Abstract
Toll-like receptors (TLRs) play important roles in inflammation and innate immune response to pathogens. TLR8 recognizes ssRNA and induces NF-κB via MyD88 signaling. TL1A is a member of the TNF superfamily that markedly enhances IFN-γ production by IL-12/IL-18-stimulated peripheral and mucosal CD4+ T cells. TL1A expression is increased in the mucosa of patients with inflammatory bowel disease (IBD) and is considered a key mediator of Crohn’s disease (CD). We have previously shown that TL1A is strongly induced by immune complexes (IC) but not TLR ligands in antigen-presenting cells. However, a potential interaction between these pro-inflammatory signaling pathways has not been investigated. IC-induced TL1A expression of monocytes was potently inhibited by a TLR8 or TLR7/8 ligand (R848) in a dose-dependent manner. Furthermore, when co-cultured with CD4+ T cells, TLR8 ligands inhibited TL1A production, resulting in almost complete inhibition of IFN-γ production by the CD4+ T cells. Furthermore, we demonstrate that IFN-α is not required for this suppressive effect by TLR8 signaling. Our data demonstrate for the first time a direct interaction between TLR and TL1A signaling pathways. TLR8 activation may be an important, novel pathway for targeted treatment of Th1-mediated diseases, such as CD.
Keywords: Monocytes, Cytokines, Fcγ Receptor, Inflammation, Signaling
Introduction
TL1A (TNFSF15) has been identified as a member of the TNF superfamily [1]and is a strong costimulator of T cells[2, 3]. TL1A binds to death domain receptor 3 (DR3, TNFRSF25) and induces NF-κB activation in cells expressing this receptor [1]. TL1A was shown to increase IL-2 responsiveness and enhance IFN-γ and GM-CSF release in anti-CD3-and anti-CD28-stimulated peripheral blood T cells. We have previously shown that DR3 expression in CCR9+CD4+ T cells is up-regulated following stimulation with IL-12 and IL-18 [4]. Furthermore, TL1A augments IFN-γ production by IL-12/IL-18-stimulated peripheral blood and mucosal CD4+CCR9+ T cells [2, 4]. Several studies have implicated the TL1A/DR3 pathway in the pathogenesis of IBD. We and others have shown that surface expression of TL1A and its receptor DR3 by mucosal T cells is increased in mucosal inflammation in Crohn’s Disease and UC [3, 5]. Furthermore, both TL1A and DR3 were up-regulated in the inflamed intestinal mucosa in two different models of chronic murine ileitis [6]. We have recently shown that TL1A is a central molecule in chronic T cell mediated colitis [7]. Neutralizing TL1A antibodies attenuated chronic colitis in this model by attenuating TH1 and TH17 responses [7]. In addition, TL1A-DR3 signaling also plays a role in other TH1, TH2, and TH17 driven pathologies, in particular in murine models of arthritis, experimental autoimmune encephalomyelitis, and allergic lung inflammation [8–10].
We recently identified the FcγR signaling pathway as a major inducer of membrane and soluble TL1A while neither TLR ligands nor IFN-γ were able to induce TL1A in antigen-presenting cells [11]. Furthermore, TL1A production in FcγR activated monocytes leads to enhancement of IFN-γ production by IL-12/IL-18 primed CD4+ T cells. Because of the important role TL1A plays as a T cell activator and its up-regulation in inflammatory conditions the elucidation of signaling pathways that potentially inhibit TL1A induction and expression is of great potential therapeutic importance.
Toll-like receptors play a central role in the initiation of innate cellular immune responses. Signaling from all TLRs except TLR3 activates MyD88 which finally leads to NF-κB activation through IL-1R-association kinase (IRAK)-1, IRAK-4, tumor necrosis factor-associated factor 6 (TRAF6) [12], and canonical IκB kinase (IKK) complex [13–15]. Activated NF-κB induces multiple pro-inflammatory cytokines genes such as TNF-α, IL-6, and IL-1β. All of these pro-inflammatory mediators have been implicated in mucosal inflammation in both CD and UC [16]. TLR7, 8, and 9 are expressed intracellularly where they associate with endosomes and are able to both activate and/or inhibit inflammatory responses [17, 18]. TLR8 can induce pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-12 from PBMCs, monocytes and DCs [19]. Moreover, TLR8 is expressed on regulatory T cells, and its ligands can reverse their suppressive activity [20]. Although TLR8 is a potent inducer of pro-inflammatory cytokines, TLR8 also induces the anti-inflammatory cytokine, IL-10, from human PBMCs [21]. The natural ligand for TLR8 is still unknown, however, uridine-rich or uridine/guanosine-rich single-stranded RNA, derived from viruses such as human immunodeficiency virus (HIV-1) and influenza virus, or synthetic antiviral imidazoquinoline component Resiquimod (R848) can bind to TLR8 [22–24] in the endosomal membrane, and then TLR8 signaling consequently activates NF-κB through the MyD88-dependent pathways. In addition to this common pathway, TLR8 also engages in another signaling pathway which proceeds via MyD88, IRAK4, IRAK1 and IRF-7 [25, 26]. Activated IRF-7 enters the nucleus and induces IFN-α/β, which mediate innate and adaptive immunity to viruses [13, 14, 27–29], but are also involved in an anti-inflammatory response during experimental colitis [30]. Recently, we have reported that TLR8 is an X-linked IBD susceptibility gene with both common predisposing and protecting haplotypes [31]. These associations further emphasize the importance of TLR8 signaling in IBD.
Here, we demonstrate that TLR8 signaling efficiently inhibits FcγR-induced expression of surface and soluble TL1A in monocytes. This inhibition leads to almost complete diminution of IFN-γ production by co-cultured autologous CD4+ T cells. We also demonstrate that this inhibition by TLR8 ligands is not mediated by IFN-α. Our data are the first to demonstrate a direct interaction between TLR and TL1A signaling pathways.
Results
TLR8 ligand is the most efficient inhibitor of FcγR induced TL1A induction in human monocytes
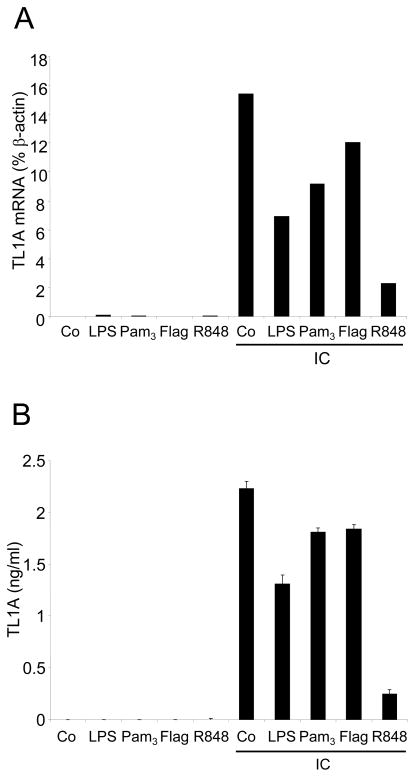
We have previously shown that IC are strong inducers of TL1A mRNA as well as surface expression and secretion [11]. To determine if pro-inflammatory signaling pathways interfere with IC-induced TL1A induction, we stimulated CD14+ monocytes with IC in combination with various TLR ligands (Figure 1). We have previously demonstrated that these TLR ligands do not induced TL1A expression ([11] and Figure 1). While the TLR ligands LPS, Pam3, and flagellin inhibited IC-induced up-regulation of TL1A mRNA and TL1A secretion, R848 was the most efficient inhibitor of IC-induced TL1A up-regulation (Figure 1A, B). Incubation of monocytes with the TLR7/8 ligand R848, in combination with IC, led to an almost complete inhibition of TL1A mRNA induction and TL1A secretion. This led us to focus on TLR8 signaling in subsequent experiments.
FIGURE 1. The TLR8 ligand R848 is an efficient inhibitor of IC-induced TL1A expression.
CD14+ monocytes were incubated with LPS, Pam3, Flagellin, or R848 in the presence or absence of IC for 16 h. The control (Co) represents incubation in medium alone. (A) TL1A mRNA was analyzed by real-time PCR. Data are expressed as % of β-Actin mRNA expression. (B) Supernatants were harvested and TL1A protein levels analyzed by ELISA. Means ± SD are shown (n=2). Shown is one representative experiment out of three independent experiments.
TLR8 mRNA is expressed in human monocytes and TLR8 agonists induce IL-6, TNF-α, and IL-10 production
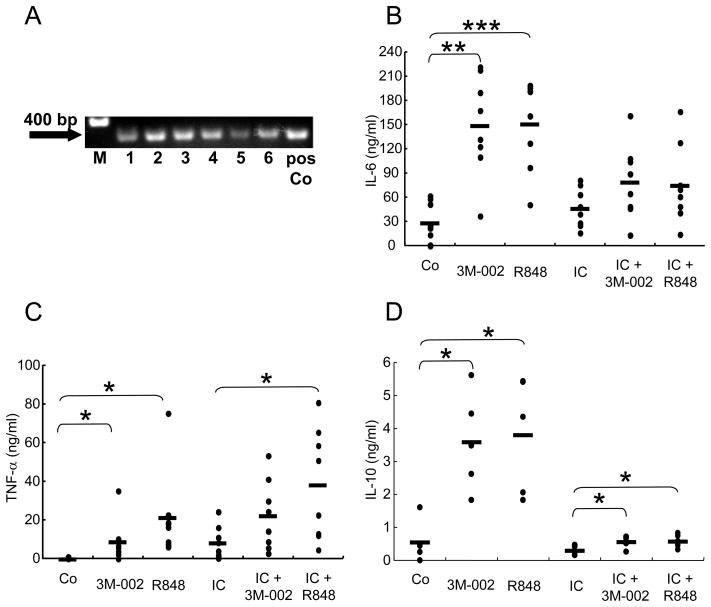
Previous publications reported that TLR8 but not TLR7 mRNA is expressed in freshly isolated human monocytes [32]. First, we confirmed that TLR8 was expressed on human monocytes from all six normal donors tested (Figure 2A). Next, we sought to determine whether stimulation of monocytes leads to secretion of pro- and anti-inflammatory cytokines. The TLR8 ligand 3M-002 or R848 (TLR7/8 ligand) directly induced IL-6 and TNF-α from monocytes (Figure 2B, 2C). In addition, when co-cultured with IC, 3M-002 and R848 induced higher levels of IL-6, and TNF-α. Furthermore, TLR7/8 lignads also enhanced IC-induced production of these cytokines. Interestingly, the anti-inflammatory cytokine, IL-10, was also directly induced from monocytes stimulated by 3M-002 or R848 (Figure 2D). We observed that IC-stimulation alone did not induce IL-10 production, while stimulation with 3M-002 or R848 and IC did induce low levels of IL-10. However, the IL-10 produced by 3M-002 or R848 plus IC is significantly lower than the IL-10 produced by the TLR8 ligands alone (Figure 2D). These data suggest that FcγR and TL1A signaling pathways are intertwined in the production of pro- and anti-inflammatory cytokines.
FIGURE 2. TLR8 expression and induction of cytokines in IC-stimulated monocytes.
(A) Monocytes were isolated from the peripheral blood of healthy donors (n=6) and incubated for 16 h. RT-PCR for TLR8 is shown. The positive control (pos Co) was purchased from InvivoGen. M represents molecular weight markers (B–D) Differential production of cytokines by monocytes isolated from individual donors and stimulated with 3M-002 (TLR8 agonist) or R848 (TLR7/8 agonist) in the presence or absence of IC. The control (Co) represents incubation in medium alone. (B) IL-6 (n=8), (C) TNF-α (n=8), and (D) IL-10 (n=5) levels in supernatants were measured by ELISA. Means are shown as black bars. *, p<0.05; **, p<0.001; ***, p<0.0001 (Student’s t-test).
TLR8 ligands inhibit FcγR signalling-induced TL1A mRNA, soluble and cell-surface TL1A expression
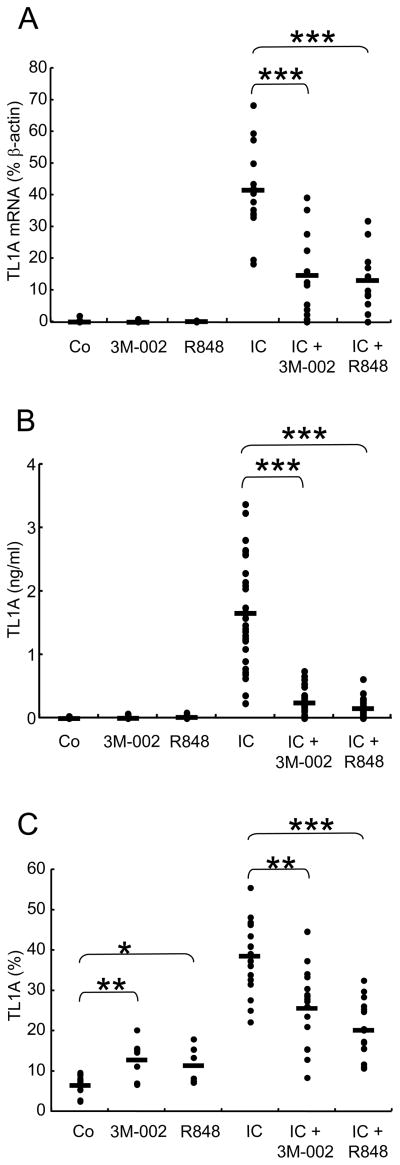
We next examined the effect of TLR8 stimulation on IC-induced TL1A expression in monocytes. TL1A has been demonstrated to be expressed as a membrane bound form and to be secreted upon stimulation with IC [11]. To determine if TLR8 ligands have an effect on IC-induced TL1A expression, we stimulated monocytes with 3M-002 or R848 in combination with IC and analyzed TL1A mRNA, secretion, and surface expression. TL1A mRNA induced by IC stimulation was significantly inhibited by TLR8 agonist or R848 (64% and 68% inhibition, respectively) (Figure 3A). The secretion of TL1A in IC-stimulated monocytes was inhibited by 84 % by 3M-002 or 89 % by R848 (Figure 3B). Although TL1A expression on the surface of monocytes was induced by TLR8 agonist or R848 alone, TL1A expression induced by IC was also inhibited by TLR8 agonist or R848 (33% and 47% inhibition, respectively) (Figure 3C). These data indicate that TLR8 ligands can inhibit both TL1A mRNA and protein expression in response to FcγR activation.
FIGURE 3.
TLR8 ligands inhibit IC-induced TL1A expression. TL1A mRNA, TL1A secretion, and TL1A membrane expression were determined following stimulation of monocytes with 10 μM 3M-002 (TLR8 agonist) or 10 μM R848 (TLR7/8 agonist) in the absence or presence of IC. The control (Co) represents incubation in medium alone. (A) TL1A transcript levels were quantified by real-time RT-PCR and expressed as percent β-actin transcript levels (n=16). (B) TL1A secretion in supernatants was measured by ELISA (n=30). (C) TL1A expression on the surface of monocytes was evaluated by flow cytometry (n=20)and the results presented as % monocytes expressing TL1A. Means are shown as black bars. *, p<0.05; **, p<0.001; ***, p<0.0001 (Student’s t-test).
TLR8 ligands inhibit IFN-γ production from CD4+ T cells through suppression of IC-stimulated monocyte TL1A production
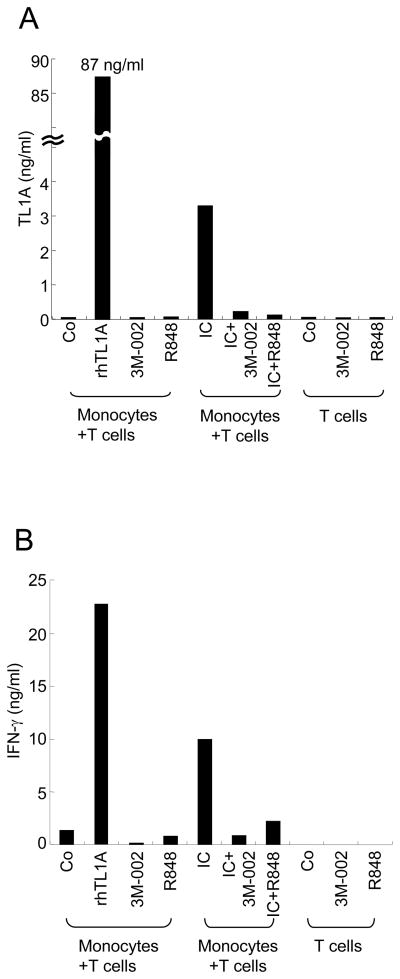
To determine whether inhibition of IC-induced TL1A production by 3M-002 or R848 has a functional consequence on T cell activation, we used an autologous monocyte T cell co-culture system [11]. Monocytes were cultured with IC alone or with addition of 3M-002 or R848. The next day, IL-12/IL-18 primed autologous CD4+ T cells were incubated with the monocytes and IFN-γ production by CD4+ T cells was measured. We have previously shown that endogenous TL1A induced by IC-stimulation of monocytes could enhance production of IFN-γ from autologous IL-12/IL-18 activated CD4+ T cells [11]. After 48 h of CD4+ T cell/monocyte coculture, TL1A and IFN-γ production were measured by ELISA. TL1A induced by IC was inhibited by the addition of TLR8 agonist or R848 (Figure 4A) while TL1A was undetectable in unstimulated monocytes or T cells alone (Figure 4A). Therefore, endogenous TL1A was induced by IC and inhibited by TLR8 stimulation. Although control monocytes co-cultured with CD4+ T cells induced low levels of IFN-γ (Figure 4B), IC-stimulated monocytes induced a 5 fold higher IFN-γ production (Figure 4B). Co-cultures of IC-stimulated monocytes with 3M-002 or R848, which blocked almost completely TL1A expression, inhibited IFN-γ production to baseline (unstimulated monocytes) levels (Figure 4B).
FIGURE 4.
TLR8 agonist and R848 abrogate IC-stimulated monocyte enhancement of IFN-γ production by autologous CD4+ T cells. CD4+ T cells were isolated and incubated overnight with IL-12 and IL-18. The next day, autologous T cells were either cultured alone or co-cultured in the presence or absence of 3M-002 (TLR8 agonist) or R848 (TLR7/8 agonist) with monocytes that had been preincubated with or without IC for 16 h. The control (Co) represents incubation in medium alone. After 48 h, supernatants were harvested and analyzed for (A) TL1A and (B) IFN-γ production by ELISA. Representative data of two experiments with similar results is shown (n=3).
Type I IFN (IFN-α) is not required for inhibition of TL1A production by TLR8 ligands
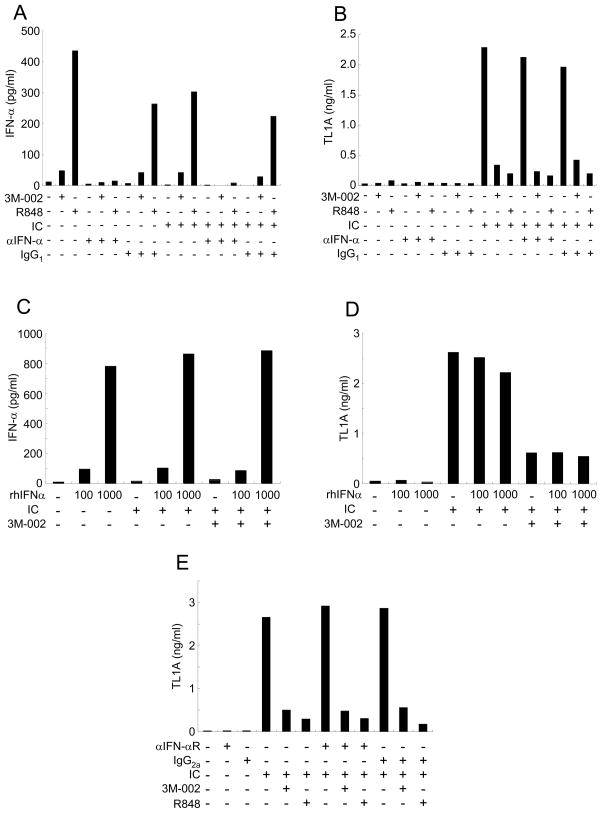
The mechanism of how TLR8 activation inhibits TL1A expression while simultaneously activating other pro-inflammatory genes is unclear. Recently it has been shown that type I IFN (IFN-α/β) protects mice from DSS-induced experimental colitis [30]. Since the stimulation with TLR8 ligands led to the induction of IFN-α, we hypothesized that IFN-α might be responsible for the inhibitory effects of TLR8 stimulation on FcγR-induced TL1A induction. To evaluate the role of IFN-α in TLR8 mediated TL1A inhibition, monocytes were stimulated with or without IC and 3M-002 or R848 in the presence or absence of neutralizing anti-IFN-α Ab. Monocytes stimulated with 3M-002 and in particular R848, induced IFN-α production, which was neutralized by anti-IFN-α antibodies (Figure 5A). However, the TLR8/R848 inhibitory effects on TL1A production were not reversed by anti-IFN-α antibodies (Figure 5B). To determine if exogenous recombinant IFN-α was able to inhibit IC-induced TL1A production, monocytes were stimulated with IC and TLR8 agonist or R848 in the presence or absence of 100 or 1000 U of recombinant IFN-α (Figure 5C, D). The effects of TLR8 agonist or R848 were not abrogated by the addition of exogenous IFN-α. Moreover, to evaluate the role of IFN-α in monocytes, we measured TL1A production in monocytes stimulated with or without IC and TLR8 agonist or R848 in the presence or absence of anti-IFN-αR antibodies. Anti-IFN-αR Ab had no effect on the inhibition of TL1A induction by TLR8 agonist or R848 (Figure 5E). Collectively, these data show that autocrine or paracrine IFN-α produced byTLR8 ligand stimulation is not responsible for TL1A inhibition by TLR8 agonist or R848 in IC-stimulated monocytes.
FIGURE 5. IFN-α is not required for TLR8 inhibition of IC-induced TL1A production.
(A) IFN-α and (B) TL1A production in monocytes stimulated with or without anti-IFN-α antibodyor control IgG1 in the presence or absence of IC and 3M-002 (TLR8 agonist) or R848 (TLR7/8 agonist) were measured by ELISA (n=3 per treatment group). (C) IFN-α and (D) TL1Aproduction in monocytes stimulated with or without recombinant IFN-α in the presence or absence of IC and 3M-002 (TLR8 agonist) were measured by ELISA. One representative experiment is shown (n=3). (E) TL1A production in monocytes stimulated with or without anti-IFN-α receptor antibody or control IgG1 in the presence or absence of IC and 3M-002 (TLR8 agonist) or R848 (TLR7/8 agonist) was measured by ELISA. One representative experiment out of two independent experiments is shown (n=3).
Furthermore, we evaluated if NF-κB signaling is involved in the inhibition of TL1A production. While TLR7/8 ligands induce the activation of NF-κB, IC is only a weak inducer of NF-κB activation in monocytes. The combination of TLR7/8 ligands and IC does not reduce NF-κB activation compared to TLR7/8 ligand stimulation alone suggesting that that the NF-κB signaling pathway is not involved in the inhibition of TL1A induction by TLR7/8 ligands (Data not shown).
Discussion
In this study we investigated the consequence of activation of TLR8 signaling pathway in regulating TL1A expression and function. We demonstrate here that TLR8 is expressed in human monocytes, and that TLR8 ligands are able to induce IL-6 and TNF-α production. FcγR stimulation, a known inducer of TL1A expression from antigen-presenting cells, also led to increased TNF-α production, as we have shown previously [11]. The co-stimulation of IC and TLR8 ligands increased TNF-α secretion by monocytes compared to IC alone. In contrast, co-stimulation of IC and TLR8 ligands inhibited IL-6 and IL-10 production by monocytes compared to TLR8-induced IL-6 and IL-10 secretion suggesting that both cytokines are not involved in the inhibition of FcγR-induced TL1A by TLR8 ligands.
We previously demonstrated that membrane and soluble TL1A is strongly induced by FcγR stimulation [11]. Surface but not soluble expression of TL1A on monocytes was stimulated by TLR8 agonist or R848 alone. However, co-stimulation of monocytes with IC and TLR8 significantly inhibited surface TL1A expression compared to that of IC-stimulated monocytes alone. Furthermore, IC-induced TL1A secretion was almost completely suppressed by TLR8 ligands while TLR2, 4, and 5 ligands had only minimal effects on TL1A inhibition. To understand whether the effect of TLR8 activation was at the level of gene expression or translation, we investigated TL1A mRNA induction. The level of TL1A mRNA induced by FcγR activation was inhibited by more than 60% by TLR8 ligands suggesting that TLR8 ligands inhibit IC-induced TL1A expression at least partially on the transcriptional level. Inthe presence of IC, TL1A surface expression increased and was inhibited by 30–50 % by TLR8 agonist or R848. TLR8 signaling significantly inhibited secretion of IC-induced TL1A by more than 80 %. Taken together, TLR8 signaling significantly inhibited TL1A mRNA, surface expression, and secretion of soluble TL1A. Furthermore, we demonstrate that TLR8 ligands also significantly inhibit IFN-γ production by CD4+ T cell when co-cultured with monocytes treated with IC plus TLR8. We have previously shown that monocyte generated TL1A can enhance IFN-γ production from CD4+ effector T cells [11]. In these co-culture experiments, we observed that monocyte-derived TL1A secretion was completely blocked by TLR8 leading to complete abrogation of TL1A-mediated CD4+ T cell IFN-γ production. Therefore, our data demonstrate that TLR8 signaling may be a potent inhibitor of the costimulatory activity of TL1A during chronic inflammation. Further experiments are necessary to determine the in vivo efficacy of TLR8 treatments in animal models of chronic intestinal inflammation.
Induction of TLR8 signaling depends entirely on MyD88. In addition to the activation of NF-κB TLR8 signaling also leads to the activation of IRF7 via IRAK-1 that ultimately leads to the induction of type I IFNs, such as IFN-α/β [13, 14, 19, 27, 29]. IFN-α is one of the characteristic cytokines induced following TLR8 activation. We, therefore, hypothesized that IFN-α may be responsible for the suppression of TL1A production. Furthermore, it has been demonstrated that TLR9-induced IFN-α/β suppresses the severity of experimental colitis in RAG−/− mice [30]. However, neither neutralization of IFN-α signaling nor recombinant IFN-α had an effect of suppression of TL1A induction by TLR8 ligands suggesting that IFN-α does not play an important role in the inhibition of FcγR-induced TL1A production by TLR8 ligands.
In this study, we have demonstrated that TLR8 signaling is a potent inhibitor of FcγR-induced TL1A production. Recent data from our laboratory defined both protective and risk haplotype variants in the TLR8 gene that are associated with Crohn’s disease [31]. Furthermore, a recent genome-wide association study of CD has provided evidence that variation in TNFSF15, the TL1A gene, contributes to CD [33, 34] and our laboratory demonstrated that risk TL1A variants lead to increased TL1A expression in antigen-presenting cells from CD patients [35]. The results from this study demonstrating that TLR8 activation functions as an inhibitor of TL1A expression might be one explanation how these two separate signaling pathways could lead to a more severe or milder form of the disease depending on the haplotype combinations in a given IBD patient. Further studies linking function of TL1A and TLR8 to relative haplotypes may well be able to address this question. If the TLR8 signaling can also suppress TL1A production in vivo, the TLR8 pathway may be important in preventing or controlling severe chronic inflammation such as is seen in CD.
Materials and Methods
Peripheral blood cell isolation and culture
Blood was obtained from normal donors after informed consent in accordance with procedures established by the Cedars-Sinai Institutional Review Board (IRB number 2673). CD14 + Monocytes were isolated from PBMC as described previously [11]. CD4+ T cells were isolated from PBMC using a human T lymphocyte Enrichment Set (BD Biosciences) and cultured for 16 h in the presence of human IL-12 (0.25 ng/ml; PeproTech) and human IL-18 (6.25 ng/ml; MBL) in RPMI complete medium.
Stimulation of monocytes
Monocytes were plated in 12-well plates and stimulated for 16 h with immune complex (IC) and TLR8 agonist (3M-002, 3M Pharmaceuticals), TLR7/8 agonists (R848, 3M Pharmaceuticals), LPS (100 ng/ml), Pam3CSK4 (InvivoGen, 300 ng/ml), Flagellin (10 μg/ml). Plate bound, cross-linked human IgG (IC) was prepared as described elsewhere [11]. For IFN-α experiments, monocytes were preincubated with recombinant human IFN-α (BioSource) at 100 or 1000 U/ml, anti-human IFN-α antibody (Chemicon) at 20 μl/ml, or, anti-human IFN-α receptor antibody (PBL Biomedical Laboratories) at 20 μg/ml for 1 h and then incubated with TLR8 agonist or R848 in the presence or absence of IC stimulation.
Co-culture of CD4+ T cells with monocytes
After 16 h stimulation of CD4+ T cells with IL-12 and IL-18, medium was replaced with fresh medium supplemented with IL-12 and IL-18 and cells were added to autologous monocytes supplemented with TLR8 agonist or R848. Before co-cultures, monocytes were incubated with TLR8 agonist or R848 in the presence or absence of IC for 16 h. At the time of co-culture, recombinant TL1A was added as positive control. Co-cultures were incubated for 48 h and supernatants were harvested and analyzed for IFN-γ and TL1A production by ELISA.
PCR and Real-time PCR analysis
Total RNA was isolated from monocytes using TRIzol (Invitrogen Life Tchnologies) according to the manufacturer’s protocol. TLR8 transcripts were amplified by reverse transcriptase PCR using TLR8 RT-primers (InvivoGen). TL1A and β-actin transcripts were anayzed by quantitative real-time RT-PCR as described previously [11].
Analysis of TL1A, IL-6, TNF-α, IFN-γ, IL–10 and IFN-α by ELISA
TL1A was quantified in supernatants from stimulated monocytes using an ELISA as described previously [11]. Human IL-6, TNF-α, or IFN-α concentrations were measured by ELISA (IL-6, TNF-α: eBioscience, IFN-α: Bender MedSystems). Human IL-10 and human INF-γ were measured by ELISA as described previously [3].
Flow Cytometry
Monocytes stimulated with IC or bacteria for 16 h were stained with the TL1A Ab as described previously [11]. Cells were analyzed on a CyAn™ ADP flow cytometer (Dako Cytomation, Carpinteria, CA) and analyzed with the Summit 4.1 software package (Dako).
Statistics
Statistical significance was determined by Student’s t test. A value of p < 0.01 was considered to be statistically significant.
Acknowledgments
The authors would like to thank Teva Pharmaceutical USA (North Wales, PA) for generously providing us with anti-TL1A antibodies. This work was supported by grants from the National Institutes of Health DK056328 and DK046763 (S.R.T.).
Abbreviations
- IC
Immune complexes
- CD
Crohn’s Disease
- UC
Ulcerative Colitis
- IBD
inflammatory bowel disease
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 2.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, Wei P, Targan SR. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 3.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, Targan SR. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Papadakis KA, Zhu D, Prehn JL, Landers C, Avanesyan A, Lafkas G, Targan SR. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174:4985–4990. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]
- 5.Bamias G, Martin C, 3rd, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, Pizarro TT, Wei P, Cominelli F. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 6.Bamias G, Mishina M, Nyce M, Ross WG, Kollias G, Rivera-Nieves J, Pizarro TT, Cominelli F. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, Braun J, Targan SR. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers A, Wang EC, Siegel RM. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, Weng S, Browning B, Scott ML, Ma L, Su L, Tian Q, Schneider P, Flavell RA, Dong C, Burkly LC. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178:4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 16.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 18.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 20.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh TK, Mickelson DJ, Fink J, Solberg JC, Inglefield JR, Hook D, Gupta SK, Gibson S, Alkan SS. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 23.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 24.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 25.Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova JL, Su B, Li X. TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- 26.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 28.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 30.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saruta M, Targan SR, Mei L, Ippoliti AF, Taylor KD, Rotter JI. High-frequency haplotypes in the X chromosome locus TLR8 are associated with both CD and UC in females. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J Immunol. 2006;176:7438–7446. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- 33.Kakuta Y, Kinouchi Y, Negoro K, Takahashi S, Shimosegawa T. Association study of TNFSF15 polymorphisms in Japanese patients with inflammatory bowel disease. Gut. 2006;55:1527–1528. doi: 10.1136/gut.2006.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki K, McGovern D, Ragoussis J, Paolucci M, Butler H, Jewell D, Cardon L, Takazoe M, Tanaka T, Ichimori T, Saito S, Sekine A, Iida A, Takahashi A, Tsunoda T, Lathrop M, Nakamura Y. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum Mol Genet. 2005;14:3499–3506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 35.Michelsen KS, Thomas LS, Taylor KD, Yu QT, Mei L, Landers CJ, Derkowski C, McGovern DP, Rotter JI, Targan SR. IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS ONE. 2009;4:e4719. doi: 10.1371/journal.pone.0004719. [DOI] [PMC free article] [PubMed] [Google Scholar]