Abstract
In the present experiment, the temporal predictability of response time was systematically manipulated to examine its effect on the time course of motor pre-programming and release of the intended movement by an acoustic startle stimulus. Participants performed a ballistic right wrist extension task in four different temporal conditions: 1) a variable foreperiod simple RT task, 2) a fixed foreperiod simple RT task, 3) a low resolution countdown anticipation timing task, and 4) a high resolution anticipation-timing task. For each task, a startling acoustic stimulus (124 dB) was presented at several intervals prior to the “go” signal (“go” −150ms, −500 ms, and −1500 ms). Results from the startle trials showed that the time course of movement pre-programming was affected by the temporal uncertainty of the imperative “go” cue. These findings demonstrate that the resolution of the timing information regarding the response cue has a marked effect on the timing of movement preparation such that under conditions of low temporal resolution, participants plan the movement well in advance in accordance with the anticipated probability of onset of the cue, whereas movement preparation is delayed until less than 500 ms prior to response time when continuous temporal information is provided.
Keywords: Anticipation-timing, motor preparation, programming, startle
Introduction
For some types of movement tasks, there may be a strategic advantage to preparing the response well in advance of when the movement is required. For example, when approaching a traffic signal that you anticipate is going to change to red, getting ready to stop can improve your reaction time. Two factors play a role in the improvements in reaction time in this context: task instruction and the predictability of the timing of the stimulus to initiate the response. In the laboratory, this type of situation can be mimicked using an instructed-delay paradigm in which the timing of task instruction (simple vs. choice reaction time tasks) or temporal predictability of the imperative “go” cue are systematically manipulated. It has been shown in circumstances where the required response is known in advance, such as in a simple reaction time (RT) task, that motor programming can be completed in advance, allowing participants to respond significantly more quickly. This is done by reducing or eliminating the motor programming that must occur after the “go” signal (e.g., Carlsen et al., 2008b; Keele, 1968; Klapp, 2003). Similarly, RTs are affected by the duration and variability of the foreperiod between a precue and response signal (Klemmer, 1956; Niemi and Näätänen, 1981). Based on these findings from psychophysical studies of reaction times, it has been assumed that reduced response times are mediated by earlier preparation of the motor response. Yet, even when the required action is known beforehand, there may be contexts in which it is more efficient not to prepare the action in advance or to at least to defer motor preparation until later depending on the temporal constraints of the task (e.g., a go-no/go task, see Carlsen et al., 2008a; Coxon et al., 2006). Therefore, even for tasks where the action can be programmed in advance, RT alone cannot reveal precisely when the motor preparation is initiated.
A variety of neurophysiological methods have been used to study advance motor planning and preparation during simple RT tasks. For example, electroencephalography (EEG) and magnetoencephalography (MEG) have been used to examine movement-related cortical potentials that precede voluntary movement under self-paced (Kornhuber and Deecke, 1965) and externally cued conditions (Walter et al., 1964). These studies have shown that movement-related activity is characterized by a slow-rising negative potential that can begin more than 1500 ms prior to movement onset under conditions in which the timing of response onset can be predicted (e.g., Cui and MacKinnon, 2009; Cunnington et al., 1995; Jahanshahi et al., 1995; Jankelowitz and Colebatch, 2002; Thickbroom and Mastaglia, 1985). Yet, when the timing of the response cue cannot be predicted in advance (e.g. foreperiod between 3.5 s and 10 s), an early movement-related potential is absent, suggesting that participants revert to a reactive mode of control (Cui and MacKinnon, 2009; Cunnington et al., 1995). These data imply that in instances when the foreperiod is too unpredictable, it may not be beneficial to prepare the motor system well in advance, and that early movement preparation only occurs under conditions in which the timing of response onset can be predicted with high probability. Interestingly, this idea was seemly contradicted by the findings of Thickbroom et al. (2000) who showed that movement-related preparatory activity (evidenced by fMRI measures of changes in blood oxygen-related dependent signal), was increased in premotor regions when the timing of the imperative “go” cue was unpredictable. In this case, the motor task was paced by a cue presented at a mean rate of 2 Hz and variability of ± 0.5 s. Based on this finding, Thickbroom et al. (2000) proposed a model of the time course of movement preparation. This model suggested that for temporally predictable (i.e. fixed interval) cues, movement-related cortical activation starts well before movement onset (> 1 s) and peak activity is timed to coincide with the predicted response time, while for semi-unpredictable cues (e.g. foreperiod = 2 ± 0.5 s), activation starts 1 sec prior to the shortest possible foreperiod (i.e. the time from a warning cue until the “go” cue), and is held constant until the “go” signal is presented.
One major drawback of the techniques typically used to estimate movement preparation (EEG, MEG, fMRI), is that the derived signal does not provide information about when the actual spatial and temporal parameters of the response sequence have been constructed, that is, what is the state of preparation of the motor plan. For example, it has been previously suggested that in an anticipation-timing type task, some of the EEG activity typically thought to be related to motoric activation may be due to non-motoric processes. Specifically, while the lateralized differences in the amplitude of the MRP are typically associated with specific lateralized motor preparation (Leuthold et al., 2004; Praamstra et al., 1996), some of the difference in activity across hemispheres was in fact due to non-motoric processing such as directed attention (Van 't Ent and Apkarian, 1998). Furthermore, while it may be possible to estimate which side has been selected or attended to based on lateralized movement-related potentials, it may not be possible to differentiate between different movement effectors (Van 't Ent and Apkarian, 1999).
It has recently been shown that a startling acoustic stimulus (SAS) can be used to probe the state of preparation of the planned and intended movement. Using simple RT tasks, it has been shown that the replacement of the imperative “go” cue with an acoustic stimulus intense enough (e.g., 124 dB) to result in a classic startle response can act to elicit the release of the planned movement at latencies markedly shorter (< 80 ms) than trials without the startle (Carlsen et al., 2004a; Carlsen et al., 2004b; Valls-Solé et al., 1995; Valls-Solé et al., 1999). A fundamental tenet of this paradigm is that the observed response speeding by startle is observed only for tasks where the response is prepared in advance of the imperative stimulus, such as in a simple RT task (Carlsen et al., 2003; Carlsen et al., 2004a; Carlsen et al., 2004b; Carlsen et al., 2007; Carlsen et al., 2009; Castellote et al., 2007; Cressman et al., 2006; MacKinnon et al., 2007; Maslovat et al., 2008; Maslovat et al., 2009; Valls-Solé et al., 1995; Valls-Solé et al., 1999; Valls-Solé et al., 2005). Subsequent studies showed that, under fixed foreperiod conditions (e.g. 3 s) the planned movement could be released at short latency when the SAS was presented as much as 1400 ms prior to the predicted response time. However, the incidence of movement release was markedly reduced compared to probing simultaneous with the imperative “go” signal, but increased as the timing of the SAS approached the “go” cue (MacKinnon et al., 2007). These findings suggest that the spatial and temporal characteristics of the planned movement are progressively constructed well in advance of the intended movement onset in accordance with the predicted timing of the response cue. However, a very different result was seen when a SAS was presented in a task where the response was to be timed accurately with the arrival of a clock hand at a target. Specifically, a SAS presented at various times in advance of the target in an anticipation-timing task, did not trigger an early response even when applied only 200 ms prior to the target, suggesting that for these types of timing tasks it may not be critically important or strategically beneficial to prepare a response well in advance of the required target (Carlsen et al., 2008b). This finding is in accord with a transcranial magnetic stimulation (TMS) study that showed that increases in corticospinal excitability and accompanying decreases in intracortical inhibition, as evidenced by changes in the motor evoked potential (MEP) amplitude, did not occur until 150 ms prior to the target (Coxon et al., 2006). Thus it may be that preparation of the motor command is delayed until just prior to response initiation when temporal uncertainty is completely removed by providing high resolution feedback of the required response timing.
The goal of the present experiment was to examine how the temporal predictability of the response cue affects the timing of motor preparation. This was done using a loud acoustic stimulus to probe the state of preparation of the planned motor response and then examining the effect of this stimulus on the incidence, timing and characteristics of released movement across four task conditions that differed with respect to the resolution of the response timing information. Specifically, participants performed a right wrist extension movement to a target under four different cueing conditions: 1) a variable foreperiod RT condition, 2) a fixed foreperiod RT condition, 3) a 1 sec. resolution countdown anticipation-timing condition, and 4) a high resolution clock-face anticipation-timing condition. In addition, a SAS was presented at one of three time points prior to the “go” signal in some trials. Electromyographic (EMG) and kinematic data were collected (see the Experimental Procedures section below for more details regarding cues and dependent measures).
It was hypothesized that when temporal resolution was very high (anticipation-timing conditions), programming of the motor output and its release by startle would not occur until less than 200 ms prior to the intended response time. However, when the temporal resolution was low (e.g. 3 s fixed interval), it was hypothesized that the planned movement would be prepared much earlier with respect to the “go” signal and thus would be susceptible to release by startle as much as 1500 ms prior to the intended response time.
Results
Task performance
Kinematic variables were analyzed using a 4 (cuing condition) × 4 (stimulus time) repeated measures (RM) ANOVA to determine if either the cuing condition or time of SAS presentation (if any) had any effect on the participants ability to perform the task. No main effects or interactions between analyzed variables were observed for Peak velocity, Time to peak velocity, Peak displacement, Time to peak displacement, Movement final position, or Movement time. This indicates that the kinematics of the movement were similar irrespective of cuing condition or whether or not the movement was elicited by startle.
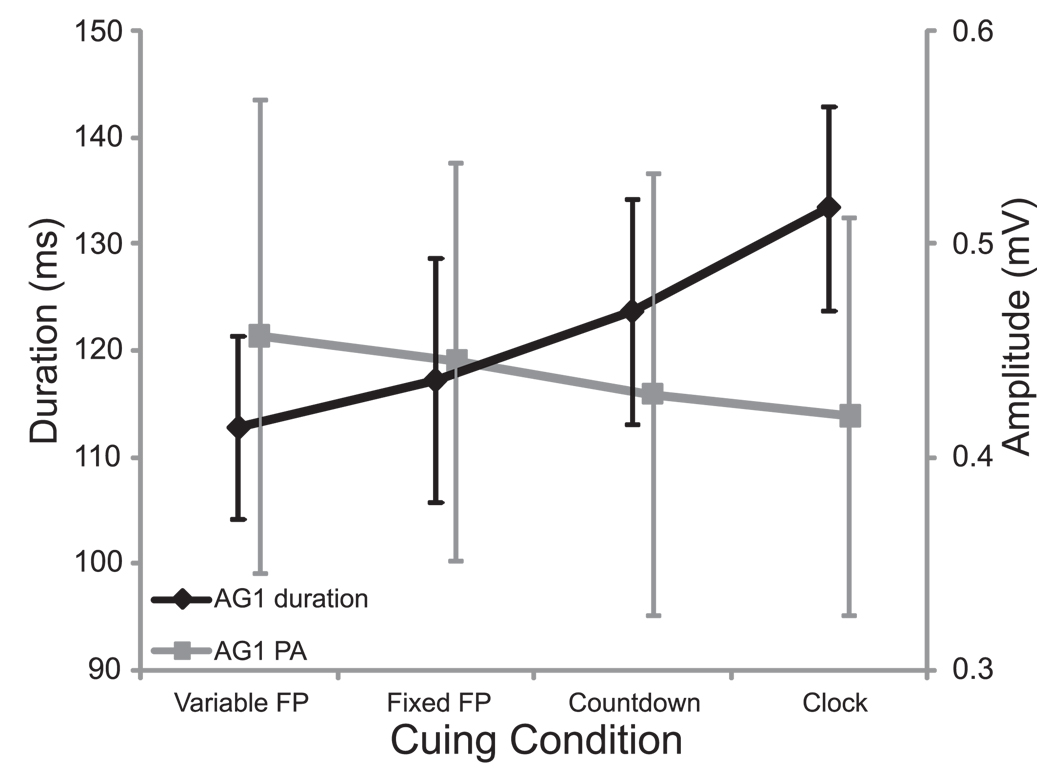
To examine if a similar movement was generated between conditions, the triphasic EMG timing characteristics associated with the movement were analyzed for differences using a 4 × 4 RM ANOVA. A main effect of cuing condition was found for duration of ECR burst 1 (i.e. initial agonist burst), F(3,21) = 6.837, p = .002, partial eta squared (ηp2) = .494. Post-hoc analysis showed that the initial agonist (ECR) burst duration was significantly (p < .05) longer (133.3 ms) in the clock condition than in the RT conditions (variable FP = 112.7 ms, fixed FP = 117.1 ms). Furthermore, a significant linear trend (p = .003) was observed for cuing condition in duration of ECR burst 1 (see Fig. 1, black). Notably, no main effect of stimulus time was seen for ECR burst 1, F(3,21) = .995, p = .415, suggesting that burst duration was unaffected by the presence of a SAS. Additionally, no main effects or interactions were observed in time from ECR burst 1 onset to FCR onset, or from ECR burst 1 to ECR burst 2 onset (p > .155). In order to determine how a difference in EMG burst duration was seen without a corresponding difference in kinematic profile, ECR burst 1 peak amplitude was analyzed. However, while it appeared that amplitude decreased as burst duration increased (see Fig. 1, grey), there were no differences in ECR burst 1 peak amplitude between cuing conditions (p = .270).
Figure 1.
Mean (+/− 1 SE) initial agonist (AG1) EMG burst duration (black) and amplitude (grey) collapsed across stimulus time for each cuing condition. Note that as duration increased across timing conditions, amplitude appeared to decrease.
Probability of early response
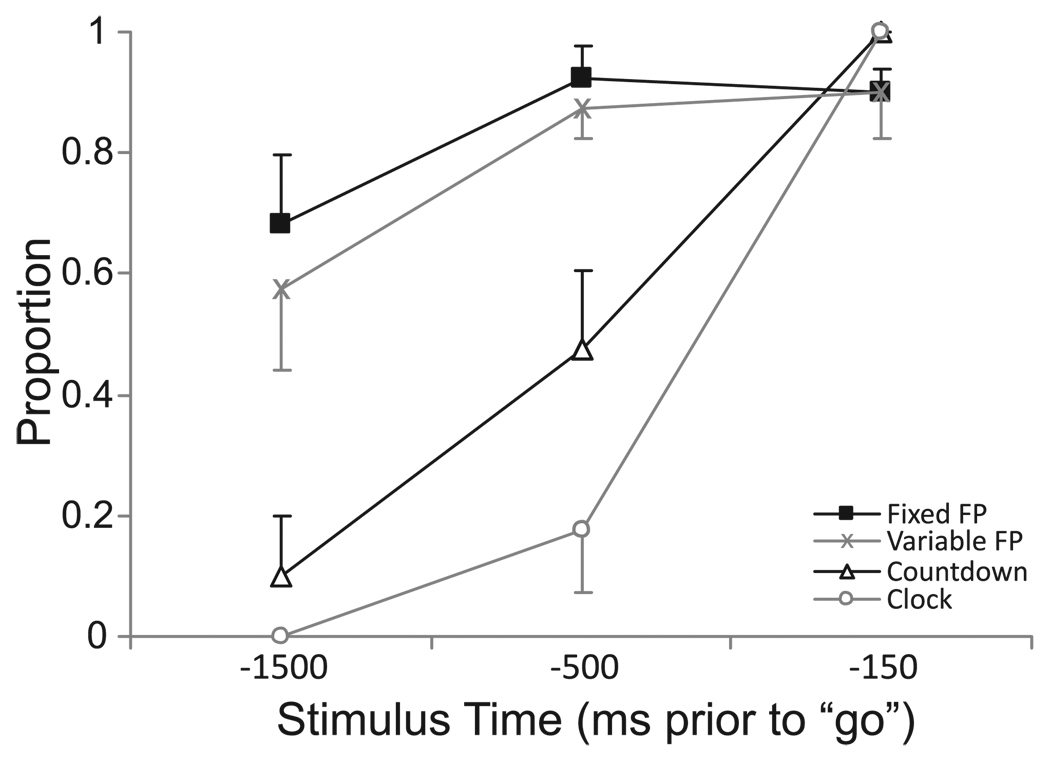
The probability of eliciting an early response due to SAS for each condition and each stimulus time is presented in Fig. 2. Proportion values were subjected to an arcsine square root transform prior to analysis using a 4 (cuing condition) × 3 (stimulus time) RM ANOVA. While main effects were observed for both cuing condition, F(3,21) = 24.708, p < .001, ηp2 = .779, and stimulus time, F(2,14) = 88.398, p < .001, ηp2 = .927, there was also a significant interaction between the variables, F(6,42) = 13.884, p < .001. Post-hoc analysis showed that when the stimulus was delivered at −1500 ms with respect to the visual “go” signal, the response was elicited significantly (p < .01) less often in the Countdown (10%) and Clock (0%) conditions, compared to the Variable (57.5%) and Fixed foreperiod (68.1%) conditions. In contrast, at −150 ms, no differences were observed between the conditions in the proportion of trials in which a response was elicited, and all were > 90%. At −500 ms, a pattern emerged in which the proportion of trials in which a response was elicited was significantly different (p < .05) between conditions whereby in the Countdown condition a response was elicited more often (47.5%) than in the Clock condition (17.5%), but less often than in the Variable (87.5%) and Fixed foreperiod (92.5%) conditions (Fig. 2).
Figure 2.
Mean (+/− 1 SE) proportion of early responses elicited by the SAS for each cuing condition (separate lines) at each stimulus time (time is with respect to imperative “go” stimulus).
Effect of startle on response latency (RT conditions)
The second way in which the effect of startle on the movement was assessed was to determine the latency between the onset of the stimulus and the initiation of response related EMG activity. That is, if the movement was made within 250 ms following a startle, what was the latency to onset of EMG? Analysis was performed using a 2 (cuing condition) × 4 (stimulus time) RM ANOVA on only the RT conditions (Variable and Fixed foreperiod). This was done because for many participants, the startle did not lead to the early production of the movement in the Countdown and Clock conditions. A significant main effect was found for stimulus time, F(3,18) = 93.613, p < .001, ηp2 = .940, while no effect was found for cuing condition, and there was no significant interaction between the variables. Post-hoc analysis showed that when a startle led to the early production of a response, EMG onset occurred at a significantly (p < .01) shorter latency following the startle stimulus (mean = 92.5 ms) than in Control trials (195.4 ms). However, for the startle elicited responses, there were no differences in the latency to onset between either the stimulus times or foreperiod conditions (Fig. 3).
Figure 3.
Mean (+/− 1 SE) premotor reaction time (RT) for the Variable (grey) and Fixed (black) foreperiod RT cues for both the Control (separated points) and startle conditions at each stimulus time (joined points). Note that for the startle conditions RT refers to the time between the acoustic stimulus and any elicited response within 250 ms.
Effect of startle on response latency (anticipation-timing conditions)
In the Countdown and Clock conditions, early response production due to startle occurred much less often when presented at either −1500 ms or −500 ms (Fig. 2), thus it was not possible to perform a full factorial analysis of stimulus time by cuing condition. However, when early response production was observed in these conditions, it also occurred at a latency (Countdown = 109.7 ms, Clock = 88.0 ms, averaged across stimulation times) comparable to that observed in the Variable and Fixed foreperiod RT conditions (cf. Fig. 3).
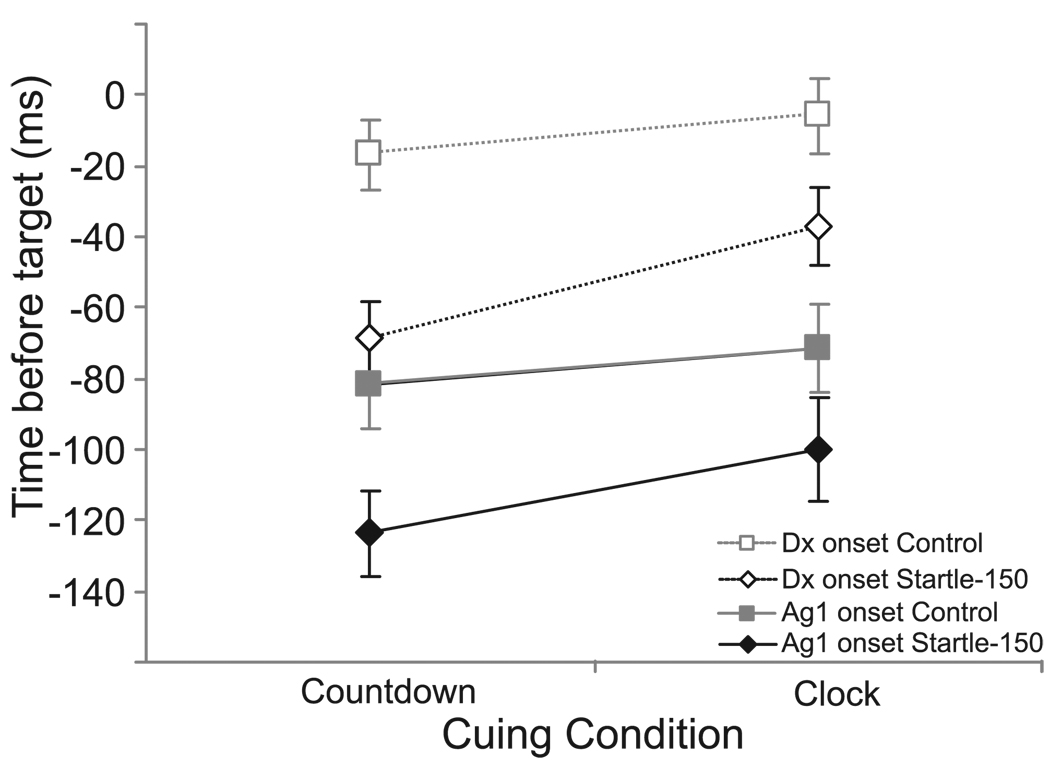
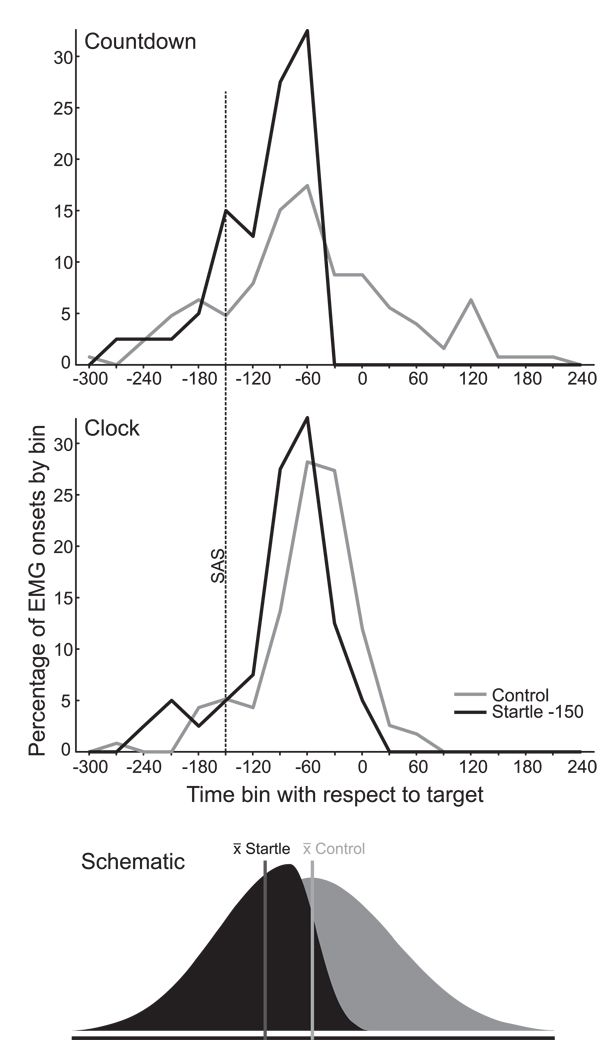
Therefore, for the Countdown and Clock conditions, EMG onset with respect to target was analyzed using a 2 (cuing condition) × 2 (stimulus) RM ANOVA to determine if presenting a startle stimulus 150 ms prior to the target led to the early production of the movement. Analysis showed that there were significant main effects for both cuing condition, F(1,7) = 13.461, p = .008, ηp2 = .658, and for stimulus, F(1,7) = 14.456, p = .007, ηp2 = .674, but there was no significant interaction between the variables (p = .340). Inspection of the data revealed that the presentation of a startling stimulus led to earlier EMG onsets for both the Countdown and Clock cues (Fig. 4). In addition, for both the Control and Startle (−150) conditions, EMG onset occurred later (i.e. closer to the target) for the Clock cue than for the Countdown cue. Similar results were observed for Displacement onset (i.e. target error) albeit with a relatively constant delay following the EMG onset (Fig. 4).
Figure 4.
Mean (+/− 1SE) initial agonist (AG1) EMG onset (filled, solid lines) and angular displacement onset (open, dashed lines) for the Countdown and Clock cues in both the Control (grey squares) condition and when a startling acoustic stimulus was presented 150 ms prior to the target (Startle-150, black diamonds).
SCM Startle response
The probability of observing a startle response in any condition was analyzed to determine whether or not the early production of the movement was associated with a startle response. The proportion of Startle trials in which SCM EMG activity occurred within 120 ms of the 124 dB stimulus was calculated, and results are shown in Figure 5. Data were subjected to an arcsine square root transform and analyzed using a 4 (cuing condition) × 3 (stimulus time) RM ANOVA. Main effects were observed for both cuing condition, F(3,21) = 7.210, p = .002, ηp2 = .507, and for stimulus time, F(2,14) = 18.625, p <.001, ηp2 = .727. Post hoc analysis showed that for both variables, only the outside values were significantly (p < .05) different. That is, when averaged across the other variable, the proportion of trials in which startle SCM activity was observed was greater at −150 ms (96.9% of trials) than at −1500 ms (70.8% of trials), and greater in the Variable foreperiod condition (94.2% of trials) than the Clock condition (71.7% of trials). However, inspection of the data revealed that irrespective of condition, when an early movement was elicited (see Fig. 2), there was always an associated startle response.
Figure 5.
Mean (+/− 1SE) proportion of startle trials (124 dB acoustic stimulus) in which a startle response (EMG activity in SCM) was observed for each cuing condition (separate lines) and each stimulus time. Note the similarity to Figure 3.
Discussion
Previous studies examining the time course of motor preparation have suggested that the predictability of a “go” signal affects how early a voluntary response is prepared (Cui and MacKinnon, 2009; Cunnington et al., 1995; Jahanshahi et al., 1995; Jankelowitz and Colebatch, 2002; Thickbroom and Mastaglia, 1985; Thickbroom et al., 2000) However, it is not known how the resolution of response timing information specifically affects advance preparation of motor output. Here we show that changes in the resolution of the timing information had a profound effect on advance motor preparation. In particular, when the precise moment that an action must be executed was known with high precision, programming was delayed until shortly before execution. However, motor programming was completed much earlier when the time of the required response was less predictable. The following discussion reviews the evidence that the temporal resolution of response timing affects motor output preparation and a hypothetical model of the timing of preparation is presented.
Experiments have shown that when the required response was known in advance of the “go” signal, a loud (124 dB) startling acoustic stimulus (SAS) capable of eliciting a classic startle response could release the preplanned action (Carlsen et al., 2003; Carlsen et al., 2007; Castellote et al., 2007; Cressman et al., 2006; Kumru et al., 2006; MacKinnon et al., 2007; Maslovat et al., 2008; Oude Nijhuis et al., 2007; Valls-Solé et al., 1999). The vast majority of these studies that have used startle to elicit a prepared movement have presented the startling stimulus in conjunction with, or in place of, the imperative “go” signal. In one study, however, the SAS was presented at 1400 ms and 100 ms prior to the “go” cue during a fixed foreperiod reaction time task (MacKinnon et al., 2007). The presentation of a SAS at these time points often resulted in the release of the planned movement sequence, but the incidence of release was markedly reduced at −1400 ms whereas movement was reliably released at −100 ms. These findings were interpreted to suggest that movements were planned well in advance of the intended action when the timing of the cue could be predicted. However, in a task where movement onset had to be precisely timed with a moving clock hand (i.e. anticipation-timing), the presentation of a SAS only 200 ms prior to the target did not result in early response triggering (Carlsen et al., 2008b). This discrepancy suggested that differences in the temporal resolution of the timing cue could have a marked impact on the timing of movement preparation.
In the current experiment the temporal resolution was varied across four cuing conditions, and a SAS was presented at 3 time points prior to the usual “go” signal. The primary finding of the current study is that there was a differential proportion of trials in which the required movement was elicited by startle depending on the cuing condition. The intended movement was released early in about 60% of RT (i.e. Variable and Fixed foreperiod condition) trials when a startling acoustic stimulus (SAS) was presented 1500 ms prior to the “go” signal. This percentage grew to about 90% at 500 ms and 150 ms prior to the “go” signal (Fig. 2). In contrast, when more precise timing information was provided (i.e. Countdown and Clock conditions) the response was much less likely to be released by startle until later. Specifically, in the Countdown and Clock conditions the response was almost never produced when a SAS was presented at −1500 ms, and always when SAS occurred at −150 ms. This pattern of results suggests that there is a fundamental difference in the motor preparatory strategy employed depending on the temporal information provided.
Proportion of trials elicited by startle (RT conditions)
The presentation of a SAS led to a high proportion of trials in which an early targeted response was elicited in the Variable and Fixed foreperiod RT conditions irrespective of when the SAS was presented, thus it appears that participants often programmed the movement soon after the precue in readiness for the imperative stimulus. Moreover, in the RT conditions, the movement was elicited at a much shorter latency following the stimulus compared to Control trials (Fig. 3). Indeed, premotor RTs observed when the SAS resulted in early response production (mean 92.5 ms, range 55 – 127 ms) were similar to those reported in studies where SAS simply replaced the usual “go” signal for upper limb tasks (e.g., Carlsen et al., 2004b; Carlsen et al., 2007; Kumru et al., 2006; Maslovat et al., 2008; Maslovat et al., 2009; Valls-Solé et al., 1995; Valls-Solé et al., 1999) and were much shorter than control trials (mean 195.4 ms, range 160 – 234 ms), suggesting that the pre-programmed movement was triggered directly by the SAS. Based on these data, it is suggested that when an early response was elicited in the current experiment, it was directly triggered by startle and unlikely to have been an (incorrect) voluntary initiation due to the presentation of an unexpected stimulus. Finally, since no differences due to startle were observed in EMG timing pattern or characteristics or movement kinematics between Control and Startle trials the response elicited appears to have been the intended and pre-programmed action.
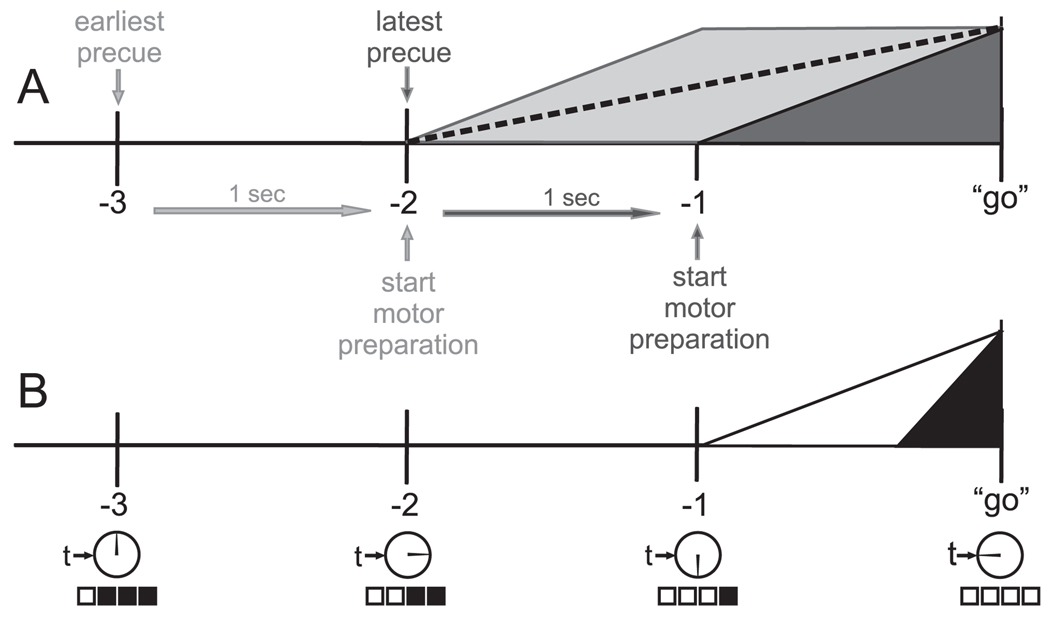
Imaging studies, including EEG, MEG and fMRI, have shown that movement-related cortical activity begins to increase well before the movement occurs (Kornhuber and Deecke, 1965; Shibasaki and Hallett, 2006; Thickbroom et al., 2000). In order to react as soon as possible following the imperative stimulus in RT tasks, particularly those with a variable foreperiod, it would be strategically beneficial to be prepared well in advance. This strategy has been modeled previously by Thickbroom et al. (2000), who suggested that movement-related cortical activation begins approximately 1 sec prior to the soonest possible “go” signal (based on a learned range of possible foreperiods), ramping up to peak activity in approximately one second. However, since it is difficult to determine exactly when the motoric components of the response preparation are completed using imaging information alone (e.g., Van 't Ent and Apkarian, 1998; Van 't Ent and Apkarian, 1999) the startle probe was used in the present experiment to verify whether the response was prepared and ready to be executed at a given time prior to the “go” signal. In our experiment, for the Variable foreperiod condition, the shortest possible foreperiod was 2 sec; thus in the case where the precue occurred at −3 sec (e.g. soonest possible “go” at −1 sec), the model would suggest that preparatory activity would start approximately 2 seconds prior to the imperative stimulus. However, if the precue occurred at −2 seconds, motor preparation would start approximately 1 sec prior to the imperative stimulus (see Fig. 6 for a modified version of the Thickbroom et al. model). Therefore, for our conditions, this model would suggest that, on average, while some motor preparation would be occurring at −1500 ms prior to the “go” signal, the programming of the motor command would be less likely to be completed at this time (see Fig. 6a, dashed line). This might explain the lower proportion of trials in which the movement was elicited by a SAS at −1500 ms in the Variable and Fixed conditions (Fig. 2). While cortical potentials associated with motor readiness have been previously shown to emerge at least 1.5 sec prior to movement (e.g., Cui and MacKinnon, 2009; Kornhuber and Deecke, 1965), there is considerable variability across subjects and it is likely that there is also marked variability within subjects across trials. Thus, it is possible that some participants prepared even earlier due to a lack of familiarity with the foreperiod duration, whereas, other participants may have consistently delayed preparation based upon a more accurate prediction of the response timing. Indeed two participants had a relatively low (20%) incidence of movement release at −1500 ms whereas others (n=4) showed a much higher probability (>80%) of release at the same time point. In the former group, it is possible that they progressively learned the timing of the response cue over the course of the experiment. in fact, it has been previously shown that with sufficient practice of a fixed foreperiod RT task participants can learn to react almost simultaneous with the stimulus (Quesada and Schmidt, 1970). Furthermore, it has been shown that the implicit temporal features of a regularly timed stimulus can be learned in few trials (Praamstra et al., 2006). Thus, although in the current experiment participants were not informed whether or not the foreperiod was fixed, and only 75 trials were performed, it is still possible that some subjects were able to learn the timing well enough to revert to a more predictive mode of control (Cressman et al., 2006; Thickbroom et al., 2000). However, it appears that for the majority of subjects performing the Fixed foreperiod condition, the task remained sufficiently unpredictable such that the start of motor preparation took place at a time similar to that seen in the Variable foreperiod condition. This is evidenced by a similar incidence of release by startle in the Variable and Fixed conditions where the model suggests that programming of the motor command would almost always be completed and be ready for initiation: At −500 ms and −150 ms, the response was elicited by startle a vast majority of the time (Fig. 2).
Figure 6.
A modified model of cortical motor preparatory activity in reaction time (panel A) and anticipation timing tasks (panel B). Panel A depicts a reaction time situation in which the earliest possible precue (light grey) occurs 3 sec prior to the imperative “go” signal, with related motor preparation initiated 1 sec later and taking 1 sec to reach a peak. The latest possible precue (dark grey) occurs 2 seconds prior to “go” with associated motor preparation 1 sec later. Mean motor preparatory activity between the two extremes shown with a dashed line. Panel B depicts cortical motor preparatory activity in the anticipation timing situations used in the current experiment. The Countdown condition is shown with motor preparation initiated following the last cue (white) and the Clock condition is shown with motor preparation initiated shortly before the “go” target. See text for further discussion.
Proportion of trials elicited by startle (anticipation-timing conditions)
In contrast to the Fixed and Variable foreperiod RT cuing conditions, participants appear to have waited until much later before programming the response in the anticipation-timing conditions. For example, when a SAS was delivered at either 1500 or 500 ms prior to target in the Clock cue, the task-specific response was rarely elicited early (0 and 17.5% of trials respectively). This was also true when a SAS was presented at 1500 ms prior to target in the Countdown cue condition (10% of trials, see Fig. 2). This would suggest that, at these latencies, the required motor output was seldom prepared in advance and the movement could not be triggered by startle. Notably however, although it was infrequent, if an early response was elicited in the Countdown or Clock conditions at either −1500ms or −500 ms, it occurred at a short latency following the SAS similar to that seen in the RT conditions (mean range 88 – 114 ms) suggesting that the response was occasionally pre-programmed and ready to be triggered in these situations.
In contrast to the early SAS probes (−1500, −500), in both the Countdown and Clock conditions the response was always produced within 250 ms following the SAS if it was presented 150 ms prior to the target. This result is not entirely surprising, in light of evidence an experiment using a similar anticipation-timing clock paradigm (Coxon et al., 2006) where it was shown that no-go responses cannot be suppressed when the “stop” signal is presented less than 150 ms prior to the required response time. Additionally, in order for movement to begin simultaneously with the target in the control trials, EMG activity had to begin approximately 62 ms in advance of the target due to electro-mechanical delay. Taking into account nerve conduction time (20–30 ms, Rothwell, 1997) this means that the response had to be initiated at least 80–90 ms in advance of the target. Nevertheless, when a SAS was presented at 150 ms prior to the target in the Countdown and Clock conditions the response was produced an average of 42 and 28 ms earlier than in control trials. Looking at the distribution of EMG onsets for control and SAS −150 in the anticipation timing conditions (Fig. 7), it is clear that while sometimes participants initiated the movement early, it is the latter part of the EMG onset distribution that was most affected by startle. In other words, for both Control and Startle trials, the response was occasionally initiated very early (up to 300 ms before the target, and sometimes well before the SAS occurred). However, if the response was not yet initiated in the startle trials, the SAS invariably led to the accelerated release of the movement. This, we suggest, provides evidence that the motor output was almost always programmed 150 ms before the presentation of the “go” signal in the anticipation timing conditions.
Figure 7.
Percentage of initial agonist EMG onsets observed at various times (30 ms bins) with respect to the target (time “0”) for the Countdown (top panel) and Clock (middle panel) cues. Grey lines are control trials, black lines are trials in which a 124 dB startling acoustic stimulus (SAS) was delivered 150 ms prior to the target (dashed line). Bottom panel is a schematic representation of how the EMG onset distribution is skewed by the presentation of a SAS. See text for further discussion.
As previously noted, for the Clock condition, a SAS presented at −500 infrequently (17.5 %) elicited the required response. Whereas when a SAS occurred at −150 ms, the response was consistently elicited earlier than in control trials, suggesting that response preparation had to begin between 150 and 500 ms prior to target. Interestingly, there was a much higher incidence of movement release by startle −500 ms in the Countdown condition (47.5 %, see Fig. 2). The higher percentage of early responses due to a SAS suggests that participants were much more likely to have programmed the movement at least 500 ms before the target in the Countdown condition. Considering the difference between the Countdown and Clock cuing conditions, it appears that the resolution of temporal information affected when participants prepared the motor system for the upcoming action. In the Countdown condition there was only a visual cue every 1 sec, whereas the clock condition provided updates as fast as the computer monitor could refresh the screen (85 Hz or about every 12 ms), appearing continuous. Thus there was much greater uncertainty with respect to timing the movement onset to coincide with the target in the Countdown condition. This temporal uncertainty appears to have resulted in participants preparing soon after the final cue in order to be ready to perform the required movement.
If we consider the nature of the task with respect to the anticipation timing conditions, it would not be strategically beneficial, or energetically efficient to prepare the motor system earlier than required based on the provided information. For example, in the Clock condition, participants can likely wait until just prior to responding before programming the movement and readying the motor system for its release. Using a startled anticipation-timing paradigm similar to that used here, it was previously shown that the movement was never elicited early when a SAS was presented up 200 ms prior to the target (Carlsen et al., 2008b). Similarly, it was shown that motor pathway excitability measured using TMS evoked MEPs did not increase until 150 ms prior to target (Coxon et al., 2006). However, these previous studies involved a “stop” signal, and while the task was somewhat different to that used here, the results appear similar in that motor preparation was delayed as long as possible prior to responding. In the Countdown condition, however, the greater temporal uncertainty (which appears to have resulted in a wider distribution of EMG onsets observed in the Countdown compared to the Clock cuing condition, Fig. 7) appears to have led to earlier motor preparation beginning soon after the last warning cue (i.e. the “1” of the “3-2-1-go” sequence). While there would be no need to prepare prior to the final warning cue, after this the task would then be similar to a 1 sec fixed foreperiod condition, and a similar mode of preparation may be employed (see Fig. 6). As such, for the Countdown condition at latencies when it was strategically inefficient to program a response (−1500 ms) startle infrequently led to early response production, whereas when it was much more likely that participants would choose to program the response (−500 ms) startle led to early response production much more often.
Startle response probability
A secondary finding of the current study was that the probability of eliciting a startle response (i.e., SCM EMG activity) in participants was modulated by whether or not the motor system was being prepared for a voluntary response. It is well known that in the absence of other factors, the startle response in humans habituates within 2 – 6 random SAS presentations (Blumenthal et al., 2005; Brown et al., 1991), although this habituation can be compensated for to a small degree by using higher intensity stimuli and providing longer intervals between startles (Blumenthal et al., 2005). However, it has been shown that the startle response can be elicited indefinitely in participants if they are preparing for an upcoming voluntary action (Carlsen et al., 2003; Valls-Solé et al., 1997). This “dishabituation” is thought to result from increased subcortical neural excitability associated with the voluntary motor preparation (Carlsen et al., 2003). Therefore, the co-expression of SCM startle activity in conjunction with early movement release following SAS, particularly after the first 5 to 6 startle trials, may be used as a marker that participants were preparing the motor system.
Based on the remarkably short latency of the reaction times of movements evoked by a SAS and the co-expression of a classic startle reflex with the released movement, it was suggested that perhaps the spatial and temporal parameters of the prepared action were represented in subcortical structures common to both the startle and voluntary response pathways (e.g. reticular formation, Rothwell 2006) and the startle response resulted in sufficient activation to release the pre-programmed response without the usual cortical trigger (Carlsen et al., 2004a; Carlsen et al., 2009; Valls-Solé et al., 1999; Valls-Solé et al., 2008). Indeed, regions of the pontomedullary reticular formation show preparatory activity that is related to both postural and focal movement aspects of the intended task (Buford and Davidson, 2004; Schepens and Drew, 2004). For this reason, it has further been argued that the SCM EMG response amplitude resulting from a loud stimulus can be used as an index of subcortical motor circuit excitability during both classic startle reflex paradigms (Yeomans and Frankland, 1996) and SAS-evoked voluntary movements (Carlsen et al., 2008a; Kumru et al., 2006; Kumru and Valls-Solé 2006). In the present experiment, a SCM response was not always elicited by the SAS, although when an early, targeted response was observed due to SAS it was always accompanied by SCM EMG activity. Furthermore, startle-related SCM activity elicited due to SAS did not always lead to the early production of the targeted response. This means that early release of the movement was always accompanied by a startle response while a startle response did not always lead to the early release of the movement. This was particularly true in the Clock condition. In fact the pattern of SCM response probability (Fig. 5) clearly resembles that of the probability of early response (c.f. Fig. 2), although it falls in a narrower range and is higher than that observed for early responses. Thus since the cuing condition also affected the probability of eliciting a SCM startle response, this suggests that the cues had an effect on the amount of subcortical motor activation. It may be suggested that since the Clock condition was always presented in the second half of testing, habituation effects may have impacted the probability of observing a SCM response. However, this is unlikely, since SCM was elicited >90% of the time at −150 ms irrespective of cuing condition (i.e. block order).
Targeted response characteristics
A final notable finding was that while the kinematics of the response were unchanged by the addition of a SAS or by cuing condition, there was a small but significant difference in the early part of the EMG pattern depending on cuing condition. Specifically, the initial EMG burst was shorter in duration in the RT conditions than in the anticipation-timing (particularly Clock) conditions. Furthermore, it appears that the amplitude of the initial EMG burst may have been modulated to result in similar kinematics between the conditions (Fig. 1). Since these differences were independent of whether or not the response was elicited by startle, it suggests that the responses were pre-programmed with slightly different characteristics. One explanation for the differences may that they are due to the reactive nature of the Variable and Fixed foreperiod tasks compared to the more self-initiated nature of the anticipation timing tasks. The reactive tasks may lead to the preparation of a stronger initial burst to comply with the task goal of initiating displacement as quickly as possible following the “go” signal. A similar distinction between internally generated and externally triggered movements has been noted previously (Obhi and Haggard, 2004), although larger EMG activity was found for internally generated actions. Nevertheless, this result is certainly suggestive of the preparation of different motor plans depending on cuing condition and may warrant further study.
Conclusions
The present study investigated how the temporal resolution of the response timing information affected the timing of movement preparation. Specifically, unpredictable response times (i.e. low temporal resolution) led to earlier response programming than when with the response timing information was provided with high resolution. In addition, some of our data suggest that the early EMG characteristics for a similar movement are modulated based on response timing resolution. These results demonstrate that, under conditions of low temporal resolution, participants plan the movement well in advance in accordance with the anticipated probability of onset of the cue, whereas movement preparation is delayed until less than 200 ms prior to response time when continuous temporal information is provided (anticipation timing conditions).
Experimental Procedure
Participants
Nine neurologically healthy volunteers (5M, 4F; ages 31 +/− 10 years) participated in the study after giving informed consent. Testing of each participant took place in a single session. All participants gave written informed consent, and the study was conducted in accordance with the ethical approval of the Institutional Research Board at Northwestern University and conformed to the Declaration of Helsinki. Data from one participant was not used due to the lack of a reliable startle response (i.e. SCM EMG activity, see below). Therefore, final analysis was based on data from eight participants.
Participant Position
The participants sat facing a computer monitor with their right forearm resting semi-prone in a brace attached to a custom made wrist manipulandum that allowed measurement of wrist angular displacement. The participant’s hand gripped a post attached to the arm of the manipulandum and the shoulder was flexed and abducted about 15 deg. The forearm was secured to the brace using Velcro straps placed proximal to the wrist joint and distal to the elbow. The wrist starting position was 10 deg of flexion and was indicated by both online visual feedback and a physical stop. (An overhead schematic of the participant position is provided in Figure 8).
Figure 8.
Overhead schematic of positioning and task. Wrist starting position was flexed 10 deg (light dashed line), with the movement target located 20 deg of angular extension from the starting position (bold dashed line). The speaker that delivered the 124 dB acoustic stimulus was located 50 cm behind the ears of the participant.
Task and Feedback
The task for the participants was to perform a right wrist extension movement as quickly and as accurately as possible to a fixed target region located at 20 degrees of angular displacement from the starting position. Wrist position feedback was given by representing the position of the manipulandum with a 1 cm tall yellow cursor line within a horizontal (1cm × 15cm) black rectangle presented on the computer screen. The starting point of the cursor was 1 cm from the left edge of the rectangle. A blue line, 2 cm from the right edge of the rectangle served as the movement target. Real time position feedback was not given during test trials, and was only provided during practice and in between trials to familiarize participants with the desired movement. The movement task was completed under four different cueing conditions described below:
Variable foreperiod RT condition: At the initiation of a trial, a grey square (3cm × 3cm) with a black outline was presented in the centre of the screen as the warning “get ready” cue. Following a variable amount of time (2 – 3 sec. foreperiod) the square changed to green as the imperative “go” signal. Participants were instructed to initiate movement (react) as quickly as possible following the onset of the green square.
Fixed foreperiod RT condition: As above except the time between the warning cue and the imperative stimulus was fixed at 3 sec.
Countdown anticipation-timing condition: Four squares (1.5 cm × 1.5 cm, lined up horizontally in the centre of the monitor and separated by 1.5 cm) appeared sequentially from left to right on the computer screen at 1000 ms intervals. As such, the time from appearance of the initial square to the final square was 3 sec. The first three squares were yellow, and the last was green. The participant was intended to try to time the onset of movement to occur coincidentally with the appearance of the green (4th) square.
Clock anticipation-timing condition: A representation of an analog clock face (10 cm diameter) was presented on the computer monitor with the exception that numbers were not placed around the circumference. The clock hand was consistently timed so that it made one revolution in 4 sec. At the start of a trial, the previously stationary clock hand then moved from the topmost position (12 o’clock) in a clockwise direction, making one full revolution. The participant was intended to try to initiate the required wrist extension movement coincidently with the clock hand reaching the 9 o’clock (3000 ms) position on the clock face (indicated by a red arrow).
The four above conditions were presented in pseudorandomized blocked order, with the two RT conditions completed before the two anticipation timing conditions. This was done so that participants would not attempt to use a timing strategy in the RT conditions to anticipate “go” signal onset. Participants performed 6 –10 practice trials and then completed 75 test trials for each condition. A rest period was provided between blocks. After each trial in the RT conditions (1 and 2 above), participants were provided with feedback on the computer monitor about their RT (in ms) and movement accuracy (error in deg.). For the anticipation timing conditions (3 and 4 above), in addition to movement accuracy, movement initiation timing error (ms) with respect to the target was provided (in condition 4 the clock hand also indicated the time movement initiation relative to the target). The experimental protocol was controlled using custom software written with LabVIEW Software (National Instruments Inc.).
Startling Acoustic Stimulus (SAS)
Several trials in each block were accompanied by a concurrent loud acoustic stimulus (124 dB), which participants were told was irrelevant to the task. The stimulus was presented at three time points prior to the “go” signal in each of the four conditions. These time points were 1500, 500 and 150 ms prior to the “go” signal (or target). The SAS was presented in five trials for each time point in each condition (total of 15 SAS trials per condition).
The acoustic stimulus (1 KHz, 40ms) was generated using a tone generator (Grass model S10CTCMA) which was amplified and presented via a loudspeaker (<1 ms rise time) placed 50 cm behind the head of the participant with an intensity of 124 dB. The stimulus intensities were measured using a sound level meter (Brüel & Kjær Impulse Precision Sound Level Meter type 2204) using the “A” measurement scale at a distance of 50 cm from the loudspeaker (approximately the distance to the ears of the participant).
Recording Equipment
Surface Electromyographic (EMG) data were collected from the muscle bellies of the following superficial muscles: right extensor carpi radialis longus (ECR), right flexor carpi radialis (FCR), and left sternocleidomastiod (SCM), using bipolar preamplified (gain = 10) surface electrodes (Delsys Bagnoli DE-2.1) connected via shielded cabling to an external amplifier system (Delsys Bagnoli 16). The recording sites were prepared and cleansed in order to decrease electrical impedance, oriented parallel to the muscle fibers, and then attached using double-sided adhesive strips. A grounding electrode was placed on the participant’s left lateral epicondyle. Wrist angular position data were collected using an optical encoder connected attached to the central axis of the manipulandum. On each trial, unfiltered EMG and position data were digitally sampled at 1 kHz (National Instruments PCI-6030E via BNC-2090) for 4 sec using a customized program written with LabVIEW software (National Instruments Inc.) and stored for offline analysis. Data collection was initiated by the computer 3000 ms prior to the imperative stimulus (or target) in all conditions.
Data Reduction
Movement onset was defined as the first point of a change of more than 0.2 deg of angular displacement from the starting position following the stimulus. For the anticipation timing conditions target error was defined as the amount of time between the clock hand arriving at the target or the final square appearing, and movement onset. Negative timing error was when movement was initiated prior the target, while positive error was when movement occurred after the target. Early movement onset due to SAS was defined as when the presentation of a startling stimulus (124 dB) led to targeted movement production within 250 ms. This time was chosen so that voluntary responses (i.e. responding to the SAS voluntarily as a “go” signal) would not be overly aggressively filtered out along with erroneous movements. This method led to a rejection rate of less than 1% of trials due to errors. Peak displacement was defined as the displacement at the point at which angular acceleration crossed zero the second time after movement onset. Movement final position was defined as the first point at which angular velocity fell below and remained below 8 deg/s for at least 150 ms. Time to peak displacement was the time from displacement onset to peak displacement. Movement time was defined as the time from displacement onset to final position.
EMG burst onsets were defined as the point at which the EMG first began sustained rise above baseline levels. The location of this point was determined by first displaying the EMG pattern on a computer monitor with a superimposed line indicating the point at which rectified, filtered EMG activity increased to more than 2 standard deviations above baseline (mean of 100 ms of EMG activity preceding onset). Onset was then verified by visually locating and manually adjusting the onset mark to the point at which the activity first increased on the raw EMG trace. This method allows for correction of errors due to the strictness of the algorithm. EMG offsets were marked in a similar fashion, with the activity between EMG onset and EMG offset being defined as a distinct burst. Peak EMG amplitudes were defined as the largest EMG amplitude, rectified and filtered with a 25 Hz low pass elliptic filter, recorded within an interval of 100 ms following EMG burst onset.
Statistical Analyses
Dependent variables were analyzed using Repeated Measures Analysis of Variance (RM ANOVA), to determine if differences existed between control and startle trials or between conditions. Prior to analysis proportion variables were subjected to an arcsine square root transform and Greenhouse-Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity. Differences with a probability of less than .05 were considered to be significant. Tukey’s Honestly Significant Differences (HSD) post-hoc tests were administered to determine the locus of any differences.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (PDF-357177 to ANC) and the National Institutes of Health (R01NS054199 to CDM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiol. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114:1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp. Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp. Brain Res. 2003;152:510–518. doi: 10.1007/s00221-003-1575-5. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J. Motor Behav. 2004a;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp. Brain Res. 2004b;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp. Brain Res. 2007;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Dakin CJ, Sanderson DJ, Inglis JT, Franks IM. Startle reveals an absence of advance motor programming in a Go/No-go task. Neurosci. Lett. 2008a;434:61–65. doi: 10.1016/j.neulet.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Motor preparation in an anticipation-timing task. Exp. Brain Res. 2008b;190:453–461. doi: 10.1007/s00221-008-1487-5. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Differential effects of startle on reaction time for finger and arm movements. J. Neurophysiol. 2009;101:306–314. doi: 10.1152/jn.00878.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellote JM, Kumru H, Queralt A, Valls-Solé J. A startle speeds up the execution of externally guided saccades. Exp. Brain Res. 2007;177:129–136. doi: 10.1007/s00221-006-0659-4. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Carlsen AN, Chua R, Franks IM. Temporal uncertainty does not affect response latencies of movements produced during startle reactions. Exp. Brain Res. 2006;171:278–282. doi: 10.1007/s00221-006-0459-x. [DOI] [PubMed] [Google Scholar]
- Cui RQ, MacKinnon CD. The effect of temporal accuracy constraints on movement-related potentials. Exp. Brain Res. 2009;194:477–488. doi: 10.1007/s00221-009-1725-5. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL, Phillips JG. Movement-related potentials in Parkinson's-disease presence and predictability of temporal and spatial cues. Brain. 1995;118:935–950. doi: 10.1093/brain/118.4.935. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG. Movement-related potentials associated with self-paced, cued and imagined arm movements. Exp. Brain Res. 2002;147:98–107. doi: 10.1007/s00221-002-1220-8. [DOI] [PubMed] [Google Scholar]
- Keele SW. Movement control in skilled motor performance. Psychol. Bull. 1968;70:387–403. [Google Scholar]
- Klapp ST. Reaction time analysis of two types of motor preparation for speech articulation: Action as a sequence of chunks. Journal of Motor Behavior. 2003;35:135–150. doi: 10.1080/00222890309602129. [DOI] [PubMed] [Google Scholar]
- Klemmer ET. Time uncertainty in simple reaction time. J. Exp. Psychol. 1956;51:179–184. doi: 10.1037/h0042317. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L. Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente. Potentiale. Pflügers Archiv Eur. J. Physiol. 1965;284:1–17. [PubMed] [Google Scholar]
- Kumru H, Urra X, Compta Y, Castellote JM, Turbau J, Valls-Solé J. Excitability of subcortical motor circuits in Go/noGo and forced choice reaction time tasks. Neurosci. Lett. 2006;406:66–70. doi: 10.1016/j.neulet.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Kumru H, Valls-Solé J. Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp. Brain Res. 2006;169:427–432. doi: 10.1007/s00221-005-0156-1. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W, Ulrich R. Preparing for action: Inferences from CNV and LRP. J. Psychophysiol. 2004;18:77–88. [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J. Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Ishimoto R, Chua R, Franks IM. Response preparation changes following practice of an asymmetrical bimanual movement. Exp. Brain Res. 2008;190:239–249. doi: 10.1007/s00221-008-1467-9. [DOI] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Chua R, Franks IM. Response preparation changes during practice of an asynchronous bimanual movement. Exp. Brain Res. 2009;195:383–392. doi: 10.1007/s00221-009-1801-x. [DOI] [PubMed] [Google Scholar]
- Niemi P, Näätänen R. Foreperiod and simple reaction-time. Psychol. Bull. 1981;89:133–162. [Google Scholar]
- Obhi SS, Haggard P. Internally generated and externally triggered actions are physically distinct and independently controlled. Exp. Brain Res. 2004;156:518–523. doi: 10.1007/s00221-004-1911-4. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Janssen L, Bloem BR, van Dijk JG, Gielen SC, Borm GF, Overeem S. Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J. Physiol. (Lond.) 2007;584:97–109. doi: 10.1113/jphysiol.2007.136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Horstink MWIM, Cools AR. Dipole source analysis suggests selective modulation of the supplementary motor area contribution to the readiness potential. Electroencephalogr. Clin. Neurophysiol. 1996;98:468–477. doi: 10.1016/0013-4694(96)95643-6. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Kwok HF, Oostenveld R. Neurophysiology of implicit timing in serial choice reaction-time performance. J. Neurosci. 2006;26:5448–5455. doi: 10.1523/JNEUROSCI.0440-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada DC, Schmidt RA. A test of the Adams-Creamer decay hypothesis for the timing of motor responses. Journal of Motor Behavior. 1970;2:273–283. doi: 10.1080/00222895.1970.10734885. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J. Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereltschaftspotential? Clin. Neurophysiol. 2006;117:2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Mastaglia FL. Cerebral events preceding self-paced and visually triggered saccades. A study of presaccadic potentials. Electroencephalogr. Clin. Neurophysiol. 1985;62:277–289. doi: 10.1016/0168-5597(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Sacco P, Ghosh S, Morris IT, Mastaglia FL. The role of the supplementary motor area in externally timed movement: the influence of predictability of movement timing. Brain Res. 2000;874:233–241. doi: 10.1016/s0006-8993(00)02588-9. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci. Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Valldeoriola F, Tolosa E, Nobbe F. Habituation of the auditory startle reaction is reduced during preparation for execution of a motor task in normal human subjects. Brain Res. 1997;751:155–159. doi: 10.1016/s0006-8993(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. Journal of Physiology-London. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegré M. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp. Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Exp. Brain Res. 2008;187:497–507. doi: 10.1007/s00221-008-1402-0. [DOI] [PubMed] [Google Scholar]
- Van 't Ent D, Apkarian P. Inter-hemispheric lateralization of event related potentials; motoric versus non-motoric cortical activity. Electroencephalogr. Clin. Neurophysiol. 1998;107:263–276. doi: 10.1016/s0013-4694(98)00068-6. [DOI] [PubMed] [Google Scholar]
- Van 't Ent D, Apkarian P. Motoric response inhibition in finger movement and saccadic eye movement: a comparative study. Clin. Neurophysiol. 1999;110:1058–1072. doi: 10.1016/s1388-2457(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Walter WG, Aldridge VJ, McCallum WC, Cooper R. Contingent negative variation : Electrocortical sign of sensori-motor association in man. Electroencephalogr. Clin. Neurophysiol. 1964;17:340–377. [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Research Reviews. 1996;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]