Abstract
This chapter will discuss the current knowledge of the contribution of systemic and local inflammation in acute and sub-chronic stages of experimental stroke in both the adult and neonate. It will review the role of specific cell types and interactions among blood cells, endothelium, glia, microglia, the extracellular matrix and neurons – cumulatively called "neurovascular unit" – in stroke induction and evolution. Intracellular inflammatory signaling pathways such as nuclear factor kappa beta and mitogen-activated protein kinases, and mediators produced by inflammatory cells such as cytokines, chemokines, reactive oxygen species and arachidonic acid metabolites, as well as the modifying role of age on these mechanisms, will be reviewed as well as the potential for therapy in stroke and hypoxic-ischemic injury.
Keywords: cerebral ischemia, hypoxia-ischemia, neurovascular unit, leukocyte, microglia, cytokine, chemokine, adhesion molecules, integrin
1. INTRODUCTION
For a long time the central nervous system was considered to be “immunologically privileged” due to shielding from access by immune cells by the blood-brain barrier (BBB) [1]. However, it is becoming apparent that leukocytes [2], as well as cytokines and chemokines [3], can cross the intact BBB and that there is a substantial cross-talk between peripheral and local immune components [4, 5]. It is well known that stroke triggers a robust inflammatory reaction characterized by peripheral leukocyte influx into the cerebral parenchyma and activation of endogenous microglia [6–11]. Following adult stroke, the generation of reactive oxygen species (ROS) and intracellular calcium accumulation in neurons and other brain cells triggers immune responses ultimately leading to inflammatory cell activation and infiltration. Ischemic cells, even ischemic neurons, secrete inflammatory cytokines that cause, among other things, adhesion molecule upregulation in the cerebral vasculature which leads to peripheral leukocyte recruitment. Brain cells are also capable of secreting chemokines, leading to further inflammatory cell chemotaxis into the ischemic lesion. Once activated, inflammatory cells can release a variety of cytotoxic agents including cytokines, matrix metalloproteinases (MMPs), nitric oxide (NO) and more ROS. These substances can contribute to more cell damage as well as disruption of the BBB and extracellular matrix [12–15]. Figure 1 demonstrates the conceptual framework for the contribution of inflammatory mechanisms to stroke. Studies in experimental stroke have also shown that the extent, timing and consequences of these processes are profoundly affected by the presence of reperfusion, by gender, genetic background, and by age.
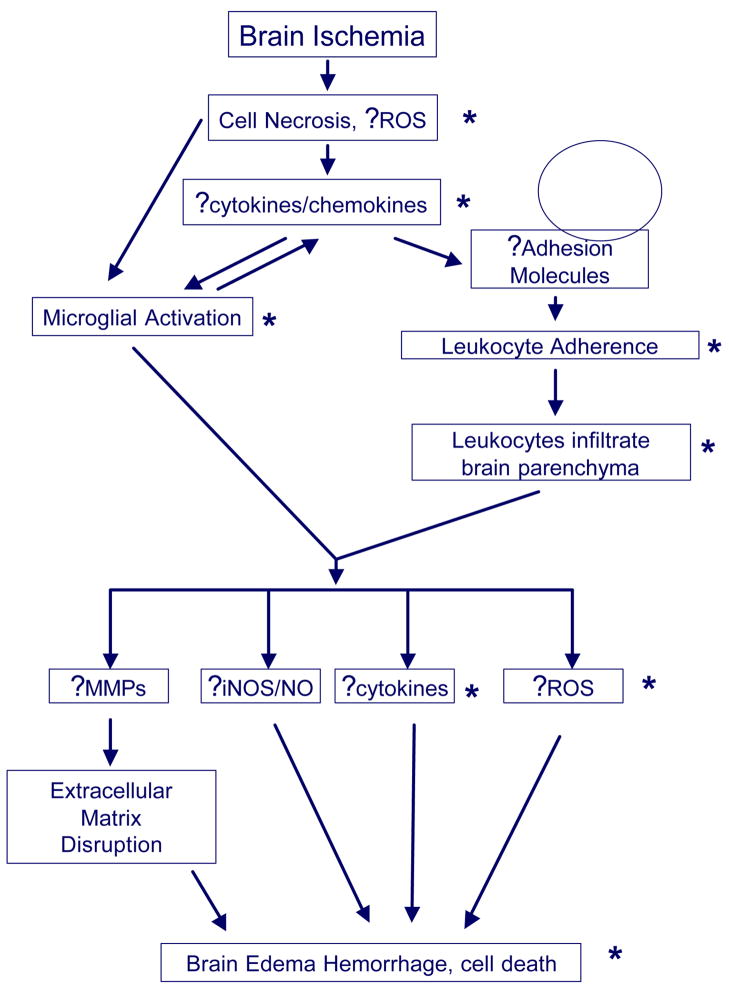
Figure 1. Inflammation following stroke.
Brain ischemia triggers inflammatory responses due to the presence of necrotic cells, generation of reactive oxygen species (ROS) and production of inflammatory cytokines even within neurons. These initiators lead to microglial activation which produce more cytokines causing upregulation of adhesion molecules in the cerebral vasculature. Chemokines lead to inflammatory cell chemotaxis to ischemic brain. Adhesion molecules mediate adhesion of circulating leukocytes to vascular endothelia and infiltration into the brain parenchyma. Once in the brain, activated leukocytes and microglia produce a variety of inflammatory mediators such as matrix metalloproteinases (MMPs), inducible nitric oxide synthase (iNOS) which generates nitric oxide (NO), cytokines and more ROS which lead to brain edema, hemorrhage and ultimately, cell death. MMPs are thought to mediate extracellular matrix disruption, a key event in brain edema and hemorrhage. Asterisks (*) are used to indicate the described differences in events in neonatal compared to adult stroke.
The field of perinatal and neonatal stroke is emerging [16, 17]. Strikingly, the incidence of arterial stroke in newborns, about 1/4,000 term babies [18], is similar to that in the elderly. Although basic mechanisms of neurodegeneration are shared across all age groups, immaturity critically affects brain susceptibility and response to ischemia-related insults (reviewed in [19–22]). The component of injury is not an exception (see recent reviews [21, 23, 24]). While signs of inflammation are rapid after both hypoxic encephalopathy and focal stroke in term babies [16, 17], data for this age group suggests different than in adult involvement of local inflammation, leukocyte-mediated injury, involvement of the BBB and enhanced susceptibility to ROS. While more limited than in adult, the amount of data on various aspects of inflammation after ischemic injury warrants more study. Known differences between adult and neonate are indicated by asterisks in Figure 1. While blocking various aspects of the inflammatory cascade has shown to ameliorate injury from experimental stroke [22, 25], this has yet to be successfully translated to human clinical trials.
2. ACUTE INJURY AFTER STROKE & INFLAMMATION
2-1. SYSTEMIC ACUTE-PHASE RESPONSE
Ischemic brain injury can be amplified by the hepatic release of regulatory acute-phase proteins [26]. The systemic acute-phase response, characterized by hepatic acute phase protein synthesis, is associated with leukocyte mobilization, and changes in serum levels of glucocorticosteroids and cytokines, events that occur even before there is any evidence of an inflammatory response in the brain [4, 26]. Accumulating data suggest that leukocytosis depends on the pattern of cytokine expression in the brain. The CXC chemokine cytokine-induced chemoattractant protein –1 (CINC-1), which amplifies the hepatic response by initiating a dose-dependent neutrophil recruitment to the brain, is one of the first acute-phase proteins to be released from the liver in response to interleukin IL-1β microinjection into the brain, whereas injection of tumor necrosis factor TNF-α induces monocytes rather than neutrophils. The contribution of an acute-phase response as an injury amplifier to stroke in humans has yet to be demonstrated [27, 28].
2-1. LEUKOCYTES
Ischemic injury to the neural parenchyma leads to local production of pro-inflammatory cytokines [29–31] and chemotactic molecules [32, 33], a subsequent increase in numbers of leukocytes in the systemic circulation [34, 35], secondary changes in the adhesion properties of the surrounding vascular endothelium, and site-specific chemotaxis of leukocytes. In adults, neutrophils [8, 31, 36, 37] accumulate in ischemic brain tissue as early as 4–6 hours after ischemia onset. Evidence that neutrophils potentiate ischemic injury include numerous studies documenting improved neurological outcome following neutrophil depletion and inhibition of adhesion molecules which facilitate neutrophil entry into injured brain (reviewed in [38, 39]). Neutrophils can enhance injury via several mechanisms, including ROS production [40], and release of MMP-9 [14], acting both from within the peripheral circulation and after extravasation into injured tissue. The existence of cytokine-specific patterns of monocytes and neutrophil recruitment [35, 41, 42] has been demonstrated by direct intracerebral injections of IL-1β, TNFα, IL-8, or NMDA. IL-1β injection triggered recruitment of neutrophils, but very few monocytes. TNFα injection induced predominantly monocyte infiltration, rather than neutrophils [41]. An excitotoxic lesion produced by NMDA, in turn, profoundly increased IL-1β, but not TNFα, and the corresponding selective infiltration of neutrophils, not monocytes [41]. Macrophage accumulation, which typically occurs within one day after stroke [31, 36], will be discussed later in this chapter. While neutrophils are the earliest leukocyte subpopulation in the brain after stroke, lymphocytes have also been documented [43]. Whether lymphocytes play an active role in ischemic brain pathogenesis is unclear. In a study of cultured primary neurons, isolated neutrophils, but not lymphocytes, potentiated neuronal injury due to excitotoxin exposure [44]. However, preventing lymphocyte trafficking into ischemic brain ameliorated injury, suggesting that, like neutrophils, lymphocytes play a deleterious role [45].
In contrast to stroke in the adult, neutrophils do not transmigrate into brains of postnatal day 7 (P7) rats following H-I within 42 h [46] or are present in the parenchyma only briefly [47]. Rather, neutrophils accumulate within vessels. Lack of transmigration is unlikely to be due to an intrinsic inability of neutrophils to transmigrate into the brain, as they do transmigrate following permanent middle cerebral artery (MCA) occlusion [48]. Circulating or marginated neutrophils, however, can contribute to hypoxic-ischemic (H-I) injury in the newborn period as was shown if neutrophils were depleted prior to H-I [49]. Recent studies [35, 50–52] have shown that the degree and spatial distribution of BBB disruption during the neonatal period is dependent on neutrophils in response to intra-striatal injection of IL-1β [4].
Lymphocytes are generally thought to play a negative role in ischemic brain pathogenesis even though there is also conflicting data. In adult rodents after focal ischemia, T and B cells are seen days after injury [53, 54], whereas in neonates infiltration of these cells following neonatal H-I and focal stroke may be less profound [47, 55] or even transient [48]. Following permanent middle cerebral artery occlusion (MCAO) in rats, lymphocytes were elevated in the ischemic lesion after neutrophils [43, 56]. Preventing lymphocyte trafficking into ischemic brain ameliorated injury, suggesting that like neutrophils, lymphocytes also play a deleterious role [45]. However, in a study of cultured primary neurons, isolated neutrophils, but not lymphocytes potentiated neuronal injury due to excitotoxin exposure [44].
There is increasing evidence for the role of mast cells after neonatal H-I and excitotoxic injury [57, 58]. Injurious effects of mast cells appear to depend on TGF-β and IL-9 [59]. Agents that inhibit histamine release or mast cell degranulation have been shown to diminish excitotoxic brain injury in P5 rats [57, 58]. Consistent with these findings, mast cell-deficient mice suffer less injury than WT controls [58].
2-3. ADHESION MOLECULES
Leukocyte extravasation is separated into discrete steps, which are associated with the interaction of selectins and their ligands, then integrins and other adhesion molecules, and finally chemokines and chemokine receptors. Adhesion molecules may represent important therapeutic targets (see reviews [60–62]), as inhibiting leukocyte adhesion by targeting various adhesion molecules, thus preventing leukocytes from entering ischemic brain, results in reduced neurologic injury [63–66]. The interaction between leukocytes and the vascular endothelium is mediated by three main groups of cell adhesion molecules: selectins (P-selectin, E-selectin, and L-selectin), the immunoglobulin superfamily (intercellular adhesion molecules, e.g. ICAM-1, 2 and vascular cell adhesion molecule-1, or VCAM-1) and integrins (CD11a-c) [61, 62, 67]. Furthermore, adhesion molecules appear most relevant in ischemia with reperfusion, as several anti-adhesion molecule approaches were ineffective when reperfusion did not occur [68–72].
2-3-1. SELECTINS
Selectins mediate the first step of leukocyte infiltration, rolling on the endothelium. P-selectin was originally described on platelets and then described on endothelial cells, and E-selectin was originally described on endothelial cells [73]. L-selectin which was originally described on lymphocytes, is present on all leukocyte populations [74, 75]. While E- and P-selectin are involved in leukocyte rolling and recruitment during the early stages of activation, L-selectin acts as a guide for unstimulated leukocytes [76]. In the presence of activated endothelium, rolling stops and L-selectin is shed from the cell membrane by proteolytic cleavage, followed by endothelial transmigration [77–79].
The induction of P- and E-selectins has been documented in different experimental stroke models [77, 80–83]. E- and P-selectin upregulation appears to be involved in promoting ischemic inflammatory responses and injury exacerbation after ischemic stroke. Mice deficient in P-selectin have smaller infarcts and less neutrophil infiltration after transient MCA occlusion compared to wildtype (WT) littermates [84]. Conversely, mice overexpressing P-selectin have larger infarcts than WT. Furthermore, treatment with antibodies or inhibitors against P- and E-selectin was similarly associated with improved neurological outcome in rodents [83–85] and baboons [86]. The role of L-selectin in brain ischemia is less clear, as preventing its shedding by a blocking antibody, and presumably its ability to mediate leukocyte transmigration, does not appear to significantly influence stroke outcome [87, 88].
Since E-selectin is almost exclusively upregulated in activated endothelium, tolerance to E-selectin could lead to suppression of immune responses and reduce trafficking of peripheral leukocytes to ischemic brain, the notion confirmed by induction of immune tolerance to brain antigens and the consequent reduction of the extent of ischemic injury by exposing animals to E-selectin intranasally [89, 90] [89, 90], or even prevention of stroke [91]. Thus, it is conceivable that this approach could lead to the development of a vaccine against stroke.
Developmental differences in E- and P-selectin may underlie the disparities observed in post-ischemic neutrophil infiltration between adult and immature brain. While some studies have shown similar levels of E- and P-selectin in immature and adults rats [92], lower surface expression of P-selectin on endothelial cells or less efficient adhesion of neonatal neutrophils to P-selectin have been described by others [93, 94] thus leaving open the question on whether selectins are accountable for the minimal in leukocyte transmigration through the BBB.
In humans, polymorphisms in the P-selectin gene were found to be independent predictors of thrombo-embolic stroke [95], and P-selectin, but not necessarily E-selectin, has also been documented in the serum of stroke patients [96], P-selectin may persist in the circulating blood for up to 90 days after stroke [97]. Soluable sE- and sL-selectin have also been detected in stroke patients [98].
2-3-2. INTEGRINS
Integrins are a family of adhesion molecules that are heterodimers consisting of a common β subunit and a variable α subunit [99]. Of the α subunits, there are three subfamilies, denoted α1–3. Members of the α1 subfamily (or VLA, very late activation) bind collagen, laminin and fibronectin and are involved in the structure of the extracellular matrix, whereas α2 integrins (CD18) are involved in leukocyte cell adhesion. β3 integrins, also known as the cytoadhesins, include the platelet glycoprotein IIb/IIIa (αIIb/α3) and the vitronectin receptor (αv/β3), factors involved in clot generation and stabilization.
Leukocyte integrins are transmembrane cell surface proteins activated by chemokines, cytokines, and other chemoattractants. To bind to activated endothelium, integrins must be expressed on the leukocyte’s surface in order to recognize endothelial cell adhesion molecules [100]. The α2 integrins bind to receptors of the immunoglobulin gene superfamily, and contain a common α2 chain with one of 3 distinct α chains (CD11a, CD11b, or CD11c). The CD11a/CD18 integrin is also referred to as LFA-1, whereas CD11b/CD18 is also called Mac-1. Of the α chains, CD11b has been the most studied in stroke models. Leukocytes and monocytes also express the α4β1 (very late antigen-4,VLA-4, CD49d) integrin, which binds to VCAM-1 and ligands of the subendothelial matrix [79].
Blocking CD11b [101–103], CD18 [104–107] or both [107–109] reduces injury from experimental stroke and is associated with decreased neutrophil infiltration. Similarly, neutrophils from mice that lack CD18 exhibited reduced leukocyte adhesion to endothelial cell monolayers, and when these mice were subjected to experimental stroke, they had improved cerebral blood flow and less neurological injury and neutrophil accumulation compared to the wildtype phenotype [68]. Blocking integrins essential for lymphocyte and monocyte trafficking may also limit damage due to reperfusion injury. Antibodies against VLA-4 given 2 h after a 3 h period of temporary MCA occlusion followed by reperfusion decreased infarct size [45].
At the clinical level, two studies examined the potential of anti-integrin therapies in acute stroke patients. In one phase III trial, a humanized CD11/CD18 antibody was administered to patients within 12 h symptom onset [110]. The second trial was a phase IIb dose escalation study of a non-antibody peptide, recombinant neutrophil inhibiting factor (rNIF) in stroke patients (Acute Stroke Therapy by Inhibition of Neutrophils or ASTIN) [111] administered within 6 h of symptom onset. Both studies were stopped prematurely due to a lack of effect on predetermined endpoints. Although both compounds appeared to be effective in preclinical studies [107, 109, 112], lack of an obvious effect in humans could be due to study design not in line with laboratory data (such as late treatment or lack of documented recanalization) or the inherent heterogeneity of clinical stroke. Another possibility is that changes in neutrophil integrins are different in acute ischemic stroke patients compared to rodents. For example, while many integrins are upregulated after stroke in humans, CD11b is actually decreased [113]; therefore, some anti-adhesion molecule approaches may not be appropriate. Regardless, it is clear that more work and possibly improved trial design are needed.
2-3-3.IMMUNOGLOBULIN SUPERFAMILY
Firm adhesion of leukocytes to the endothelial cells as well as leukocyte activation is mediated by receptors of the immunoglobulin superfamily. These molecules cause stronger attachment of leukocytes to the endothelium than the selectins. This family includes 5 molecules that are expressed by endothelial cells: ICAM-1 and ICAM-2, VCAM-1, platelet-endothelial cell adhesion molecule-1 (PECAM-1), and the mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1) [114]. These molecules are involved in leukocyte adhesion at relatively low shear forces. ICAM-1 (CD54) is constitutively present in low levels on the cell membranes found on endothelial cells, leukocytes, epithelial cells, and fibroblasts. Its expression greatly increases upon stimulation by cytokines. ICAM-2 (CD102) is an endothelial cell membrane receptor that does not increase after stimulation, whereas VCAM-1 (CD106) is induced by TNF-α and IL-1. PECAM-1 (CD31) is involved in the attachment of endothelial cells to each other, and leukocyte transmigration across the endothelium.
ICAM-1 has been the most studied target within immunoglobin superfamily adhesion molecules in cerebral ischemia. Increased expression of ICAM-1 in ischemic brain occurs within hours of stroke onset, peaks at about 12–24 h, and appears to precede leukocyte infiltration [115–117]. Several studies have now shown that blocking ICAM-1 with antibodies [103, 106] or inhibiting ICAM-1 mRNA with antisense oligonucleotides [118] improves outcome from experimental stroke. Consistent with these findings, mice deficient in ICAM-1 had smaller infarcts compared to wildtype mice [119–121]. Furthermore, combination treatment with recombinant tissue plasminogen activator (rt-PA) and anti-ICAM-1 antibodies led to reduced infarct size compared to rt-PA treatment alone [71]. Anti-ICAM-1 treatment appears to generally require reperfusion to be effective [72], but one study in ICAM-1 deficient mice demonstrated smaller infarcts in both permanent and transient MCA occlusion [119].
The role of VCAM-1 in stroke is less clear. While increases in VCAM-1 mRNA after cerebral ischemia have been observed by some investigators [122], others have not [118]. One study [123] showed that unfractionated heparin led to reduced infarct size in experimental stroke, and was associated with a reduced inflammatory response including decreased VCAM-1 expression. However, treatment with anti-VCAM-1 antibodies did not have any effect on stroke outcome [124], suggesting that VCAM-1 may not play a significant role in ischemic brain injury.
In humans with stroke, elevated soluble intercellular and vascular cell adhesion molecules-1 (sICAM-1 and sVCAM-1) have been documented [98], and VCAM-1 expression has been observed in autopsied brains of stroke victims within cerebral vessels and astrocytes [122]. Furthermore, stroke patients treated with unfractionated heparin were found to have blunting of the typical rise in serum sVCAM-1 [125]. However, anti-ICAM therapy for stroke was not only ineffective in one phase III clinical trial, but it significantly worsened outcome [126]. The interpretation of this study may have been confounded by the use of a murine antibody in humans, with subsequent neutrophil and complement activation [127].
3. THE NEUROVASCULAR UNIT & INFLAMMATION
The concept of the “neurovascular unit” includes the brain microvasculature, glia, neurons and the extracellular matrix. It has emerged from the understanding that focusing on mechanisms of neuronal death without taking into account the microenvironment which provides support for neurons and their connections, may have hampered successful translation of animal data to human stroke. This concept is especially relevant to the understanding of inflammation and ischemic brain injury.
3-1. BLOOD-BRAIN BARRIER (BBB)
The integrity of the BBB is controlled by a number of different and partially independent components, including the presence of an extracellular matrix, tight junctions, pericytes and astrocyte endfeet [128–130], which provides exclusion of circulating cells and macromolecules. Tight junctions, together with adherens junctions, form junctional complexes and play a central role in the control of paracellular permeability and maintenance of cell polarity [130]; endothelial cells have a poor capacity for pinocytosis precluding trans-cellular movement of solutes in the absence of endothelial fenestrae. As a result, trafficking of leukocytes [2] and solutes [131] occurs in the controllable way. It is well known that in the adult brain, the BBB is disrupted after stroke, with the extent of temporo-spatial damage dependent on both the systemic and local inflammatory reactions. Peripheral leukocytes, especially neutrophils, can contribute to the opening of the BBB [14, 36, 37] and can infiltrate through the disrupted barrier and produce injury by releasing toxic mediators [8, 132]. Many factors affect BBB breakdown [11, 133]. Besides leukocyte activation [131, 134], increased levels of adhesion molecules and degradation of components of the basal lamina by MMPs [135] leads to disruption of the BBB after adult stroke. Microglia [136] and locally and systemically produced cytokines [10, 137] also potentiate damage to BBB constituents.
Emerging evidence suggests that the early postnatal BBB is not as permeable as commonly thought. Although mechanisms of BBB function in the fetus are different than those in adult [138], tight junctions are present early in embryonic development [139], restricting entrance of proteins into the brain in a controllable fashion, and by birth the BBB is functional with no fenestrations [140]. Studies using intra-striatal injections of inflammatory cytokines IL-1β or TNFα [35, 50–52] have shown that regulation of the BBB is age-dependent even during the first 3 weeks of life, with the BBB being more permeable at postnatal day 21 (P21) than in adult or one day old pups, demonstrating that the relationship between BBB integrity and brain maturation is not linear. Importantly, the BBB remains more integrant in P7 than in adult rats at least within 24 hr post-transient MCA occlusion [141]. Mechanisms that keep the BBB relatively preserved are not well understood but may be related to the very limited [46, 47, 49] transmigration of neutrophils in the injured parenchyma during the neonatal period.
3-2. MATRIX METALLOPROTEINASES (MMP) AND EXTRACELLULAR MATRIX
The MMP family of zymogen proteases are involved in the degradation and remodeling of the extracellular matrix under normal conditions. MMPs are found in the cytosol in a pro- or inactivated state, and are cleaved by proteases such as plasmin or other MMPs to their active state. MMP-9 appears to be the most relevant studied in stroke. Expression of the pro- and active MMP-9 is elevated within hours to days after focal stroke in rats [128, 142], mice [143, 144] and humans [145], and is temporally and spatially correlated with a loss of BBB integrity (reviewed in [135, 146]). The damaging role of MMP-9 has been established by reduction of postischemic BBB disruption after MMP inhibition [147] and MMP-9 gene deletion [14, 144, 148]. While postischemic MMP-9 can be produced by various brain cell types, including neurons, astroglia and microglia, and endothelial cells [128, 149], neutrophils contain abundant quantities of pro-MMP-9 in secretory granules [150], and can produce large quantities of MMP-9 upon activation and degranulation [14]. Recently, it was shown that neutrophil, rather than parenchyma derived MMP-9 participate in BBB disruption and injury after experimental stroke using bone marrow chimeras. When MMP-9 deficient bone marrow was transplanted into wildtype (WT) mice, these chimeras demonstrated less injury and BBB disruption compared to WT mice transplanted with WT marrow, indicated that only MMP-9 in circulating immune cells are involved in ischemic injury [14]. Microglia, another major source of the MMPs following ischemia, are also necessary to stimulate astrocytes to generate active MMPs [151]. Importantly, upregulation of brain MMP-9 levels by thrombolytic therapy with tissue plasminogen activator (tPA) poses risks of cerebral hemorrhage after ischemic stroke[152, 153]. In contrast to MMP-9, MMP-2 seems to have no effect on acute brain injury after focal ischemia [154] but may play a role in recovery. The role of tissue inhibitors of metalloproteinases, TIMPs, in regulating activity of MMPs and their effects on neuronal cell death repair after cerebral ischemia, particularly during angiogenesis, has been recently reviewed [12, 146].
3-3. MICROGLIA/MACROPHAGES
Macrophages in ischemic brain lesions derive from three distinct sources, including parenchymal microglial cells, infiltrating macrophages derived from blood monocytes, and perivascular monocytes. Microglia, the resident macrophages of the brain, are the main cell type that provides immuno-surveillance [155]. Microglia populate the developing brain by birth [156] and gradually ramify during the first two weeks of life, as on going programmed cell death during development declines [157]. Microglial cells undergo a graded process of activation in response to injury [158], which involves morphological transformation, increased migratory activity, proliferation, secretion of cytokines, proteases, and other inflammatory mediators, and antigen presentation [158–160]. Different types of insults can activate distinct receptor types and elicit particular subsets of these functions which, in turn, can serve to protect or exacerbate injury [156, 161]. While studies in tissue slices, dissociated cells, and animal models suggest that populations of resting or activated microglia are very diverse morphologically and functionally [160, 162, 163], it is not clear what distinguishes one subpopulation from the other.
Brain ischemia induces activation of microglia through various mechanisms [114, 155]. The number of receptor families on microglia shown to contribute to injury mediated by these cells keeps growing [33, 164, 165] but interestingly, effects are in part mediated by CD14 and toll-like receptor 4 (TLR4) following experimental[166] and human stroke [167]. Activated microglia secrete or express inflammatory cytokines and chemokines [4, 168–172], FAS receptor [55, 173], and iNOS [61, 174, 175], which further activate microglia and propage local inflammatory responses. Microglial cells can damage other cells, including endothelial cells [136], oligodeondrocytes [176], astrocytes [136] and neurons [177, 178], in part via superoxide produced by NADPH oxidase. Activation of microglial cells is often associated with proliferation of these cells [179–181], further amplifying microglial-dependent aspects of injury. In vivo data suggest a general rule that a more severe ischemic brain insult is generally associated with higher macrophage densities. Pharmacological inhibition of cytokine accumulation, such as IL-1 receptor antagonist (IL-1ra) [168], or deficiency of inflammatory cytokines, such as IL-18, IL-6, or deletion of the FAS receptor, reduce injury and diminish various signs of inflammation, including microglial activation [168, 170, 173]. Minocycline, a tetracycline family antibiotic, was shown to provide significant protection against brain ischemia by inhibiting of microglial activation and proliferation in adult [136, 182–186] but show mixed results in neonatal rodents after focal stroke or H-I [187–189].
The relative contribution of resident macrophages (fully activated microglia) versus invading peripheral macrophages after ischemia is unclear, and hampered by a lack of tools to differentiate these two cell populations. Invaded monocytes and microglia (which once activated, become virtually indistinguishable from circulating macrophages) are nearly impossible to distinguish, although some studies have used a pattern of common leukocyte antigen CD45 expression to separate the two populations [43, 159, 190]. Regardless, depletion of peripheral macrophages using liposome encapsulated clodronate failed to affect infarct size [191], suggesting that brain, rather than circulating macrophages are important in ischemia pathogenesis.
Compared to the adult, microglial activation in neonates is much more rapid following transient focal ischemia [192, 193] and excitotoxic injury[194], and continues for weeks following focal ischemia [48, 189] and excitotoxic injury [194]. While it is still unknown why there are such differences between microglial activation in the mature and immature brain, several studies have suggested that distinct steps of maturation and differentiation [195] and the propensity of neurons to undergo apoptosis [196, 197] in the developing brain may contribute to this age-dependency of the microglial response. Activated microglia/macrophages readily accumulate in injured tissue following H-I [47, 171, 198, 199], produce inflammatory cytokines [171], high levels of NO [200] and MMPs [59]. Reduction of microglia/macrophage associated injury is diminished [187, 194] [194] if microglial activation is inhibited [189], thus supporting the idea that microglia contribute to ischemic and excitotoxic injury in the immature brain as well.
While microglial cells can adversely affect acute ischemic injury by secreting toxic inflammatory mediators, they may also be involved in repair and neurogenesis [201]. Microglia/macrophages have the potential to actually protect cells by secreting factors such as transforming growth factor-beta1 (TGF-beta1) and glial cell line-derived neurotrophic factor (GDNF) [202, 203]. Interestingly, administering isolated microglia intracerebroventricularly improved behavioral and histologic outcome in an experimental stroke model [204].
3-4. ASTROCYTES
Astrocytes play an important role within the neurovascular unit. These cells contribute to neuronal homeostasis and the structure and maintenance of the BBB. It has been increasingly recognized that astrocytes also have immune modulating roles in brain and contribute to brain inflammation by expressing major histocompatibility complex (MHC) and costimulatory molecules, developing Th2 (anti-inflammatory) immune responses and suppressing interleukin-12 (IL-12) expression [205]. After cerebral ischemia astrocytes become a major source of inflammatory mediators such as cytokines, chemokines [206–208] and inducible nitric oxide synthase (iNOS) [209, 210]. Death of astrocytes in ischemic core shortly cerebral ischemia may have important implications for excitotoxicity, anti-oxidative defenses and integrity of the BBB. So-called "reactive gliosis" which is characterized by specific structural and functional changes, is observed acutely in ischemic penumbra and, later, in the core [211]. While the function of glial fibrillary acidic protein (GFAP) in brain ischemia is controversial, GFAP-null mice had significantly larger cortical infarct volumes than their wild-type littermates, suggesting a beneficial role of GFAP [212]. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis factor superfamily, can stimulate proinflammatory molecule production by interaction with its Fn14 receptor found on astrocytes [213]. Expression of TWEAK and Fn14 has been documented in a murine model of stroke, and a soluble decoy to Fn14 markedly reduced infarct volume [214]. These data may suggest that while astrocytes normally play important roles in neuron maintenance and function, activated astrocytes have the potential to pose harm to ischemic brain.
Similar to that observed in adult ischemia models, GFAP immunoreactive astrocytes are rarely seen within the ischemic core but are abundant in penumbra regions 24 hr after transient focal ischemia in P7 rats [192]. While mechanisms of astrocytic death in the immature post-ischemic brain are not well understood, at least a subpopulation of these cells is dying in a caspase-3 dependent manner [48, 215]. Caspase-3-dependent death of GFAP –immunoreactive astrocytes is seen early after permanent MCA occlusion and transient CCA occlusion in P7 rats [48, 215] and is associated with increased BAX expression [48], cytochrome c release from mitochondria, DNA fragmentation and PARP cleavage [216, 217]. The fate of astrocytes has been shown to be affected by microglial activation after H-I in immature rodents [218].
4. MEDIATORS OF INFLAMMATION
4-1. CYTOKINES
Cytokines are upregulated in the brain after a variety of insults including stroke. They are expressed not only in cells of the immune system, but also through endogenous production by resident brain cells, including glia and neurons [219, 220]. The most studied cytokines related to inflammation in stroke are IL-1, TNF-α, IL-6, IL-10 and transforming growth factor- β (TGF- β) [25].
4-1-1. IL-1
IL-1 is a 17-kDa polypeptide and exists two isoforms, IL-1α and IL-1β and its endogenous inhibitor, IL-1 receptor antagonist (IL-1ra) [10]. In experimental stroke, IL-1beta mRNA elevations have been documented within 15–30 min after ischemia [221, 222], with protein detected a few hours later [223]. Much of the data to date suggests that IL-1 potentiates brain injury in experimental stroke [224]. Increased brain damage occurred when IL-1beta was administered to rats [219], and mice deficient in IL-1 have smaller infarcts compared to wildtype mice. In rat models of focal ischemia, administration of IL-1ra [225] or its overexpression by adenoviral vectors [226] has been shown to reduce neurologic deficits and infarct size. IL-1 has two receptors, IL-1R1 and IL-1R2, but only the former is involved in signal transduction [223]. However, IL-1β does not appear to act through IL-1R1 in brain ischemia, as IL-1β administration increased infarct volume even in IL-1R1 deficient mice [224, 227]. Focal transient ischemia in neonates induces a rapid and sustained increase of IL-1β in circulation and then in the brain [189, 228].
4-1-2. TNFα
TNFα is also upregulated in the adult brain after ischemia with similar expression patterns as IL-1β initial increases are seen 1–3 h after ischemia onset [220, 229, 230]. TNFα expression was initially observed in neurons [220], then later in microglia and some astrocytes [231]. Expression is also biphasic with a second increase appearing at 24 and 48 h [229]. TNFα appears to have pleiotropic functions in the ischemic brain [229]. Inhibition of TNFα reduces ischemic brain injury [29, 232], while administration of recombinant TNF-α protein after stroke onset worsens ischemic brain damage [233]. However, TNFα may also protect the brain under certain circumstances. TNFα appears to be involved in the phenomenon of ischemic tolerance [234], and mice deficient in TNF receptors have larger infarcts [235, 236].
The reasons for this disparity might be due to different pathways through which TNFα signals. There are at least two TNFα receptors: TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). Most effects induced by TNFα are mediated by TNFR1. TNFR1 contains a death domain (DD) that interacts directly with TNFR1 and may act as a bifurcation point for signaling related to cell death or cell survival. By reacting with Fas-associated death domain (FADD) and caspase-8, TNFα may lead to apoptosis. Reacting with TNF-receptor associating factor 2 and receptor-interacting protein may lead to anti-inflammatory and anti-apoptotic functions (reviewed in [8, 9]). Whether and how this applies in brain ischemia has yet to be clearly elucidated.
The pathophysiological role of TNFα in neonatal stroke is assumed but yet to be proven. While neonatal mice lacking functional Fas death receptors are resistant to H-I brain injury [173], perhaps in part due to a dependence on TNFα, but no change in TNFα levels was observed in injured ischemia-reperfused brain of animals of the same age [189].
4-1-3
IL-6 is largely thought of as a pro-inflammatory cytokine, but whether it plays a significant role in ischemic stroke is far from clear. IL-6 deficient mice have similar sized infarcts compared to wildtype suggesting that it does not participate in ischemic pathogenesis [237]. However, other studies suggest either a beneficial [238] or detrimental role [239]. IL-6 appears transiently several hours after ischemia [169, 170], and blocking its actions protects the neonatal brain [168].
4-1-4
IL-10 is an anti-inflammatory cytokine and acts by inhibiting IL-1 and TNFα and also by suppressing cytokine receptor expression, and inhibiting receptor activation. It is synthesized in the CNS and is upregulated following experimental stroke [240]. Exogenous administration [241, 242] and gene transfer of IL-10 [243] in cerebral ischemia models both appear to have a protective effect. It also reduced excitotoxic brain lesions in neonatal mice [244]. This pleotrophic Th2 cytokine can act on both hematopoietic and non-hematopoietic cells and can reverse injury caused by IL-1β, TNF-a and IL-6 [244]. Importantly, protection is seen only when IL-10 is administered post-insult, whereas treatment prior to or at the time of insult is ineffective [244]. IL-10 also reduces macrophage accumulation in the injured brain.
4-1-5
IL-18 is a less studied pro-inflammatory cytokine. IL-18 becomes active upon cleavage by caspase-1 in a way similar to that for IL-1β, and occurs predominantly in activated microglia and monocytes. One study in stroke patients suggested that IL-18 is involved in stroke-induced inflammation and that initial serum IL-18 levels may be predictive of stroke outcome [245]. In experimental stroke models, IL-18 levels increase in injured tissue [189]. The role of this cytokine is still unclear. Deletion of the IL-18 gene differentially affected injury in adult and neonatal rodents. There was no effect on infarct volume in adults [246], but there was reduced H-I injury in neonates [170].
4-1-6
TGF-β1, a member of a family of multifunctional peptide growth factors, is considered an anti-inflammatory cytokine that can affect survival of cells of the CNS. TGF-β1 is involved in the modulation of cell growth, differentiation, angiogenesis, immune function, extra-cellular matrix production, cell chemotaxis, apoptosis and hematopoiesis [247]. Its expression has been reported in microglia and astrocytes, with low levels in neurons. Overexpression of TGF- 1 using an adenoviral vector protected mouse brains from ischemic stroke and reduced the accompanying inflammatory response [248]. A recent study showed that cultured neurons may be protected from ischemia-like insults by microglia-secreted TGF-β1 [203].
4-2. CHEMOKINES
Chemoattractant cytokines, or chemokines, and their receptors exert a variety of physiological functions, including control of cell migration, proliferation, differentiation and angiogenesis under normal and disease states [33]. An important role for β (CC), α (CXC) and δ (CXC3) classes of chemokines has been documented in many animal models of neurodegeneration, including stroke [249, 250]. They are classified into four classes based on the positions of key cysteine residues (C): C, CC, CXC, and CX3C, and act through specific and shared receptors belonging to the superfamily of G-protein-coupled receptors [32]. While chemokines play a central role in leukocyte physiology by controlling inflammatory cell trafficking, the functional consequences of chemokine receptor activation are not limited to leukocyte chemotaxis, but include cell activation, gene transcription, mitogenic effects, apoptosis, an increase in the respiratory burst, degranulation, phagocytosis, and lipid mediator synthesis (reviewed by Horuk [251, 252]). In the CNS, activated glial cells are almost the exclusive source of intra-parenchymal cytokine production [253]. Accumulated data indicate that a variety of chemokines are induced in the animal models of focal cerebral ischemia.
4-2-1. MCP-1
The central role of MCP-1 and its only known receptor, CCR2 [254], in monocyte transmigration into the brain was demonstrated by profound deficits in the recruitment of monocytes to sites of inflammation and injury in CCR2 deleted mice [255, 256], but increased brain cytokine production and brain injury in CCR2 overexpressing mice [257]. Increases in MCP-1 expression in the CNS is seen in a variety of acute insults, including ischemia [206, 258, 259]. A variety of cells has been shown to express MCP-1 after focal ischemia [206]. Consistent with a deleterious role, its inhibition or deficiency is associated with reduced injury [260, 261], whereas overexpression of MCP-1 exacerbats injury [259].
In newborn rodents, MCP-1 is expressed following H-I [262], transient focal ischemia [189] and excitotoxic insults [263]. The pathophysiologic role of MCP-1 in neonatal brain injury was evident by protection of the neonatal brain by functional inactivation of MCP-1 post-insult [263] or in mice with depleted intrelukin-1 converting enzyme [262].
4-2-2. MIP-1α
Macrophage inflammatory protein-1α (MIP-1α) is induced in the animal models of focal cerebral ischemia in adults [253, 264–266] and in neonates [171]. It is thought to contribute to ischemic injury by regulating monocyte and microglial recruitment and activation and disruption of the BBB [267].
4-2-3. IL-8, CINC-1/KC
These ELR+ CXC chemokines exhibit potent chemoattractant activities towards neutrophils and show angiogenic activities [268, 269]. All ELR+ CXC chemokines except IL-8 bind to CXCR2 with high affinity, while IL-8 binds to both CXCR1 and CXCR2. While two functional high affinity CXC receptors for IL-8 are identified in human [270, 271], only CXCR2 is described in rat [272]. Both CXCR1 and CXCR2 are predominantly expressed in immune cells, and to a lesser extent by a variety of cells, including glial cells [273] and cerebellar and cortical neurons [252, 274]. Transient increase of the CXCR2 ligand, CINC-1 has been reported acutely following transient ischemia in adult [250]. IL-8 [42] and CINC-1/KC [35] are thought responsible for the neutrophil-mediated BBB disruption after brain injury.
4-2-4. Fractalkine
The CX3C chemokine, fractalkine is expressed almost exclusively in neurons. Following ischemia, expression has been localized to viable neurons in the infarct periphery as well as some endothelial cells. Interestingly, expression of its receptor, CX3CR1 was observed only on microglia/macrophages [275]. The significance of these observations are not entirely clear, but fractalkine deficiency is associated with reduced infarct size suggesting that fractalkine may mediate neuron-microglial interactions and cell death [276].
4-2-5. SDF-1
With recent interest in the area of cell based therapy for stroke, chemokines may also play an important role in honing stem cells to regions of injury. Several chemokines such as MCP-1 and SDF-1 and/or their receptors have been observed in the penumbra [132, 277] and at the interface of ischemic tissue and cell transplants [132, 278]. Inhibiting these chemokines reduced stem cell migration into ischemic tissue [278, 279].
4-3. REACTIVE OXYGEN SPECIES
Once activated, the inflammatory cells in the brain generate ROS as a defense mechanism against microbes. Inflammatory cells generate ROS via several enzyme systems. Superoxide is generated via COX, xanthine dehydrogenase, xanthine oxidase and NADPH oxidase, whereas myeloperoxidase (MPO) and monoamine oxidase (MAO) generate hypochlorous acid and H2O2.. Superoxide anion is a major oxidant generated in the brain parenchyma after MCA occlusiob, and is well known to cause direct injury to ischemic brain as well as to react with NO to generate peroxynitrite [280].
NADPH oxidase is a multicomponent enzyme that consists of two membrane bound subunits, gp91 and p22, and three cytosolic subunits, p67, p47 and p40 plus Rac, a small GTPase [281]. With appropriate stimuli, the cytosolic subunits translocate to the membrane where they interact with the membrane bound subunits to transfer electrons from NADPH to oxygen to form superoxide. Prior work has shown that NADPH oxidase deficient mice have smaller infarcts than wild type mice. Furthermore, when bone marrow from NADPH oxidase deficient mice were transplanted into either wildtype or deficient mice, wildtype mice transplanted with deficient marrow suffered the same amount of injury as wildtype mice transplanted with wildtype marrow. These latter data would suggest that NADPH oxidase generated within endogenous brain cells such as microglia, rather than circulating leukocytes, are responsible for the worsened ischemic damage [282]. Studies in brain slices [283] and co-cultured oligodendrocytes and microglial cells [176] also suggest the direct injurious role of microglial NADPH oxidase.
MPO, found in neutrophils and monocytes but not macrophages, is thought to mediate bactericidal killing through H2O2 and hypochlorous acid. However, very little has been directly investigated regarding its role in ischemic brain pathophysiology. One study subjected MPO deficient mice to focal cerebral ischemia and found that infarct size was paradoxically increased [284]. The authors subsequently found that these MPO deficient mice also had increased products of nitrosylation within the ischemic brain. Subsequent work suggested that MPO‘s protective effect may be due to its ability to scavenge nitrotyrosine (a by product of peroxynitrite reactions) in the presence of glutathione [284, 285]. Related work has also shown that MPO contributes to the termination of neutrophil influx, as MPO-deficient leukocytes can exhibit a stronger and more prolonged respiratory burst [286]. Therefore, it is possible that MPO may actually limit the extent of ROS-mediated tissue injury.
ROS-mediated injury is a major cause of injury in the developing brain [287, 288] due to imbalanced brain antioxidative defense mechanisms, high concentrations of unsaturated fatty acids [289], high rates of oxygen consumption, low concentrations of antioxidants, an imbalance between anti-oxidative enzymes, including catalase, CuZn-superoxide dismutase (SOD1), mitochondrial SOD2 and glutathione peroxidase (Gpx) [290–293], and availability of unbound iron. All of these factors contribute to the vulnerability of the immature brain to oxidative damage, as described in several recent reviews [20, 22, 24, 294].
4-4. NITRIC OXIDE/NITRIC OXIDE SYNTHASE
Nitric oxide (NO) is a small, relatively stable, free-radical gas that readily diffuses into cells and cell membranes where it reacts with molecular targets. NO is produced via conversion of L-arginine by three different isoforms of nitric oxide synthase (NOS). Inducible NOS (iNOS), produced by several cell types following brain injury [295–297] but is acutely found in activated microglia [61, 298]. NO generation can have opposing roles in the process of ischemic injury, protective when generated by endothelial NOS (eNOS) and detrimental when produced by neuronal NOS (nNOS) and iNOS [299, 300], making nonspecific NOS inhibitors undesirable [301]. NO that accumulates locally in the brain can directly damage cell constituents, produce a number of toxic species, such as peroxynitrate [302], activate COX-2 [303], or activate inflammatory cells [298]. Consistent with the notion that iNOS is injurious in ischemia, deletion of iNOS [304] or iNOS inhibition [296, 305, 306] is neuroptrotective in neonatal and adult animals. The spatial and temporal patterns of iNOS expression and activity are not uniform following ischemia-reperfusion. While some studies in adult rats found increased iNOS expression/activity in penumbra but not in ischemic core within 6–48 hr post 2 hr MCA occlusion [307], other studies showed a more delayed onset of iNOS up-regulation [308].
The pathophysiological role of iNOS in neonatal brain injury has been established by data that showed both elevated iNOS message and/or protein in rodent models of birth asphyxia [293], neonatal excitotoxic damage [295] and H-I [200, 309], as well as the ability to ameliorate ischemic injury and NO synthesis in rat brain by pharmacologic inhibition of iNOS [200, 309]. In contrast, following focal ischemia-reperfusion in neonatal rats, iNOS inhibition attenuated activation of mediators of neuronal death without affecting activation or proliferation of microglial cells or reducing injury volume [175]. Inhibition of iNOS, appeared to have deleterious effects in neonatal meningitis model [310].
4-5. ARACHIDONIC ACID METABOLITES
The arachidonic acid (AA) cascade is initiated by the activation of phospholipase A2 (PLA2) [311]. Energy failure due to disruption of blood flow can result in calcium accumulation in brain cells. Calcium then activates PLA2 which hydrolyses glycerophospholipids to release AA. The cytosolic form, cPLA2 is found in reactive astrocytes and microglia following cerebral ischemia [312]. It is an important enzyme in ischemia, as cPLA2 deficient mice had smaller infarcts and developed less brain edema with fewer neurological deficits than their wild type littermates [313]. Arachidonic acid metabolites are potent mediators that contribute to post-ischemic brain inflammation and circulatory disorders [314]. AA is metabolized through two different pathways via cyclooxygenase (COX) or lipoxygenase (LOX).
4-5-1. Cyclooxygenase pathway
Arachidonic acid released from brain phospholipids during ischemia/reperfusion is converted to prostaglandin H2 (PGH2) by cyclooxygenase (COX). Among the two isoforms, COX-1 is constitutively expressed in many cells types, including microglia and leukocytes during brain injury [315] and COX-2 is an inducible isoform increased following ischemia [316, 317]. COX-1 deficient mice have increased vulnerability to brain ischemia, and would support a protective role possibly due to an effect on maintaining cerebral blood flow [11]. PGH2 is further metabolized to a variety of prostaglandins such as prostacyclin (PGI2) and thromboxane A2.(TXA2). The roles of various COX metabolites are protean, but accumulated data suggest that those downstream of COX-2 are likely deleterious. Several studies have now shown that treatment with COX-2 inhibitors improve neurological outcome after stroke [318–321]. COX-2 deficient mice have reduced injury after N-methyl-D-aspartate (NMDA) exposure [322], whereas COX-2 overexpression exacerbates brain injury [323]. COX-2 activation at least in part depends on iNOS activation [303]. Interestingly, COX-2 mediates its toxic effect through PGE2 rather than ROS, even though COX-2 can generate both [324].
4-5-2. 5-Lipoxygenase pathway
Compared to the COX pathway, less work has been done to study interventions in the lipoxygenase pathway, but like COX, modulators of lipoxygenase have the potential to improve the stroke outcome as well. AA can be converted to 5-hydroperoxyeicosatetraenoic acid (5-HPETE) by 5-lipoxygenase (5-LOX) which is metabolized to leukotriene A4 (LTA4), a precursor of cysteinyl leukotrienes (cysLTs). cysLTs are proinflammatory lipid mediators and consist of leukotriene C4 (LTC4), D4 (LTD4) and E4 (LTE4). LTC4 synthase is found exclusively in inflammatory cells of hematopoietic origin, and converts LTA2 to LTC4 after conjugation with glutathione [325]. LTC4 is a potent chemoattractant that has been implicated in the BBB dysfunction, edema and neuronal death after ischemia/reperfusion. During brain ischemia/reperfusion, biphasic AA and LTC4 elevations have been documented and appear to correspond to biphasic patterns of BBB disruption [326]. Pretreatment with a 5-lipoxygenase inhibitor, AA861 resulted in significant attenuation of LTC4 levels and reduction in brain edema and cell death [327]. Although little work has been done to study interventions in the lipoxygenase pathway, but have the potential to improve the stroke outcome.
5. INTRACELLULAR INFLAMMATORY SIGNALING MECHANISMS
Cerebral ischemia upregulates gene expression [328–331], including rapid transcriptional activation of pro-inflammatory factors. Some of these transcription factors are particularly involved in the inflammatory response, and will be discussed here. (Figure 2).
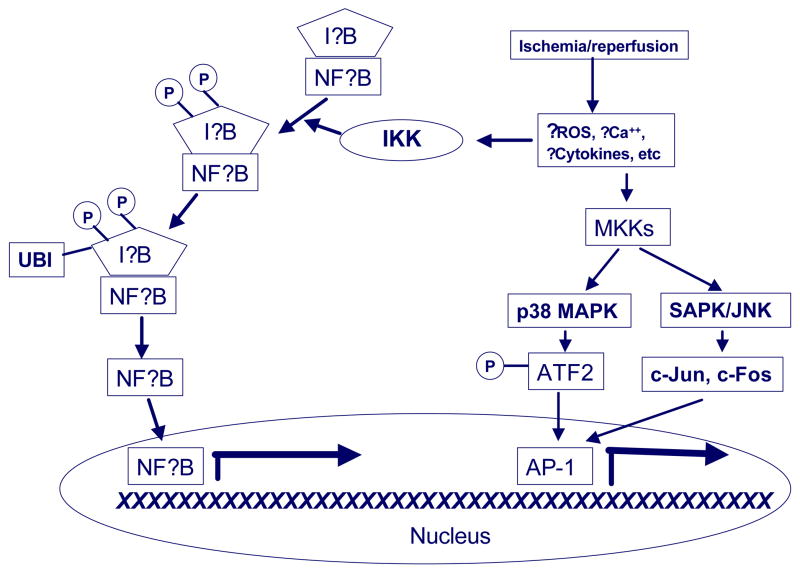
Figure 2. Transcriptional activation of inflammation following ischemia.
Brain ischemia leads to the generation of a variety of substances that are capable of activating various inflammatory transcription factors (see text section 3 for details). Ischemia triggers the activation of the following inflammation relevant transcription factors: nuclear factor kappa B (NFkB), the p38 mitogen activated protein kinase (p38 MAPK), and stress activated protein kinase (SAPK/JNK). NFkB is normally contained in the cytosol by its inhibitor protein (IkB). When phosphorylated by its kinase, IKK (P), IkB is ubiquitinated (UBI) and degraded in the proteasome. Once liberated from IkB, NFkB translocates to the nucleus where it binds to consensus DNA sequences. p38 MAPK and SAPK/JNK are activated in a tiered fashion by MAPK kinase (MKK). Activated p38 MAPK phosphorylates activating transcription factor (ATF2), while SAPK/JNK leads to c-Jun and c-Fos expression. These three factors make up the transcription factor, activator protein-1 (AP-1).
5-1. NUCLEAR FACTOR kappa Beta (NF-kB)
NF-kB, is a dimeric transcription factor consisting of subunits of the Rel family, and are involved in the regulation of inflammation [332, 333]. The most common form of NF-kB is a heterodimer composed of Rel A (p65) and p50. NF-kB is normally located in the cytoplasm bound to its endogenous inhibitor protein, known as IkB. Phosphorylation of IkB at serines 32 and 36 by an upstream IkB kinase (IKK) leads to IkB phosphorylation, ubiquitination and degradation in the 26S proteasome. This liberates NF-kB and allows it to translocate to the nucleus. Once in the nucleus, NF-kB binds to κB sites, specific domains within the promoters of downstream genes to activate their transcription. Many genes involved in inflammation contain functional kB sites, such as TNF-α, ICAM-1, COX-2, iNOS and IL-6. Following experimental stroke, IKK and NF-kB activation have been documented, and these processes appear to be correlated to the anti-inflammatory effect of mild hypothermia [334]. However, the function of NF-kB in stroke is still controversial [335]. Mice deficient in NF-kB’s p50 subunit are protected from experimental stroke [336], consistent with a death-promoting role of NF-kB in focal ischemia. However, another study demonstrated that rats given diethyldithiocarbamate (DDTC), a NF-kB inhibitor, had enhanced neuronal DNA fragmentation and larger infarct sizes compared to controls, suggesting a beneficial role [337]. A potential role for IKK inhibitors in stroke therapy has been demonstrated by data where mice that contain a targeted deletion of IkBkb (which encodes IKK2) in mouse neurons and mice that express a dominant inhibitor of IKK in neurons have markedly reduced infarct size, whereas constitutive activation of IKK2 enlarges infarct size [338].
5-2. MITOGEN-ACTIVATED PROTEIN KINASE (MAPK)
Mitogen-activated protein kinases play an important role in transducing stress-related signals by a cascade of intracellular kinase phosphorylation and transcription factor activation that regulate inflammatory gene production among other functions (for reviews, see [339-342]). There are three well characterized, interlinked signaling pathways which have been documented following brain ischemia: the stress-activated protein kinases/c-Jun N-terminal kinases (SAPK/JNK), the p38 MAPKs and extracellular signal-regulated kinases (ERKs) [340, 342–344]. Of these, p38 MAPK appears to be the best studied in terms of promoting inflammatory signals in the ischemic brain [339]. p38 MAPK promotes the stabilization and enhanced translation of mRNAs encoding proinflammatory proteins [342]. p38 plays a role in the induction of the inflammatory and immune response via the regulation of NF-kB recruitment to selected targets [345]. It is activated by inflammatory cytokines such as IL-1 and TNF, both of which have been documented in the ischemic brain. Following activation and phosphorylation by its upstream kinase, MAPK kinase (MKK), p38 phosphorylates and activates the transcription factor, activating transcription factor (ATF2), whereas phosphorylation of JNK induces c-Jun. The role of JNK in neurodegeneration was recently reviewed [346].
Following forebrain ischemia in rodents, phosphorylated p38 MAPK was detected in the hippocampus within neuron- [344] and microglia-like [347] cells, suggesting its role in the endogenous inflammatory response. Furthermore, p38 MAPK inhibitors reduced brain injury and neurological deficits in focal cerebral ischemia as well as ischemia-induced cytokine expression. SB239063, a second generation inhibitor also reduced lipopolysaccharide (LPS)-induced plasma TNF production [348].
While p38 inhibitors showed some promise in adult stroke models, the role of this kinase is less clear in neonatal ischemic injury. While increased p38 phosphorylation was observed after HI in neonatal rats brain [349], profound reduction of p38 phosphorylation was observed in injured brain after transient focal ischemia [189].
5-3. ACTIVATOR PROTEIN -1 (AP-1)
AP-1 is a heterodimer comprised of bZIP transcription factors (e.g., c-Jun and JunD), activating transcription factor 2 (ATF2) and c-Fos. Upstream activation of AP-1 components is mediated through the JNK/SAPK cascade. c-Fos was found to be up regulated as early as 30 minutes after stroke onset [350]. The Fos protein contains a DNA binding region and a leucine zipper. The leucine zipper forms an α-helix which can align with other proteins (such as Jun) containing like structures to form dimers. These dimers bind to specific DNA regions known as the AP-1 domain, which regulates the expression of a number of target genes (collectively referred to as late response genes). Combinations of c-Fos and c-Jun family proteins form different dimers consisting of various subunits depending on the circumstances. The composition of the dimer may determine whether the late response gene is turned on or off [351]. ATF2 is a member of the cAMP response element binding protein (CREB) subfamily of bZIP transcription factors. AP-1 heterodimers containing ATF transcription factors can bind both to the TPA (12-O-tetradecanoylphorbol-13-acetate) responsive element (TRE) and to the cAMP response element (CRE) [352]. AP-1 is an important activator of a number of inflammatory genes including the genes for IL-1 and TNF. In addition, AP-1 participates in the transcriptional induction of cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and E-selectin which are involved in leukocyte recruitment [342, 352, 353].
6. SUMMARY
Stroke triggers a robust inflammatory response in both adult and neonatal brain. An overwhelming number of publications have linked post-ischemic inflammation to the progression of brain damage. Thus, anti-inflammatory therapies may be effective against stroke. However, this has yet to be shown at the clinical level. Furthermore, inflammation is also a host defense against pathogens, and inflammation plays a role in angiogenesis, tissue remodeling and regeneration [201, 354]. Though the potential benefits of post-ischemic inflammation have not been well documented, at least some cytokines or other inflammatory mediators produced by inflammatory cells showed to be neuroprotective after stroke. Whether inflammation is destructive or protective may depend on how severe the ischemia is, how inflammatory cells are activated and the stage of stroke in which inflammatory responses contribute. For the developing brain, studies are also needed to determine whether anti-inflammatory agents have adverse effects on the long-term brain development. Future work should address the optimal timing of inflammation modulating interventions as well as elucidating how the immune system moves from damaging to protective/restorative responses and how these stages are affected by genetic background, age or gender.
Acknowledgments
This work was supported by NIH NINDS grants RO1 NS44025 (ZSV), P50 NS35902 (ZSV), RO1 NS40516 (MAY), P01 NS37520 (MAY), & P50 NS014543 (MAY), American Heart Association Established Investigator Award (MAY), United Cerebral Palsy Grant R766-04 (ZSV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabry Z, Raine CS, Hart MN. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994;15(5):218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 3.Pan W, Kastin AJ. Changing the chemokine gradient: CINC1 crosses the blood-brain barrier. J Neuroimmunol. 2001;115(1–2):64–70. doi: 10.1016/s0165-5728(01)00256-9. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, et al. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166(5):1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18(5):407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Davies CA, Loddick SA, Stroemer RP, Hunt J, Rothwell NJ. An integrated analysis of the progression of cell responses induced by permanent focal middle cerebral artery occlusion in the rat. Exp Neurol. 1998;154(1):199–212. doi: 10.1006/exnr.1998.6891. [DOI] [PubMed] [Google Scholar]
- 7.Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327(1):123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- 8.Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl. 1996;66:27–31. doi: 10.1007/978-3-7091-9465-2_5. [DOI] [PubMed] [Google Scholar]
- 9.Lovering F, Zhang Y. Therapeutic potential of TACE inhibitors in stroke. Curr Drug Targets CNS Neurol Disord. 2005;4(2):161–168. doi: 10.2174/1568007053544147. [DOI] [PubMed] [Google Scholar]
- 10.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 11.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14(1):89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29(10):2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 13.Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22(12):1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 14.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289(2):H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 15.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3(3):150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 18.deVeber G, Roach ES, Riela AR, Wiznitzer M. Stroke in children: recognition, treatment, and future directions. Semin Pediatr Neurol. 2000;7(4):309–317. doi: 10.1053/spen.2000.20074. [DOI] [PubMed] [Google Scholar]
- 19.Vexler ZS, Ferriero DM. Molecular and biochemical mechanisms of perinatal brain injury. Semin Neonatol. 2001;6(2):99–108. doi: 10.1053/siny.2001.0041. [DOI] [PubMed] [Google Scholar]
- 20.Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207(Pt 18):3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- 21.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28(6):405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Vexler ZS, Ferriero DM. Mechanisms of Ischemic Cell Death in the Developing Brain. In: Chan P, editor. Handbook of Neurochemistry. 2006. [Google Scholar]
- 23.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18(2):117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- 24.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30(4):227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4(5):522–529. [PubMed] [Google Scholar]
- 26.Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab. 2002;22(3):318–326. doi: 10.1097/00004647-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Palasik W, Fiszer U, Lechowicz W, Czartoryska B, Krzesiewicz M, Lugowska A. Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. Eur Neurol. 2005;53(4):188–193. doi: 10.1159/000086355. [DOI] [PubMed] [Google Scholar]
- 28.Marquardt L, Ruf A, Mansmann U, Winter R, Buggle F, Kallenberg K, et al. Inflammatory response after acute ischemic stroke. J Neurol Sci. 2005;236(1–2):65–71. doi: 10.1016/j.jns.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Feuerstein G, Wang X, Barone FC. Cytokines in brain ischemia--the role of TNF alpha. Cell Mol Neurobiol. 1998;18(6):695–701. doi: 10.1023/A:1020690004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19(8):819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991;29(3):336–345. doi: 10.1002/jnr.490290309. [DOI] [PubMed] [Google Scholar]
- 32.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 33.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2(2):108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 34.Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, et al. CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. Faseb J. 2003;17(9):1168–1170. doi: 10.1096/fj.02-0757fje. [DOI] [PubMed] [Google Scholar]
- 35.Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, et al. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8(16):923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- 36.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144(1):188–199. [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, Kogure K. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab. 1995;15(6):941–947. doi: 10.1038/jcbfm.1995.119. [DOI] [PubMed] [Google Scholar]
- 38.Hartl R, Schurer L, Schmid-Schonbein GW, del Zoppo GJ. Experimental antileukocyte interventions in cerebral ischemia. J Cereb Blood Flow Metab. 1996;16(6):1108–1119. doi: 10.1097/00004647-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26(8):884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 40.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, et al. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114(1):49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blond D, Campbell SJ, Butchart AG, Perry VH, Anthony DC. Differential induction of interleukin-1beta and tumour necrosis factor-alpha may account for specific patterns of leukocyte recruitment in the brain. Brain Res. 2002;958(1):89–99. doi: 10.1016/s0006-8993(02)03473-x. [DOI] [PubMed] [Google Scholar]
- 42.Veldhuis WB, Floris S, van der Meide PH, Vos IM, de Vries HE, Dijkstra CD, et al. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J Cereb Blood Flow Metab. 2003;23(9):1060–1069. doi: 10.1097/01.WCB.0000080701.47016.24. [DOI] [PubMed] [Google Scholar]
- 43.Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932(1–2):110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- 44.Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A. 2004:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32(1):206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- 46.Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J. The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res. 1997;41(5):607–616. doi: 10.1203/00006450-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Bona E, Andersson AL, Blomgren K, Gilland E, Puka-Sundvall M, Gustafson K, et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatr Res. 1999;45(4 Pt 1):500–509. doi: 10.1203/00006450-199904010-00008. [DOI] [PubMed] [Google Scholar]
- 48.Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal Rat. Stroke. 1999;30(9):1916–1923. doi: 10.1161/01.str.30.9.1916. discussion 1923–1914. [DOI] [PubMed] [Google Scholar]
- 49.Palmer C, Roberts RL, Young PI. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic-ischemic brain injury. Pediatr Res. 2004;55(4):549–556. doi: 10.1203/01.PDR.0000113546.03897.FC. [DOI] [PubMed] [Google Scholar]
- 50.Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120 ( Pt 3):435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 51.Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58(3):245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci. 2000;20(21):8153–8159. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 54.Catania A, Lipton JM. Peptide modulation of fever and inflammation within the brain. Ann N Y Acad Sci. 1998;856:62–68. doi: 10.1111/j.1749-6632.1998.tb08313.x. [DOI] [PubMed] [Google Scholar]
- 55.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21(6):1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li GZ, Zhong D, Yang LM, Sun B, Zhong ZH, Yin YH, et al. Expression of interleukin-17 in ischemic brain tissue. Scand J Immunol. 2005:481–486. doi: 10.1111/j.1365-3083.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 57.Patkai J, Mesples B, Dommergues MA, Fromont G, Thornton EM, Renauld JC, et al. Deleterious effects of IL-9-activated mast cells and neuroprotection by antihistamine drugs in the developing mouse brain. Pediatr Res. 2001;50(2):222–230. doi: 10.1203/00006450-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Mesples B, Fontaine RH, Lelievre V, Launay JM, Gressens P. Neuronal TGF-beta1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice. Neurobiol Dis. 2005;18(1):193–205. doi: 10.1016/j.nbd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Hedtjarn M, Mallard C, Hagberg H. Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2004;24(12):1333–1351. doi: 10.1097/01.WCB.0000141559.17620.36. [DOI] [PubMed] [Google Scholar]
- 60.Sughrue ME, Mehra A, Connolly ES, Jr, D'Ambrosio AL. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. Inflamm Res. 2004;53(10):497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- 61.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10(1):95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeGraba TJ. The role of inflammation after acute stroke: utility of pursuing anti-adhesion molecule therapy. Neurology. 1998;51(3 Suppl 3):S62–68. doi: 10.1212/wnl.51.3_suppl_3.s62. [DOI] [PubMed] [Google Scholar]
- 63.Kishimoto TK, Rothlein R. Integrins, ICAMs, and selectins: role and regulation of adhesion molecules in neutrophil recruitment to inflammatory sites. Adv Pharmacol. 1994;25:117–169. doi: 10.1016/s1054-3589(08)60431-7. [DOI] [PubMed] [Google Scholar]
- 64.Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. Time course of ICAM-1 expression and leukocyte subset infiltration in rat forebrain ischemia. Mol Chem Neuropathol. 1995;26(3):213–230. doi: 10.1007/BF02815139. [DOI] [PubMed] [Google Scholar]
- 65.Clark WM, Lauten JD, Lessov N, Woodward W, Coull BM. The influence of antiadhesion therapies on leukocyte subset accumulation in central nervous system ischemia in rats. J Mol Neurosci. 1995;6(1):43–50. doi: 10.1007/BF02736758. [DOI] [PubMed] [Google Scholar]
- 66.Clark WM, Zivin JA. Antileukocyte adhesion therapy: preclinical trials and combination therapy. Neurology. 1997;49(5 Suppl 4):S32–38. doi: 10.1212/wnl.49.5_suppl_4.s32. [DOI] [PubMed] [Google Scholar]
- 67.Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114(4):1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 68.Prestigiacomo CJ, Kim SC, Connolly ES, Jr, Liao H, Yan SF, Pinsky DJ. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30(5):1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 69.Clark WM, Madden KP, Rothlein R, Zivin JA. Reduction of central nervous system ischemic injury by monoclonal antibody to intercellular adhesion molecule. J Neurosurg. 1991;75(4):623–627. doi: 10.3171/jns.1991.75.4.0623. [DOI] [PubMed] [Google Scholar]
- 70.Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788(1–2):25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- 71.Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30(3):624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 72.Zhang RL, Chopp M, Jiang N, Tang WX, Prostak J, Manning AM, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26(8):1438–1442. doi: 10.1161/01.str.26.8.1438. discussion 1443. [DOI] [PubMed] [Google Scholar]
- 73.Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25(1):202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- 74.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84(7):2068–2101. [PubMed] [Google Scholar]
- 75.Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137(2):69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- 76.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180(5):1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang RL, Chopp M, Zhang ZG, Phillips ML, Rosenbloom CL, Cruz R, et al. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 1996;16(6):1126–1136. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Ohmori K, Kanda K, Mitsuoka C, Kanamori A, Kurata-Miura K, Sasaki K, et al. P- and E-selectins recognize sialyl 6-sulfo Lewis X, the recently identified L-selectin ligand. Biochem Biophys Res Commun. 2000;278(1):90–96. doi: 10.1006/bbrc.2000.3768. [DOI] [PubMed] [Google Scholar]
- 79.Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33(8):2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H, Abe K, Tojo S, Kimura K, Mizugaki M, Itoyama Y. A change of P-selectin immunoreactivity in rat brain after transient and permanent middle cerebral artery occlusion. Neurol Res. 1998;20(5):463–469. doi: 10.1080/01616412.1998.11740549. [DOI] [PubMed] [Google Scholar]
- 81.Haring HP, Berg EL, Tsurushita N, Tagaya M, del Zoppo GJ. E-selectin appears in nonischemic tissue during experimental focal cerebral ischemia. Stroke. 1996;27(8):1386–1391. doi: 10.1161/01.str.27.8.1386. discussion 1391–1382. [DOI] [PubMed] [Google Scholar]
- 82.Huang J, Kim LJ, Mealey R, Marsh HC, Jr, Zhang Y, Tenner AJ, et al. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285(5427):595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 83.Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31(12):3047–3053. [PubMed] [Google Scholar]
- 84.Connolly ES, Jr, Winfree CJ, Prestigiacomo CJ, Kim SC, Choudhri TF, Hoh BL, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81(3):304–310. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki H, Hayashi T, Tojo SJ, Kitagawa H, Kimura K, Mizugaki M, et al. Anti-P-selectin antibody attenuates rat brain ischemic injury. Neurosci Lett. 1999;265(3):163–166. doi: 10.1016/s0304-3940(99)00229-3. [DOI] [PubMed] [Google Scholar]
- 86.Mocco J, Choudhri T, Huang J, Harfeldt E, Efros L, Klingbeil C, et al. HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ Res. 2002;91(10):907–914. doi: 10.1161/01.res.0000042063.15901.20. [DOI] [PubMed] [Google Scholar]
- 87.Yenari MA, Sun GH, Kunis DM, Onley D, Vexler V. L-selectin inhibition does not reduce injury in a rabbit model of transient focal cerebral ischemia. Neurol Res. 2001;23(1):72–78. doi: 10.1179/016164101101198154. [DOI] [PubMed] [Google Scholar]
- 88.Bednar MM, Gross CE, Russell SR, Fuller SP, Ellenberger CL, Schindler E, et al. Humanized anti-L-selectin monoclonal antibody DREG200 therapy in acute thromboembolic stroke. Neurol Res. 1998;20(5):403–408. doi: 10.1080/01616412.1998.11740538. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003;100(25):15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin targets immunomodulation to activating vessel segments and prevents ischemic and hemorrhagic stroke. Ernst Schering Res Found Workshop. 2004;47:117–132. doi: 10.1007/978-3-662-05426-0_7. [DOI] [PubMed] [Google Scholar]
- 91.Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33(9):2156–2163. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- 92.Bernardes-Silva M, Anthony DC, Issekutz AC, Perry VH. Recruitment of neutrophils across the blood-brain barrier: the role of E- and P-selectins. J Cereb Blood Flow Metab. 2001;21(9):1115–1124. doi: 10.1097/00004647-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Lorant DE, Li W, Tabatabaei N, Garver MK, Albertine KH. P-selectin expression by endothelial cells is decreased in neonatal rats and human premature infants. Blood. 1999;94(2):600–609. [PubMed] [Google Scholar]
- 94.Tcharmtchi MH, Smith CW, Mariscalco MM. Neonatal neutrophil interaction with P-selectin: contribution of P-selectin glycoprotein ligand-1 and sialic acid. J Leukoc Biol. 2000;67(1):73–80. doi: 10.1002/jlb.67.1.73. [DOI] [PubMed] [Google Scholar]
- 95.Zee RY, Cook NR, Cheng S, Reynolds R, Erlich HA, Lindpaintner K, et al. Polymorphism in the P-selectin and interleukin-4 genes as determinants of stroke: a population-based, prospective genetic analysis. Hum Mol Genet. 2004;13(4):389–396. doi: 10.1093/hmg/ddh039. [DOI] [PubMed] [Google Scholar]
- 96.Shyu KG, Chang H, Lin CC. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischaemic stroke. J Neurol. 1997;244(2):90–93. doi: 10.1007/s004150050055. [DOI] [PubMed] [Google Scholar]
- 97.Cha JK, Jo WS, Shin HC, Bae HR, Ho JM, Kim JW. Increased platelet CD63 and P-selectin expression persist in atherosclerotic ischemic stroke. Platelets. 2004;15(1):3–7. doi: 10.1080/09537100310001644024. [DOI] [PubMed] [Google Scholar]
- 98.Simundic AM, Basic V, Topic E, Demarin V, Vrkic N, Kunovic B, et al. Soluble adhesion molecules in acute ischemic stroke. Clin Invest Med. 2004;27(2):86–92. [PubMed] [Google Scholar]
- 99.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 100.Smith CW. Leukocyte-endothelial cell interactions. Semin Hematol. 1993;30(4 Suppl 4):45–53. [PubMed] [Google Scholar]
- 101.Chen H, Chopp M, Zhang RL, Bodzin G, Chen Q, Rusche JR, et al. Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994;35(4):458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 102.Chopp M, Zhang RL, Chen H, Li Y, Jiang N, Rusche JR. Postischemic administration of an anti-Mac-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in rats. Stroke. 1994;25(4):869–875. doi: 10.1161/01.str.25.4.869. discussion 875–866. [DOI] [PubMed] [Google Scholar]
- 103.Chopp M, Zhang ZG. Anti-adhesion molecule and nitric oxide protection strategies in ischemic stroke. Curr Opin Neurol. 1996;9(1):68–72. doi: 10.1097/00019052-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Zhang RL, Zhang ZG, Chopp M. Increased therapeutic efficacy with rt-PA and anti-CD18 antibody treatment of stroke in the rat. Neurology. 1999;52(2):273–279. doi: 10.1212/wnl.52.2.273. [DOI] [PubMed] [Google Scholar]
- 105.Zhang ZG, Chopp M, Tang WX, Jiang N, Zhang RL. Postischemic treatment (2–4 h) with anti-CD11b and anti-CD18 monoclonal antibodies are neuroprotective after transient (2 h) focal cerebral ischemia in the rat. Brain Res. 1995;698(1–2):79–85. doi: 10.1016/0006-8993(95)00830-j. [DOI] [PubMed] [Google Scholar]
- 106.Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45(4):815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- 107.Jiang N, Moyle M, Soule HR, Rote WE, Chopp M. Neutrophil inhibitory factor is neuroprotective after focal ischemia in rats. Ann Neurol. 1995;38(6):935–942. doi: 10.1002/ana.410380615. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, Zhang ZG, Zhang RL, Lu M, Krams M, Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34(7):1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- 109.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, et al. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153(2):223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 110.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18 (Suppl 2):s18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- 111.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34(11):2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 112.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43(6):1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 113.Caimi G, Canino B, Ferrara F, Montana M, Musso M, Porretto F, et al. Granulocyte integrins before and after activation in acute ischaemic stroke. J Neurol Sci. 2001;186(1–2):23–26. doi: 10.1016/s0022-510x(01)00495-6. [DOI] [PubMed] [Google Scholar]
- 114.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62(2):127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 115.Wang X, Siren AL, Liu Y, Yue TL, Barone FC, Feuerstein GZ. Upregulation of intercellular adhesion molecule 1 (ICAM-1) on brain microvascular endothelial cells in rat ischemic cortex. Brain Res Mol Brain Res. 1994;26(1–2):61–68. doi: 10.1016/0169-328x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Feuerstein GZ. Induced expression of adhesion molecules following focal brain ischemia. J Neurotrauma. 1995;12(5):825–832. doi: 10.1089/neu.1995.12.825. [DOI] [PubMed] [Google Scholar]
- 117.Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, et al. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682(1–2):182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- 118.Vemuganti R, Dempsey RJ, Bowen KK. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35(1):179–184. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- 119.Kitagawa K, Matsumoto M, Mabuchi T, Yagita Y, Ohtsuki T, Hori M, et al. Deficiency of intercellular adhesion molecule 1 attenuates microcirculatory disturbance and infarction size in focal cerebral ischemia. J Cereb Blood Flow Metab. 1998;18(12):1336–1345. doi: 10.1097/00004647-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 120.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97(1):209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soriano SG, Lipton SA, Wang YF, Xiao M, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are less susceptible to cerebral ischemia-reperfusion injury. Ann Neurol. 1996;39(5):618–624. doi: 10.1002/ana.410390511. [DOI] [PubMed] [Google Scholar]
- 122.Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercelluar adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul Fibrinolysis. 1999;10(5):277–284. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 123.Cervera A, Justicia C, Reverter JC, Planas AM, Chamorro A. Steady plasma concentration of unfractionated heparin reduces infarct volume and prevents inflammatory damage after transient focal cerebral ischemia in the rat. J Neurosci Res. 2004;77(4):565–572. doi: 10.1002/jnr.20186. [DOI] [PubMed] [Google Scholar]
- 124.Justicia C, Martin A, Rojas S, Gironella M, Cervera A, Panes J, et al. Anti-VCAM-1 antibodies did not protect against ischemic damage either in rats or in mice. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600198. [DOI] [PubMed] [Google Scholar]
- 125.Chamorro A, Cervera A, Castillo J, Davalos A, Aponte JJ, Planas AM. Unfractionated heparin is associated with a lower rise of serum vascular cell adhesion molecule-1 in acute ischemic stroke patients. Neurosci Lett. 2002;328(3):229–232. doi: 10.1016/s0304-3940(02)00518-9. [DOI] [PubMed] [Google Scholar]
- 126.Enlimomab AST. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57(8):1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 127.Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, et al. Anti-ICAM-1 monoclonal antibody R6.5 (Enlimomab) promotes activation of neutrophils in whole blood. J Immunol. 1999;162(4):2353–2357. [PubMed] [Google Scholar]
- 128.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29(5):1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 129.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86(4):1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 130.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275(27):20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 131.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63(1):84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 133.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 (Suppl 1):S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 135.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 136.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37(4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 137.Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNFalpha transport across the blood-brain barrier. Exp Neurol. 2006;198(1):222–233. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 138.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. 1999;26(2):85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- 139.Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96(1–2):229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- 140.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314(1):119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 141.Wendland M, Derugin N, Manabat C, Fox CK, Ferriero DM, Vexler ZS. The blood-brain barrier is more preserved in neonatal versus adult rats following transient focal cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2003;23(suppl 1):169. [Google Scholar]
- 142.Yong VW. The potential use of MMP inhibitors to treat CNS diseases. Expert Opin Investig Drugs. 1999;8(3):255–268. doi: 10.1517/13543784.8.3.255. [DOI] [PubMed] [Google Scholar]
- 143.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842(1):92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 144.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 145.Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23(5):406–415. [PubMed] [Google Scholar]
- 146.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 147.Fernandez-Patron C, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1–32] Faseb J. 2001;15(12):2230–2240. doi: 10.1096/fj.01-0178com. [DOI] [PubMed] [Google Scholar]
- 148.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 149.Maier CM, Hsieh L, Yu F, Bracci P, Chan PH. Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke. 2004;35(5):1169–1174. doi: 10.1161/01.STR.0000125861.55804.f2. [DOI] [PubMed] [Google Scholar]
- 150.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69(6):851–859. [PubMed] [Google Scholar]
- 151.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893(1–2):104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 152.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9(10):1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 153.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 154.Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12(13):3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 155.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 156.Carson MJ, Sutcliffe JG. Balancing function vs. self defense: the CNS as an active regulator of immune responses. J Neurosci Res. 1999;55(1):1–8. doi: 10.1002/(SICI)1097-4547(19990101)55:1<1::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 157.Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20(9):1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 158.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30(1):77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 159.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22(1):72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 160.Bohatschek M, Kloss CU, Kalla R, Raivich G. In vitro model of microglial deramification: ramified microglia transform into amoeboid phagocytes following addition of brain cell membranes to microglia-astrocyte cocultures. J Neurosci Res. 2001;64(5):508–522. doi: 10.1002/jnr.1103. [DOI] [PubMed] [Google Scholar]
- 161.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 162.Brockhaus J, Moller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16(1):81–90. doi: 10.1002/(SICI)1098-1136(199601)16:1<81::AID-GLIA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 163.Bechmann I, Nitsch R. Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia. 1997;20(2):145–154. doi: 10.1002/(sici)1098-1136(199706)20:2<145::aid-glia6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 164.Trebst C, Staugaitis SM, Kivisakk P, Mahad D, Cathcart MK, Tucky B, et al. CC chemokine receptor 8 in the central nervous system is associated with phagocytic macrophages. Am J Pathol. 2003;162(2):427–438. doi: 10.1016/S0002-9440(10)63837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Losy J, Zaremba J, Skrobanski P. CXCL1 (GRO-alpha) chemokine in acute ischaemic stroke patients. Folia Neuropathol. 2005;43(2):97–102. [PubMed] [Google Scholar]
- 166.Saito S, Matsuura M, Tominaga K, Kirikae T, Nakano M. Important role of membrane-associated CD14 in the induction of IFN-beta and subsequent nitric oxide production by murine macrophages in response to bacterial lipopolysaccharide. Eur J Biochem. 2000;267(1):37–45. doi: 10.1046/j.1432-1327.2000.00956.x. [DOI] [PubMed] [Google Scholar]
- 167.Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126(1–2):107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 168.Hagberg H, Gilland E, Bona E, Hanson LA, Hahin-Zoric M, Blennow M, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res. 1996;40(4):603–609. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 169.Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26(6):1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- 170.Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22(14):5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cowell RM, Xu H, Galasso JM, Silverstein FS. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke. 2002;33(3):795–801. doi: 10.1161/hs0302.103740. [DOI] [PubMed] [Google Scholar]
- 172.Cowell RM, Plane JM, Silverstein FS. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci. 2003;23(28):9459–9468. doi: 10.1523/JNEUROSCI.23-28-09459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O'Riordan DP, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17(1):89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 174.Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: delayed gene expression and strategies for neuroprotection. Ann N Y Acad Sci. 1997;835:203–217. doi: 10.1111/j.1749-6632.1997.tb48631.x. [DOI] [PubMed] [Google Scholar]
- 175.Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Aminoguanidine inhibits caspase-3 and calpain activation without affecting microglial activation following neonatal transient ischemia. Journal of Neurochemistry. 2006;96:1467–1479. doi: 10.1111/j.1471-4159.2006.03672.x. [DOI] [PubMed] [Google Scholar]
- 176.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102(28):9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Flavin MP, Ho LT. Propentofylline protects neurons in culture from death triggered by macrophage or microglial secretory products. J Neurosci Res. 1999;56(1):54–59. doi: 10.1002/(SICI)1097-4547(19990401)56:1<54::AID-JNR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 178.Flavin MP, Coughlin K, Ho LT. Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience. 1997;80(2):437–448. doi: 10.1016/s0306-4522(97)00078-x. [DOI] [PubMed] [Google Scholar]
- 179.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20(3):269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 180.Graeber MB, Lopez-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, et al. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res. 1998;813(2):241–253. doi: 10.1016/s0006-8993(98)00859-2. [DOI] [PubMed] [Google Scholar]
- 181.Ladeby R, Wirenfeldt M, Dalmau I, Gregersen R, Garcia-Ovejero D, Babcock A, et al. Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia. 2005;50(2):121–131. doi: 10.1002/glia.20159. [DOI] [PubMed] [Google Scholar]
- 182.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21(8):2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26(4):515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lai AY, Todd KG. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia. 2006 doi: 10.1002/glia.20335. [DOI] [PubMed] [Google Scholar]
- 187.Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52(1):54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- 188.Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189(1):58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 189.Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25(9):1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 191.Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia Stroke. 1997;28(2):382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]
- 192.Derugin N, Wendland M, Muramatsu K, Roberts T, Gregory G, Ferriero D, et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rat. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- 193.Derugin N, Dingman A, Wendland M, Fox C, ZSV Magnetic Resonance Imaging as a Surrogate Measure for Histological Sub-Chronic Endpoint in a Neonatal Rat Stroke Model. Brain Res. 2005;1066:49–56. doi: 10.1016/j.brainres.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 194.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121(3):619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 195.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A. 2001;98(11):6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 197.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12(4):488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McRae A, Gilland E, Bona E, Hagberg H. Microglia activation after neonatal hypoxic-ischemia. Brain Res Dev Brain Res. 1995;84(2):245–252. doi: 10.1016/0165-3806(94)00177-2. [DOI] [PubMed] [Google Scholar]
- 199.Ivacko JA, Sun R, Silverstein FS. Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res. 1996;39(1):39–47. doi: 10.1203/00006450-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 200.Tsuji M, Higuchi Y, Shiraishi K, Kume T, Akaike A, Hattori H. Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat [In Process Citation] Pediatr Res. 2000;47(1):79–83. doi: 10.1203/00006450-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 201.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 202.Watanabe H, Abe H, Takeuchi S, Tanaka R. Protective effect of microglial conditioning medium on neuronal damage induced by glutamate. Neurosci Lett. 2000;289(1):53–56. doi: 10.1016/s0304-3940(00)01252-0. [DOI] [PubMed] [Google Scholar]
- 203.Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373(2):159–164. doi: 10.1016/j.neulet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 204.Kitamura Y, Yanagisawa D, Inden M, Takata K, Tsuchiya D, Kawasaki T, et al. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci. 2005;97(2):289–293. doi: 10.1254/jphs.sc0040129. [DOI] [PubMed] [Google Scholar]
- 205.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 206.Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902(2):171–177. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- 207.Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, et al. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19(11):1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 208.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9(3–4):259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 209.Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia. 1997;21(1):142–153. doi: 10.1002/(sici)1098-1136(199709)21:1<142::aid-glia16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 210.Iadecola C, Xu X, Zhang F, el-Fakahany EE, Ross ME. Marked induction of calcium-independent nitric oxide synthase activity after focal cerebral ischemia. J Cereb Blood Flow Metab. 1995;15(1):52–59. doi: 10.1038/jcbfm.1995.6. [DOI] [PubMed] [Google Scholar]
- 211.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 212.Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM. High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab. 2000;20(7):1040–1044. doi: 10.1097/00004647-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 213.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, et al. TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32(1):102–107. [PubMed] [Google Scholar]
- 214.Yepes M, Brown SA, Moore EG, Smith EP, Lawrence DA, Winkles JA. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166(2):511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Benjelloun N, Joly LM, Palmier B, Plotkine M, Charriaut-Marlangue C. Apoptotic mitochondrial pathway in neurones and astrocytes after neonatal hypoxia-ischaemia in the rat brain. Neuropathol Appl Neurobiol. 2003;29(4):350–360. doi: 10.1046/j.1365-2990.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 216.Ducrocq S, Benjelloun N, Plotkine M, Ben-Ari Y, Charriaut-Marlangue C. Poly(ADP-ribose) synthase inhibition reduces ischemic injury and inflammation in neonatal rat brain. J Neurochem. 2000;74(6):2504–2511. doi: 10.1046/j.1471-4159.2000.0742504.x. [DOI] [PubMed] [Google Scholar]
- 217.Joly LM, Benjelloun N, Plotkine M, Charriaut-Marlangue C. Distribution of Poly(ADP-ribosyl)ation and cell death after cerebral ischemia in the neonatal rat. Pediatr Res. 2003;53(5):776–782. doi: 10.1203/01.PDR.0000059751.00465.F6. [DOI] [PubMed] [Google Scholar]
- 218.McRae A, Bona E, Hagberg H. Microglia-astrocyte interactions after cortisone treatment in a neonatal hypoxia-ischemia model. Brain Res Dev Brain Res. 1996;94(1):44–51. doi: 10.1016/0165-3806(96)00043-0. [DOI] [PubMed] [Google Scholar]
- 219.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26(4):676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- 220.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 221.Buttini M, Sauter A, Boddeke HW. Induction of interleukin-1 beta mRNA after focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 1994;23(1–2):126–134. doi: 10.1016/0169-328x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 222.Yabuuchi K, Minami M, Katsumata S, Yamazaki A, Satoh M. An in situ hybridization study on interleukin-1 beta mRNA induced by transient forebrain ischemia in the rat brain. Brain Res Mol Brain Res. 1994;26(1–2):135–142. doi: 10.1016/0169-328x(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 223.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23(12):618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 224.Touzani O, Boutin H, LeFeuvre R, Parker L, Miller A, Luheshi G, et al. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor. J Neurosci. 2002;22(1):38–43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of Interleukin-1 Receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138(2):206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- 226.Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15(4):547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- 227.Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann N Y Acad Sci. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 228.Vexler ZS. Inflammation and ischemia in the developing brain. In: Yenari MA, Giffard RG, editors. Glia and inflammation in neurodegenerative disease. Nova Science Publishers, Inc; 2006. [Google Scholar]
- 229.Ohtaki H, Yin L, Nakamachi T, Dohi K, Kudo Y, Makino R, et al. Expression of tumor necrosis factor alpha in nerve fibers and oligodendrocytes after transient focal ischemia in mice. Neurosci Lett. 2004;368(2):162–166. doi: 10.1016/j.neulet.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 230.Saito K, Suyama K, Nishida K, Sei Y, Basile AS. Early increases in TNF-alpha, IL-6 and IL-1 beta levels following transient cerebral ischemia in gerbil brain. Neurosci Lett. 1996;206(2–3):149–152. doi: 10.1016/s0304-3940(96)12460-5. [DOI] [PubMed] [Google Scholar]
- 231.Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M. Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17(5):491–499. doi: 10.1097/00004647-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 232.Yang GY, Gong C, Qin Z, Ye W, Mao Y, Bertz AL. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport. 1998;9(9):2131–2134. doi: 10.1097/00001756-199806220-00041. [DOI] [PubMed] [Google Scholar]
- 233.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28(6):1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 234.Ginis I, Jaiswal R, Klimanis D, Liu J, Greenspon J, Hallenbeck JM. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cereb Blood Flow Metab. 2002;22(2):142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 235.Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18(12):1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 236.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2(7):788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 237.Clark WM, Rinker LG, Lessov NS, Hazel K, Hill JK, Stenzel-Poore M, et al. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31(7):1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- 238.Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, et al. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23(4):406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 239.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4(1):2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21(5):427–449. [PubMed] [Google Scholar]
- 241.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251(3):189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 242.Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158(2):444–450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- 243.Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111(7):913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- 244.Mesples B, Plaisant F, Gressens P. Effects of interleukin-10 on neonatal excitotoxic brain lesions in mice. Brain Res Dev Brain Res. 2003;141(1–2):25–32. doi: 10.1016/s0165-3806(02)00636-3. [DOI] [PubMed] [Google Scholar]
- 245.Zaremba J, Losy J. Interleukin-18 in acute ischaemic stroke patients. Neurol Sci. 2003;24(3):117–124. doi: 10.1007/s10072-003-0096-0. [DOI] [PubMed] [Google Scholar]
- 246.Wheeler RD, Boutin H, Touzani O, Luheshi GN, Takeda K, Rothwell NJ. No role for interleukin-18 in acute murine stroke-induced brain injury. J Cereb Blood Flow Metab. 2003;23(5):531–535. doi: 10.1097/01.WCB.0000059587.71206.BA. [DOI] [PubMed] [Google Scholar]
- 247.Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54(1):71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 248.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factorss1 expression. Stroke. 2001;32(2):544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 249.Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22(3):147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 250.Yamasaki Y, Matsuo Y, Matsuura N, Onodera H, Itoyama Y, Kogure K. Transient increase of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in ischemic brain areas after focal ischemia in rats. Stroke. 1995;26(2):318–322. doi: 10.1161/01.str.26.2.318. discussion 322–313. [DOI] [PubMed] [Google Scholar]
- 251.Horuk R, Ng HP. Chemokine receptor antagonists. Med Res Rev. 2000;20(2):155–168. doi: 10.1002/(sici)1098-1128(200003)20:2<155::aid-med3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 252.Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, et al. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158(6):2882–2890. [PubMed] [Google Scholar]
- 253.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23(21):7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91(7):2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103(6):773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Huang D, Tani M, Wang J, Han Y, He TT, Weaver J, et al. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22(24):10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Minami M, Satoh M. Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life Sci. 2003;74(2–3):321–327. doi: 10.1016/j.lfs.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 259.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003;23(6):748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 260.Kumai Y, Ooboshi H, Takada J, Kamouchi M, Kitazono T, Egashira K, et al. Anti-monocyte chemoattractant protein-1 gene therapy protects against focal brain ischemia in hypertensive rats. J Cereb Blood Flow Metab. 2004;24(12):1359–1368. doi: 10.1097/01.WCB.0000143534.76388.3C. [DOI] [PubMed] [Google Scholar]
- 261.Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22(3):308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 262.Xu H, Barks JD, Schielke GP, Silverstein FS. Attenuation of hypoxia-ischemia-induced monocyte chemoattractant protein-1 expression in brain of neonatal mice deficient in interleukin-1 converting enzyme. Brain Res Mol Brain Res. 2001;90(1):57–67. doi: 10.1016/s0169-328x(01)00087-0. [DOI] [PubMed] [Google Scholar]
- 263.Galasso JM, Miller MJ, Cowell RM, Harrison JK, Warren JS, Silverstein FS. Acute excitotoxic injury induces expression of monocyte chemoattractant protein-1 and its receptor, CCR2, in neonatal rat brain. Exp Neurol. 2000;165(2):295–305. doi: 10.1006/exnr.2000.7466. [DOI] [PubMed] [Google Scholar]
- 264.Takami S, Minami M, Nagata I, Namura S, Satoh M. Chemokine receptor antagonist peptide, viral MIP-II, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21(12):1430–1435. doi: 10.1097/00004647-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 265.Takami S, Minami M, Katayama T, Nagata I, Namura S, Satoh M. TAK-779, a nonpeptide CC chemokine receptor antagonist, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2002;22(7):780–784. doi: 10.1097/00004647-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 266.Nishi T, Maier CM, Hayashi T, Saito A, Chan PH. Superoxide dismutase 1 overexpression reduces MCP-1 and MIP-1alpha expression after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600124. [DOI] [PubMed] [Google Scholar]
- 267.McMahon EJ, Cook DN, Suzuki K, Matsushima GK. Absence of macrophage-inflammatory protein-1alpha delays central nervous system demyelination in the presence of an intact blood-brain barrier. J Immunol. 2001;167(5):2964–2971. doi: 10.4049/jimmunol.167.5.2964. [DOI] [PubMed] [Google Scholar]
- 268.Popivanova BK, Koike K, Tonchev AB, Ishida Y, Kondo T, Ogawa S, et al. Accumulation of microglial cells expressing ELR motif-positive CXC chemokines and their receptor CXCR2 in monkey hippocampus after ischemia-reperfusion. Brain Res. 2003;970(1–2):195–204. doi: 10.1016/s0006-8993(03)02343-6. [DOI] [PubMed] [Google Scholar]
- 269.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68(1):1–8. [PubMed] [Google Scholar]
- 270.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 271.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 272.Dunstan CA, Salafranca MN, Adhikari S, Xia Y, Feng L, Harrison JK. Identification of two rat genes orthologous to the human interleukin-8 receptors. J Biol Chem. 1996;271(51):32770–32776. doi: 10.1074/jbc.271.51.32770. [DOI] [PubMed] [Google Scholar]
- 273.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150(2):617–630. [PMC free article] [PubMed] [Google Scholar]
- 274.Giovannelli A, Limatola C, Ragozzino D, Mileo AM, Ruggieri A, Ciotti MT, et al. CXC chemokines interleukin-8 (IL-8) and growth-related gene product alpha (GROalpha) modulate Purkinje neuron activity in mouse cerebellum. J Neuroimmunol. 1998;92(1–2):122–132. doi: 10.1016/s0165-5728(98)00192-1. [DOI] [PubMed] [Google Scholar]
- 275.Tarozzo G, Campanella M, Ghiani M, Bulfone A, Beltramo M. Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur J Neurosci. 2002;15(10):1663–1668. doi: 10.1046/j.1460-9568.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 276.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125(1–2):59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 277.Bonavia R, Bajetto A, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol Lett. 2003;139(2–3):181–189. doi: 10.1016/s0378-4274(02)00432-0. [DOI] [PubMed] [Google Scholar]
- 278.Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, et al. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30(7):831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- 279.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7(2):113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 280.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 281.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386(Pt 3):401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28(11):2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 283.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 284.Takizawa S, Aratani Y, Fukuyama N, Maeda N, Hirabayashi H, Koyama H, et al. Deficiency of myeloperoxidase increases infarct volume and nitrotyrosine formation in mouse brain. J Cereb Blood Flow Metab. 2002;22(1):50–54. doi: 10.1097/00004647-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 285.Ichimori K, Fukuyama N, Nakazawa H, Aratani Y, Koyama H, Takizawa S, et al. Myeloperoxidase has directly-opposed effects on nitration reaction--study on myeloperoxidase-deficient patient and myeloperoxidase-knockout mice. Free Radic Res. 2003;37(5):481–489. doi: 10.1080/1071576031000099830. [DOI] [PubMed] [Google Scholar]
- 286.Arnhold J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry (Mosc) 2004;69(1):4–9. doi: 10.1023/b:biry.0000016344.59411.ee. [DOI] [PubMed] [Google Scholar]
- 287.Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, et al. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44(3):357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 288.Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004;56(4):656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- 289.Palmer C. Hypoxic-ischemic encephalopathy. Therapeutic approaches against microvascular injury, and role of neutrophils, PAF, and free radicals. Clin Perinatol. 1995;22(2):481–517. [PubMed] [Google Scholar]
- 290.Aspberg A, Tottmar O. Development of antioxidant enzymes in rat brain and in reaggregation culture of fetal brain cells. Brain Res Dev Brain Res. 1992;66(1):55–58. doi: 10.1016/0165-3806(92)90139-n. [DOI] [PubMed] [Google Scholar]
- 291.Buard A, Clement M, Bourre JM. Developmental changes in enzymatic systems involved in protection against peroxidation in isolated rat brain microvessels. Neurosci Lett. 1992;141(1):72–74. doi: 10.1016/0304-3940(92)90337-7. [DOI] [PubMed] [Google Scholar]
- 292.de Haan JB, Newman JD, Kola I. Cu/Zn superoxide dismutase mRNA and enzyme activity, and susceptibility to lipid peroxidation, increases with aging in murine brains. Brain Res Mol Brain Res. 1992;13(3):179–187. doi: 10.1016/0169-328x(92)90025-7. [DOI] [PubMed] [Google Scholar]
- 293.Fernandez AP, Alonso D, Lisazoain I, Serrano J, Leza JC, Bentura ML, et al. Postnatal changes in the nitric oxide system of the rat cerebral cortex after hypoxia during delivery. Brain Res Dev Brain Res. 2003;142(2):177–192. doi: 10.1016/s0165-3806(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 294.Vexler ZS. In: Inflammation and ischemia in the developing brain. Yenari MA, Giffard RG, editors. 2006. [Google Scholar]
- 295.Acarin L, Peluffo H, Gonzalez B, Castellano B. Expression of inducible nitric oxide synthase and cyclooxygenase-2 after excitotoxic damage to the immature rat brain. J Neurosci Res. 2002;68(6):745–754. doi: 10.1002/jnr.10261. [DOI] [PubMed] [Google Scholar]
- 296.Niwa M, Inao S, Takayasu M, Kawai T, Kajita Y, Nihashi T, et al. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol Med Chir (Tokyo) 2001;41(2):63–72. doi: 10.2176/nmc.41.63. discussion 72–63. [DOI] [PubMed] [Google Scholar]
- 297.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140(2):205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 298.Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29(1):1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 299.Lipton SA. Neuronal protection and destruction by NO. Cell Death Differ. 1999;6(10):943–951. doi: 10.1038/sj.cdd.4400580. [DOI] [PubMed] [Google Scholar]
- 300.Cheung WS, Bhan I, Lipton SA. Nitric oxide (NO. ) stabilizes whereas nitrosonium (NO+) enhances filopodial outgrowth by rat retinal ganglion cells in vitro. Brain Res. 2000;868(1):1–13. doi: 10.1016/s0006-8993(00)02161-2. [DOI] [PubMed] [Google Scholar]
- 301.Willmot MR, Bath PM. The potential of nitric oxide therapeutics in stroke. Expert Opin Investig Drugs. 2003;12(3):455–470. doi: 10.1517/13543784.12.3.455. [DOI] [PubMed] [Google Scholar]
- 302.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92(16):7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci U S A. 1998;95(18):10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17(23):9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995;268(1 Pt 2):R286–292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 306.Peeters-Scholte C, Koster J, Veldhuis W, van den Tweel E, Zhu C, Kops N, et al. Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke. 2002;33(9):2304–2310. doi: 10.1161/01.str.0000028343.25901.09. [DOI] [PubMed] [Google Scholar]
- 307.Zhu DY, Deng Q, Yao HH, Wang DC, Deng Y, Liu GQ. Inducible nitric oxide synthase expression in the ischemic core and penumbra after transient focal cerebral ischemia in mice. Life Sci. 2002;71(17):1985–1996. doi: 10.1016/s0024-3205(02)01970-7. [DOI] [PubMed] [Google Scholar]
- 308.Iadecola C, Zhang F, Casey R, Clark HB, Ross ME. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke. 1996;27(8):1373–1380. doi: 10.1161/01.str.27.8.1373. [DOI] [PubMed] [Google Scholar]
- 309.Higuchi Y, Hattori H, Kume T, Tsuji M, Akaike A, Furusho K. Increase in nitric oxide in the hypoxic-ischemic neonatal rat brain and suppression by 7-nitroindazole and aminoguanidine. Eur J Pharmacol. 1998;342(1):47–49. doi: 10.1016/s0014-2999(97)01524-0. [DOI] [PubMed] [Google Scholar]
- 310.Leib SL, Kim YS, Black SM, Tureen JH, Tauber MG. Inducible nitric oxide synthase and the effect of aminoguanidine in experimental neonatal meningitis. J Infect Dis. 1998;177(3):692–700. doi: 10.1086/514226. [DOI] [PubMed] [Google Scholar]
- 311.Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10(1):113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, et al. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27(2):110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 313.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390(6660):622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 314.Sanchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 2004;35(1):163–168. doi: 10.1161/01.STR.0000105391.62306.2E. [DOI] [PubMed] [Google Scholar]
- 315.Schwab JM, Beschorner R, Meyermann R, Gozalan F, Schluesener HJ. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96(5):892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- 316.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17(8):2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yokota C, Kaji T, Kuge Y, Inoue H, Tamaki N, Minematsu K. Temporal and topographic profiles of cyclooxygenase-2 expression during 24 h of focal brain ishemia in rats. Neurosci Lett. 2004;357(3):219–222. doi: 10.1016/j.neulet.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 318.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Wide therapeutic time window for nimesulide neuroprotection in a model of transient focal cerebral ischemia in the rat. Brain Res. 2004;1007(1–2):98–108. doi: 10.1016/j.brainres.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 319.Nagayama M, Niwa K, Nagayama T, Ross ME, Iadecola C. The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab. 1999;19(11):1213–1219. doi: 10.1097/00004647-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 320.Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32(10):2370–2375. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 321.Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res. 2003;960(1–2):273–276. doi: 10.1016/s0006-8993(02)03805-2. [DOI] [PubMed] [Google Scholar]
- 322.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci U S A. 2001;98(3):1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, et al. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54(2):155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- 324.Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, et al. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55(5):668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- 325.Lam BK. Leukotriene C(4) synthase. Prostaglandins Leukot Essent Fatty Acids. 2003;69(2–3):111–116. doi: 10.1016/s0952-3278(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 326.Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, et al. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129(1):199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- 327.Baskaya MK, Hu Y, Donaldson D, Maley M, Rao AM, Prasad MR, et al. Protective effect of the 5-lipoxygenase inhibitor AA-861 on cerebral edema after transient ischemia. J Neurosurg. 1996;85(1):112–116. doi: 10.3171/jns.1996.85.1.0112. [DOI] [PubMed] [Google Scholar]
- 328.Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(7):1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 329.Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23(7):786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- 330.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362(9389):1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 331.Hedtjarn M, Mallard C, Eklind S, Gustafson-Brywe K, Hagberg H. Global gene expression in the immature brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2004;24(12):1317–1332. doi: 10.1097/01.WCB.0000141558.40491.75. [DOI] [PubMed] [Google Scholar]
- 332.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 333.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 334.Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23(5):589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- 335.Cechetto DF. Role of nuclear factor kappa B in neuropathological mechanisms. Prog Brain Res. 2001;132:391–404. doi: 10.1016/S0079-6123(01)32090-3. [DOI] [PubMed] [Google Scholar]
- 336.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5(5):554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 337.Hill WD, Hess DC, Carroll JE, Wakade CG, Howard EF, Chen Q, et al. The NF-kappaB inhibitor diethyldithiocarbamate (DDTC) increases brain cell death in a transient middle cerebral artery occlusion model of ischemia. Brain Res Bull. 2001;55(3):375–386. doi: 10.1016/s0361-9230(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 338.Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, et al. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11(12):1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- 339.Barone FC, Parsons AA. Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Investig Drugs. 2000;9(10):2281–2306. doi: 10.1517/13543784.9.10.2281. [DOI] [PubMed] [Google Scholar]
- 340.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22(6):631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 341.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9(2):180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 342.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 343.Irving EA, Barone FC, Reith AD, Hadingham SJ, Parsons AA. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res. 2000;77(1):65–75. doi: 10.1016/s0169-328x(00)00043-7. [DOI] [PubMed] [Google Scholar]
- 344.Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, et al. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci. 2000;20(12):4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 346.Liu G, Rondinone CM. JNK: bridging the insulin signaling and inflammatory pathway. Curr Opin Investig Drugs. 2005;6(10):979–987. [PubMed] [Google Scholar]
- 347.Walton KM, DiRocco R, Bartlett BA, Koury E, Marcy VR, Jarvis B, et al. Activation of p38MAPK in microglia after ischemia. J Neurochem. 1998;70(4):1764–1767. doi: 10.1046/j.1471-4159.1998.70041764.x. [DOI] [PubMed] [Google Scholar]
- 348.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, et al. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001;296(2):312–321. [PubMed] [Google Scholar]
- 349.Hee Han B, Choi J, Holtzman DM. Evidence that p38 mitogen-activated protein kinase contributes to neonatal hypoxic-ischemic brain injury. Dev Neurosci. 2002;24(5):405–410. doi: 10.1159/000069046. [DOI] [PubMed] [Google Scholar]
- 350.Lu XC, Williams AJ, Yao C, Berti R, Hartings JA, Whipple R, et al. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res. 2004;77(6):843–857. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- 351.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 352.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9(2):240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 353.Munoz C, Castellanos MC, Alfranca A, Vara A, Esteban MA, Redondo JM, et al. Transcriptional up-regulation of intracellular adhesion molecule-1 in human endothelial cells by the antioxidant pyrrolidine dithiocarbamate involves the activation of activating protein-1. J Immunol. 1996;157(8):3587–3597. [PubMed] [Google Scholar]
- 354.Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36(12):2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]