Cell therapy approaches for lung diseases continue to evolve at a rapid pace. Early studies suggested that stem or progenitor cells derived from adult bone marrow or other adult tissues might engraft as lung epithelium in numbers sufficient to effect structural lung repair or replacement of defective or damaged airway or alveolar epithelial cells in cystic fibrosis, emphysema, and other conditions.1 However, it is now recognized that structural engraftment is generally a rare occurrence of uncertain significance, although recent studies suggest that engraftment of adult or placental origin cells might be induced to occur at higher levels.2,3 In this issue of Molecular Therapy, a study by Wang and colleagues4 shows that intratracheal instillation of human embryonic stem cells (hESCs), previously differentiated in culture into cells with phenotypic markers of type II alveolar epithelial cells—the surfactant-producing cells of the lungs—improved survival and ameliorated lung inflammation and fibrosis in mice with experimentally induced lung injury. This is the first available information demonstrating that cells derived from ESCs can have beneficial effects in lung injury, and it provides a platform for potential therapeutic use of ESCs in a variety of lung diseases.
With adult stem cells, the focus has shifted primarily to analysis of paracrine and immunomodulatory effects in the absence of significant engraftment, notably with mesenchymal stromal (stem) cells (MSCs) and endothelial progenitor cells (EPCs). Studies in animal models demonstrate that administration of bone marrow–derived EPCs can stimulate angiogenesis in experimental models of destructive pulmonary vascular diseases such as pulmonary hypertension.5 Although the mechanisms for the EPC effects are not yet completely understood, the results of two recent clinical trials in China suggested both safety and efficacy of autologous bone marrow–derived EPC administration in both adult and pediatric patients with pulmonary hypertension.6 A comparable trial of autologous EPC administration in adults with pulmonary hypertension is under way in Canada.7 MSCs have potent immunomodulatory effects as well as limited expression of MHCs and other immunostimulatory markers, allowing allogeneic administration.8 Both intratracheal and systemic administration of MSCs can ameliorate inflammatory and fibrotic lung injuries in mouse models, and recently MSC administration has been demonstrated to ameliorate inflammatory injury in isolated perfused human lungs.9,10,11 Anti-inflammatory and antifibrotic mediators released by the MSCs have been implicated, but the mechanisms are not yet fully defined. A trial to assess the effect of systemic allogeneic MSC administration on decreasing pulmonary and systemic inflammation in patients with moderate to severe chronic obstructive pulmonary disease is under way in the United States.12 A recent 6-month interim analysis of this trial demonstrated safety of MSC administration and yielded promising results indicating both decreased systemic inflammation and a trend toward improved quality of life in treated patients.
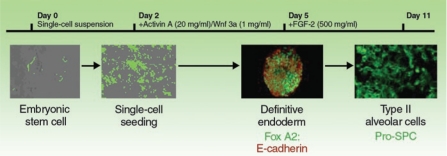
However, progress utilizing ESCs for lung regeneration or repair has been slower. Type II alveolar epithelial cells—which, in addition to producing surfactants, are the precursors of the type I alveolar–type epithelial cells responsible for gas exchange in the lung—are attractive targets for regeneration using ESCs. In studies over approximately the past 5 years, several laboratories have demonstrated that both mouse and human ESCs can be induced in culture to acquire phenotypic markers of type II alveolar epithelial cells, including expression of surfactant proteins.13,14,15,16 However, this occurred, in general, at a low level unless the ESCs were transduced to select for surfactant protein–expressing cells.17 More recent protocols and manipulation of cell signaling pathways guiding embryological lung development have yielded more robust in vitro derivation of cells with phenotypic characteristics of type II alveolar epithelial cells from murine ESCs (Figure 1).17,18,19,20,21 Derivation of airway epithelial cells from ESCs has proven even more elusive, although development of cells with phenotypic markers of airway epithelial cells has been demonstrated following culture of the ESCs under air–liquid interface conditions.21,22 Moreover, studies of ESC engraftment or paracrine effects in lung are sorely lacking. Endotracheal administration of mouse ESC–derived type II alveolar epithelial cells to fetal mice resulted in survival of the cells in the lungs and maintenance of surfactant expression over a 24-hour period.20 However, whether the cells truly engrafted or had functional significance was not determined. Furthermore, the long-term fate of the ESCs in lung and comparable observations following administration of ESCs to adult lungs have not been explored.
Figure 1.
Schematic of protocol utilized to differentiate embryonic stem cells into cells with phenotypic characteristics of type II alveolar epithelial cells. FGF-2, fibroblast growth factor 2; Pro-SPC, pro-surfactant protein C. From ref. 20.
It is in this context that the current work of Wang and colleagues represents a significant advance in the potential utility of ESCs for lung repair.4 Utilizing one of the approved human ESC lines developed at the University of Wisconsin (line H9.2), these investigators had previously utilized surfactant protein C promoter–driven neomycin expression to develop a >99% pure population of ESC-derived cells with phenotypic characteristics of type II alveolar epithelial cells (hES-ATII).17 For the current studies, the investigators developed two additional H9.2 hESC-derived cell lines expressing the transcriptional promoters for the type I alveolar epithelial cell markers aquaporin 5 (AQP5) and T1α, each upstream of the LacZ gene. Type II alveolar epithelial cells in culture can differentiate into type I alveolar epithelial cells, and the appearance of β-galactosidase expression was observed in hES-ATII cells derived from the AQP5 and T1α hESC lines. This suggests that type I alveolar epithelial cells can be derived from the cultured hES-ATII cells. However, the novelty and significance of these studies center on observations made following intratracheal administration of the hES-ATII cells 1 or 2 days following induction of acute lung injury resulting from intratracheal bleomycin administration to immunocompromised severe combined immunodeficient mice. Bleomycin administration causes acute lung inflammation and apoptotic death of alveolar epithelial cells, and it provides a potential opportunity for engraftment of exogenously administered cells. Accordingly, a substantial number of hES-ATII cells appeared to have engrafted in lung and could be found as long as 9 days later. Notably, up to 20% of the total surfactant protein C–expressing cells appeared to be of hES-ATII origin. A few hES-ATII cells also appeared to have differentiated into type I cells in vivo. No apparent engraftment was observed when hES-ATII cells were administered to naive uninjured mice or when a control cell population was administered. In parallel, bleomycin-induced lung injury was significantly reduced in mice receiving hES-ATII cells but not vehicle or cell controls. Reduced injury was measured by both qualitative and quantitative measures, including, importantly, functional physiological measurements of lung capacity and gas exchange.
To our knowledge, these findings are the first to demonstrate amelioration of lung injury by ESC administration. Whether the observed amelioration resulted from structural engraftment of the administered cells or reflect a heretofore unsuspected paracrine effect of the hES-derived cells is not yet clear. Future studies will help answer these and other questions. Nonetheless, the results of Wang and colleagues open a new window on the use of ESCs for repair of lung injury. This has several ramifications, including the study and potential use of ESCs in genetic lung diseases. For example, hESC lines derived from embryos with cystic fibrosis have been established in England and Belgium.23,24 These cells exhibit normal morphology and protein expression compared with other hESC lines but have not been studied in detail. With the new loosening of restrictions on study of hESCs in the United States, it is anticipated that there will be additional rapid advances in research on ESCs in lung injury and repair.
REFERENCES
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Kolls JK, Ortiz LA, Panoskaltis-Mortari A., and , Prockop DJ. Stem cells and cell therapy approaches for lung diseases. Conference report. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, et al. Identification of a bone marrow–derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Morales JE, Calame DG, Alcorn JL., and , Wetsel RA. Transplantation of human embryonic stem cell–derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q., and , Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow–derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Ward MR, Stewart DJ., and , Kutryk MJ. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv. 2007;70:983–998. doi: 10.1002/ccd.21302. [DOI] [PubMed] [Google Scholar]
- Nauta AJ., and , Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V., and , Matthay MA. Intrapulmonary delivery of bone marrow–derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Resp Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V., and , Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin–induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiris Therapeutics (2009) Osiris therapeutics reports interim data for COPD stem cell studyPress release < < http://investor.osiris.com/releasedetail.cfm?ReleaseID=391580 > ( 23 June 2009).
- Samadikuchaksaraei A., and , Bishop AE. Derivation and characterization of alveolar epithelial cells from murine embryonic stem cells in vitro. Methods Mol Biol. 2006;330:233–248. doi: 10.1385/1-59745-036-7:233. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M., and , Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Denham M, Cole TJ., and , Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1210–L1215. doi: 10.1152/ajplung.00427.2005. [DOI] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E., and , Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vranken BE, Rippon HJ, Samadikuchaksaraei A, Trounson AO., and , Bishop AE.2007 JulyThe differentiation of distal lung epithelium from embryonic stem cells Curr Protoc Stem Cell BiolChapter 1Unit 1G.1. [DOI] [PubMed] [Google Scholar]
- Winkler ME, Mauritz C, Groos S, Kispert A, Menke S, Hoffmann A, et al. Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells. Cloning Stem Cells. 2008;10:49–64. doi: 10.1089/clo.2007.0075. [DOI] [PubMed] [Google Scholar]
- Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute L, De Block G, Liebaers I, Sermon K., and , De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ, et al. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod Biomed Online. 2005;10:390–397. doi: 10.1016/s1472-6483(10)61801-9. [DOI] [PubMed] [Google Scholar]
- Mateizel I, De Temmerman N, Ullmann U, Cauffman G, Sermon K, Van de Velde H, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]