Abstract
Measles virus Edmonston strain (MV-Edm) is thought to have remarkable oncolytic activity that selectively destroys human tumor cells. The P/V/C protein of wild-type MV was shown to resist the antiviral effects of interferon (IFN)-α. Here, we engineered new MVs by arming MV-Edm tag strain (a V-defective vaccine-lineage strain, MV-Etag) with the P or N, P, and L genes of wild-type MV (MV-P and MV-NPL, respectively). The oncolytic activities of the MVs were determined in human renal cell carcinoma (RCC) cell lines and primary human RCC cells by the MTT assay. The antitumor efficacy of the MVs was evaluated in A-498 xenografts in nude mice. IFN-α effectively inhibited the replication of MV-Etag and MV-P, but not MV-NPL. MV-NPL more efficiently induced cytopathic effects (CPEs) in OS-RC-2 cells, even in the presence of human IFN-α. MV-NPL replicated more rapidly than MV-P and MV-Etag in A-498 cells. Apoptosis was induced earlier in A-498 cells by MV-NPL than MV-Etag and MV-P. MV-NPL showed more significant antitumoral effects and had prolonged replication compared to MV-Etag and MV-P. In this study, we demonstrated that the newly engineered MV-NPL has more effective oncolytic activity and may help establish an innovative cancer therapy.
Introduction
Oncovirotherapy, which uses replication-competent viruses as a cancer therapy, is attracting much interest.1,2,3,4 Recently, several reports confirmed that these live-attenuated viruses can induce rapid and lytic infections in tumor cells.5,6,7,8,9,10 Furthermore, some viruses are being used as cancer therapies in current clinical trials.4,11,12 Measles virus Edmonston strain (MV-Edm) has potent antineoplastic activity against various human cancers, including lymphoma, ovarian cancer, mesothelioma, breast cancer, and hepatocellular carcinoma.11,13,14,15,16 It selectively induces potent cytopathic effects (CPEs), notably intercellular fusion in cancer cells, but causes minimal damage in normal cells.9,17 In addition, the MV genome is very stable and the vaccine strains have never reverted to pathogenic forms, making MV highly suitable for further development as an oncolytic agent.
Measles virus is a negative-strand RNA virus of the Morbillivirus genus in the Paramyxoviridae family. A polymerase (L) and its cofactor (P) associate with the viral RNA and N protein to form a ribonucleoprotein. This complex is surrounded by the M protein. The P gene encodes the P protein and two nonstructural accessory proteins, C and V.18 The two MV envelope glycoproteins H and F work in concert to induce virus–cell membrane fusion. CD46 and CD150 were identified as two MV receptors. CD150 expression is confined to immune cells, whereas CD46 is expressed ubiquitously in nucleated cells.19,20,21 CD46 is abundantly expressed in cancer cells,22,23 but minimally expressed in normal cells such as fibroblasts and peripheral blood lymphocytes,9,17 making cancer cells a suitable target for MV oncolytic therapy.
Type I interferon (IFN-α/β) is a powerful innate antiviral defense. MV vaccine strains can induce significantly higher levels of type I IFN than wild-type MV.24 To combat the cellular innate immune response, many viruses encode antagonistic molecules that block some steps of the type I IFN antiviral response.25 The P proteins of wild-type MV have been shown to resist type I IFN. Furthermore, an engineered MV-Edm tag strain (MV-P), whose P gene was replaced with the comparable wild-type gene, induces significantly less IFN-α in tumor cells and has enhanced oncolytic potency against human multiple myeloma compared to the parental virus.13 The major function of the N protein is to surround the genomic RNA, encapsidate the viral genome, and support its replication and transcription.26,27 The N, P, and L proteins are assembled into the ribonucleoprotein, which is the viral replication unit. Therefore, we reasoned that combining a safe targeted therapy mechanism that destroys the host antiviral genetic program and enhances viral genome replication in cancer cells would generate a novel and innovative cancer gene therapy.
In this study, we generated a newly engineered MV, MV-NPL, which is based on the Edm tag strain but is armed with the N, P, and L genes of the wild-type strain. We demonstrated that MV-NPL has enhanced oncolytic activity against human renal cell carcinoma (RCC) cell lines in vitro and in vivo compared to MV-Edm tag and MV-P. We found that MV-NPL had faster replication and transcription than MV-Edm tag and MV-P in RCC cell lines in vitro and RCC cell line xenografts in vivo. In addition, MV-NPL efficiently proliferated and killed RCC cell line even in the presence of IFN-α. Furthermore, this oncolytic activity was specific as MV-NPL caused minimal cytopathic effects in normal human cell line.
Results
CD46 is overexpressed in human RCC cell lines
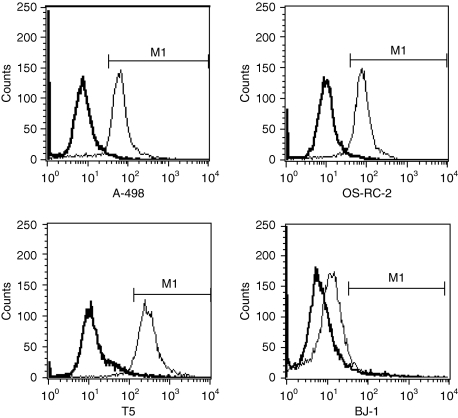
CD46 expression in the human RCC cell lines A-498 and OS-RC-2, primary human RCC cells of T5, normal human skin fibroblast cell line BJ-1 was analyzed by flow cytometry. CD46 was expressed on the surface of most human RCC cell lines and primary human RCC cells: 93.8% in A-498, 93.7% in OS-RC-2, and 92–95% in primary human RCC cells (n = 3). However, only 8.7% in BJ-1 demonstrated positive expression of CD46 (Figure 1). These results demonstrated that human RCC cell lines and primary RCC cells expressed higher levels of CD46 than normal cells.
Figure 1.
CD46 receptor expression on the human renal cell carcinoma (RCC) cell lines A-498 and OS-RC-2, primary human RCC cells of T5, and normal human cell line BJ-1. CD46 receptor was highly expressed on the human RCC cell lines A-498 and OS-RC-2 as well as primary human RCC cells of T5, but was minimally expressed on the normal human cell line BJ-1. The analysis was performed by flow cytometry. CD46 expression in isotype control is 1%. The thick histograms show the measured fluorescence of cells incubated with an isotype control (detailed) and the thin histograms represent cells labeled with an anti-CD46 fluorescein isothiocyanate antibody.
MV-NPL induces stronger CPEs than MV-P and MV-Etag in human RCC cell lines and primary human RCC cells
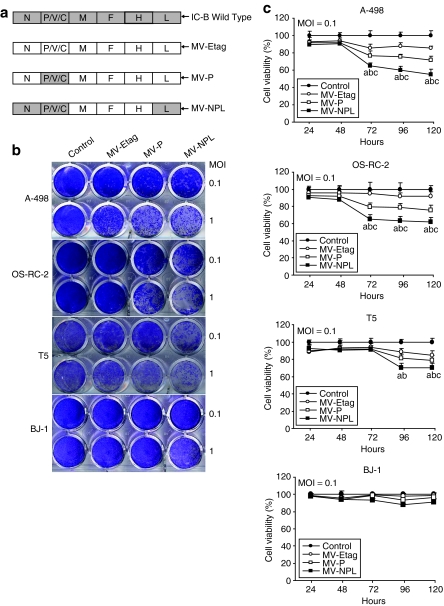
Schematic representation of the genome of MV-Etag (a V-defective vaccine-lineage strain) strain, engineered MV-Etag strain that expresses the wild-type P gene (MV-P) and the engineered MV-Etag strain that expresses the wild-type N, P, and L genes (MV-NPL) is shown in Figure 2a. They were rescued and efficiently propagated in Vero cells and used for the following experiments. We studied the CPEs associated with each MV in the human RCC cell lines A-498 and OS-RC-2, primary RCC cells (n = 3), the normal human skin fibroblast cell line BJ-1. Cells were infected with the various viruses at multiplicities of infection (MOIs) of 1 and 0.1 for 120 hours and the stained with crystal violet. Compared to MV-P and MV-Etag, MV-NPL demonstrated more dramatic CPEs in an MOI-dependent manner (n = 3; Figure 2b). The CPEs appeared at 72 hours postinfection with each MV at an MOI of 0.1 in both A-498 and OS-RC-2 cells and primary RCC cells (data not shown). However, normal human cell line BJ-1 showed minimal CPEs after each MV infection (Figure 2b). We further determined the cell viability after infection with the various viruses using the Cell-Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay. Analyses were performed every 24 hour for 120 hours. Compared with MV-P and MV-Etag, MV-NPL demonstrated a greater reduction of proliferation in A-498, OS-RC-2, and primary RCC cells from 72 or 96 hours to 120 hours at an MOI of 0.1 (n = 3; Figure 2c).
Figure 2.
Induction of cytopathic effects (CPEs) and cell death in human renal cell carcinoma (RCC) cell lines A-498, 0S-RC-2, primary RCC cells T5, and normal human cell line BJ-1 by MV-Etag, MV-P, and MV-NPL. (a) At 120 hours after infection with each MV at multiplicities of infection (MOIs) of 1 and 0.1, the cells were stained with crystal violet. (b) Cells were infected with each MV at an MOI of 0.1 and cell viability was analyzed using the MTT assay. Each value is normalized to the control (untreated cells), which was set at 100%, and represents the mean ± SD (a, P < 0.01, MV-NPL versus control group; b, P < 0.01, MV-NPL versus MV-Etag group; c, P < 0.01, MV-NPL versus MV-P group).
MV-NPL induces faster cell lysis in A-498 cells than MV-P and MV-Etag
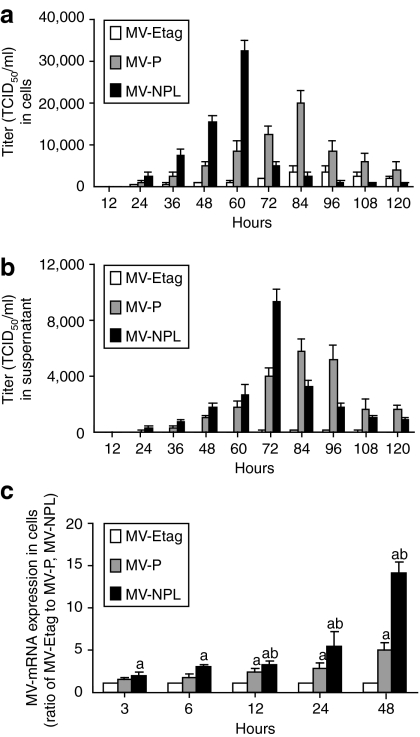
A-498 cells were plated in 6-well plates at a density of 2 × 105 cells/well. The cells were infected with various viruses at an MOI of 0.1 and the supernatants and cells were collected from 12 to 120 hours. The intracellular viruses were released by two cycles of freezing/thawing. The viral titers were determined as the TCID50 (50% tissue culture infective dose) in Vero cells in a 96-well plate. The intracellular MV-NPL viral titer of A-498 cells peaked at 60 hours postinfection (Figure 3a). Compared with MV-NPL, intracellular MV-P virus demonstrated slower replication and the viral titer peaked at 84 hours (Figure 3a). In the culture supernatant, the MV-NPL viral titer peaked at 72 hours (Figure 3b). Similar to the intracellular viral titer, the extracellular MV-P titer peaked with delayed kinetics at 84–96 hours compared to MV-NPL. We also found that after the intracellular viral titer peaked, the viral titer in the culture supernatant peaked in A-498 cells infected with MV-NPL. Real-time PCR analyses revealed a time-dependent increase in measles viral mRNA in A-498 cells, and compared with other viruses, cells infected with MV-NPL demonstrated higher viral mRNA levels (Figure 3c).
Figure 3.
Production of MV-Etag, MV-P, or MV-NPL in the human renal cell carcinoma (RCC) cell line A-498. Cells were infected with each MV at an multiplicity of infection (MOI) of 0.1. (a) Cells and (b) supernatants were harvested at the indicated times. The viral titers were determined on Vero cells and expressed as TCID50/ml. (c) Total RNA from A-498 cells was isolated at the indicated times. The viral mRNA levels were measured using real-time PCR. Each value is normalized to that in MV-Etag, which was set a ratio = 1, and represents the mean ± SD (a, P < 0.05, MV-NPL and MV-P versus MV-Etag; b, P < 0.05, MV-NPL versus MV-P). TCID50, 50% tissue culture infective dose.
MVs induced human IFN-α production from human normal skin fibroblast cells of BJ-1, and human RCC cells of A-498 and OS-RC-2. MV-NPL more effectively evaded to the antiviral defense of IFN-α in Vero cells than MV-P and MV-Etag.
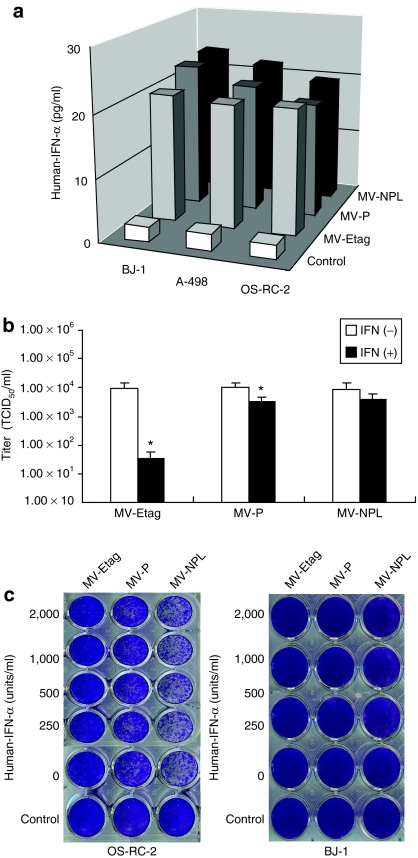
The IFN family, particularly type I IFN (IFN-α/β), induces a powerful innate antiviral response. IFN-α production induced by human normal and tumor cells after infection by MVs was quantified using IFN-specific enzyme-linked immunosorbent assay (ELISA) kits (Figure 4a). We examined the sensitivity of the MV viruses to human IFN-α. Vero cells were infected with different MVs at an MOI of 0.001, and then treated with human IFN-α (1,000 IU/ml) 2 hours after infection. The viral titers were determined at 48 hours postinfection. Human IFN-α effectively inhibited MV-Etag proliferation (Figure 4b). To some extent, IFN-α also suppressed MV-P proliferation, whereas it had no apparent effect on MV-NPL proliferation (Figure 4b). To investigate whether IFN-α can prevent the CPEs of MV, OS-RC-2 cells were infected with each MV at an MOI of 0.1. Different concentrations of human IFN-α (250–2,000 IU/ml) were added to the infected cells and crystal violet staining was performed at 120 hours after infection. Compared to MV-P and MV-Etag, MV-NPL more effectively induced CPE even in the presence of IFN-α. However, there were no obvious CPEs in BJ-1 cells (Figure 4c).
Figure 4.
Different sensitivities of MV-Etag, MV-P, and MV-NPL to human INF-α. (a) BJ-1, A-498 and OS-CR-2 cells were infected with MV-Etag, MV-P, or MV-NPL at an multiplicity of infection (MOI) of 0.1. After 48-hour infection, the production of IFN-α was determined using human IFN-α enzyme-linked immunosorbent assay kit. (b) Vero cells were infected with each MV at an MOI of 0.001 and cultured in the presence or absence of 1,000 IU/ml recombinant IFN-α. Viral titers at 48 hours were determined by titrating the TCID50 on Vero cells, *P < 0.05 versus IFN(+). (c) OS-RC-2 cells and BJ-1 cells in 24-well plates were infected with each MV at an MOI of 0.1. Two hours after infection, human IFN-α was added to the cells at the indicated concentrations. At 120 hours after infection, the cells were stained with crystal violet. IFN, interferon; TCID50, 50% tissue culture infective dose.
MV-NPL induces more apoptosis in human RCC cells than MV-P and MV-Etag
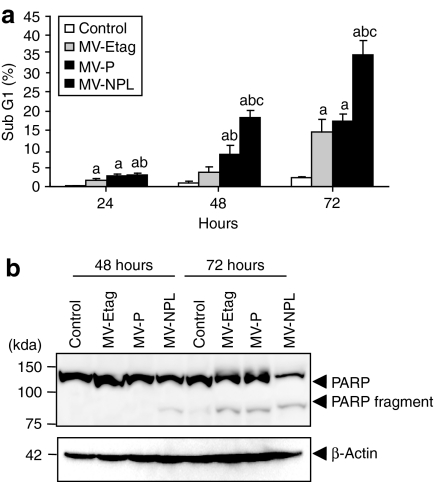
A-498 cells were infected with each MV at an MOI of 0.1, and apoptotic cells were analyzed by propidium iodide staining and subsequent flow cytometry. Upon infection with the MVs, the number of cells in sub-G1 increased in a time-dependent manner (Figure 5a). MV-NPL induced apoptosis in ~20 and 40% of cells at 48 and 72 hours at an MOI of 0.1, respectively (Figure 5a). However, at the same time points, MV-P and MV-Etag only induced apoptosis in ~10 and 15% of cells (Figure 5a). We further examined poly(ADP-ribose) polymerase expression and found that the 85-kd cleaved poly(ADP-ribose) polymerase fragment (85 kd) was more rapidly expressed in A-498 cells infected with MV-NPL than those infected with MV-P or MV-Etag (Figure 5b).
Figure 5.
Apoptosis induced by MV-Etag, MV-P or MV-NPL in the human renal cell carcinoma (RCC) cell line A-498. Cells were infected with each MV at an multiplicity of infection (MOI) of 0.1. (a) Adherent and detached cells were harvested at 24, 48, and 72 hours postinfection. The percentage of sub-G1 cells was measured by fluorescence-activated cell sorting (a, P < 0.05, MV-NPL, MV-P and MV-Etag versus control; b, P < 0.05, MV-NPL and MV-P versus MV-Etag; c, P < 0.05, MV-NPL versus MV-P). (b) Whole-cell lysates of A-498 cells that were infected with each MV were subjected to western blot analysis using anti-PARP and β-actin antibodies. PARP, poly(ADP-ribose) polymerase.
Intratumoral administration of MV-NPL induces regression of A-498 xenografts
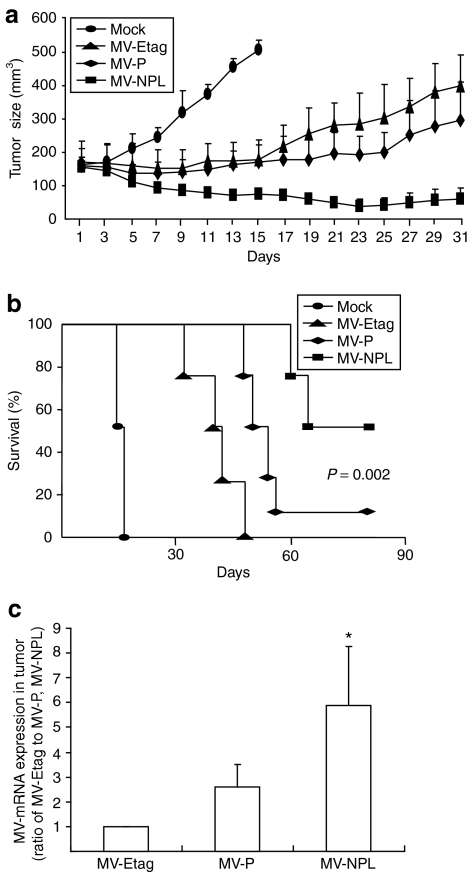
Each MV was given intratumorally to nude mice bearing established (0.5–0.6 cm in diameter) subcutaneous human A-498 tumor xenografts. Intratumoral administration of MV-Etag or MV-P (10 doses of 1.0 × 105 TCID50/dose) effectively suppressed the growth of A-498 xenografts. Compared with MV-Etag or MV-P, intratumoral injection of MV-NPL caused even more regression of the A-498 xenografts (Figure 6a). At 80 days after injection, the survival rate was significantly improved in the MV-NPL-injected group (55%), compared to the control group (0%), MV-Etag-injected group (0%), and MV-P-injected group (11%) (Figure 6b). A-498 xenografts infected with MV-NPL had the highest mRNA expression of the M gene of MV, indicating that MV-NPL was more effectively replicated in the xenografts than MV-P at 19 days after the injection when the former significantly suppressed the tumor growth than the latter (P < 0.05) (Figure 6c).
Figure 6.
Therapeutic efficacy of MV-Etag, MV-P, or MV-NPL for renal cell carcinoma (RCC) xenografts in vivo. (a) A-498 cells (5 × 106 in 100 µl phosphate-buffered saline) were injected subcutaneously. When tumors reached a diameter of 0.5–0.6 cm, the virus was injected intratumorally every other day for a total of 10 doses (1.0 × 105 TCID50/dose) over 19 days. The first day of injection represents day 1, and the tumor volumes were measured every other day (n = 9/group). (b) Kaplan–Meier survival analysis are shown for treated mice and mock-treated mice (P = 0.002, MV-NPL versus MV-P). (c) Intratumoral administration of each MV was initiated with a 1.0 × 105 TCID50 injection. Nineteen days after injection, the tumors were harvested and the M gene viral mRNA levels were determined by real-time PCR, *P < 0.05 versus MV-P. TCID50, 50% tissue culture infective dose.
Discussion
Oncovirotherapy using replication-competent viruses for cancer treatment has recently attracted considerable attention. Engineering replication-competent viruses for cancer therapy is a novel and promising strategy. Live-attenuated MV has a potent and tumor-specific oncolytic activity against a variety of human tumors.9,13,28,29 In clinical trials, the MV vaccine strain has been shown to mediate regression of T-cell lymphomas when administered intratumorally.11 However, there is no report on the oncolytic effects of measles virus on human RCCs. In this study, we report for the first time that measles virus has potent antitumor activity against human RCC cells in vitro and in vivo.
Numerous factors in the tumor microenvironment, such as the stromal architecture and surrounding innate immune system, could potentially restrict viral replication and spread in vivo.30 Human type I IFNs such as IFN-α/β have been shown to inhibit gene expression and the production of progeny virions of the measles virus vaccine strain, including Edmonston tag strain.31,32,33 In order to more effectively control tumor growth and eventually eradicate tumors by direct viral spread and oncolysis, an attenuated replication-competent virus must be able to evade the host innate immune response.13,34 The P/V/C protein of the MV wild-type strain encoded by the P gene was shown to block IFN-α induced-signaling, allowing the virus to evade the innate immune response.13,18,31,35,36 The N, P and L proteins assemble into the ribonucleoprotein, which serves as the MV replication unit. In our study, we found that wild-type N protein provided virus with resistance to IFN similar to P (data no shown). Moreover, the L protein gene of wild type can more effectively induce viral RNA and protein synthesis than vaccine strain.37 In this study, we engineered a novel MV-Edm by replacing the N, P, and L genes with those of the wild-type MV strain to create a virus that rapidly replicates on human renal cancer cells. Compared to MV-P and MV-Etag, MV-NPL exhibited more efficient replication and a potent killing effect in human renal cancer cells in vitro and in vivo. In our study, we demonstrated that human IFN-α effectively inhibited the replication of MV-Etag and MV-P, but not MV-NPL, at an MOI of 0.001 in Vero cells in vitro. Furthermore, MV-NPL exhibited more efficient cytopathic effects than MV-Etag and MV-P in OS-RC-2 cells even in the presence of IFN-α.
Several mechanisms could account for the enhanced antitumoral effects of the engineered virus in vitro, such as faster replication kinetics, enhanced cell killing, or evasion of host antiviral mechanisms.13,34 In this study, we demonstrated that MV-NPL replicated faster than MV-P and MV-Etag. In addition, RCCs infected with MV-NPL produced more viruses than those infected with MV-P or MV-Etag. Measles M mRNA was detected earlier in A-498 cells infected with MV-NPL than with MV-P or MV-Etag, which resulted in more substantial upregulation of viral protein production compared to the other viruses in a time-dependent manner (Figure 3). Therefore, we considered that the rapid oncolysis of cancer cells induced by MV-NPL is due to rapid viral mRNA transcription followed by abundant intracellular viral protein production and accelerated cancer cells lysis, causing the cells to necrose after viral infection. To determine the mechanism of MV-induced cell death, we used sub-G1 staining. Our results demonstrated that MV-NPL induced apoptosis in infected tumor cells faster than MV-Etag and MV-P. Therefore, we concluded that not only necrosis but also apoptosis was an important mode in MV-induced cell death.
MV has been shown to use two receptors, SLAM (CD150) and CD46, for entry into cells. SLAM (CD150) is a signaling lymphocyte activation molecule and its expression profile is confined to immune cells. CD46 is ubiquitously expressed in nucleated cells. The MV-Edm strain can infect cells via CD46, which is expressed more frequently in human cancer cells than normal cells. The most important issue to consider when developing an effective oncovirotherapy is that the oncolytic virus needs to selectively infect tumor cells but not normal cells. Our data demonstrated that CD46 was overexpressed in human RCC cell lines as well as cultured primary human RCC cells with 11-fold higher expression than in the normal human BJ-1 cell line. Compared to cancer cells, MV induces minimal CPEs in normal human cell line. These results suggested that cancer cells are suitable targets for MV infection.
Compared with oncolytic DNA viruses, RNA viruses do not require host nuclear transcription factors, and must rely on an alternative mechanism to preferentially replicate in tumor cells.30 Furthermore, the MV genome is very stable, and the vaccine strains have never reverted to pathogenic forms. The Edmonston strain has been successfully used as a vaccine with an excellent safety profile. These data suggest that the MV vaccine strain has high tumor selectivity and safety, even though additional safety studies should be performed before starting clinical trials. In this study, we demonstrated that even low intratumoral doses of the engineered MV in vivo are sufficient to induce tumor regression.
We also demonstrated that MV-NPL efficiently induced tumor regression and showed the highest viral mRNA expression in the mouse model compared to MV-Etag and MV-P. The oncolysis of cancer cells has been clearly shown in vitro, so we suspected that the same mechanism occurs in our in vivo model. In current oncolytic virus research, intratumoral, intravenous, or intraperitoneal injections have been used to treat immune-deficient mice bearing human tumor xenografts. Among these treatments, intratumorally injected virus can efficiently escape circulating MV–neutralizing antibodies; therefore, this method is considered to be more desirable.
Currently, some additional immune mechanisms have been implicated in oncovirotherapy-mediated therapeutic effects. Several studies have shown that CD8 T cells are related to the efficiency of herpes simplex virus–induced,38 vesicular stomatitis virus–induced,39 and MV-induced14,40 virotherapies. However, adult patients infected with measles virus have significantly higher levels of regulatory T cells, IFN-γ, and interleukin-10 (ref. 41). Furthermore, the phagocytosis of apoptotic MV-infected mesothelioma cells induced spontaneous DC maturation and activation and significant CD8 T-cell amplification. Collectively, oncovirotherapy may exhibit multiple clinical effects, including tumor lysis followed by the appearance and persistence of antitumor immunity.
In summary, our current results demonstrated that newly engineered MV-NPL has more effective oncolytic activity than the parental virus or MV-P as a systemic therapy for human RCC. The engineered virus caused CPEs in human RCCs, but had no toxic effects on normal human cells. Furthermore, MV-NPL replicated faster and more effectively resisted IFN-α than MV-Etag and MV-P, allowing the virus to escape the innate immune response. Although additional safety issues should be investigated, these properties of MV-NPL may help establish an innovative cancer therapy in the future.
Materials and Methods
Construction of engineered viruses. The plasmids p(+)MV323 (ref. 42) and p(+)MV2A (ref. 43), which encode the full-length antigenomic complementary DNA of the IC-B wild-type strain and the Edmonston tag strain, respectively, were used in this study. We inserted restriction enzyme sites (SnaBI-N-SplI-P-Eco47III-M-Nrul-F-PacI-H-SpeI-L-PmeI) into the noncoding region of the p(+)MV323 genome using PCR with specific primers. Using the appropriate restriction enzymes, a series of genomic regions of p(+)MV2A were replaced with identical regions in p(+)MV323, which generated plasmids carrying the full-length genomes of recombinant engineered viruses (Figure 3a). Engineered MVs were rescued from cloned viral genome complementary DNA with a highly efficiently reverse genetics system as described previously.44 The engineered MVs were propagated in Vero cells, an African green monkey kidney epithelial cell line, and passage three viral stocks were used in this study.
Cell line culture. The human RCC cell lines A-498 and OS-RC-2, the normal human skin fibroblast cell line BJ-1 were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Japan Bioserum; Sigma, Steinheim, Germany). Vero cells were used to produce measles virus and maintained in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum (Japan Bioserum). All media used in this study contained 100 U/ml of penicillin–streptomycin. All cell lines used in this study were cultured in a humidified atmosphere with 5% CO2 at 37 °C.
Primary cell culture. Primary human RCC tissues were established using surgical specimens immediately after resection from Kyushu University Hospital after institutional review board approval and informed patient consent. Tissues were sliced, minced, treated with collagenase (GIBCO, Invitrogen, Carlsbad, CA) at 37 °C for 2 hours on a shaker, and then filtered through a nylon mesh (100-µm diameter) to obtain single cell suspensions. Harvested cells were cultured in Minimum Essential Medium α medium (GIBCO, Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT) and 4 µg/ml of Gentamicin Reagent Solution (GIBCO, Invitrogen). All cells used in this study were cultured in a humidified atmosphere with 5% CO2 at 37 °C.
Flow cytometry. CD46 expression and the subdiploid status of cells (sub-G1) were determined by flow cytometry. To measure CD46 expression, the cells were harvested with Cell Dissociation Buffer (GIBCO, Invitrogen), washed twice with phosphate-buffered saline (PBS), and incubated with a fluorescein isothiocyanate–labeled monoclonal mouse anti-human CD46 or control antibodies (BD Biosciences, Pharmingen) for 1 hour on ice. The cells were washed twice with PBS and then 10,000 cells per sample were analyzed using a FACScan (BD Biosciences, San Jose, CA). For sub-G1, A-498 cells were plated in 6-well plates, and then treated with each MV at an MOI of 0.1. Adherent and detached cells were harvested at 24, 48, and 72 hours after infection and fixed in ice-cold 70% ethanol for at least 1 hour. Cell pellets were washed twice with PBS and then incubated for 30 minutes at room temperature in 1 ml PBS containing 50 µg propidium iodide (Sigma-Aldrich, St Louis, MO), 0.1% Triton X-100, 1 mmol/l EDTA, and 0.5 mg RNaseA. After staining, 10,000 events per sample were analyzed using a FACScan (BD Biosciences). Fragmented apoptotic nuclei were recognized by their sub-G1 DNA content. The percentage of sub-G1 cells was recorded for each sample. All flow cytometry data were analyzed using the Mod Fit LT software (Verity Software House, Topsham, MN).
Evaluation of CPEs in vitro. A-498, OS-RC-2, T5 (cultured primary human RCC cells), and BJ-1 cells were cultured in 24-well plates at a density of 2 × 104 cells/well. The cells were infected with each MV at an MOI of 1 or 0.1 in 0.2 ml of Opti-MEM I (GIBCO, Invitrogen) for 2 hours. The virus suspension was removed, and 1 ml of fresh medium was added to each well with or without the noted concentrations of human IFN-α. At 120 hours after infection, the cells were gently washed twice with PBS, and the remaining cells were fixed with 0.5% glutaraldehyde in PBS for 15 minutes. Then, cells were washed with PBS and stained with 0.1% crystal violet solubilized in 2% ethanol–distilled water. The stained product was subsequently washed twice with distilled water, air-dried, and then photographed.
Western blot analysis and ELISA. Infected cells were harvested and solubilized in a Nonidet P-40-based lysis buffer [20 mmol/l Tris (pH 7.4), 250 mmol/l NaCl, 1% Nonidet P-40, 1 mmol/l EDTA, 50 mg/ml leupeptin, and 1 mmol/l phenylmethylsulfonyl fluoride). After incubating on ice for 5 minutes, the cell lysates were clarified by centrifugation at 13,000 g for 30 minutes at 4 °C. The protein concentrations in the lysates were quantified using Multiskan spectrum. The samples were separated on precast 4–12% gradient MOPS polyacrylamide gels (NOVEX, San Diego, CA), and then transferred to nitrocellulose membranes (BIO-RAD, Hercules, CA). The membranes were pretreated with Tris-buffered saline containing 5% dry milk and 0.05% Triton X-100 (TBST) for 1 hour at room temperature and then incubated with monoclonal antiproteolytic cleavage of poly(ADP-ribose) polymerase (Biovision, Mountain View, CA) and a rabbit anti-β-actin (CHEMICON International, Temecula, CA) antibodies for 1 hour at room temperature. After several washes in TBST, the membranes were probed with rabbit or mouse peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 hour. After a final wash with TBST, the immune-reactivity of the blots was detected using an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). ELISA specific for IFN-α was performed using a human IFN-α ELISA kit (PBL Biomedical Laboratories) as per manufacturer's instructions.
Cell proliferation assay. The Cell-Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI) was used in this study. A-498, OS-RC-2, T5, and BJ-1 cells were plated in 96-well plates at a density of 1 × 104 cells/well. Twelve hours after seeding, the cells were infected with each MV at an MOI of 0.1 for different time intervals and then incubated with 20 µl of MTS reagent for 2 hours at 37 °C. The absorbance at 490 nm was recorded using an ELISA plate reader.
Assessment of MV replication in a human RCC cell line. The human RCC cell line A-498 was seeded in 6-well plates at a density of 2.0 × 104 cells/well. Twelve hours after plating, the cells were infected with each MV at an MOI of 0.1 in Opti-MEM I. The cells and supernatants were collected at different time intervals. The viruses were released by two cycles of freezing and thawing. The viral titers in the cells and supernatants were determined by titrating the TCID50 on Vero cells.
Human IFN-α sensitivity of MVs. Vero cells were infected with each MV at an MOI of 0.001. Two hours after infection, human IFN-α A/D (Sigma, St Louis, MO) was added to the cells at a concentration of 1,000 IU/ml. At 48 hours postinfection, the cells were harvested together with the culture media. The viral titers in both the intracellular samples and the culture supernatants were determined by titrating the TCID50 on Vero cells.
In vivo xenograft experiments. A-498 cells (5 × 106 in 100 µl PBS) were injected subcutaneously into the right flanks of 4-week-old female BALB/c nude mice using a 27-gauge needle. The length and width of the tumors in each mouse were measured daily with calipers. Mice were randomly divided into four groups: MV-Etag, MV-P, MV-NPL, or control (n = 9/group). Intratumoral administration of each MV was initiated when the tumors reached a diameter of 0.5–0.6 cm. The mice were injected with each MV (1 × 105 TCID50 in 50 µl Opti-MEM I), and each mouse received 10 MV doses on days 1, 3, 5, 7, 9, 11, 13, 15, 17, and 19. Control mice (mock therapy group) were injected with equal volumes of Opti-MEM I containing no virus. The tumor volume was calculated as length × width × width/2. Mice were killed if they lost >20% of their body weight or the tumor diameter exceeded 1.0 cm. All mouse experiments were approved by the Committee of the Ethics on Animal Experiments in the Faculty of Medicine, Kyushu University and carried out following the Guidelines for Animal Experiments in the Faculty of Medicine, Kyushu University, Fukuoka, Japan and The Law and Notification of the Government.
Statistical analysis. Each experiment was repeated three different times, and data are presented as means ± SD. Where indicated, the data were analyzed by a one-way analysis of variance with Bonferroni's post hoc test using SPSS 15.0 software (SPSS, Chicago, IL). P values <0.05 were considered statistically significant.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (17016053).
REFERENCES
- Cattaneo R, Miest T, Shashkova EV., and , Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH., and , Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Liu TC., and , Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- Aghi M., and , Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Kim M, Chung YH., and , Johnston RN. Reovirus and tumor oncolysis. J Microbiol. 2007;45:187–192. [PubMed] [Google Scholar]
- Nettelbeck DM, Rivera AA, Balagué C, Alemany R., and , Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- Connor JH, Naczki C, Koumenis C., and , Lyles DS. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J Virol. 2004;78:8960–8970. doi: 10.1128/JVI.78.17.8960-8970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csatary LK, Gosztonyi G, Szeberenyi J, Fabian Z, Liszka V, Bodey B, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67:83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and , Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Fu X, Tao L., and , Zhang X. An oncolytic virus derived from type 2 herpes simplex virus has potent therapeutic effect against metastatic ovarian cancer. Cancer Gene Ther. 2007;14:480–487. doi: 10.1038/sj.cgt.7701033. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H., and , Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ., and , Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvrit A, Brandler S, Sapede-Peroz C, Boisgerault N, Tangy F., and , Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, et al. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ, et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R., and , Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kadota SI, Takeda M, Miyajima N., and , Nagata K. Measles virus V protein blocks interferon (IFN)-α/β but not IFN-γ signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 2003;545:177–182. doi: 10.1016/s0014-5793(03)00528-3. [DOI] [PubMed] [Google Scholar]
- Dörig RE, Marcil A, Chopra A., and , Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K., and , Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Anderson BD, Nakamura T, Russell SJ., and , Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, et al. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34:713–720. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Naniche D, Yeh A, Eto D, Manchester M, Friedman RM., and , Oldstone MB. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of α/β interferon production. J Virol. 2000;74:7478–7484. doi: 10.1128/jvi.74.16.7478-7484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandvaux N, tenOever BR, Servant MJ., and , Hiscott J. The interferon antiviral response: from viral invasion to evasion. Curr Opin Infect Dis. 2002;15:259–267. [Google Scholar]
- Bourhis JM, Canard B., and , Longhi S. Structural disorder within the replicative complex of measles virus: functional implications. Virology. 2006;344:94–110. doi: 10.1016/j.virol.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Sato H, Inoue Y, Watanabe A, Yoneda M, Ikeda F, et al. Phosphorylation of measles virus nucleoprotein upregulates the transcriptional activity of minigenomic RNA. Proteomics. 2008;8:1871–1879. doi: 10.1002/pmic.200701051. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9:961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- Ohno S, Ono N, Takeda M, Takeuchi K., and , Yanagi Y. Dissection of measles virus V protein in relation to its ability to block α/β interferon signal transduction. J Gen Virol. 2004;85 Pt 10:2991–2999. doi: 10.1099/vir.0.80308-0. [DOI] [PubMed] [Google Scholar]
- Shingai M, Ebihara T, Begum NA, Kato A, Honma T, Matsumoto K, et al. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. J Immunol. 2007;179:6123–6133. doi: 10.4049/jimmunol.179.9.6123. [DOI] [PubMed] [Google Scholar]
- Haller O, Kochs G., and , Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LF, Gu JF, Tang WH, Fan JK, Wei N, Zou WG, et al. Significant antitumor activity of oncolytic adenovirus expressing human interferon-β for hepatocellular carcinoma. J Gene Med. 2008;10:983–992. doi: 10.1002/jgm.1231. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Parisien JP., and , Horvath CM. STAT2 is a primary target for measles virus V protein-mediated α/β interferon signaling inhibition. J Virol. 2008;82:8330–8338. doi: 10.1128/JVI.00831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Saito H, Kubota T, Yokosawa N, Amano K., and , Fujii N. Measles virus suppresses interferon-α signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-α receptor complex. Virology. 2003;306:135–146. doi: 10.1016/s0042-6822(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Takeda M, Ohno S, Tahara M, Takeuchi H, Shirogane Y, Ohmura H, et al. Measles viruses possessing the polymerase protein genes of the Edmonston vaccine strain exhibit attenuated gene expression and growth in cultured cells and SLAM knock-in mice. J Virol. 2008;82:11979–11984. doi: 10.1128/JVI.00867-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt G, Berg K., and , Blixenkrone-Møller M. Measles virus-induced modulation of host-cell gene expression. J Gen Virol. 2002;83 Pt 5:1157–1165. doi: 10.1099/0022-1317-83-5-1157. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Dunster LM, Schneider-Schaulies S., and , ter Meulen V. Pathogenetic aspects of measles virus infections. Vet Microbiol. 1995;44:113–125. doi: 10.1016/0378-1135(95)00004-t. [DOI] [PubMed] [Google Scholar]
- Niewiesk S, Götzelmann M., and , ter Meulen V. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc Natl Acad Sci USA. 2000;97:4251–4255. doi: 10.1073/pnas.060012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XL, Cheng YM, Shi BS, Qian FX, Wang FB, Liu XN, et al. Measles virus infection in adults induces production of IL-10 and is associated with increased CD4+ CD25+ regulatory T cells. J Immunol. 2008;181:7356–7366. doi: 10.4049/jimmunol.181.10.7356. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takeuchi K, Miyajima N, Kobune F, Ami Y, Nagata N, et al. Recovery of pathogenic measles virus from cloned cDNA. J Virol. 2000;74:6643–6647. doi: 10.1128/jvi.74.14.6643-6647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara M, Takeda M., and , Yanagi Y. Contributions of matrix and large protein genes of the measles virus Edmonston strain to growth in cultured cells as revealed by recombinant viruses. J Virol. 2005;79:15218–15225. doi: 10.1128/JVI.79.24.15218-15225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, et al. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]