Abstract
Certain components of a graft that provoke alloimmunity may not be vital for graft function or critical as targets of rejection. Corneal transplantation is an example of this since graft epithelium plays a role in allosensitization, while corneal graft endothelium—which shares the same alloantigens—is the critical target in allorejection. In this study, we found that exploiting this biology by replacing donor epithelium of an allograft with an allodisparate 3rd-party epithelium yields a marked enhancement in transplant survival. Such “chimeric” allografts consisted of a C3H/He (H-2k) corneal epithelium over a C57BL/6 (H-2b) epithelial-denuded cornea (or v.v.) and orthotopically placed on BALB/c (H-2d) hosts. Conventional corneal allografts (C3H/He, or C57BL/6) or isografts (BALB/c) were also transplanted on BALB/c hosts. Alloreactive T cell frequencies (CD4+ IFN-gamma+) primed to graft endothelium were strongly diminished in chimeric relative to conventionally allografted hosts. This was corroborated by decreased T cell infiltration (p=0.03) and a marked enhancement of allograft survival (p=0.001). Our results represent the first successful demonstration of chimeric tissue, epithelial-denuded allograft plus 3rd-party allodisparate epithelium, in the promotion of allograft survival. Moreover, chimeric grafting can be readily performed clinically, whereby corneal allograft rejection remains a significant problem particularly in inflamed graft beds.
Introduction
All components of an allograft do not contribute equally to immune rejection, as certain cell types of a graft that provoke alloimmunity may not be vital for graft function or critical as targets of rejection. An example of this biology can be found in the immune rejection of corneal transplants (1-6). The graft epithelium, which is comprised of the majority of corneal cells, is thought to bear the crux of the immunogenic load and has been implicated in the induction of alloimmunity (2, 3, 5, 6). Immune rejection of this tissue layer, however, is mostly self-limiting as it is eventually repopulated by host epithelial cells (1, 4). In contrast, vital to maintaining corneal clarity is the corneal endothelium—which is comprised of a cellular monolayer distinct from the vascular endothelium and derived from the neural crest. Because corneal endothelial cells are not capable of replicating, the death of these cells leads to irreversible graft opacity and subsequent failure. Hence, while the corneal graft epithelium is implicated in induction of alloimmunity, the graft endothelium is the critical target of immune rejection.
Interestingly, transplantation of epithelial denuded allografts has shown no beneficial effect on graft survival, either in mice or humans (5, 6). While the reasons for this observation are not completely understood, it is believed that the barrier function of the corneal epithelium as well as its high expression of angiostatic (6, 7) and immunomodulatory factors (i.e., TGF-b, IL-1Ra, and PDL-1) (8-10) constitutively suppress inflammation (3, 6). Hence, it is conceivable that the epithelium of a corneal graft acts as a ‘double-edged sword’ in transplantation: on the one hand it is considered to bear the crux of the alloantigen load, while on the other hand its constitutive anti-inflammatory properties can suppress alloimmunity.
We therefore hypothesized that the transplantation of epithelial denuded allografts reconstituted ex vivo with a 3rd-party allodisparate donor, or “chimeric” grafts, would promote allograft survival. In this setting, we reasoned that 3rd-party graft epithelium is unable to sensitize the host to alloantigens expressed by the allodisparate donor endothelium—the critical target in rejection. Moreover, providing a healthy graft epithelium at the time of transplantation, albeit allogeneic to the host, would afford barrier function and a compliment of immunoregulatory factors that maximally promote allograft survival. To test our hypothesis, BALB/c (H-2d) recipients were transplanted with chimeric grafts, which consisted of epithelial denuded C3H/He (H-2k) allografts reconstituted ex vivo with C57BL/6 (H-2b) epithelium. In companion experiments, BALB/c recipients were transplanted with the reverse grafting scheme: chimeric grafts which consisted of epithelial denuded C57BL/6 allografts reconstituted ex vivo with C3H/He epithelium. We present data herein demonstrating that transplant survival rates were markedly higher for chimeric grafts as compared to conventional allografts, and this was associated with diminished alloreactivity to graft endothelium.
In addition to providing a better understanding of the dynamics of corneal alloimmunity, the use of such chimeric grafts could have considerable utility and is highly feasible in the clinical realm where corneal allograft rejection is a clinically significant problem particularly in inflamed graft beds. Moreover, use of 3rd-party epithelium circumvents the inconvenience in using recipient-derived corneal epithelium to this end (6), as harvesting this tissue may not be available if the contralateral eye is not healthy or avoids the need to obtain donor-matched tissue.
Materials and Methods
Animals and Anesthesia
Eight- to 12-wk-old C57BL/6, BALB/c and C3H/He male mice were obtained from Taconic Farms. Mice were housed in a specific pathogen-free environment at the Schepens Eye Research Institute animal facility. All animals were treated according to guidelines established by the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and the Public Health Policy on Humane Care and Use of Laboratory Animals (US Public Health Review), and all procedures were approved by the Institutional Animal Care and Use Committee. Anesthesia was administered intraperitonealy by Ketamine/Xylazine suspensions at a dose of 120 mg/Kg of body weight and 20 mg/Kg of body weight, respectively.
Corneal Transplantation and Assessment of Graft Survival
This procedure has been detailed elsewhere (11). Briefly, the central cornea (2mm diameter) was excised from a donor mouse using Vannas scissors (Storz Instruments, San Dimas, CA) and placed on ice in Optisol-GS (Bausch and Lomb, Rochester, NY). The graft bed was prepared by excising a 1.5-mm site in the central cornea of a recipient mouse. A donor button was then placed onto the recipient bed and secured with eight interrupted 11-0 nylon sutures (Sharpoint, Reading, PA). Graft survival was evaluated using a slit-lamp biomicroscope and was performed twice a week over the course of 8 weeks. We employed a standardized opacity-grading (ranges, 0–5+) scheme to identify rejection (11). This was defined as two consecutive time-points with a score of 2+—indicated by a level of graft opacity that occludes clear recognition of iris detail
Assembly of Chimeric Corneal Grafts
Aspects of this procedure were adapted and modified from Gibson and Grill and Hori et al (12, 6). Donor corneal buttons were incubated in 20 mM EDTA at 37°C for 45 minutes followed by a wash with sterile PBS (without Ca2+ and Mg2+). Under a dissecting microscope, corneal epithelium (EPI) was subsequently peeled off as an intact sheet from the subjacent stroma-endothelium (S-E) and tissues were kept moist with PBS in a sterile Petri dish (Fig. 1a, b). Chimeric grafts were then assembled by gently placing EPI over identically sized S-E (Fig. 1b). Chimeric grafts were preserved in Optisol-GS on ice until the time of surgery the same day. To demonstrate the donor disparity within a transplanted chimeric graft via confocal analysis, donor GFP(+) S-E was reconstituted with donor GFP(-) EPI and orthotopically placed onto a GFP(-) host (Fig. 1c). Other chimeric grafts underwent biomicroscopic examination (Fig. 1d)—including assessment of graft opacity and epithelial stability—which indicated that the creation of chimeric grafts yielded no undesirable effects post-transplantation, such as primary graft failure.
Figure 1. Standardization of chimeric graft transplantation.
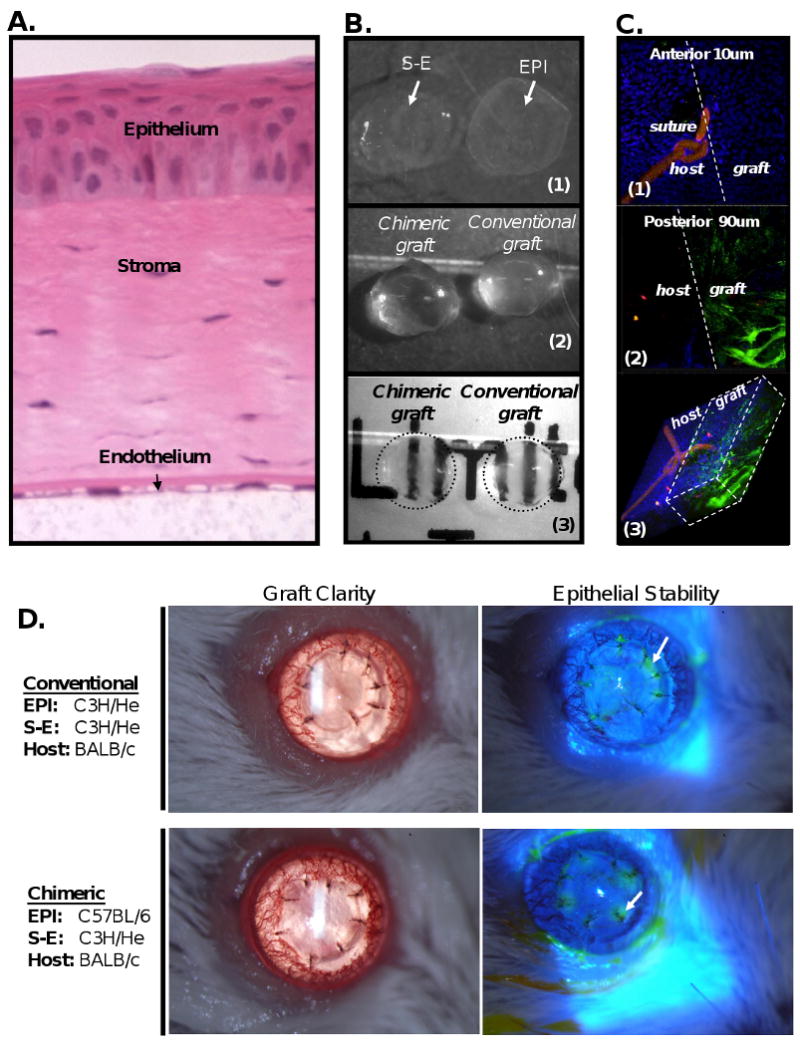
(A) Full-thickness cross-section of a mouse cornea demonstrates the high cellularity of the corneal epithelium (EPI) versus the corneal stroma and endothelium (S-E). (B) Ex vivo microsurgical separation of EPI from subjacent S-E (B1) and subsequent chimeric reconstitution with an allodisparate S-E does not affect graft clarity (B2, 3). (C) Post-transplant demonstration of the donor disparity within a chimeric graft, which consisted of GFP(-) EPI over GFP(+) S-E grafted onto a GFP(-) host. En face confocal analysis of a corneal flat-mount focusing on the graft-host junction (dashed line), captured the anterior 10um (C1), the subjacent 90um of the chimeric engrafted cornea (C2), and a micrograph of the full-thickness cornea which was digitally reconstructed (C3). (D) Transplantation of chimeric grafts yields no undesirable effects, such as primary graft failure. Biomicroscopic evaluation revealed chimeric graft clarity post-transplantation and expected epithelial deficiency (green) only around the sutures (arrow) identified via cobalt blue illumination with topical fluorescein application.
Mixed Lymphocyte Reaction (MLR)
Recipient T cells were harvested from ipsilateral draining lymph nodes (submandibular and cervical) 3 weeks post-transplantation, as these are the critical sites for allosensitization to corneal allografts (13, 14). T cells were magnetically sorted with CD90 antibody (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Stimulator cells consisted of harvested naïve spleens, whereby red blood cells were lysed and T cells depleted magnetically (CD90 antibody). The high purities (>90%) of both T cell populations as well as stimulators cells were confirmed via FACS analyses. Viable cells were enumerated (trypan blue exclusion assay) and set at 1.0 × 106 cells in 1mL of complete RPMI medium (BioWhittaker Inc, Walkersville, MD) with 10% FBS. T cells were subsequently plated in triplicate wells in a 96-well U-bottom plate (BD Falcon), at a 1:1 ratio (stimulators to T cells) at 37°C and 5.0% CO2.
T Cell Proliferation and ELISA Assays
After 4 days of incubation, MLR cultures were pulsed with BrdU (Chemicon International). Harvested cells underwent fixation/permeabilization according to the manufacturer's instructions (eBioscience), Fc receptor blockade via α-CD16/CD32 IgG2b (BD Pharmingen), and subsequently incubated in DNase with FITC-conjugated α-BrdU IgG1 (Fast Immune, BD Pharmingen) at room temperature for 30 minutes in 1% BSA. Other cell aliquots were incubated with the appropriate isotype control (BD Pharmingen). Cells were thoroughly washed and analyzed via EPICS XL flow cytometer (Beckman Coulter). In other MLR cultures, the supernatants were harvested after 5 days of incubation, as peak IFN-γ levels were observed at this time-point. A colorimetric sandwich ELISA against mouse IFN-γ (Quantikine, R&D Systems) was used to assay culture supernatants according to the manufacturer's instructions. Plates were then analyzed via spectrophotometer (Bio-Tek Instruments Inc). Triplicate wells were used to calculate the standard error of the mean (±SEM).
Intracellular FACS Analysis
After 4 days of incubation, MLR cells were stimulated with PMA (50ng/mL, Sigma Aldrich) and ionomycin (1μg/mL, Sigma Aldrich) for 10 hours with the addition of 0.6μL/mL Brefeldin A (Golgi Plug, BD Pharmingen). Cells were harvested and thoroughly washed, followed by Fc receptor blockade and subsequently incubated in PECy5-conjugated α-CD4 IgG2a (BD Pharmingen) for 30 minutes at 4°C in FACS buffer and thoroughly washed. Cells then underwent fixation/permeabilization (eBioscience), thoroughly washed, and incubated with PE-conjugated α-IFN-γ IgG21 (BD Pharmingen) for 30 minutes in FACS buffer at 4°C. Other cells were incubated with the appropriate isotype control for extracellular and intracellular Abs (BD Pharmingen). Cells were thoroughly washed and analyzed via EPICS XL flow cytometer (Beckman Coulter).
Cornea Digestion and FACS Analysis
Single-cell suspensions were prepared from the corneal samples by collagenase digestion, as previously described (10). Briefly, corneal buttons were removed and minced into small fragments, followed by digestion with 2 mg/ml collagenase type IV (Sigma-Aldrich) and 0.05 mg/ml DNase I (Roche) for 1 hour at 37°C with agitation. The suspension was then triturated through a 30-gauge needle to homogenize the remaining tissue, and filtered through a 70-μm cell strainer. Trypan blue exclusion assay confirmed cell viability. Cells underwent Fc receptor blockade and then incubated with PE-conjugated α-CD45 IgG2b and PE-conjugated α-CD3 IgG1 (BD Pharmingen). Other cells were incubated with the appropriate isotype controls for extracellular Abs (BD Pharmingen). Cells were thoroughly washed and analyzed via EPICS XL flow cytometer (Beckman Coulter).
Computations and Statistical Analyses
For all MLR experiments, various parameters of T cell alloreactivity were carried out—which included the level of: (a) T cell proliferation (% BrdU incorporation); (b) IFN-y secretion (pg/ml); and (c) % CD4+ IFN-y+ cells (FACS analysis). Comparisons of primed alloreactivity to the endothelium of conventional versus chimeric allografts included the subtraction of naïve T cell responses to the respective recall alloantigen. This is because naïve T cells also respond in vitro to recall allostimulation Hence, the % Reduction in alloreactivity for each parameter was calculated as follows:
Regarding statistical analyses, error bars displayed in the figures were calculated from the ±SEM. Various statistical tests including Student's t test and ANOVA were performed throughout the study, and are indicated in the respective figure captions. In addition, Kaplan-Meier analysis constructed survival curves and respective log-rank tests compared the rates of corneal graft survival. P values of <0.05 were considered significant.
Results
Alloreactivity of host T cells to graft corneal endothelium is diminished in chimeric transplantation
Since 3rd-party graft epithelium in the chimeric setting is unable to sensitize the host to alloantigens expressed by an allodisparate donor endothelium, we hypothesized that host alloreactivity would therefore be reduced to donor endothelium—the critical target in corneal allograft rejection. To test this, we employed 3 mouse strains fully-mismatched at the H-2 loci, which included: C3H/He (H-2k), C57BL/6 (H-2b), and BALB/c (H-2d). BALB/c (B/c) hosts were transplanted with chimeric grafts, which consisted of C57BL/6 (B6) EPI + C3H/He (C3H) S-E (Fig. 2a). Conventional allografts were also transplanted for direct comparison which consisted of C3H EPI + C3H S-E, and these grafts were similarly separated and reconstituted ex vivo throughout this study. Isografts (B/c) were transplanted as a background control. To measure alloreactivity to graft endothelium, MLR assays were employed for measurement of T cell proliferation (BrdU incorporation) and IFN-y secretion (ELISA). Recall alloantigen matched with graft endothelium (C3H) was used to stimulate host T cells (Fig. 2a). This experiment was also carried out for the reverse transplantation scheme to control for the different donor strains; thus, chimeric grafts consisting of C3H EPI + B6 S-E were compared to B6 EPI + B6 S-E conventional allografts and T cells were in turn stimulated to B6 recall alloantigen (Fig. 2a).
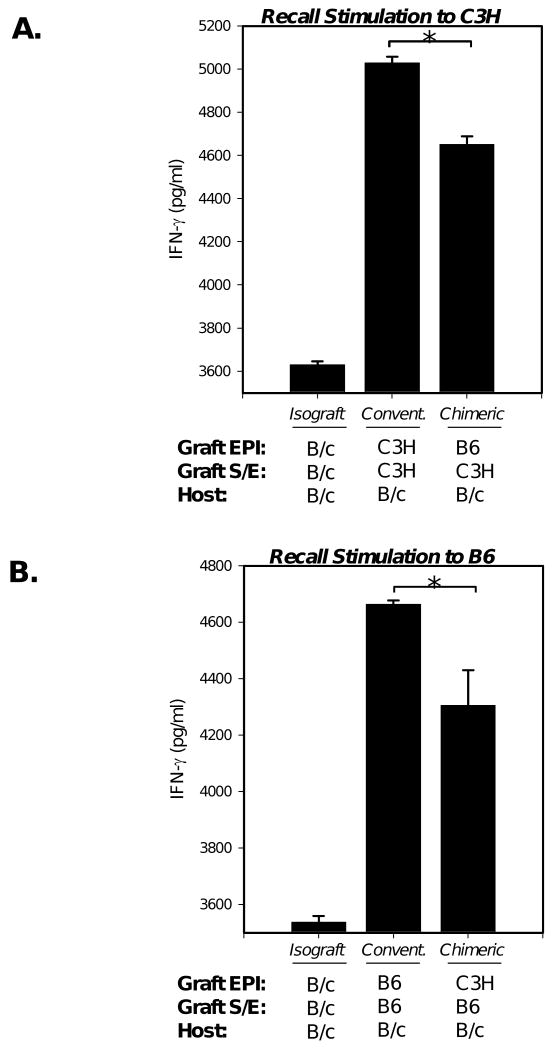
Figure 2. Orthotopic transplantation of chimeric grafts leads to decreased levels of T cell proliferation to alloantigens of the corneal graft endothelium.
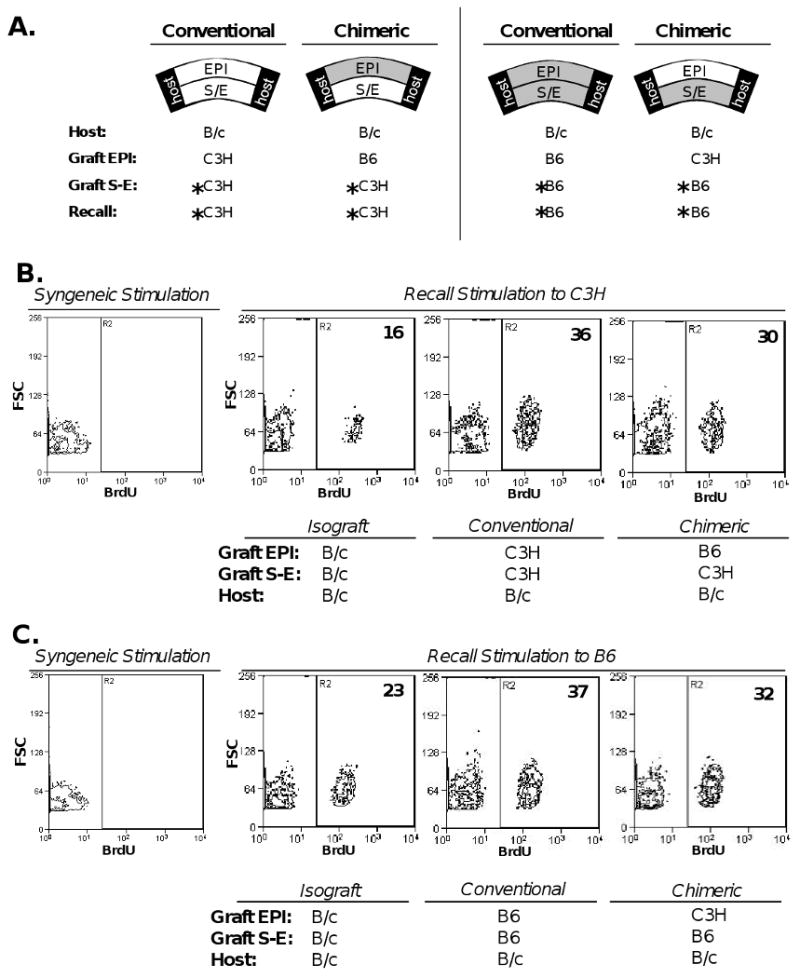
(A) B/c mice transplanted with C3H S-E + B6 EPI chimeric grafts were compared to recipients of C3H S-E + C3H EPI conventional allografts. The reverse transplantation scheme was also carried out, whereby B/c recipients of B6 S-E + C3H EPI chimeric grafts were compared to recipients of B6 S-E + B6 EPI conventional allografts. Isografts (B/c) transplanted onto recipients as a background control. Three weeks post-transplantation, unfractionated T cells harvested from host draining lymph nodes (n=5 recipients per group) were stimulated to recall alloantigen (C3H, or B6) matched with respective graft endothelium (*). (B, C) T cell proliferation was measured via FACS analysis of BrdU incorporation. % Reduction of primed alloreactivity to graft endothelium was calculated by subtracting naïve T cell response to recall allostimulation, as described in Material and Methods. There was a 30% (B) and 35% (C) reduction in T cell proliferation in chimeric engrafted hosts relative to respective conventional allografts. The data shown are representative of 2 independent experiments.
As described in Material and Methods, % Reduction was calculated to accurately compare primed alloreactivity yielded to the allogeneic graft endothelium, by subtracting the proportion of naïve T cells that also respond in vitro to recall allostimulation. Using this analysis, we found a 30% (Fig. 2b) and 35% (Fig. 2c) reduction in T cell proliferation in chimeric engrafted hosts relative to respective conventional allografts. We also measured culture supernatants via ELISA for IFN-γ secretion—a cytokine secreted by TH1 T cells important in corneal graft rejection (15). This also revealed a statistically significant reduction of 27% (Fig. 3a) and 31% (Fig. 3b) from chimeric engrafted hosts relative to respective conventional allografts.
Figure 3. Chimeric grafts lead to decreased levels of IFN-γ secretion to graft alloantigens.
(A) B/c recipients of C3H S-E + B6 EPI chimeric grafts were compared to those receiving C3H S-E + C3H EPI conventional allografts. (B) The reverse transplantation scheme was also carried out, whereby recipients B/c recipients of B6 S-E + C3H EPI chimeric grafts were compared to those receiving B6 S-E + B6 EPI conventional (convent.) allografts. Isografts (B/c) were also transplanted as a background control. Unfractionated T cells harvested from host draining lymph nodes (n=5 recipients per group) were stimulated with recall alloantigen (C3H, or B6) matched with respective graft endothelium. Supernatant was collected to measure IFN-γ levels via ELISA and the % Reduction was calculated, indicating a 27% (A) and 31% (B) reduction in chimeric engrafted hosts relative to respective conventional allografts. An asterisk (*) indicates statistical significance (p<0.05) calculated via post hoc test (Newman-Keuls) following ANOVA. The data shown are representative of 2 independent experiments.
Alloreactivity of the host CD4+ T cell compartment to graft endothelium is diminished in chimeric transplantation
The CD4+ T cell compartment is critical in mediating corneal graft rejection. While CD8+ T cells are primed (15), these lymphocytes are not necessary for acute corneal graft rejection (16). We therefore assessed whether a diminishment in alloreactivity exists in the CD4+ T cell compartment of chimeric engrafted hosts. FACS analysis was used to measure alloreactive CD4(+) IFN-γ(+) T cell frequencies emanating from MLR stimulation to recall alloantigens (C3H, or B6). T cells placed in MLR cultures were harvested from B/c hosts transplanted with chimeric grafts (B6 EPI + C3H S-E) and compared with hosts transplanted with conventional allografts (C3H EPI + C3H S-E) (Fig. 4a). The reverse transplantation scheme was also carried out for companion experimentation, whereby other B/c recipients received chimeric grafts (C3H EPI + B6 S-E) and compared to those receiving conventional allografts (B6 EPI + B6 S-E) (Fig. 4b). Isografts (B/c) were transplanted as a background control. Alloreactivity was diminished in chimeric relative to conventional allografted hosts, as measured by reduced frequencies of alloreactive CD4(+) IFN-γ(+) T cells. This reduction was 53% (Fig. 4a) and 40% (Fig. 4b). Interestingly, in both chimeric engrafted groups, this percent reduction was greatest in the CD4(+) IFN-γ(+) rather than in the CD4(-) IFN-γ(+) population (data not presented).
Figure 4. Chimeric grafts result in diminished CD4+ T cell alloreactivity.
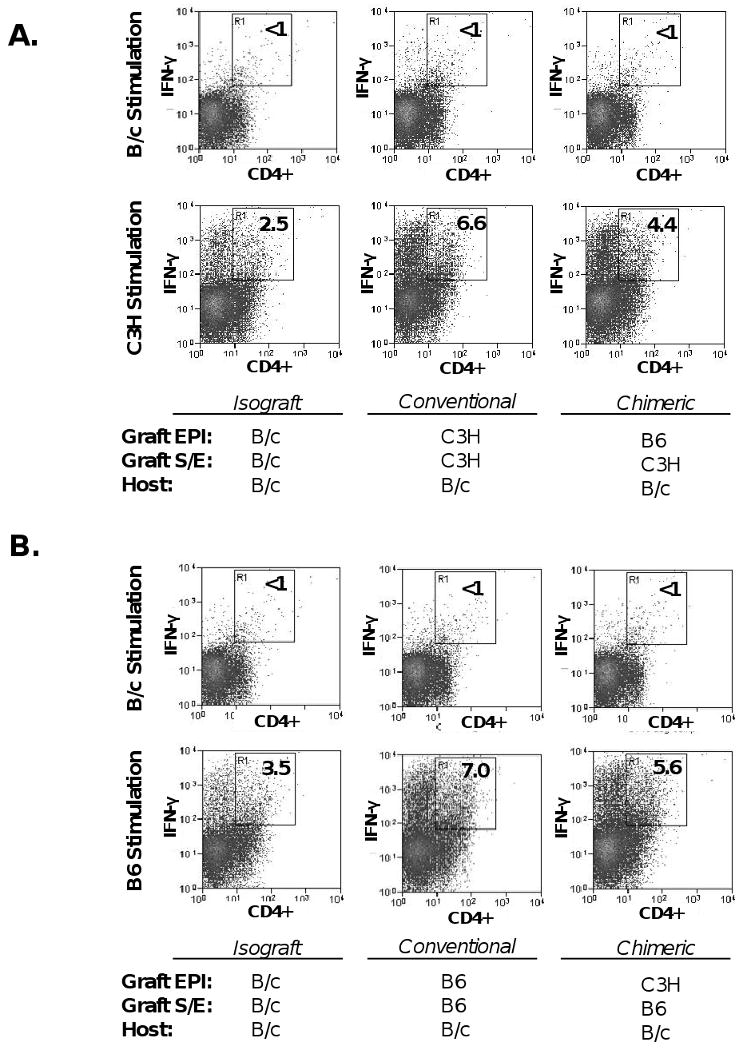
(A) B/c recipients of C3H S-E + B6 EPI chimeric grafts were compared to those transplanted with C3H S-E + C3H EPI conventional allografts. (B) The reverse transplantation scheme was also carried out, whereby B/c recipients of B6 S-E + C3H EPI chimeric grafts were compared to those transplanted with B6 S-E + B6 EPI conventional allografts. Isografts were also transplanted as a background control. Unfractionated T cells harvested from host draining lymph nodes (n=5 recipients per group) were stimulated with recall alloantigen (C3H, or B6) matched with respective graft endothelium. Syngeneic (B/c) stimulation was also included as a negative control. Two-color FACS analysis measured the frequencies of alloreactive CD4(+) IFN-γ(+) T cells and the % Reduction was calculated, indicating a 53% (A) and 40% (B) reduction in chimeric engrafted hosts relative to respective conventional allografts.
T cell infiltration is reduced in chimeric engrafted corneas
We also assessed the effector T cell response to chimeric grafts by first measuring T cell infiltration of engrafted corneas. Corneas were digested into single-cell suspensions for double-staining with CD45-FITC plus CD3-PE and subsequent FACS enumeration of infiltrating T cells (Fig. 5a). B/c recipients transplanted with chimeric grafts consisting of B6 EPI + C3H S-E were compared with those receiving C3H EPI + C3H S-E conventional allografts (Fig. 5b). In companion experiments, other B/c recipients transplanted with chimeric grafts consisting of C3H EPI + B6 S-E, were compared with those receiving B6 EPI + B6 S-E conventional allografts (Fig. 5c). Isografts were also transplanted, but showed marginal levels of T cell infiltration which were identical to those of naïve corneas (data not presented). We found that T cell infiltration in chimeric engrafted cornea was reduced relative to respective conventional allografts by approximately 53% (Fig. 5b) and 45% (Fig. 5c) at week 3 post-transplantation. These data indicate a reduced T cell infiltration of chimeric engrafted cornea and therefore suggest that effector T cell alloreactivity is diminished to donor cornea endothelium.
Figure 5. Diminished T cell infiltration in chimeric engrafted corneas.
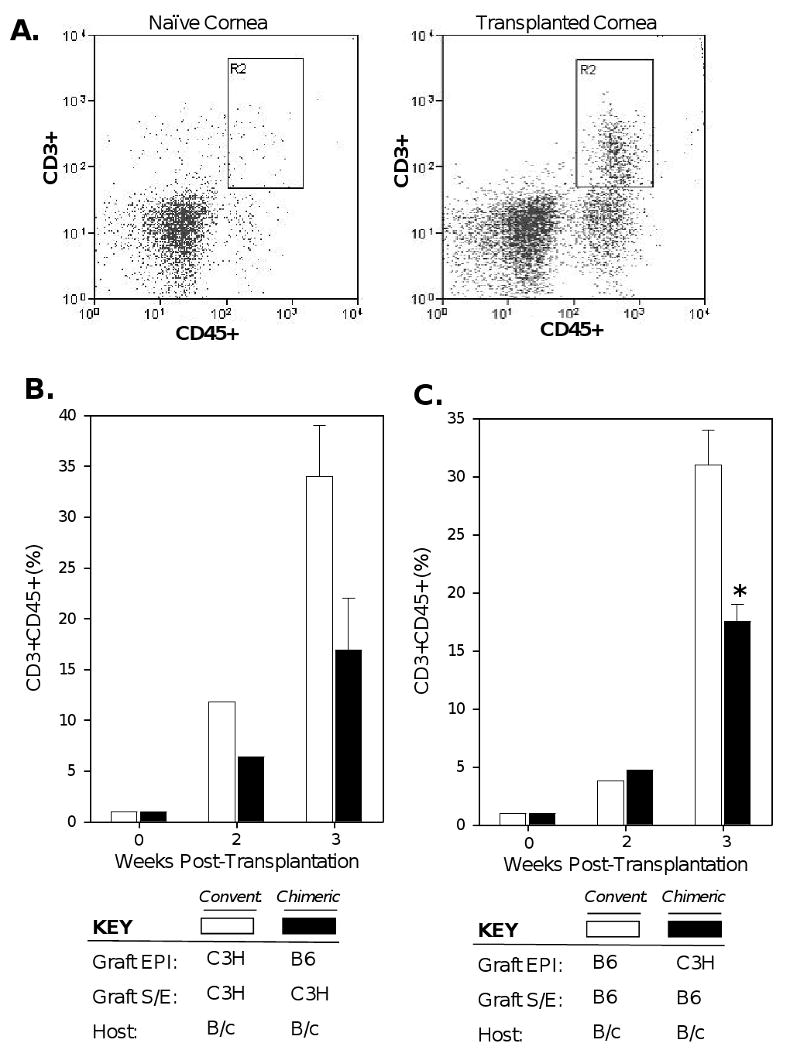
(A) Corneas were digested into single-cell suspensions for double-staining with CD45-FITC + CD3-PE and subsequent FACS enumeration of infiltrating T cells. (B) B/c recipients of C3H S-E + B6 EPI chimeric grafts were compared to those transplanted with C3H S-E + C3H EPI conventional allografts. (C) The reverse transplantation scheme was also carried out, whereby recipients B/c recipients of B6 S-E + C3H EPI chimeric grafts were compared to those transplanted with B6 S-E + B6 EPI conventional allografts. Corneas were pooled (n≥3 per group) at each of the indicated time-points; data for week 3 post-transplantation are the mean of 3 independent experiments. An asterisk (*) indicates a statistical significance (p = 0.03) as calculated by Student's t test.
Evaluation of transplant survival with chimeric corneal allografts
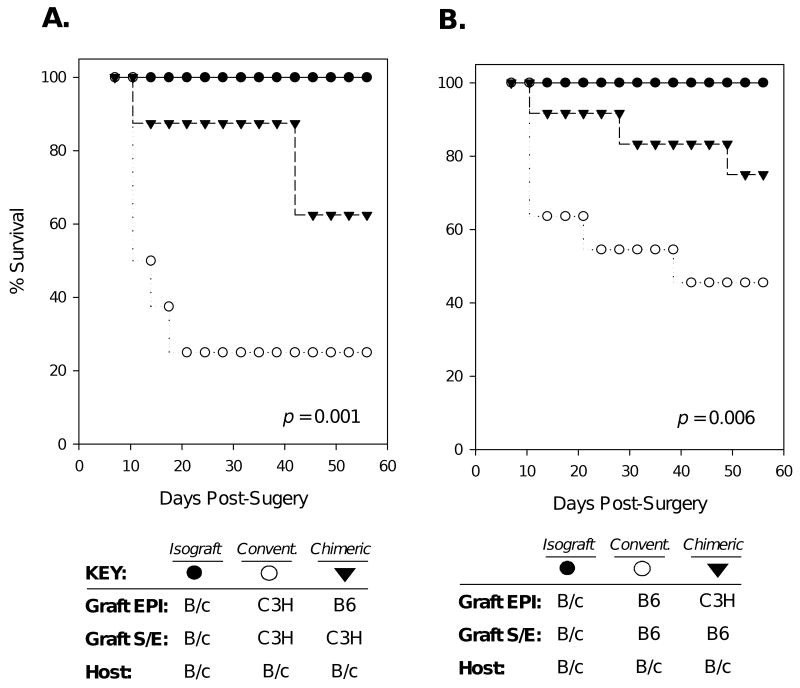
Because the graft endothelium is the critical target in immune rejection, a diminishment in alloreactivity to donor cornea endothelium, as indicated in both chimeric graft schemes, could lead to enhanced transplant survival. To test this, B/c recipients of chimeric grafts consisting of C3H S-E + B6 EPI were compared to those receiving C3H S-E + C3H EPI conventional allografts (Fig. 6a). In addition, other B/c recipients of chimeric grafts consisting of B6 S-E + C3H EPI were compared to those receiving B6 S-E + B6 EPI conventional allografts (Fig. 6b). Isografts (B/c) were transplanted in other recipients as well. Graft survival was judged using a standard scoring method via biomicroscopic examination and assessed twice a week out to 8 weeks post-transplantation (11). We found that survival was markedly increased with chimeric grafts in both settings. The survival rate of C3H S-E + B6 EPI chimeric grafts was 62%, while C3H S-E + C3H EPI conventional allografts was 25% (p=0.001). Similarly, the survival rate of B6 S-E + C3H EPI chimeric grafts was 75%, while B6 S-E + B6 EPI conventional allografts was 45% (p= 0.006).
Figure 6. Survival of chimeric allografts is markedly enhanced.
Graft survival was assessed biomicroscopically, and data are presented as Kaplan-Meier survival curves. (A) B/c recipients were transplanted with isografts (n=8), C3H S-E + B6 EPI chimeric grafts (n=8), or C3H S-E + C3H EPI (n=8) conventional allografts. (B) The reverse transplantation scheme was also carried out, whereby isografted B/c recipients (n=9), were compared to those receiving B6 S-E + C3H EPI chimeric grafts (n=11), or B6 S-E + B6 EPI conventional allografts (n=12). Log-rank analysis of Kaplan-Meier survival curves indicated statistical significance for both graft schemes (A. p=0.001, B. p=0.006).
Discussion
To the best of our knowledge, this is first time chimeric graft tissue, assembled from allogeneic plus 3rd-party allodisparate components, has successfully been used to demonstrate enhanced allograft survival. The current study employed the corneal transplantation model with chimeric grafts of allogeneic stroma-endothelia plus 3rd-party allodisparate epithelia. Our data indicate that in this setting, an inhibition of effector T cell activity is led by a diminishment in allosensitization to the graft endothelium. This was tested by employing a recall MLR assay, whereby host T cells were stimulated with alloantigen matching the graft endothelium. With a direct comparison to MLR recall stimulation from conventionally allografted hosts, we observed a reduction in T cell proliferation levels in chimeric engrafted hosts. This was corroborated by similar levels of reduction in IFN-γ secretion sampled from culture supernatants. Boisgerault et al. (15) and others (17) have previously shown the importance of TH1 cytokines, specifically IFN-γ, secreted by draining lymph node T cells in effecting corneal allograft rejection. Additionally, several studies including Yamada et al., have demonstrated an association of corneal allograft survival with a reduction in T cell proliferation (18). In the current study, not only did we find an inhibition of T cell proliferation and IFN-y secretion to graft endothelium, but this suppressive effect was consistently observed following the transplantation for both chimeric graft schemes (C3H EPI + B6 S-E, and B6 EPI + C3H S-E grafts). This was the case, interestingly, in spite of the fact that the C3H strain (I-Ek) is more allogenic than the B6 strain (I-Enull)—as demonstrated here both by heightened T cell infiltration and graft rejection of conventional C3H versus B6 allografts. Hence, the observed consistency in both chimeric graft schemes emphasizes the robust suppressive effect afforded by the chimeric nature of these experimental grafts.
Given the previous studies published which have highlighted the alloimmunogenic nature of donor corneal epithelium (2-6), we were somewhat perplexed that the overall reduction in total alloreactivity to donor endothelium following chimeric transplantation was not more profound. Interestingly, however, by focusing on the CD4+ compartment we found a substantially larger reduction than in total T cell alloreactivity. This was evidenced by an approximate 50% reduction in CD4(+) IFN-γ(+) T cell frequencies in recall MLR assays, which was up to 3-fold greater than CD4(-) IFN-γ(+) frequencies in some cases. In addition, this was associated with both a significant reduction in T cell infiltration and a marked enhancement in the survival of chimeric allografts. Indeed, graft survival is an important indicator of host CD4(+) alloreactivity, as the main mechanism for allorecognition corneal transplantation occurs by indirect presentation via MHC class II to CD4(+) T cells (20). Several independent studies have shown that in the absence of recipient CD4+ T cells—i.e., by gene deletion (16), or antibody blockade (20)—a robust impairment in acute corneal graft rejection results.
It is nonetheless apparent throughout our experimentation that alloreactivity to graft endothelium was not fully abrogated in the chimeric setting, which suggests that alloantigens expressed by donor stroma-endothelium still play a considerable role in allosensitization. This was also demonstrated by Hori et al (6), showing that even epithelial denuded allografts resurfaced with epithelium of recipient origin can incur rejection, albeit significantly less that full-thickness allografts. This is made possible as corneal stromal cells can upregulate MHC expression particularly under inflamed conditions (21), and minor histocompatibility (H) antigens expressed exclusively by graft stroma-endothelium may also contribute to allosensitization. Regarding minor H alloantigens, Sonoda et al. (11) and others (22) have indeed shown that high rejection rates can occur amongst corneal grafts confronted only with these alloantigens. Likewise, it is also possible that the 3rd-party epithelium, despite being MHC allodisparate, may share and thereby sensitize the host to minor H antigens of the graft stroma-endothelium, given the wide array of minor antigens expressed by grafted tissues.
In the aggregate, we hereby conclude that corneal transplant survival is markedly enhanced with the use of chimeric grafts, as 3rd-party allodisparate epithelium does not contribute to host sensitization against MHC alloantigens borne by the graft stroma-endothelium. This effect promotes survival because graft endothelium is the critical target in corneal graft rejection; while in contrast, rejection of graft epithelium is mostly self-limiting as these cells are repopulated by the host. Our findings represent a significant advancement in the current understanding in the dynamics of corneal alloimmunity, as we have shown direct evidence for the first time that graft epithelium alone is paramount for allosensitization of host CD4+ T cells. Moreover, chimeric corneal grafts may have direct and feasible applications to the clinical setting where immune rejection remains a significant problem particularly in high-risk recipients (23). It has recently been shown that various partial thickness allograft techniques, referred to as posterior lamellar endothelial keratoplasty, may also reduce the incidence of rejection (24). Such current microsurgical techniques could readily lend themselves to the preparation and transplantation of chimeric grafts in humans. Moreover, application and preparation of chimeric grafts may be further synergized with the recent advancements in the expansion of epithelial sheets ex vivo, which is currently being used in the clinic for ocular surface reconstruction (25).
Acknowledgments
The authors would like to thank Drs. Ammon Peck and Sharmila Masli for their excellent discussions during the development of this study. This work was funded in part by the National Institutes of Health grants EYRO1-12963 (R. Dana) and EYT32-07156 (D.R. Saban), as well as a joint grant funded by the Fight for Sight and American Society of Cataract and Refractive Surgeons, PD06009 (D.R. Saban).
Grant Information: Supported in part by: NIH/NEI RO1-12963 (Dana); T32-07156 (Saban); FFS/ASCRS PD06009 (Saban);
References
- 1.Khodadoust AA, Silverstein AM. Transplantation and rejection of individual cell layers of the cornea. Invest Ophthalmol. 1969 Apr;8(2):180–95. [PubMed] [Google Scholar]
- 2.Treseler PA, Foulks GN, Sanfilippo F. The expression of HLA antigens by cells in the human cornea. Am J Ophthalmol. 1984 Dec 15;98(6):763–72. doi: 10.1016/0002-9394(84)90696-2. [DOI] [PubMed] [Google Scholar]
- 3.Hori J, Joyce N, Streilein JW. Epithelium-deficient corneal allografts display immune privilege beneath the kidney capsule. Invest Ophthalmol Vis Sci. 2000 Feb;41(2):443–52. [PubMed] [Google Scholar]
- 4.Hori J, Streilein JW. Dynamics of donor cell persistence and recipient cell replacement in orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2001 Jul;42(8):1820–8. [PubMed] [Google Scholar]
- 5.Stulting RD, Waring GO, 3rd, Bridges WZ, Cavanagh HD. Effect of donor epithelium on corneal transplant survival. Ophthalmology. 1988 Jun;95(6):803–12. doi: 10.1016/s0161-6420(88)33120-9. [DOI] [PubMed] [Google Scholar]
- 6.Hori J, Streilein JW. Role of recipient epithelium in promoting survival of orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 2001 Mar;42(3):720–6. [PubMed] [Google Scholar]
- 7.Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, Pytowski B, Persaud K, Wu Y, Streilein JW, Dana R. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006 Jul 25;103(30):11405–10. doi: 10.1073/pnas.0506112103. Epub 2006 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy MC, Rosenbaum JT, Brown J, Planck SR, Huang X, Armstrong CA, Ansel JC. Novel production of interleukin-1 receptor antagonist peptides in normal human cornea. J Clin Invest. 1995 Jan;95(1):82–8. doi: 10.1172/JCI117679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishima H, Nakamura M, Murakami J, Nishida T, Otori T. Transforming growth factor-beta modulates effects of epidermal growth factor on corneal epithelial cells. Curr Eye Res. 1992 Jul;11(7):691–6. doi: 10.3109/02713689209000742. [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007 Sep 15;179(6):3672–9. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice—evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992 Oct;54(4):694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982 Aug;23(2):269–73. [PubMed] [Google Scholar]
- 13.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001 May;42(6):1293–8. [PubMed] [Google Scholar]
- 14.Plsková J, Duncan L, Holán V, Filipec M, Kraal G, Forrester JV. The immune response to corneal allograft requires a site-specific draining lymph node. Transplantation. 2002 Jan 27;73(2):210–5. doi: 10.1097/00007890-200201270-00010. [DOI] [PubMed] [Google Scholar]
- 15.Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001 Aug 15;167(4):1891–9. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 16.Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2614–21. [PubMed] [Google Scholar]
- 17.Qian Y, Boisgerault F, Benichou G, Dana MR. Blockade of CD40-CD154 costimulatory pathway promotes survival of allogeneic corneal transplants. Invest Ophthalmol Vis Sci. 2001 Apr;42(5):987–94. [PubMed] [Google Scholar]
- 18.Yamada J, Hamuro J, Sano Y, Maruyama K, Kinoshita S. Allogeneic corneal tolerance in rodents with long-term graft survival. Transplantation. 2005 May 27;79(10):1362–9. doi: 10.1097/01.tp.0000159869.55962.94. [DOI] [PubMed] [Google Scholar]
- 19.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004 Oct 1;173(7):4464–9. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 20.Coupland SE, Krause L, Karow AC, Bartlett RR, Lehmann M, Hoffmann F. Delay in corneal allograft rejection due to anti-CD4 antibody given alone and in combination with cyclosporin A and leflunomide. Ger J Ophthalmol. 1995 Sep;4(5):294–301. [PubMed] [Google Scholar]
- 21.Young E, Stark WJ, Prendergast RA. Immunology of corneal allograft rejection: HLA-DR antigens on human corneal cells. Invest Ophthalmol Vis Sci. 1985 Apr;26(4):571–4. [PubMed] [Google Scholar]
- 22.Sano Y, Ksander BR, Streilein JW. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl Immunol. 1996 Mar;4(1):53–6. doi: 10.1016/s0966-3274(96)80035-9. [DOI] [PubMed] [Google Scholar]
- 23.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000 Sep;19(5):625–43. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Allan BD, Terry MA, Price FW, Jr, Price MO, Griffin NB, Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007 Oct;26(9):1039–42. doi: 10.1097/ICO.0b013e31812f66e5. [DOI] [PubMed] [Google Scholar]
- 25.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004 Sep 16;351(12):1187–96. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]