Abstract
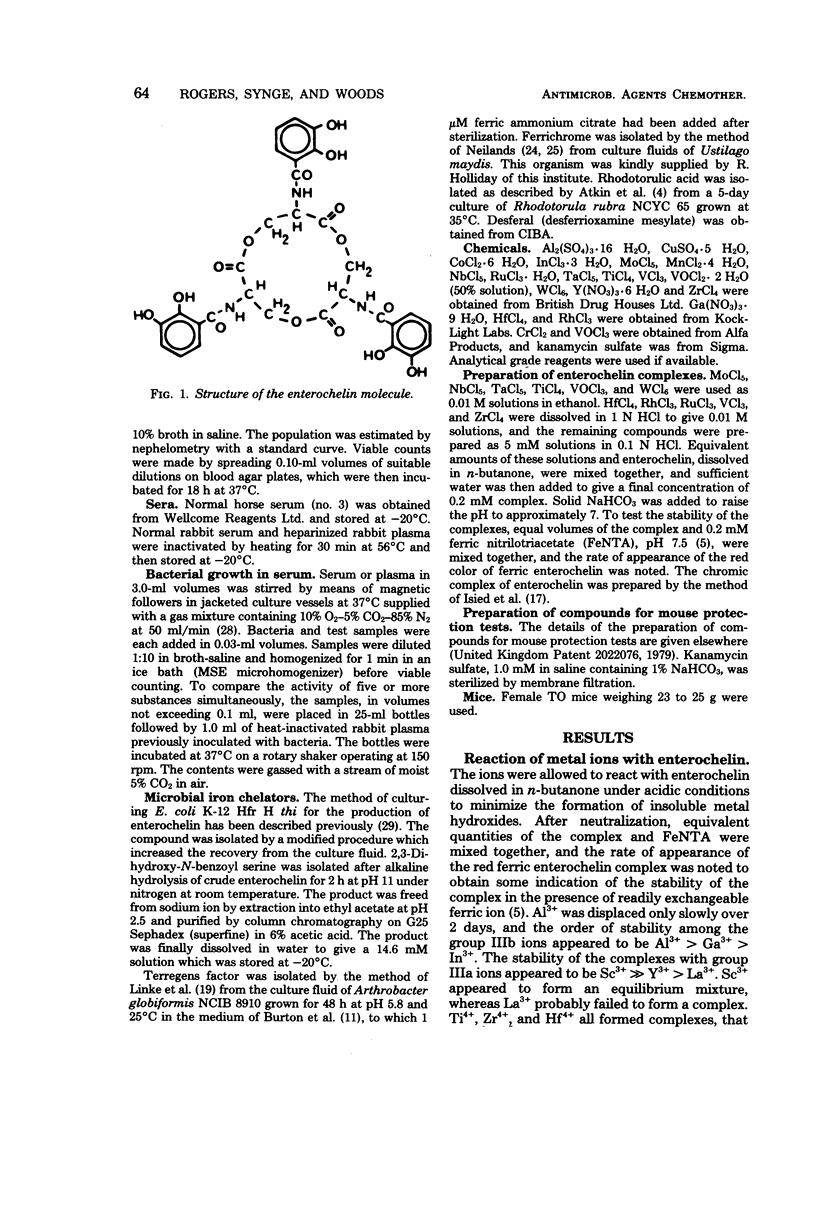
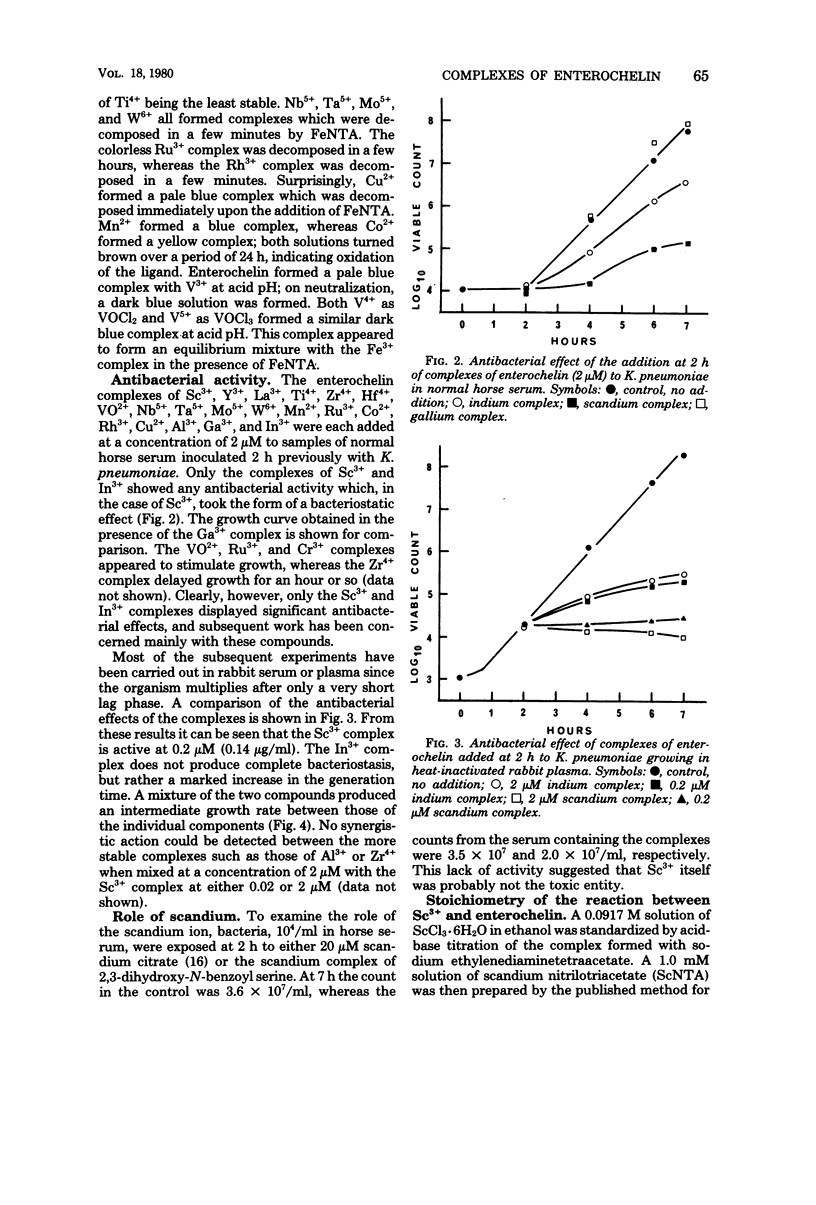
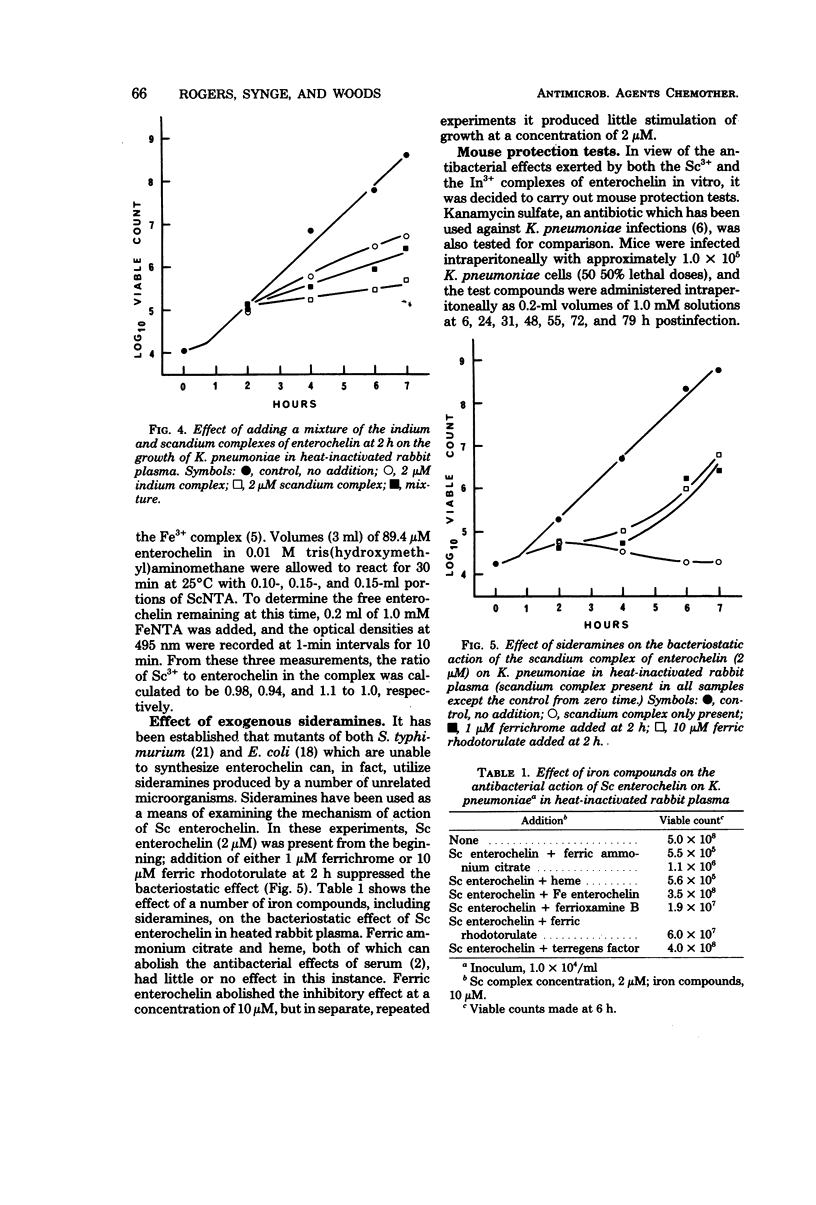
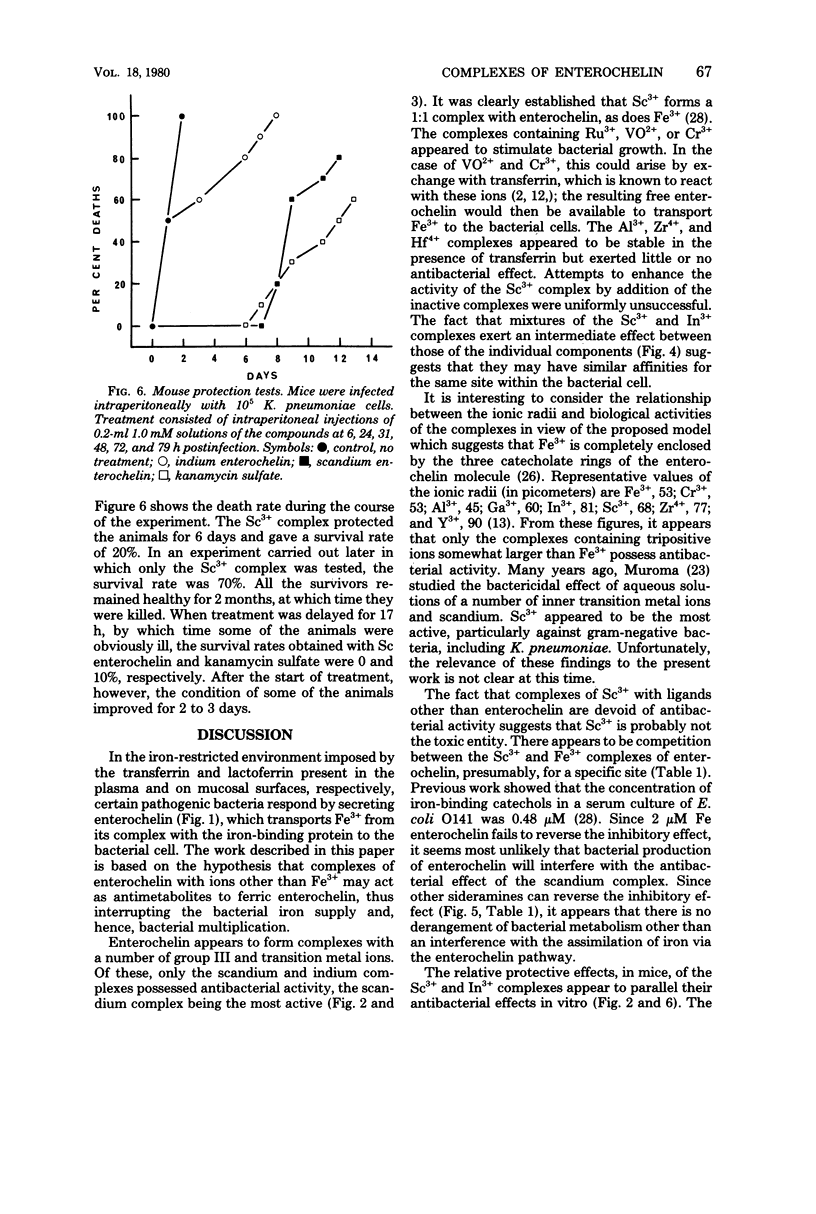
A number of studies point to the conclusion that enterochelin, the iron chelator produced by a number of pathogenic enterobacteria, may be an essential metabolite for bacterial multiplication within the host. The compound removes iron from complexes with the host iron-binding proteins transferrin and lactoferrin, and the resulting ferric enterochelin is assimilated by the bacterial cell. It was reasoned that complexes of enterochelin with ions other than Fe3+ might act as antimetabolites and inhibit bacterial multiplication by interfering with the assimilation of ferric enterochelin. Enterochelin forms complexes with a number of group III and transition metal ions. The complex containing scandium exerts a bacteriostatic effect on Klebsiella pneumoniae in serum, whereas the indium complex induces a large increase in the generation time. The Fe3+ complexes of other microbial iron-transporting compounds are capable of reversing the bacteriostatic effect of the Sc3+ complex of enterochelin, suggesting that the compound acts solely by interfering with the enterochelin system of iron transport. Preliminary experiments show that the Sc3+ complex probably acts as a competitive inhibitor of ferric enterochelin. The Sc3+ complex of enterochelin exerts a therapeutic effect on intraperitoneal K. pneumoniae infections in mice similar to that obtained with kanamycin sulfate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Aisen P., Aasa R., Redfield A. G. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969 Sep 10;244(17):4628–4633. [PubMed] [Google Scholar]
- Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972 Feb 29;257(2):314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Berendt R. F., Long G. G., Walker J. S. Treatment of respiratory Klebsiella pneumoniae infection in mice with aerosols of kanamycin. Antimicrob Agents Chemother. 1975 Nov;8(5):585–590. doi: 10.1128/aac.8.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Cannon J. C., Chasteen N. D. Nonequivalence of the metal binding sites in vanadyl-labeled human serum transferrin. Biochemistry. 1975 Oct 21;14(21):4573–4577. doi: 10.1021/bi00692a003. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald S. P., Rogers H. J. Bacteriostatic effect of serum: role of antibody to lipopolysaccharide. Infect Immun. 1980 Feb;27(2):302–308. doi: 10.1128/iai.27.2.302-308.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Perkins D. J. The binding of scandium ions to transferrin in vivo and in vitro. Eur J Biochem. 1971 Jul 15;21(1):55–59. doi: 10.1111/j.1432-1033.1971.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Isied S. S., Kuo G., Raymond K. N. Coordination isomers of biological iron transport compounds. V. The preparation and chirality of the chromium(III) enterobactin complex and model tris(catechol)chromium(III) analogues. J Am Chem Soc. 1976 Mar 31;98(7):1763–1767. doi: 10.1021/ja00423a021. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke W. D., Crueger A., Diekmann H. Stoffwechselprodukte von Miktoorganismen. 106. Zur Konstitution des Terregens-Faktors. Arch Mikrobiol. 1972;85(1):44–50. [PubMed] [Google Scholar]
- Liu P. V., Shokrani F. Biological activities of pyochelins: iron-chelating agents of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):878–890. doi: 10.1128/iai.22.3.878-890.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUROMA A. Studies in the bactericidal action of salts of certain rare earth metals. Ann Med Exp Biol Fenn. 1958;36(Suppl 6):1–54. [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- NEILANDS J. B. Factors affecting the microbial production of ferrichrome. J Biol Chem. 1953 Dec;205(2):647–650. [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Synge C., Kimber B., Bayley P. M. Production of enterochelin by Escherichia coli 0111. Biochim Biophys Acta. 1977 Apr 27;497(2):548–557. doi: 10.1016/0304-4165(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



