cartilaginous phenotype of mature cells and chondrogenic differentiation of stem cells may vary and need further studying.
Our results of the TMJ disc cell pellets contradict those from the study by Allen and Athanasiou (2006), who reported that pellet culture is rather detrimental for porcine TMJ disc cells. They reported that the impact of growth factor supplementation, including TGF-β1, in typical TMJ disc gene expression maintenance remained insignificant, as indicated by the decreased expression of aggrecan, type I collagen, decorin and biglycan in pellets in comparison with monolayer cultures (Allen & Athanasiou 2006). Type II collagen expression was low or undetectable, whereas in our study, type II collagen expression in the pellets of TMJ disc cells under chondrogenic conditions increased remarkably, exceeding even the level in the TMJ disc tissue by week 6. The follow-up in Allen and Athanasiou's study was short, only 24 h, which, among the other factors in the experimental design, may partly explain the controversy found. According to our findings, we suggest that the pellet format is a suitable high-density culture method, at least in the rabbit model, for studying the effects of different growth factors and the biology of TMJ disc cells in vitro.
TMJ disc-isolated cells have been demonstrated to hold good differentiation potential in vitro (Thomas et al. 1991; Allen & Athanasiou 2007), but their natural tendency to differentiate along the hyaline cartilage pathway during TMJ disc degeneration or in the laboratory, as demonstrated in this study with highly activated type II collagen upregulation in TMJ disc cell pellets in chondrogenic medium, may compromise their utility for cell-based replacements of the TMJ disc (Detamore & Athanasiou 2003). Moreover, the TMJ disc undergoes both morphological and cellular changes during ageing and degenerative processes, making the performance of these cells in tissue engineering unpredictable (Kurita et al. 1989; Ali & Sharawy 1996a; Minarelli & Liberti 1997; Berkovitz & Pacy 2000). Because of their mesenchymal origin, ASCs possess the capacity to differentiate to both fibroblastic and chondrogenic lineages and would serve as a good alternative for TMJ disc reconstruction (Zuk et al. 2001; Erickson et al. 2002; Awad et al. 2003; Huang et al. 2004).
5. Conclusions
The non-woven PLA discs are suitable platforms for chondrogenic differentiation of ASCs in vitro. Further, the pellet format is a suitable high-density culture method for studying the effects of different growth factors and the biology of TMJ disc-derived tissue, as well as mesenchymal stem cells, in vitro.
To the best of our knowledge, this is the first study demonstrating ASCs as a potential alternative cell source for TMJ disc engineering. Still further study is needed to determine the formation of sGAG and collagens quantitatively and to improve the fibrochondrogenic differentiation conditions. The assessment of the developed TMJ disc implant for its mechanical performance is also important in addition to in vivo studies to define the optimal time of implantation prior to clinical use.
Acknowledgements
The authors thank Mrs Hilkka Mäkinen and Mrs Mirja Hyppönen for expert technical assistance. The work was supported by TEKES, the Finnish Funding Agency for Technology and Innovation, the competitive research funding of the Pirkanmaa Hospital District, the University of Tampere and the Finnish Cultural Foundation.
Footnotes
These authors contributed equally to the study.
References
- Ali A. M., Sharawy M. 1996a. Histochemical and immunohistochemical studies of the effects of experimental anterior disc displacement on sulfated glycosaminoglycans, hyaluronic acid, and link protein of the rabbit craniomandibular joint. J. Oral Maxillofac. Surg. 54, 992–1004. ( 10.1016/S0278-2391(96)90399-7) [DOI] [PubMed] [Google Scholar]
- Ali A. M., Sharawy M. M. 1996b. An immunohistochemical study of collagen types III, VI and IX in rabbit craniomandibular joint tissues following surgical induction of anterior disk displacement. J. Oral Pathol. Med. 25, 78–85. ( 10.1111/j.1600-0714.1996.tb00197.x) [DOI] [PubMed] [Google Scholar]
- Allen K. D., Athanasiou K. A. 2006. Growth factor effects on passaged TMJ disk cells in monolayer and pellet cultures. Orthod. Craniofac. Res. 9, 143–152. ( 10.1111/j.1601-6343.2006.00370.x) [DOI] [PubMed] [Google Scholar]
- Allen K. D., Athanasiou K. A. 2007. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng. 13, 101–110. ( 10.1089/ten.2006.0094) [DOI] [PubMed] [Google Scholar]
- Allen K. D., Athanasiou K. A. 2008. Scaffold and growth factor selection in temporomandibular joint disc engineering. J. Dent. Res. 87, 180–185. ( 10.1177/154405910808700205) [DOI] [PubMed] [Google Scholar]
- Almarza A. J., Athanasiou K. A. 2004a. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 32, 2–17. ( 10.1023/B:ABME.0000007786.37957.65) [DOI] [PubMed] [Google Scholar]
- Almarza A. J., Athanasiou K. A. 2004b. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 10, 1787–1795. ( 10.1089/ten.2004.10.1787) [DOI] [PubMed] [Google Scholar]
- Almarza A. J., Athanasiou K. A. 2005. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann. Biomed. Eng. 33, 943–950. ( 10.1007/s10439-005-3311-8) [DOI] [PubMed] [Google Scholar]
- Awad H. A., Halvorsen Y. D., Gimble J. M., Guilak F. 2003. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 9, 1301–1312. ( 10.1089/10763270360728215) [DOI] [PubMed] [Google Scholar]
- Ballock R. T., Reddi A. H. 1994. Thyroxine is the serum factor that regulates morphogenesis of columnar cartilage from isolated chondrocytes in chemically defined medium. J. Cell Biol. 126, 1311–1318. ( 10.1083/jcb.126.5.1311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovitz B. K. 2000. Crimping of collagen in the intra-articular disc of the temporomandibular joint: a comparative study. J. Oral Rehabil. 27, 608–613. ( 10.1046/j.1365-2842.2000.00605.x) [DOI] [PubMed] [Google Scholar]
- Berkovitz B. K., Pacy J. 2000. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch. Oral Biol. 45, 987–995. ( 10.1016/S0003-9969(00)00067-4) [DOI] [PubMed] [Google Scholar]
- DeLise A. M., Fischer L., Tuan R. S. 2000. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334. ( 10.1053/joca.1999.0306) [DOI] [PubMed] [Google Scholar]
- Denker A. E., Nicoll S. B., Tuan R. S. 1995. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-beta 1. Differentiation 59, 25–34. ( 10.1046/j.1432-0436.1995.5910025.x) [DOI] [PubMed] [Google Scholar]
- Detamore M. S., Athanasiou K. A. 2003. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 9, 1065–1087. ( 10.1089/10763270360727991) [DOI] [PubMed] [Google Scholar]
- Detamore M. S., Athanasiou K. A. 2004. Effects of growth factors on temporomandibular joint disc cells. Arch. Oral Biol. 49, 577–583. ( 10.1016/j.archoralbio.2004.01.015) [DOI] [PubMed] [Google Scholar]
- Detamore M. S., Orfanos J. G., Almarza A. J., French M. M., Wong M. E., Athanasiou K. A. 2005. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 24, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detamore M. S., Hegde J. N., Wagle R. R., Almarza A. J., Montufar-Solis D., Duke P. J., Athanasiou K. A. 2006. Cell type and distribution in the porcine temporomandibular joint disc. J. Oral Maxillofac. Surg. 64, 243–248. ( 10.1016/j.joms.2005.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroulis G. 2005. The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. J. Oral Maxillofac. Surg. 63, 173–178. ( 10.1016/j.joms.2004.06.051) [DOI] [PubMed] [Google Scholar]
- Dolwick M. F. 1997. The role of temporomandibular joint surgery in the treatment of patients with internal derangement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 83, 150–155. ( 10.1016/S1079-2104(97)90106-2) [DOI] [PubMed] [Google Scholar]
- Erickson G. R., Gimble J. M., Franklin D. M., Rice H. E., Awad H., Guilak F. 2002. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 290, 763–769. ( 10.1006/bbrc.2001.6270) [DOI] [PubMed] [Google Scholar]
- Eriksson L., Westesson P. L. 1992. Temporomandibular joint diskectomy. No positive effect of temporary silicone implant in a 5-year follow-up. Oral Surg. Oral Med. Oral Pathol. 74, 259–272. ( 10.1016/0030-4220(92)90056-V) [DOI] [PubMed] [Google Scholar]
- Eriksson L., Westesson P. L. 2001. Discectomy as an effective treatment for painful temporomandibular joint internal derangement: a 5-year clinical and radiographic follow-up. J. Oral Maxillofac. Surg. 59, 750–758. Discussion 758-9 ( 10.1053/joms.2001.24288) [DOI] [PubMed] [Google Scholar]
- Estabrooks L. N., Fairbanks C. E., Collett R. J., Miller L. 1990. A retrospective evaluation of 301 TMJ Proplast-Teflon implants. Oral Surg. Oral Med. Oral Pathol. 70, 381–386. ( 10.1016/0030-4220(90)90164-N) [DOI] [PubMed] [Google Scholar]
- Estes B. T., Wu A. W., Guilak F. 2006. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 54, 1222–1232. ( 10.1002/art.21779) [DOI] [PubMed] [Google Scholar]
- Feinberg S. E., McDonnell E. J. 1995. The use of a collagen sheet as a disc replacement in the rabbit temporomandibular joint. J. Oral Maxillofac. Surg. 53, 535–542. Discussion 543 ( 10.1016/0278-2391(95)90066-7) [DOI] [PubMed] [Google Scholar]
- Follmar K. E., Decroos F. C., Prichard H. L., Wang H. T., Erdmann D., Olbrich K. C. 2006. Effects of glutamine, glucose, and oxygen concentration on the metabolism and proliferation of rabbit adipose-derived stem cells. Tissue Eng. 12, 3525–3533. ( 10.1089/ten.2006.12.3525) [DOI] [PubMed] [Google Scholar]
- Freed L. E., Marquis J. C., Nohria A., Emmanual J., Mikos A. G., Langer R. 1993. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 27, 11–23. ( 10.1002/jbm.820270104) [DOI] [PubMed] [Google Scholar]
- Girdler N. M. 1998. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand. J. Rheumatol. 27, 446–453. ( 10.1080/030097498442280) [DOI] [PubMed] [Google Scholar]
- Hennig T., Lorenz H., Thiel A., Goetzke K., Dickhut A., Geiger F., Richter W. 2007. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J. Cell. Physiol. 211, 682–691. ( 10.1002/jcp.20977) [DOI] [PubMed] [Google Scholar]
- Huang J. I., Zuk P. A., Jones N. F., Zhu M., Lorenz H. P., Hedrick M. H., Benhaim P. 2004. Chondrogenic potential of multipotential cells from human adipose tissue. Plast. Reconstr. Surg. 113, 585–594. ( 10.1097/01.PRS.0000101063.27008.E1) [DOI] [PubMed] [Google Scholar]
- Johns D. E., Athanasiou K. A. 2007. Improving culture conditions for temporomandibular joint disc tissue engineering. Cells Tissues Organs 185, 246–257. ( 10.1159/000102173) [DOI] [PubMed] [Google Scholar]
- Kurita K., Westesson P. L., Sternby N. H., Eriksson L., Carlsson L. E., Lundh H., Toremalm N. G. 1989. Histologic features of the temporomandibular joint disk and posterior disk attachment: comparison of symptom-free persons with normally positioned disks and patients with internal derangement. Oral Surg. Oral Med. Oral Pathol. 67, 635–643. ( 10.1016/0030-4220(89)90001-7) [DOI] [PubMed] [Google Scholar]
- Landesberg R., Takeuchi E., Puzas J. E. 1996. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch. Oral Biol. 41, 761–767. ( 10.1016/S0003-9969(96)00068-4) [DOI] [PubMed] [Google Scholar]
- Li W. J., Tuli R., Okafor C., Derfoul A., Danielson K. G., Hall D. J., Tuan R. S. 2005. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26, 599–609. ( 10.1016/j.biomaterials.2004.03.005) [DOI] [PubMed] [Google Scholar]
- Mercuri L. G., Giobbie-Hurder A. 2004. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J. Oral Maxillofac. Surg. 62, 1088–1096. ( 10.1016/j.joms.2003.10.012) [DOI] [PubMed] [Google Scholar]
- Mercuri L. G., Edibam N. R., Giobbie-Hurder A. 2007. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J. Oral Maxillofac. Surg. 65, 1140–1148. ( 10.1016/j.joms.2006.10.006) [DOI] [PubMed] [Google Scholar]
- Mills D. K., Fiandaca D. J., Scapino R. P. 1994. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J. Orofac. Pain 8, 136–154. [PubMed] [Google Scholar]
- Minarelli A. M., Liberti E. A. 1997. A microscopic survey of the human temporomandibular joint disc. J. Oral Rehabil. 24, 835–840. ( 10.1046/j.1365-2842.1997.00595.x) [DOI] [PubMed] [Google Scholar]
- Nakano T., Scott P. G. 1989. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch. Oral Biol. 34, 749–757. ( 10.1016/0003-9969(89)90082-4) [DOI] [PubMed] [Google Scholar]
- Nakano T., Scott P. G. 1996. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch. Oral Biol. 41, 845–853. ( 10.1016/S0003-9969(96)00040-4) [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelacher W. C., Wisser J., Vacanti C. A., Ferraro N. F., Jaramillo D., Vacanti J. P. 1994. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral Maxillofac. Surg. 52, 1172–1177. Discussion 1177–8 ( 10.1016/0278-2391(94)90538-X) [DOI] [PubMed] [Google Scholar]
- Richardson S. M., Curran J. M., Chen R., Vaughan-Thomas A., Hunt J. A., Freemont A. J., Hoyland J. A. 2006. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-l-lactic acid (PLLA) scaffolds. Biomaterials 27, 4069–4078. ( 10.1016/j.biomaterials.2006.03.017) [DOI] [PubMed] [Google Scholar]
- Roelen B. A., Dijke P. 2003. Controlling mesenchymal stem cell differentiation by TGFbeta family members. J. Orthop. Sci. 8, 740–748. ( 10.1007/s00776-003-0702-2) [DOI] [PubMed] [Google Scholar]
- Rucklidge G. J., Milne G., Robins S. P. 1996. Collagen type X: a component of the surface of normal human, pig, and rat articular cartilage. Biochem. Biophys. Res. Commun. 224, 297–302. ( 10.1006/bbrc.1996.1024) [DOI] [PubMed] [Google Scholar]
- Scapino R. P., Obrez A., Greising D. 2006. Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs 182, 201–225. ( 10.1159/000093969) [DOI] [PubMed] [Google Scholar]
- Springer I. N., Fleiner B., Jepsen S., Acil Y. 2001. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials 22, 2569–2577. ( 10.1016/S0142-9612(01)00148-X) [DOI] [PubMed] [Google Scholar]
- Tacchetti C., Tavella S., Dozin B., Quarto R., Robino G., Cancedda R. 1992. Cell condensation in chondrogenic differentiation. Exp. Cell Res. 200, 26–33. ( 10.1016/S0014-4827(05)80067-9) [DOI] [PubMed] [Google Scholar]
- Tanaka E., Detamore M. S., Tanimoto K., Kawai N. 2008. Lubrication of the temporomandibular joint. Ann. Biomed Eng. 36, 14–29. ( 10.1007/s10439-007-9401-z) [DOI] [PubMed] [Google Scholar]
- Thomas M., Grande D., Haug R. H. 1991. Development of an in vitro temporomandibular joint cartilage analog. J. Oral Maxillofac. Surg. 49, 854–856. Discussion 857 ( 10.1016/0278-2391(91)90015-E) [DOI] [PubMed] [Google Scholar]
- Tucker M. R., Spagnoli D. B. 1996. Autogenous dermal and auricular cartilage grafts for temporomandibular joint repair. Atlas Oral Maxillofac. Surg. Clin. North Am. 4, 75–92. [PubMed] [Google Scholar]
- Wang Y., Kim U. J., Blasioli D. J., Kim H. J., Kaplan D. L. 2005. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 26, 7082–7094. ( 10.1016/j.biomaterials.2005.05.022) [DOI] [PubMed] [Google Scholar]
- Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228. ( 10.1089/107632701300062859) [DOI] [PubMed] [Google Scholar]