Abstract
During spatial navigation, lesion and functional imaging studies suggest that the right hemisphere has a unique functional role. However, studies of direct human brain recordings have not reported interhemisphere differences in navigation-related oscillatory activity. We investigated this apparent discrepancy using intracranial electroencephalographic recordings from 24 neurosurgical patients playing a virtual taxi driver game. When patients were virtually moving in the game, brain oscillations at various frequencies increased in amplitude compared with periods of virtual stillness. Using log-linear analysis, we analyzed the region and frequency specificities of this pattern and found that neocortical movement-related gamma oscillations (34–54 Hz) were significantly lateralized to the right hemisphere, especially in posterior neocortex. We also observed a similar right lateralization of gamma oscillations related to searching for objects at unknown virtual locations. Thus, our results indicate that gamma oscillations in the right neocortex play a special role in human spatial navigation.
Introduction
Spatial navigation is a basic part of everyday life for both humans and animals. Because navigation is so ubiquitous throughout the animal world, examining the neural basis of spatial processing is especially useful because it is informative about neural mechanisms across various species. Furthermore, research on spatial navigation may also be informative about other related cognitive processes, such as episodic memory (Buzsáki, 2005). Consequently, a growing number of researchers have recently begun examining the neural basis of spatial navigation in both humans and animals.
One of the most direct ways to examine neuronal activity underlying spatial processing is to directly record electrical activity from the brain of an animal during navigation. This technique is often used to examine neuronal activity in rodents navigating laboratory mazes. Among the most robust findings of these studies is that hippocampal theta oscillations (3–12 Hz) increase in amplitude during navigational behaviors such as movement and foraging (for a review, see Buzsáki, 2002). First discovered in rodents, navigation-related theta oscillations were recently observed in humans engaged in virtual navigation tasks—first from intracranial electroencephalographic (iEEG) recordings of neurosurgical patients (Caplan et al., 2003; Caplan, Madsen, Raghavachari, & Kahana, 2001; Kahana, Sekuler, Caplan, Kirschen, & Madsen, 1999) and subsequently from magneto-encephalographic recordings in healthy subjects (Cornwell, Johnson, Holroyd, Carver, & Grillon, 2008; de Araujo, Baffa, & Wakai, 2002). Consequently, although much human navigation research focuses on theta oscillations to parallel rodent studies, many human studies also report navigation-related brain oscillations at a broad range of frequencies, including the gamma band (Cornwell et al., 2008; Ekstrom et al., 2005; Caplan et al., 2001). Both theta and gamma oscillations have been implicated in a wide range of human cognitive processes including perception, attention, learning, and memory (Kahana, 2006).
In a largely parallel line of research, a number of researchers have examined the functional roles of different brain regions in navigation via patient lesion and fMRI techniques. The results from these studies emphasize the role of the hippocampus and related medial-temporal lobe structures, along with regions in parietal and frontal cortices, in tasks that require spatial information processing (for a review, see Burgess, Maguire, & O'Keefe, 2002; Kessels, de Haan, Kappelle, & Postma, 2001). In particular, parts of right frontal, temporal, and parietal cortex specifically activate when participants engage in spatial processing (Gramann, Muller, Schonebeck, & Debus, 2006; Neggers, van der Lubbe, Ramsey, & Postma, 2006), and impaired spatial memory performance is associated with right hemisphere lesions (van Asselen et al., 2006). Furthermore, studies show that the magnitude of right hippocampus activation positively correlates with navigation accuracy (Maguire et al., 1998) and that the size of a subject's right hippocampus is positively correlated with their navigation experience (Maguire et al., 2000). Thus, a critical hypothesis emerging from these studies is that certain types of spatial processing, particularly those underlying navigation and knowledge of spatial locations, are lateralized to the right hemisphere (Leung, Oh, Ferri, & Yi, 2007; Diaz-Asper, Dopkins, Potolicchio, & Caputy, 2006; Gramann et al., 2006; Neggers et al., 2006; van Asselen et al., 2006; Weniger & Irle, 2006; Parslow et al., 2005; Wolbers & Buchel, 2005; Bohbot, Iaria, & Petrides, 2004; Voermans et al., 2004; Kessels, Kappelle, de Haan, & Postma, 2002; Spiers, Burgess, et al., 2001; Worsley et al., 2001; Nunn, Graydon, Polkey, & Morris, 1999; Abrahams, Pickering, Polkey, & Morris, 1997; Smith & Milner, 1989).
In recent years, a number of methodical studies reported close relations between fMRI data and direct recordings of brain oscillations (Mukamel et al., 2005; Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). To the extent that navigation-related brain oscillations are related to patterns observed in fMRI and lesion studies, one would expect to find that navigation-related brain oscillations are reliably more prevalent in the right hemisphere. The present study aims both to test this hypothesis and to more fully characterize the oscillatory correlates of spatial navigation across various brain regions and frequency bands.
We recorded iEEG signals from 1,447 electrodes placed in widespread brain regions of 24 neurosurgical patients. During recording, patients played a video game in which they drive a taxi throughout a virtual city. Because iEEG recordings are largely impervious to eye movements and motor artifacts (Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008; Pfurtscheller & Cooper, 1975), this technique is well suited for studying the electrophysiological correlates of spatial navigation. Each trial of the game has two phases: First, the patient searches the environment to locate a passenger and drives that passenger to the requested destination. By repeating this process over many trials, patients learn the environment's spatial layout (Newman et al., 2007; Caplan et al., 2003). In this study, we examine the relation between patients' behavior in the task and their simultaneously recorded iEEG oscillatory activity in various brain regions and frequencies.
Methods
Patient Data and Electrophysiology
Twenty-four patients (ages 11–48 years) undergoing surgical treatment for refractory epilepsy participated in our virtual navigation study (see Table 1). Results from 12 of these participants were reported by Caplan et al. (2003), and results from 5 were reported by Ekstrom et al. (2005). Data from seven of the participants have not been reported prior to this article.
Table 1.
Patient Demographics
| Patient | Age | Sex | No. of Electrodes | Implantation Types | Resection Region | Hospital |
|---|---|---|---|---|---|---|
| 1 | 14 | Female | 47 | Subdural | Left medial-temporal | C |
| 2 | 15 | Male | 105 | Subdural | Right frontal/temporal | C |
| 3 | 15 | Male | 63 | Both | Left temporal | C |
| 4 | 18 | Male | 51 | Subdural | Left medial-temporal | C |
| 5 | 15 | Male | 118 | Subdural | Left anterior frontal | C |
| 6 | 42 | Female | 50 | Both | Right temporal | F |
| 7 | 28 | Male | 66 | Both | Right frontal/temporal | F |
| 8 | 33 | Male | 77 | Both | Right temporal/occipital | F |
| 9 | 32 | Female | 49 | Both | Left temporal/occipital | F |
| 10 | 22 | Male | 27 | Both | Right hippocampus | F |
| 11 | 29 | Male | 34 | Both | Right frontal | F |
| 12 | 25 | Male | 84 | Both | None | F |
| 13 | 12 | Male | 73 | Subdural | Left temporal | C |
| 14 | 13 | Male | 58 | Subdural | Left anterior temporal | C |
| 15 | 11 | Male | 107 | Subdural | Right frontal/parietal | C |
| 16 | 36 | Male | 71 | Both | Left temporal | P |
| 17 | 25 | Male | 48 | Both | Right temporal | P |
| 18 | 18 | Female | 62 | Both | None | P |
| 19 | 27 | Male | 58 | Subdural | Right temporal | B |
| 20 | 38 | Male | 58 | Depth | Left medial-temporal | U |
| 21 | 48 | Male | 36 | Depth | Left medial-temporal | U |
| 22 | 42 | Female | 31 | Depth | Left medial-temporal | U |
| 23 | 41 | Male | 44 | Depth | Left medial-temporal | U |
| 24 | 45 | Male | 30 | Depth | Left medial-temporal | U |
“Implantation type” indicates the type of electrodes implanted (subdural = subdural grids and strips; depth = depth electrodes; both = both subdural and depth electrodes). “Hospital” indicates where data collection took place: C = Children's Hospital; F = Neurozentrum, Universitat Freiburg; P = Hospital of the University of Pennsylvania; U = UCLA Medical Center; and B = Brigham and Women's Hospital.
Each patient was implanted for 1–3 weeks with multiple subdural and/or depth electrodes to map functional regions and to localize their seizure focus for potential subsequent surgical resection. Each participant contributed data between 27 and 118 electrodes. After excluding electrodes that were placed in white matter, we were able to analyze data from 1,447 electrodes. The iEEG signal from subdural and depth electrodes was recorded by means of a Telefactor, Bio-Logic, XLTek, Neurofile, or Nicolet EEG amplifier system, depending on the testing site. Depending on the amplifier, the signals were sampled at 200, 256, 400, or 512 Hz and band-pass filtered between 0.3 and 70 Hz orbetween 0.1 and 100 Hz. Data were subsequently notch filtered at 50 (Europe) or 60 Hz (United States) to eliminate electrical noise.
To synchronize the electrophysiological recordings with behavioral events, the experimental computer sent optically isolated synchronization pulses into an unused recording channel. The time stamps associated with these pulses aligned the experimental computer's clock with the iEEG clock to a precision (<4 msec) under the sampling interval of the iEEG recording. For all participants, the locations of the electrodes were determined by means of coregistered postoperative computed tomography scans and preoperative MRIs or from postoperative MRIs. Hippocampal recordings were obtained from contacts that terminated in the hippocampus proper, as directly verified by clinical teams.
Our research protocol was approved by the appropriate institutional review boards at each of five hospital sites (Children's Hospital, Boston, MA; Neurozentrum, Universitat Freiburg, Freiburg, Germany; Hospital of the University of Pennsylvania, Philadelphia, PA; Brigham and Women's Hospital, Boston, MA; and UCLA Medical Center, Los Angeles, CA). Informed consent was obtained from the patients or their guardians (in case of children).
Behavioral Methods
The behavioral methods were similar to those described in Ekstrom et al. (2005) and Caplan et al. (2003). Briefly, participants played a virtual spatial-navigation game called Yellow Cab on a laptop computer at their bedside while undergoing long-term monitoring in the hospital. In the game, patients play the role of a taxi driver, picking up and delivering passengers to various destination stores, as they navigated virtual environments using a handheld joystick or by pressing arrow keys on the computer's keyboard. Virtual cities in the game consisted of six unlabeled, nontarget buildings and three labeled, target stores (Figure 1). Each city was laid out on a 3 × 3 grid, surrounded by an outer wall. All stores and buildings were at fixed locations and were separated from each other and the outer wall by a traversable road. Stores, buildings, and passengers appeared the same from all viewing angles; stores are labeled with bright signs bearing their name.
Figure 1.
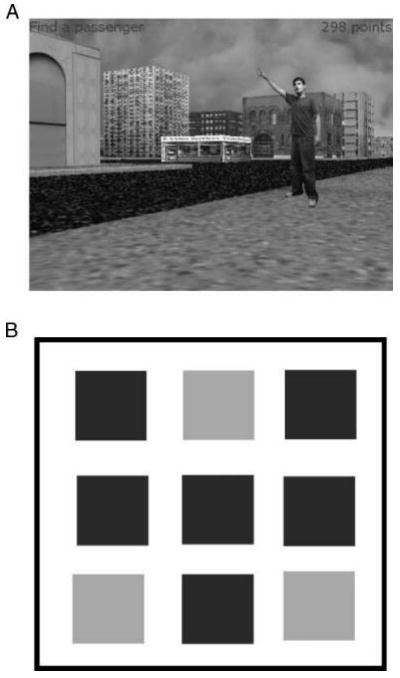
The Yellow Cab task. Participants navigated a virtual city arranged in a 3 × 3 grid, picking up randomly positioned passengers and delivering them to their specified destination stores. (A) Screen shot of the task from a first-person perspective, as seen by participants during navigation. (B) Overhead view of a sample city layout with six buildings (dark gray) and three stores (light gray).
Each trial of the game began with a “searching” phase in which the patient searched the virtual environment for a randomly located passenger and picked them up by driving the taxi near them. After pickup, on-screen text informed the patient where to deliver the passenger. Next, during the “goal-seeking” phase, the patient tried to accurately navigate the taxi to the passenger's desired destination store. (On-screen text reminded the patient of the desired destination throughout the delivery.) Upon arrival at a store (accomplished by driving into it), on-screen text informed the patient if they reached the correct destination store. Upon a successful passenger delivery, this process repeats and the patient began searching the virtual city for a new passenger. Throughout the task, a point total was displayed in the corner of the screen to encourage efficient navigation. Participants gained points for each successful delivery and lost points for each second spent driving. Patients repeat this process of picking up passengers and delivering them to their requested destinations for the duration of the ≈30-min task.
Data Analysis
We quantified oscillatory activity using the Pepisode oscillatory episode detection method (van Vugt, Sederberg, & Kahana, 2007; Caplan et al., 2001, 2003). This approach identifies epochs of iEEG signal that exhibit high oscillatory power at a particular frequency lasting several cycles. The method excludes much of the EEG's background nonoscillatory activity, by estimating the EEG's intrinsic 1/f power spectrum, and helps to exclude spikes, sharp waves, and nonrhythmic changes in power. Pepisode(f) indicates the percentage of an epoch occupied by oscillations at frequency f that exceeded a three-cycle duration threshold and the 1/f background spectrum. We computed this quantity separately for every epoch and electrode, at 24 logarithmically spaced frequencies between 1 and 54 Hz.
We labeled each moment of the recording session according to the participant's current behavior in the task. Specifically, we defined periods of virtual movement as epochs when a participant pressed a keyboard button or pushed the joystick to drive the virtual taxi forward or to turn left or right. These actions caused optic flow as the patient traversed the virtual environment. Conversely, standing-still epochs were defined as periods when the virtual taxi cab was not moving or turning (i.e., no optic flow) but did not include times when the participant was reading text instructions. Searching (for passengers) epochs were defined as periods used to locate passengers to pickup. Goal-seeking (for stores) epochs were defined as periods spent driving a passenger to their desired destination store.
For each delivery trial, we calculated four separate mean Pepisode values corresponding to that trial's epochs of moving, standing still, searching, or goal seeking. We computed each mean Pepisode value by separately averaging across the epochs of each type within that trial, including only epochs of duration 0.5–30 sec. (When computing Pepisode for searching and goal seeking, we excluded periods when the patient was standing still.) To determine whether oscillatory power at an electrode significantly changed as a result of virtual movement, we performed a Wilcoxon rank-sum test to compare the distributions of mean Pepisode values corresponding to moving and standing still. To determine whether oscillatory power was significantly modulated by whether the patient was searching or goal seeking, a similar comparison was performed between the distributions of mean Pepisode values corresponding to searching and goal seeking. For all comparisons, we pooled recordings from multiple testing sessions at a given electrode (for a single patient), as in previous work (Ekstrom et al., 2005; Caplan et al., 2003). We carried out all statistical comparisons at every electrode and frequency band.
We performed these analyses at five frequency bands: delta (1–3.5 Hz), theta (4–8 Hz), alpha (9–14 Hz), beta (16–27 Hz), and gamma (32–54 Hz). We considered an electrode as exhibiting a significant effect in a frequency band if the effect was present in at least one sampled frequency within the band at p < .001. Then, we summarized the results using an ROI approach. We calculated the count of electrodes showing significant effects in each of 10 brain regions (left and right frontal, parietal, temporal, and occipital cortices and left and right hippocampi).
Of the 1,447 recording sites, 318 were identified by neurologists as exhibiting epileptiform activity and were designated as being located within epileptic zones. (counts of epileptic recording sites in right hemisphere: hippocampal, 13; temporal, 74; parietal, 37; frontal, 47; occipital, 10. Left hemisphere: hippocampal, 25; temporal, 89; parietal, 4; frontal, 18; occipital, 1). Because we found that electrodes from nonepileptic and epileptic regions show qualitatively similar changes in navigation-related oscillatory power when analyzed separately (see Results), we analyzed data from both sets of electrodes.
Our primary concern was to examine how the prevalence of movement and search-related oscillatory activity varies throughout the brain. A traditional way of performing this type of data analysis is the χ2 test, which is often used to assess differences in the prevalence of a phenomenon among responses (electrodes) from one of several categories. However, χ2 analysis can be problematic when each electrode may be grouped according to more than one categorization scheme and especially when it is of interest to examine interactions between multiple categories. These issues are critical in our study, where each electrode's activity is labeled according to several orthogonal categorization schemes: hemisphere, brain region, frequency range, and whether the electrode is located in an epileptic zone. In addition, here we are also interested in analyzing interactions among these categories.
To overcome these obstacles and to examine this data set more systematically, we used log-linear analysis (LLA). LLA is a form of the generalized linear model specialized for Poisson-distributed count data (Howell, 1997). We used LLA to examine how the proportions of electrodes exhibiting movement- and search-related oscillatory patterns vary according to four categorical factors: Hemisphere (two levels), Brain Region (five levels), Frequency Band (five levels), and Epileptic Zone (two levels), and their two-way interactions. In our study, the critical feature of LLA is that it simultaneously examines the relative importance of all factors, including interactions, in a single statistical framework, similar to multiple regression analysis.
The LLA model was computed using Akaike information criterion (R function stepAIC; Venables & Ripley, 2002), so that factors that did not significantly improve the model fit were discarded. When a well-fitting LLA model is found, it rejects the omnibus hypothesis that there is no relation between the categorical factors and the prevalence of oscillatory activity. (This procedure obviates the need for a multiple-comparison correction.) For each factor in the LLA model, we determined unstandardized and standardized (z score) parameter estimates; these indicate the relative importance of particular effects in the LLA model. We computed the odds ratio (OR) as a measure of effect size. The OR indicates the factor's effect on the probability that an electrode exhibits navigation-related oscillatory activity. For example, OR = 2 indicates that the presence of a particular factor doubles the probability of observing a navigation-related oscillatory pattern and OR = 0.5 indicates a halving of that probability. We used contrasts in computing the effects of each factor in the model as follows: The effects of factors Region and Frequency at each level were computed relative to a baseline consisting of the average across all other levels. For factor Hemisphere, the effect in the left hemisphere was compared with that in the right hemisphere. For factor Epileptic, the effects at electrodes located in neurologist-designated epileptic zones was compared with the effects at electrodes in nonepileptic zones. Because the LLA models proportions of electrodes exhibiting navigation-related oscillations, it is unaffected by unequal electrode sampling throughout the brain. When describing fitted LLA models in Tables 3 and 5, we list all effects with p < .1 and also note effects that are more robust statistically.
Table 3.
LLA of the Effects of Hemisphere, Region, Frequency, and Epileptic Zone on the Prevalence of Movement-related Oscillations
| Factor | Level | Unstd. Par. | z | OR |
|---|---|---|---|---|
| Main Effects | ||||
| Region | Frontal | 0.69 | 6.76 | 2.0** |
| Parietal | 0.64 | 3.73 | 1.9** | |
| Occipital | −0.95 | −3.91 | 0.4** | |
| Hippo. | −0.29 | −1.77 | 0.8 | |
| Frequency | Theta | 0.16 | 5.31 | 1.2** |
| Beta | 0.17 | 5.67 | 1.2** | |
| Delta | −0.19 | −5.61 | 0.8** | |
| Gamma | −0.18 | −5.17 | 0.8** | |
| Epileptic | Epileptic | 0.15 | 1.84 | 1.2 |
| Interactions | ||||
| Hemisphere × Frequency | Right gamma | 0.12 | 3.47 | 1.1** |
| Left delta | 0.16 | 4.67 | 1.2** | |
| Hemisphere × Region | Right temporal | 0.31 | 3.50 | 1.4** |
| Right occipital | 0.53 | 2.21 | 1.7* | |
| Left frontal | 0.22 | 2.16 | 1.3* | |
| Left parietal | 0.47 | 2.77 | 1.6* | |
| Region × Epileptic | Frontal epileptic | 0.26 | 2.52 | 1.3* |
| Hippo. nonepileptic | 0.53 | 3.28 | 1.7* | |
| Parietal epileptic | 0.55 | 3.23 | 1.8* | |
| Temporal nonepileptic | 0.15 | 1.78 | 1.2 |
Positive parameter estimates (OR > 1) indicate that the factor/level increases the probability of movement-related oscillations; negative parameter estimates (OR < 1) indicate that the factor/level decreases the probability of movement-related oscillations. This table lists all effects with p < .1. Unstd. Par. = unstandardized parameter estimate; z = standardized parameter estimate; hippo. = hippocampus.
p < .05.
p < .001.
Table 5.
LLA of the Effects of Hemisphere, Region, Frequency, and Epileptic Zone on the Prevalence of Search-related Oscillations
| Factor | Level | Unstd. Par. | z | OR |
|---|---|---|---|---|
| Main Effects | ||||
| Region | Frontal | 0.35 | 2.77 | 1.4* |
| Temporal | 0.27 | 2.21 | 1.3* | |
| Hippo. | −0.75 | −2.35 | 0.5* | |
| Hemisphere | Right | 0.26 | 2.26 | 1.3* |
| Frequency | Delta | 0.51 | 4.80 | 1.7** |
| Theta | 0.66 | 6.30 | 1.9** | |
| Beta | −0.28 | −2.09 | 0.8* | |
| Gamma | −1.0 | −4.65 | 0.4** | |
| Epileptic | Epileptic | 0.25 | 3.78 | 1.3** |
| Interactions | ||||
| Hemisphere × Frequency | Right gamma | 0.49 | 2.27 | 1.6* |
| Left delta | 0.20 | 1.88 | 1.2 | |
| Hemisphere × Region | Right hippo. | 0.63 | 1.97 | 1.9* |
| Left parietal | 0.44 | 2.34 | 1.6* | |
| Hemisphere × Epileptic | Left epileptic | 0.21 | 3.19 | 1.2* |
Positive parameter estimates (OR > 1) indicate that the factor/level increases the probability of search-related oscillations; negative parameter estimates (OR < 1) indicate that the factor/level decreases the probability of search-related oscillations. Table displays all effects with p < .1. Unstd. Par. = unstandardized parameter estimate; z = standardized parameter estimate; hippo. = hippocampus.
p < .05.
p < .001.
Results
Previous work had shown that oscillatory activity increased during periods of virtual movement and that oscillatory activity also increased during periods of passenger searching as compared with store seeking (Ekstrom et al., 2005; Caplan et al., 2003). To determine whether these movement- and search-related oscillatory effects localized to a specific brain hemisphere or even to certain brain regions, we aggregated recordings across a total of 1,447 electrodes from 12 patients reported by Caplan et al. (2003), 5 patients reported by Ekstrom et al. (2005), and 7 additional patients collected for the present report. Because each patient's electrode coverage was dictated by clinical considerations, a relatively large number of participants is needed to provide sufficient electrode coverage to make generalizations about the regional specificities of these effects (see Sederberg et al., 2007). Critically, because this manuscript analyzes a larger data set than existing studies of navigation-related brain oscillations—the largest of its kind—it allows us to examine new patterns that were not observable previously. Specifically, we now analyzed how the prevalence of navigation-related oscillations varied across brain hemisphere, neocortex lobe, frequency band, the presence of epileptiform activity, and their interactions.
Behaviorally, patients reported few problems performing the task. On average, in each trial, patients spent 73 sec searching for a randomly placed passenger and 36 sec driving each passenger to their desired destination. Because the time spent driving passengers to previously seen destinations was significantly shorter than the random search time (p < .005, t test), it demonstrates that patients in this task were able to learn environments' spatial layouts successfully.
Comparison of Oscillatory Activity between Movement and Standing Still
We observed a greater prevalence of oscillatory activity during virtual movement as compared with standing still at electrodes in various neocortical brain regions as well as in both hippocampi. Table 2 illustrates this pattern by showing the counts of electrodes exhibiting changes in movement-related oscillatory power in 10 major brain regions (left and right frontal; temporal, parietal, and occipital cortices; and left and right hippocampi) and 5 major frequency bands (delta, theta, alpha, beta, and gamma).
Table 2.
Brain Regions Electrodes Exhibiting Significant “Movement versus Standing-still” Oscillatory Effects
| Delta | Theta | Alpha | Beta | Gamma | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M > S | S > M | M > S | S > M | M > S | S > M | M > S | S > M | M > S | S > M | All Cells | |
| Electrode Counts | |||||||||||
| Right | |||||||||||
| Hippocampus | 28** | 0 | 34** | 0 | 27** | 0 | 35** | 0 | 19** | 0 | 57 |
| Temporal | 129** | 0 | 198** | 1 | 168** | 3 | 206** | 1 | 141** | 0 | 296 |
| Parietal | 30** | 0 | 85** | 0 | 92** | 0 | 112** | 1 | 92** | 0 | 152 |
| Frontal | 124** | 0 | 181** | 0 | 185** | 0 | 189** | 1 | 159** | 0 | 270 |
| Occipital | 14** | 1 | 28** | 0 | 28** | 1 | 39** | 6 | 27** | 1 | 61 |
| All | 325** | 1 | 526** | 1 | 500** | 4 | 581** | 9 | 438** | 1 | 836 |
| Left | |||||||||||
| Hippocampus | 11** | 0 | 13** | 0 | 14** | 0 | 11** | 0 | 6** | 0 | 27 |
| Temporal | 128** | 0 | 179** | 0 | 136** | 1 | 152** | 1 | 91** | 0 | 294 |
| Parietal | 28** | 0 | 29** | 0 | 12** | 0 | 30** | 0 | 11** | 0 | 36 |
| Frontal | 139** | 0 | 166** | 1 | 156** | 0 | 163** | 0 | 124** | 0 | 229 |
| Occipital | 4 | 0 | 5 | 0 | 6* | 0 | 7* | 0 | 4 | 0 | 25 |
| All | 310** | 0 | 392** | 1 | 324** | 1 | 363** | 1 | 236** | 0 | 611 |
| Movement-Effect Laterality | |||||||||||
| Hippocampus | – | – | – | – | – | ||||||
| Temporal | – | – | – | Right* | Right* | ||||||
| Parietal | Left** | – | Right** | – | Right* | ||||||
| Frontal | Left* | – | – | – | – | ||||||
| Occipital | – | – | – | Right* | Right* | ||||||
| All | Left** | – | – | Right* | Right** | ||||||
Counts of electrodes are shown separately for each frequency band and brain region. The “move > still” effect is denoted by “M > S” and the “still > move” effect is denoted by “S > M.” Rows labeled “All” combine data across all regions. In the Electrode counts section, for each region-frequency group, the number of electrodes exhibiting the M > S effect is compared with the number of electrodes exhibiting the S > M oscillatory effect with a χ2 test. The “All Cells” column indicates the total number of electrodes in each region. In the Movement-effect laterality section, for each region-frequency group, the counts of electrodes exhibiting the M > S effect are compared between the left and the right hemispheres (χ2 test), and the hemisphere where the effect is significantly more prevalent is indicated.
p < .05.
p < .001.
In individual brain regions, we observed that as many as 83% of electrodes exhibited significant increases (rank-sum tests, p < .001) in the prevalence of oscillations during movement (“movement > still”). Overall, this effect was remarkably unidirectional, with less than 1% of all electrodes exhibiting a significantly greater prevalence of oscillations during stillness (“still > movement”). This pattern appeared across all cortical and hippocampal regions at all observed frequency bands. In all but three (of 50) region-frequency comparisons, the number of electrodes exhibiting the “movement > still” effect exceeded the number of electrodes displaying the “still > movement” effect (based on χ2 tests at p < .05).
Next, we used LLA to systematically examine how the proportions of electrodes exhibiting movement-related (“move > still”) oscillations varied according to the factors Hemisphere, Brain Region, Frequency Band, and Epileptic Zone and their two-way interactions. LLA is analogous to multiple regression or ANOVA, except it is appropriate for Poisson-distributed count data like our data set. Table 2 illustrates the raw electrode counts that were input to the LLA, and Table 3 details the significant patterns the LLA analysis identified. We describe these patterns below, beginning with the main effects. Overall, movement-related oscillations were observed throughout the brain, but the magnitude of this effect significantly varied across brain regions. Specifically, movement-related oscillations at all frequencies were prevalent in frontal and parietal regions (factor Region; p's < .001) and were less frequently observed in the occipital cortex (p < 10−4) and in the hippocampus (p < .08). Across all regions, movement-related oscillatory patterns were prevalent at theta and beta frequencies and were less frequently observed in the delta and gamma bands (factor Frequency; p's < 10−6).
We next used the LLA model to test our primary hypothesis that the oscillatory correlates of human navigational activity were lateralized to the right hemisphere. Consistent with this hypothesis, we observed several hemisphere-specific oscillatory patterns in the LLA model. In the gamma band, movement-related oscillations were prominent in the right hemisphere, and in the delta band, movement-related oscillations were prevalent in the left hemisphere (factor Hemisphere × Frequency; both p's < .001). These two hemisphere-wide patterns were statistically robust, which we confirmed with post hoc tests, right hemisphere gamma, χ2(1) = 27, p < .001, left hemisphere delta, χ2(1) = 20, p < .001 (see Table 2). Separate post hoc χ2 tests also specifically confirmed that movement-related gamma oscillations in temporal, parietal, and occipital cortices all exhibited significant right lateralization (all p's < .05). Within the right hemisphere, movement-related oscillations at various frequencies were prominent in temporal and occipital cortices, and within the left hemisphere, they were prevalent in the frontal and parietal cortex (factor Hemisphere× Region, all p's < .03). In the hippocampus, we did not observe significantly lateralized movement-related oscillations at any frequency (post hoc χ2 tests, p > .05).
Comparison of Oscillatory Activity between Searching and Goal Seeking
During the experiment, participants alternated between two primary modes of navigation: searching for passengers (“searching”) and seeking for stores (“goal seek”). In an analysis of intracranial EEG data from 12 patients, Caplan et al. (2003) reported that many electrodes had a greater prevalence of oscillatory activity during searching than during goal seeking. Caplan et al. (2003) noted that participants quickly learned the simple town layouts used in Yellow Cab, including the static store identities and locations, but that the passenger locations are unlearnable because they change on each trial. As such, they argued that searching for passengers requires greater attentional resources, including sustained visual attention, than goal seeking. These attention increases during searching could have accounted for the observed search-related increase in oscillatory activity, especially in the theta and gamma frequency bands (Kahana, 2006).
As expected, at many electrodes, we observed increased oscillatory power during searching for passengers as compared with goal seeking for stores. Table 4 shows the counts of electrodes that exhibited significant searching versus goal-seeking effects (rank-sum test, p < .001). In some region-frequency groups, as many as 16% of electrodes exhibited increases in oscillatory power during searching (“search > goal seek”). In sixteen region-frequency groups, the number of electrodes exhibiting this “search > goal-seek” effect significantly exceeded the number of electrodes exhibiting the opposite “goal-seek > search” effect (χ2 tests at p < .05). Overall, the search-related effect was less widespread than the movement-related effect and appeared almost entirely in frontal and temporal neocortices. However, the prevalence of search-related oscillations we observed in the current study is consistent with previous accounts. Overall, we observed that 10% of neocortical electrodes exhibited increases in search-related theta oscillations, which is comparable to the 9% figure reported by Caplan et al. (2003).
Table 4.
Brain Regions Exhibiting Significant “Searching versus Goal-seeking” Oscillatory Effects
| Delta | Theta | Alpha | Beta | Gamma | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S > G | G > S | S > G | G > S | S > G | G > S | S > G | G > S | S > G | G > S | All Cells | |
| Electrode Counts | |||||||||||
| Right | |||||||||||
| Hippocampus | 4 | 0 | 4 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 57 |
| Temporal | 32** | 0 | 46** | 0 | 18** | 0 | 17** | 0 | 14** | 0 | 296 |
| Parietal | 5 | 0 | 8 | 4 | 4 | 4 | 3 | 4 | 8* | 0 | 152 |
| Frontal | 27** | 0 | 52** | 2 | 18* | 3 | 7 | 7 | 7 | 1 | 270 |
| Occipital | 1 | 0 | 5 | 0 | 4 | 2 | 2 | 3 | 1 | 0 | 61 |
| All | 69** | 0 | 105** | 6 | 47** | 9 | 32* | 15 | 30** | 1 | 836 |
| Left | |||||||||||
| Hippocampus | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 27 |
| Temporal | 19** | 0 | 13* | 3 | 10* | 1 | 10* | 2 | 3 | 2 | 294 |
| Parietal | 4 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 36 |
| Frontal | 12** | 0 | 15** | 0 | 13** | 0 | 4 | 1 | 1 | 1 | 229 |
| Occipital | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 25 |
| All | 37** | 0 | 33** | 3 | 25* | 1 | 16* | 4 | 4 | 3 | 611 |
| Searching-Effect Laterality | |||||||||||
| Hippocampus | – | – | – | – | – | ||||||
| Temporal | – | Right** | – | – | Right* | ||||||
| Parietal | – | – | – | – | – | ||||||
| Frontal | – | Right* | – | – | – | ||||||
| Occipital | – | – | – | – | – | ||||||
| All | – | Right** | – | – | Right** | ||||||
Counts of electrodes exhibiting each effect are shown separately for each frequency band and ROI. The “searching > goal-seeking” effect is denoted by “S > G” and the “goal-seeking > searching” effect is denoted by “G > S”. In the Electrode counts section of the table, at each region/frequency band, the number of electrodes exhibiting the S > G effect was compared with the number of electrodes exhibiting the G > S effect using a χ2 test. The “All Cells” column of the table indicates the total number of electrodes observed in each region. In the Searching-effect laterality section of the table, for region-frequency group, the counts of electrodes exhibiting the (S > G) effect were compared between the left and the right hemispheres (χ2 test). When this test indicated a significant difference, the appropriate table entry indicates the hemisphere where the effect was more prevalent.
p < .05.
p < .001.
To further examine how search-related oscillatory activity varies with factors Hemisphere, Brain Region, Frequency Band, and Epileptic Zone and their two-way interactions, we again used LLA. The raw data for this analysis are described in Table 4, and the fit model is detailed in Table 5. Across all frequencies, search-related oscillations were relatively prevalent in frontal and temporal regions and were less frequently observed in hippocampus (factor Region, all p's < .03). Furthermore, search-related activity was often observed at low-frequency bands (factor Frequency: p's < 10−5) and infrequently observed at high frequencies (p's < .05). In terms of our primary hypothesis regarding the right lateralization of navigation-related oscillatory activity, the LLA model found that search-related oscillatory patterns at all frequencies were more prevalent in the right hemisphere than in the left (factor Hemisphere, p < .03).
In addition to the main effects described above, the LLA also identified additional lateralized search-related oscillatory patterns. Specifically, in the gamma band, search-related oscillations were especially prominent in the right hemisphere, as opposed to the left (factor Hemisphere × Frequency, p < .03). A post hoc test confirmed that search-related gamma-oscillations were significantly right lateralized, χ2(1) = 13, p < .001. In the hippocampus, the LLA model suggested right lateralization, but this pattern was not statistically reliable according to post hoc χ2 tests (p > .05). In parietal cortex, the LLA found that search-related oscillatory patterns were prevalent in the left hemisphere (factor Hemisphere × Region, p < .05).
Effects of Epileptic Zones
Many previous studies examining the electrophysiology of cognition in epilepsy patients immediately discard recordings from electrodes exhibiting epileptiform activity (e.g., Lachaux et al., 2005; Caplan et al., 2003). Here we directly examined whether the movement- and search-related oscillatory phenomena related to each electrode's classification as recording from an epileptic or a non-epileptic zone. When considered separately, electrodes from epileptic and nonepileptic zones showed similar frequency and regional patterns of oscillatory effects. In fact, our LLA revealed that electrodes in epileptic zones actually exhibited slightly increased levels of both movement-related (factor Epileptic, p = .065; Table 3) and search-related (p < .001; Table 5) oscillations. To specifically examine whether epileptic zones played a role in the apparent right lateralization of movement-and search-related gamma oscillations, we performed separate post hoc χ2 tests including only electrodes from nonepileptic zones. These tests confirmed (both p's < .001) that the right-lateralized movement- and search-related gamma activity was not related to hemisphere differences in epileptic activity. Overall, this increased prevalence of navigational oscillations in epileptic tissue was unexpected, given the conventional view that epileptic brain regions have diminished involvement in typical behavior (Elger, Helmstaedter, & Kurthen, 2004). In fact, epileptic regions often exhibit high-frequency oscillations that are not related to behavior (Jirsch et al., 2006), and we had expected that these epileptiform oscillations might obscure any navigation-related oscillatory patterns. Clearly, more research is needed to fully explain the potential interaction between epilepsy- and cognition-related neuronal activity.
Age-related Effects
Previous work found that there is less right lateralization of navigation-related neuronal activity in adolescents than in adults (Pine et al., 2002). Our complete data set contained recordings from 7 adolescents (ages 11–16 years) and 17 adults (ages 18–48 years). Thus, we asked whether the patterns of right-lateralized navigation-related gamma oscillations that we observed were present both in adolescents and in adults. Overall, both groups performed the task at comparable levels, as we did not observe significant age-related differences between the durations of trials' searching and goal-seeking phases (t tests, p's > .4). Examining movement-related gamma oscillations, we found that this phenomenon was significantly right lateralized both in adolescents, χ2(1) = 58, p < 10−6, and in adults, χ2(1) = 4, p < .05. Furthermore, search-related gamma oscillations were also significantly right lateralized both in adolescents, χ2(1) = 11.2, p < .001, and in adults, χ2(1) = 3.6, p = .05. Thus, our finding of right-lateralized navigation-related gamma oscillations appeared in both adolescents and adults.
Discussion
Analyzing intracranial recordings from 1,447 electrodes, we examined movement- and search-related brain oscillations recorded while subjects performed our virtual navigation task. By aggregating data across 24 neurosurgical patients, each of whom had electrodes recording particular subsets of their brain, we examined whether navigation-related oscillatory activity was lateralized to either the left or the right hemisphere.
In accord with previous work (Ekstrom et al., 2005; Caplan et al., 2003; de Araujo et al., 2002), we observed significant movement- and search-related oscillations—spanning delta, theta, alpha, beta, and gamma bands—in various brain regions across both hemispheres. However, both effects were generally more prevalent in the right hemisphere, especially in the gamma band. In particular, right-lateralized movement-related gamma oscillations were present in temporal, parietal, and occipital cortices. This is important because these posterior brain regions are particularly implicated in neural theories of spatial cognition (Byrne, Becker, & Burgess, 2007; Frings, Wagner, Quiske, et al., 2006). Because this right lateralization appeared across multiple neocortex lobes, we do not believe that the findings were directly driven by motor activity, such as joystick movements or keypresses. (Previous work showed that motor-related activity observed in human intracranial recordings is exclusively limited to motor cortex; see Miller et al., 2007.) Surprisingly, this right lateralization was evident in neocortex but not in hippocampus (cf. O'Keefe & Nadel, 1978). Our finding of right-lateralized gamma oscillations during a virtual spatial-navigation task complements previous demonstrations of right hemisphere dominance in a variety of neuroimaging and lesion studies employing spatial-navigation and spatial-memory paradigms (Leung et al., 2007; Diaz-Asper et al., 2006; Gramann et al., 2006; Neggers et al., 2006, van Asselen et al., 2006; Weniger & Irle, 2006; Parslow et al., 2005; Wolbers & Buchel, 2005; Bohbot et al., 2004; Voermans et al., 2004; Kessels et al., 2002; Spiers, Burgess, et al., 2001; Worsley et al., 2001; Nunn et al., 1999; Abrahams et al., 1997; Smith & Milner, 1989).
We showed that both movement- and search-related gamma oscillations were significantly right lateralized. This suggests that the right hemisphere neocortical gamma oscillations observed in our spatial-navigation task play a role in multiple navigational behaviors. This is similar to the previously reported association of right-lateralized fMRI activations with a wide variety of spatial tasks. Together, these two findings are compatible with recent reports that the fMRI BOLD signal correlates with gamma-band EEG oscillations in neocortex recordings from humans and nonhuman primates (Lachaux et al., 2007; Mukamel et al., 2005; Logothetis et al., 2001). In contrast to these right-lateralized gamma oscillations, we found that delta movement-related oscillations were lateralized to the left hemisphere. Although we had not specifically anticipated that delta and gamma oscillations would have different spatial characteristics, other recent studies also reported task-related oscillatory brain patterns at different frequency bands with distinct spatial localizations (e.g., Jacobs, Hwang, Curran, & Kahana, 2006).
In humans and other animals, gamma oscillations are a general marker of local neuronal activation and often indicate that information is being exchanged between brain regions (Fries, Nikolić, & Singer, 2007; Jensen, Kaiser, & Lachaux, 2007). Thus, the localization of navigation-related gamma oscillations to the right hemisphere suggests that neurons in this area are especially active and involved in large-scale neuronal processing during our task. Theories suggest that the hippocampal representation of the present location is continuously updated during movement based on perceptual and vestibular information from occipital and parietal cortices (Byrne et al., 2007; Andersen, Shenoy, Snyder, Bradley, & Crowell, 1999). Our finding that movement-related gamma oscillations lateralized to the right neocortex suggests that the right hemisphere plays a unique role in this spatial updating.
We see two possible physiological interpretations for the right lateralization of search-related gamma oscillations. One possibility is that some of the right hemisphere sites that responded to movement exhibited even greater oscillatory activity during searching. This might occur if searching for unknown objects required increased attention to basic navigational processes because there is a well-established relation between gamma oscillations and attention (Jensen et al., 2007; Bauer, Oostenveld, Peeters, & Fries, 2006; Tallon-Baudry, Bertrand, Henaff, Isnard, & Fischer, 2005). A second possibility is that searching behaviors, in which patients scan for new percepts at random locations, involve a unique set of right hemisphere neuronal assemblies. (Note that these two possibilities are not mutually exclusive.)
Although our analyses revealed greater search- and movement-related oscillations in right neocortex, we failed to observe significant oscillatory differences between the left and the right hippocampi. This finding is not unprecedented because, in addition to the extensive literature on the role of the right hemisphere in navigation, there is evidence that left brain structures also play a role in spatial tasks, albeit in a different capacity. For instance, the left hippocampus has been implicated in context-dependent episodic memory (Burgess et al., 2002; Burgess, Maguire, Spiers, & O'Keefe, 2001; Maguire et al., 1998), in learning a new environment's spatial layout (Cornwell et al., 2008), and in the use of verbal strategies in spatial-processing tasks (Frings, Wagner, Unterrainer, et al., 2006). Spiers, Maguire, and Burgess (2001) found that patients with right temporal lobectomies were primarily impaired in topographical memory, whereas patients with left temporal lobectomies primarily showed deficits in episodic memory in a large-scale virtual environment. Bohbot et al. (1998) also reported that patients with right hippocampal lesions were impaired on tasks requiring visuospatial memory, whereas patients with left hippocampal lesions were impaired on verbal and episodic memory tasks. Thus, one possible explanation for the bilateral hippocampal activity we observed was that our paradigm is a complex task that involves remembering not only spatial information, which activates the right hippocampus, but also various nonspatial details (e.g., the current passenger's pickup location and destination and the past sequence of passenger pickups and destinations), which activate the left hippocampus.
Our analyses support the view that the right neocortex is especially involved in human spatial navigation and shows how neuronal oscillations are a useful tool for mapping the functional roles of different brain regions. Furthermore, because the right-lateralized patterns we observed were especially prominent in the gamma band, they support the view that oscillations in certain frequency ranges have distinct functional roles (e.g., Jacobs et al., 2006; Sederberg, Kahana, Howard, Donner, & Madsen, 2003) in addition to broadband EEG phenomena spanning extremely wide frequency ranges (Miller et al., 2007). We also found clear lateralization differences across different lobes of the neocortex. For example, posterior lobes of the neocortex exhibited right-lateralized movement-related oscillations, but this pattern did not appear in frontal cortex—this is consistent with the view that oscillations in frontal cortex are generally less prominent than in other brain regions (Siapas, Lubenov, & Wilson, 2005).
Acknowledgments
This work was supported by the National Institutes of Health research grants MH61975, MH062196, NS50067, F31AG031100, F32NS50067, and NS054575; the NSF grant SBE0354378; and the Deutsche Forschungsgemeinschaft (SFB 780, Synaptic Mechanisms, TPC3).
References
- Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35:11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Andersen R, Shenoy K, Snyder L, Bradley D, Crowell J. The contributions of vestibular signals to the representations of space in the posterior parietal cortex. Annals of the New York Academy of Sciences. 1999;871:282–292. doi: 10.1111/j.1749-6632.1999.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. Journal of Neuroscience. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot V, Iaria G, Petrides M. Hippocampal function and spatial memory: Evidence from functional neuroimaging in healthy participants and performance of patients with medial temporal lobe resections. Neuropsychology. 2004;18:418–425. doi: 10.1037/0894-4105.18.3.418. [DOI] [PubMed] [Google Scholar]
- Bohbot V, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire E, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14:439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychological Review. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. Journal of Neurophysiology. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. Journal of Neuroscience. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B, Johnson L, Holroyd T, Carver F, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual morris water maze. Journal of Neuroscience. 2008;28:5983. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo DB, Baffa O, Wakai RT. Theta oscillations and human navigation:A magnetoencephalography study. Journal of Cognitive Neuroscience. 2002;14:70–78. doi: 10.1162/089892902317205339. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper C, Dopkins S, Potolicchio S, Caputy A. Spatial memory following temporal lobe resection. Journal of Clinical and Experimental Neuropsychology. 2006;28:1462–1481. doi: 10.1080/13803390500434359. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan J, Ho E, Shattuck K, Fried I, Kahana M. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Elger C, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurology. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikoli´ D, Singer W. The gamma cycle. Trends in Neurosciences. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Frings L, Wagner K, Quiske A, Schwarzwald R, Spreer J, Halsband U. Precuneus is involved in allocentric spatial location encoding and recognition. Experimental Brain Research. 2006;173:661–672. doi: 10.1007/s00221-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A. Gender-related differences in lateralization of hippocampal activation and cognitive strategy. NeuroReport. 2006;17:417–421. doi: 10.1097/01.wnr.0000203623.02082.e3. [DOI] [PubMed] [Google Scholar]
- Gramann K, Muller HJ, Schonebeck B, Debus G. The neural basis of ego- and allocentric reference frames in spatial navigation: Evidence from spatio-temporal coupled current density reconstruction. Brain Research. 2006;1118:116–129. doi: 10.1016/j.brainres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Howell D. Statistical methods for psychology. Belmont, CA: Duxbury Press; 1997. [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. Neuroimage. 2006;15:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux J. Human mgamma-frequency oscillations associated with attention and memory. Trends in Neurosciences. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jirsch J, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. The cognitive correlates of human brain oscillations. Journal of Neuroscience. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: A meta-analysis on the effects of hippocampal lesions. Brain Research, Brain Research Reviews. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Kappelle LJ, de Haan EHF, Postma A. Lateralization of spatial-memory processes: Evidence on spatial span, maze learning, and memory for object locations. Neuropsychologia. 2002;40:1465–1473. doi: 10.1016/s0028-3932(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O. Relationship between task-related gamma oscillations and bold signal: New insights from combined fMRI and intracranial EEG. Human Brain Mapping. 2007 doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Leung HC, Oh H, Ferri J, Yi Y. Load response functions in the human spatial working memory circuit during location memory updating. Neuroimage. 2007;35:368–377. doi: 10.1016/j.neuroimage.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maguire E, Burgess N, Donnett JG, Frackowiak SJ, Frith CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire E, Gadian D, Johnsrude I, Good C, Ashburner J, Frackowiak R. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, U S A. 2000;97:4398. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Leuthardt E, Schalk G, Rao R, Anderson N, Moran D. Spectral changes in cortical surface potentials during motor movement. Journal of Neuroscience. 2007;27:2424. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Neggers SFW, van der Lubbe RHJ, Ramsey NF, Postma A. Interactions between ego- and allocentric neuronal representations of space. Neuroimage. 2006;31:320–331. doi: 10.1016/j.neuroimage.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Newman EL, Caplan JB, Kirschen MP, Korolev IO, Sekuler R, Kahana MJ. Learning your way around town: How virtual taxicab drivers learn to use both layout and landmark information. Cognition. 2007;104:231–253. doi: 10.1016/j.cognition.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Nunn JA, Graydon FJ, Polkey CE, Morris RG. Differential spatial memory impairment after right temporal lobectomy demonstrated using temporal titration. Brain. 1999;122:47–59. doi: 10.1093/brain/122.1.47. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Parslow D, Morris R, Fleminger S, Rahman Q, Abrahams S, Recce M. Allocentric spatial memory in humans with hippocampal lesions. Acta Psychologica. 2005;118:123–147. doi: 10.1016/j.actpsy.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Cooper R. Frequency dependence of the transmission of the EEG from cortex to scalp. Electroencephalography and Clinical Neurophysiology. 1975;38:93–96. doi: 10.1016/0013-4694(75)90215-1. [DOI] [PubMed] [Google Scholar]
- Pine D, Grun J, Maguire E, Burgess N, Zarahn E, Koda V. Neurodevelopmental aspects of spatial navigation: A virtual reality fMRI study. Neuroimage. 2002;15:396–406. doi: 10.1006/nimg.2001.0988. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Siapas A, Lubenov E, Wilson M. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: Encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cerebral Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RPC, Neggers SFW, Kappelle LJ, Frijns CJM, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44:1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. Journal of Neuroscience Methods. 2007;162:49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer-Verlag; 2002. [Google Scholar]
- Voermans NC, Petersson KM, Daudey L, Weber B, Spaendonck KPV, Kremer HPH. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Weniger G, Irle E. Posterior parahippocampal gyrus lesions in the human impair egocentric learning in a virtual environment. European Journal of Neuroscience. 2006;24:2406–2414. doi: 10.1111/j.1460-9568.2006.05108.x. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. Journal of Neuroscience. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley CL, Recce M, Spiers HJ, Marley J, Polkey CE, Morris RG. Path integration following temporal lobectomy in humans. Neuropsychologia. 2001;39:452–464. doi: 10.1016/s0028-3932(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]


