Abstract
We previously demonstrated that direct intramuscular injection of rAAV2 or rAAV6 in wild-type dogs resulted in robust T-cell responses to viral capsid proteins, and others have shown that cellular immunity to adeno-associated virus (AAV) capsid proteins coincided with liver toxicity and elimination of transgene expression in a human trial of hemophilia B. Here, we show that the heparin-binding ability of a given AAV serotype does not determine the induction of T-cell responses following intramuscular injection in dogs, and identify multiple epitopes in the AAV capsid protein that are recognized by T cells elicited by AAV injection. We also demonstrate that noninvasive magnetic resonance imaging (MRI) can accurately detect local inflammatory responses following intramuscular rAAV injection in dogs. These studies suggest that pseudotyping rAAV vectors to remove heparin-binding activity will not be sufficient to abrogate immunogenicity, and validate the utility of enzyme-linked immunosorbent spot (ELISpot) assay and MRI for monitoring immune and inflammatory responses following intramuscular injection of rAAV vectors in preclinical studies in dogs. These assays should be incorporated into future human clinical trials of AAV gene therapy to monitor immune responses.
Introduction
One obstacle in using viral-derived vectors for in vivo gene delivery is the development of host immune responses to both viral and transgene products. Recombinant vectors based on a subset of adeno-associated virus (rAAV) serotypes have attracted increasing attention due to their high tropism for various cell types including postmitotic cells,1,2,3 absence of serious pathology associated with AAV infections in humans, and the perceived lack of strong immunogenicity of AAV capsids enabling sustained transgene expression.1,3,4,5 However, in a recent clinical trial of hepatic rAAV2-mediated delivery of the factor IX gene in hemophilia B patients, transient transaminitis developed after vector administration, which was accompanied by detection of AAV2 capsid-specific CD8+ T cells, and a decline in factor IX transgene expression to subtherapeutic levels by 8 weeks.6
We have focused on the development of gene therapy for Duchenne muscular dystrophy using a canine model, and have found that T-cell responses to rAAV6 and rAAV2 vectors were induced after intramuscular injection.7 Immune responses were elicited independent of the transgene constructs used, vector dose, or vector preparation. Furthermore, comparably robust cellular immune responses were also observed against empty AAV6 capsids. In line with the findings from the canine studies and the clinical hemophilia B trial, recent studies in various strains of mice and in nonhuman primates have demonstrated induction of CD8+ T cells specific to epitopes within AAV2 or AAV8 capsids after either direct injection of rAAV vectors or immunization with adenoviral vectors expressing AAV capsid protein.8,9,10 Thus, immunity to AAV-mediated therapeutic gene delivery has emerged as a significant barrier for successful long-term gene therapy. Therefore, unless a nonimmunogenic serotype of AAV is identified, future human clinical trials will need to incorporate transient immunosuppressive therapy and validated methods of monitoring immune responses to AAV.
Several different AAV serotypes with distinct patterns of tissue tropism have been isolated from human and nonhuman primates,11 and it is conceivable that some serotypes might not induce immune responses due to serotype-specific biological characteristics. Studies in mice and nonhuman primates have suggested that AAV serotypes that cannot bind heparan sulfate proteoglycans (HSPGs) due to sequence alterations of the capsid were less able to activate T cells after intramuscular injection.10 Therefore, in this study, we compared the ability of rAAV serotypes containing or lacking the HSPG-binding motif to induce immune responses in the canine model. In addition to monitoring local and systemic T-cell responses using histology and interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays, we examined the ability of magnetic resonance imaging (MRI) to serve as a noninvasive method for monitoring inflammatory responses after AAV injection into the muscle.
Results
Overall experimental design
Individual dogs from a random-bred colony received intramuscular injections of 5 × 1011 vector genomes of recombinant rAAV expressing canine factor IX (cFIX) at several distinct locations, each marked with a sterile suture. A total of four dogs were injected, two with rAAV6 and one each with rAAV1 and with the modified rAAV1 (rAAV1-E531K, described below). At 4 weeks after intramuscular injections, a time point previously shown to have the maximal immune infiltration,7 the injected site(s) were imaged by MRI, and, after the MRI scan, one of the AAV-injected sites for each individual was biopsied for histology. Similarly, at 8 weeks after injection, MRI scan was performed on the injected regions prior to biopsy of a second site. Cells were collected before and 4 weeks after the intramuscular injections from a subset of the dogs for T-cell assays. In this report, we will first describe the histology, then the T-cell assays, and finally the MRI studies.
Intramuscular injections of HSPG-binding and nonbinding serotypes induced cellular immune responses in dogs
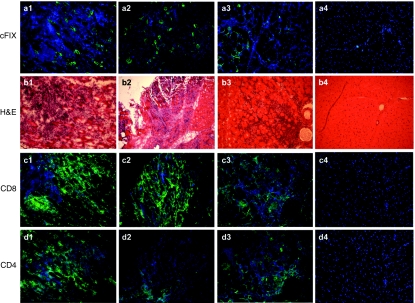
It has been suggested that activation of T cells after AAV injection in mice is due to binding of the virion to HSPG, which facilitates its uptake by dendritic cells.10 Our prior studies showed that both AAV2 and AAV6, which contain distinct HSPG-binding motifs, were immunogenic in dogs. AAV1 shares both a high degree of sequence homology (~99.2%) and tropism for muscle with AAV6 (refs. 12,13); however, AAV1 does not bind HSPG due to a single amino-acid change at position 531, with an E in AAV1 versus a K in AAV6 (ref. 14). Therefore, we prepared rAAV6, rAAV1, and rAAV1 modified to contain K at position 531 to confer binding to HSPG (AAV1-E531K), to determine the effects of HSPG binding on immunogenicity in dogs receiving intramuscular vector injections. All vectors expressed a canine factor IX transgene driven by a CMV promoter (CMV-cFIX). An equal amount of each vector preparation (5 × 1011 vector genomes) was injected into hindlimb muscles of different wild-type dogs (one animal for each vector preparation). Muscle biopsies obtained 4 weeks after vector administration showed uniformly similar robust infiltrations of CD8+ and CD4+ T cells (Figure 1) for each of the three vectors. Thus, the ability of AAV capsid to bind HSPG did not affect the induction of a local T-cell infiltrate after intramuscular injection.
Figure 1.
Immunity to rAAV6, rAAV1-E531K, and rAAV1 at week 4 following intramuscular injection. rAAV6-cFIX: panels a1, b1, c1, and d1; rAAV1-cFIX: panels a2, b2, c2, and d2; rAAV1-E531K: panels a3, b3, c3, and d3; buffer control: panels a4, b4, c4, and d4. Panels a1–a4, immunofluorescence staining with anti-cFIX antibody (green); b1–b4, H&E staining; c1–c4, immunofluorescence with anti-CD8 (green) and DAPI-stained nuclei (blue); d1–d4, immunofluorescence with anti-CD4 (green) and DAPI-stained nuclei (blue). cFIX, canine factor IX.
Detection of AAV capsid-specific T cells in PBMC
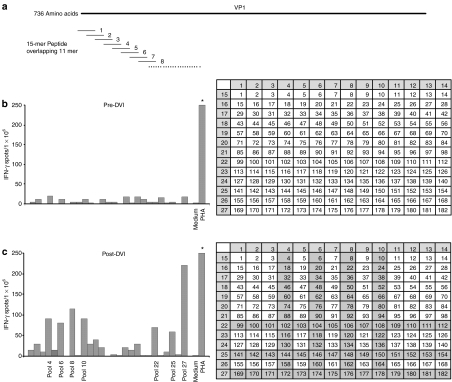
To determine whether the cellular responses detected by histological analysis correlated with induction of T-cell reactivity to viral capsids, we measured antigen-induced T-cell secretion of IFN-γ, as a marker of T-cell reactivity in peripheral blood mononuclear cell (PBMC), using a quantitative ELISpot assay15 in the dog injected with rAAV6 above. The AAV virion is composed of three capsid proteins, VP1, VP2, and VP3 in a ratio of 1:1:10, which are produced from the same open reading frame through alternative splicing.1,16,17 VP1 is the full-length protein; therefore, a peptide library spanning the entire capsid protein VP1 and consisting of 15 mer peptides each overlapping by 11 amino acids with adjacent peptides was generated (Figure 2a). The peptides were organized into 27 pools and arranged in a grid such that pools 1–14 each consisted of peptides from its corresponding column, and pools 15–27 each consisted of peptides from its corresponding row. Therefore, each peptide was present in two individual pools (Figure 2b,c). PBMC collected before and after intramuscular injection of rAAV6 vectors (from the dog used above) were stimulated with each individual pool and IFN-γ secretion was measured. AAV6 peptide-specific IFN-γ secretion was not detected before vector injection (Figure 2b), but was detected 4 weeks after injection (Figure 2c). Positive results after stimulation with pools 4, 6, 8, 10, 22, 25, and 27 predicted overlapping peptides: 102, 104, 106, 108, 144, 146, 148, 150, 172, 174, 176, and 178 as potentially immunogenic. To confirm this result, PBMC obtained before and after vector injections were expanded by incubation for 10 days with each of the six pools containing the potential immunogens (Supplementary Figure S1). The cells were then subjected to subsequent ELISpot assays with each individual peptide from the pools. A T-cell response to peptide 174 (sequence: KRWNPEVQYTSNYAK) was confirmed by expansion with pool 6, and responses to peptides 144 (sequence: ATERFGTVAFNLQSS), 174 (sequence: KRWNPEVQYTSNYAK), and 178 (sequence: ANVDFTVDNNGLYTE) were confirmed by expansion with pools 25 and 27, respectively.
Figure 2.
Enzyme-linked immunosorbent spot assay with rAAV6 peptide pools. The number of positive IFN-γ spots measured in 1 × 106 cells is shown. Medium and PHA were used as negative and positive controls, respectively. (a) A total of 182 peptides was synthesized, each of which is 15 amino acids long and overlapping by 11 amino acids with adjacent peptides. Twenty-seven peptide pools are shown in yellow. (b) Preinjection PBMCs were stimulated with individual peptide pools. (c) Postinjection PBMCs at week 4 were stimulated with individual pools. DVI, direct viral injection; IFN-γ, interferon-γ PBMC, peripheral blood mononuclear cell; PHA, phytohemagglutinin.
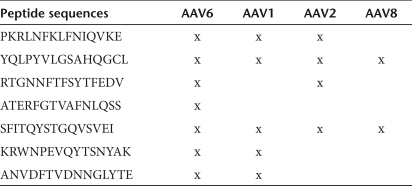
In order to validate the assay, we used the same strategy to identify immunogenic peptides in a second wild-type dog following intramuscular injection with rAAV6. In the second dog, five immunogenic peptides were identified, including one common peptide (peptide 144, ATERFGTVAFNLQSS) that was recognized by both dogs. The sequences of the immunogenic peptides in both dogs are listed in Table 1, as well as whether the individual peptides are present in other commonly used AAV serotypes (Table 2). Therefore, the results showed that AAV6 capsid proteins were recognized by T-cell responses induced after intramuscular injection of AAV in dogs, and confirmed the utility of ELISpot assays for detecting such cellular responses in peripheral blood.
Table 1.
Summary of immunogenic AAV6 epitopes identified from two dogs
Table 2.
Individual peptides conserved in commonly used AAV serotypes
Noninvasive MRI can detect AAV-induced inflammation in canine muscle
AAV vectors are currently being used in human trials for intramuscular delivery of transgenes, so it will be important to develop noninvasive methods of monitoring inflammatory responses to minimize the need for repeated muscle biopsies.18 MRI provides anatomic information and identifies inflammatory processes and progressive fibrosis by contrast derived from differences in local water proton relaxation time of transverse (T2) magnetization, as changes in T2 values and in intensity of T2 signals on T2-weighted images.19 Therefore, we tested whether MRI could be used as a noninvasive method to detect inflammatory reactions resulting from intramuscular injection of rAAV vectors by performing MRI studies on the dogs injected with different vectors (rAAV6, rAAV1, and rAAV1-E531K) described above.
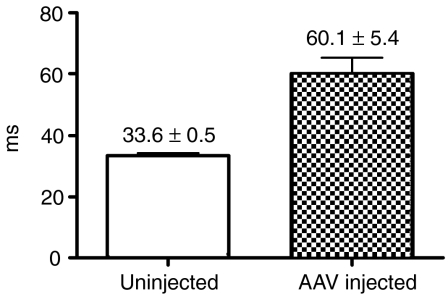
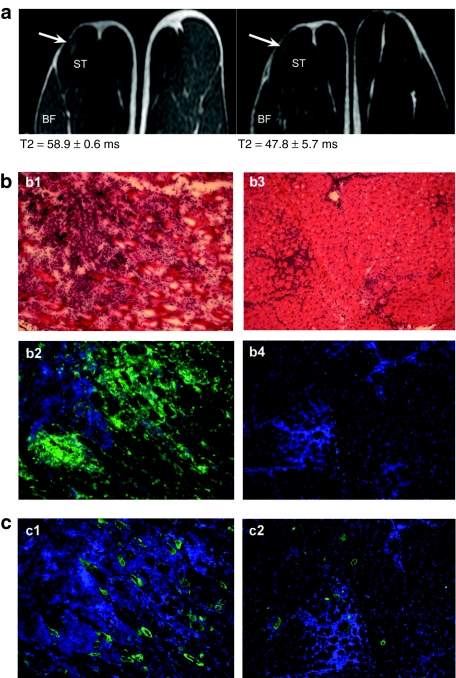
Our prior studies demonstrated that the maximum lymphocytic infiltration was consistently present 4 weeks after vector administration but was reduced by week 8 and negligible by week 12. In order to correlate MRI findings with histopathology, each dog received intramuscular rAAV injections at two different muscle sites, and MRI examinations were performed before muscle biopsies of one site at week 4 and the second site at week 8. Contralateral hindlimbs were either injected with buffer or left untreated to serve as controls. We analyzed T2 values 4 weeks after vector administration in the four wild-type dogs, given either rAAV6 (two dogs, one of which used in HSPG study and see Figure 1 for histology), rAAV1 or rAAV1-E531K (see Figure 1 for histology), all carrying the same CMV-cFIX construct. The mean T2 value was significantly higher (P < 0.0003) at the sites of AAV injection (60.1 ± 5.4) than that from the uninjected or buffer-injected control muscles (33.6 ± 0.5) in the contralateral or the same limbs (Figures 3 and 4). In Figure 4, a and b showed T2 map and T2 values from the dogs injected with rAAV6 and rAAV1-E351K, respectively, in the left biceps, and buffer or no injection in the right biceps 4 weeks after injection. Figure 4c showed T2 map and T2 values from the dog injected with rAAV1 and buffer in the same muscle. Hyperintense regions were observed on T2 maps of rAAV-injected biceps (Figure 4 a1, b1, and c1), but not buffer-injected ones (Figure 4 a1, 3 and 4; and 1 in 4c1); and T2 values were increased in rAAV-injected biceps compared to controls (Figure 4). The hyperintense regions were manually contoured to define regions of interest for the T2 measurements and compared to similar-sized regions away from the injection sites. The injection sites had been marked by taping a vitamin E capsule and agarose (1%) sample to the incision scar (labeled as 5 and 6, respectively, in Figure 4 a1). Histological analysis (Figure 1) following MRI at the 4-week time point showed a significant inflammatory response in the regions demonstrating a hyperintense T2 signal, but not in the buffer-injected control sites (Figure 1 b4, c4, and d4).
Figure 3.
Magnetic resonance imaging T2 values at week 4. Median T2 values in rAAV-injected and uninjected muscles of contralateral limb from four wild-type dogs (P < 0.0003).
Figure 4.
Magnetic resonance imaging and corresponding muscle biopsy analysis at week 4 in dogs receiving different AAV vectors. (a) rAAV6-CMV-cFIX. a1, T2 map; a2, T2 values. 1 and 2: rAAV6-injected sites; 3 and 4: buffer-injected sites; 5 and 6: vitamin E capsule and agarose (1%) sample, respectively, as markers. BF, biceps femoris; ST, semitendinosus. (b) rAAV1-E351K. b1, T2 map; b2, T2 values. (c) rAAV1. c1, T2 map; c2, T2 values (see Figure 1 for immunohistological analysis on muscle biopsy injected with corresponding vectors). ROI, regions of interest.
Eight weeks after the intramuscular injection, MRI showed reduced T2 intensity at the site of AAV injection, compared to the signal at week 4 (Figure 5a). This observation correlated with reduced inflammation and reduced cFIX expression by histological analysis (Figure 5b,c) of corresponding muscle tissues. These results demonstrated that MRI was a sensitive and potentially useful noninvasive modality for monitoring AAV-induced inflammatory responses.
Figure 5.
Progression of inflammatory responses to rAAV6 from week 4 to week 8 after intramuscular injection in dog. (a) T2-weighted images at 4 weeks (a1) and 8 weeks (a2) after vector injection. Arrows point at the two rAAV-induced inflammatory sites. (b) Muscle biopsy analysis at week 4 (b1-H&E, and b2-CD8 in green), and week 8 (b3-H&E, and b4-CD8, DAPI-stained nuclei (blue). (c) cFIX expression at week 4 (c1-green) and week 8 (c2-green).
Discussion
Vectors derived from AAV are in general less immunogenic than adenoviral and other viral vector systems, and have shown promise as delivery vehicles for human gene therapy.1,2,3,4,20,21 However, recent studies in dogs, nonhuman primates, and humans have shown development of T-cell immunity against different AAV serotypes after either intramuscular or intravenous administration.6,8,9,10,22,23 In line with these reports, our previous studies in dogs demonstrated robust T-cell-mediated immune responses to AAV capsid following intramuscular injection.7
Differences in capsid sequences among various AAV serotypes can influence the biodistribution of vectors, vector interaction with antigen-presenting cells, vector uncoating, and intracellular vector trafficking and processing. These differences might account for the lower immunogenicity reported for some AAV serotype capsids compared to others. It has been suggested that rAAV2 is more immunogenic than rAAV8 because rAAV2 capsids contain a heparin-binding motif that facilitates rAAV2 binding to HSPG on dendritic cells with resultant AAV2 antigen uptake, processing, and major histocompatibility complex class I presentation. One study both in mice and nonhuman primates showed that high-frequency, capsid-specific T cells were only elicited against natural or recombinant AAV vectors that contained a heparin-binding motif.10 On the other side, recent studies from the High and Mendell groups showed immunogenicity of AAV1 capsids following intramuscular injection in humans, another nonheparin-binding serotype;22 however, in the Mendell study,23 the immune response apparently did not diminish transgene expression. In another report from the High group,24 humans were shown to have pre-existing CD8+ memory T cells specific for AAV capsids. The cells were at a frequency too low for detection by a direct ex vivo assay. However, these T cells could be expanded by culturing in the presence of rAAV vectors and were broadly cross-reactive with different serotypes, including those that lacked HSPG binding.24 These results extended previous observation of immune responses to additional AAV serotype, AAV1, and suggested that HSPG binding might not be critical in activation of memory T cells, and that immune responses to AAV would be a barrier for gene therapy using rAAV in patients with pre-existing immunity, and for readministration of therapeutic vectors. In the current study, we compared the immunogenicity of capsids from AAV1, a serotype without a heparin-binding domain; AAV6, with a natural heparin-binding motif; and AAV1-E531K with an introduced heparin-binding site, and found that immune responses were elicited independent of HSPG binding, which is in line with the recent published results on AAV1 (ref. 22) in which immunogenic epitopes within AAV1 capsids were identified following intramuscular injection in humans. Different factors including vector dosage as indicated in Mingozzi et al.22 and Mendell et al.,23 and the species (mouse, human, and canine) studied may affect the magnitude of the immune responses and/or the outcome of intramuscular injection. It will be interesting to examine the issue of kinetics of the response in the blood in relation to vector dose in the dog model in future studies. Canine parvovirus capsid protein has between 10 and 60% of identity to that of AAV6; however, we have found that vaccination with canine parvovirus in these dogs did not induce a humoral immune response to AAV capsid proteins (Z. Wang and S.J. Tapscott, unpublished results). There was limited/no homology between canine parvovirus and the epitopes of AAV6 that were identified to be immunogenic in our study, and we did not detect epitope-specific T-cell responses after ex vivo expansion for 10 days of PBMC obtained before vector injection, suggesting our observation in dogs was independent of memory T cells. The data suggest that factors other than HSPG binding contributed to induction of cellular responses. Additional studies of immunogenicity of AAV vectors are warranted so that strategies could be developed to permit long-term transgene expression.
The immunohistochemistry of injected muscle clearly showed infiltration with both CD4 and CD8 T cells, indicating that both subsets are responding to AAV antigen. Our results supported the use of IFN-γ ELISpot assays as a sensitive method for detecting T-cell responses in general to AAV, and showed a correlation with the detection of AAV-specific T cells in the blood and histological findings of local inflammation in muscles after rAAV6 injection. Given the high sequence homology of the capsid between many AAV serotypes, it is not surprising that T cells specific for one serotype frequently crossreacted with other serotypes.24 In our study, multiple capsid epitopes were recognized in individual dogs treated with rAAV6, and many of these epitopes are also contained in other serotypes. For example, five of the seven capsid epitopes identified as immunogenic in AAV6 are entirely conserved in AAV1; four are in AAV2, and two are in AAV8. It is possible that common epitopes between AAV6 and other serotypes might behave differently in vivo and different epitopes may be identified from different AAV serotypes. This information, together with recent findings in human trials,22,23 support the notion that it is unlikely that alternative serotypes would escape host immune surveillance or that modification to the capsid sequence would eliminate immunogenicity. Thus, alternative means to blunt the host immune reaction are required. In this regard, we have demonstrated that a brief course of immunosuppression could permit sustained transgene expression in muscles of both wild-type and dystrophic dogs.18 These studies focused on intramuscular delivery of AAV; it might require intravascular delivery of AAV vectors to skeletal muscle for translational studies into human patients. Thus, future studies will be necessary to determine immune responses to AAV following intravascular delivery. A recent publication by Gregorevic et al.25 indicated that a similar immunosuppression regimen improved transgene delivery following intravascular AAV administration as well.
An important component of future human clinical trials will be methodologies for noninvasive monitoring of inflammation at sites of gene delivery. Normal and pathologic tissues can be distinguished based on relative changes in signal intensity using different MRI imaging parameters. The usefulness of MRI in evaluating lesions in muscle has been explored. Experimental injury, such as mechanical or chemical disruption of muscle membranes, or inflammatory processes such as idiopathic inflammatory myopathy, resulted in increased T2 values (measuring inflammation and fibrosis), which correlated well with muscle damage assessed by histological studies.19,26,27 Similarly, in our current study, MRI T2-weighted images and increased T2 values correlated well with the degrees of lymphocytic infiltration in muscle after rAAV administration. These preclinical MRI studies in the dogs were performed using the instrument and coils used for human clinical studies, so the protocols developed in dogs can be directly extrapolated for noninvasive monitoring in future human trials. MRI might be useful for a variety of other applications in muscular dystrophy. It has been explored for assessing muscle integrity in dystrophic muscle because ongoing inflammation and progressive fibrosis, which could theoretically be identified as hyperintense regions on T2-weighted images, are major features of dystrophic muscle.28,29,30 MRI could also be used to assess the effectiveness of gene therapy by monitoring for reduced inflammation and fibrosis as measures of improvement in muscle integrity.
We conclude that the degree of cellular immune response to rAAV vectors following intramuscular injection in dogs is not determined by the heparin-binding capability of the capsids; ELISpot can readily detect capsid-specific T-cell responses in PBMC; and MRI is a sensitive technique for serially monitoring local inflammation noninvasively. These methodologies should be considered for prescreening of Duchenne muscular dystrophy patients and monitoring immune responses in future human trials. The dog model represents an ideal preclinical model system for developing methods that can be readily translated into the human setting to characterize and monitor immunity associated with AAV-mediated gene therapy.
Materials and Methods
Animals. Research was performed according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. Dogs were housed in kennels certified by the American Association for Accreditation of Laboratory Animal Care. This study was approved by the Institutional Animal Care and Use Committee of the FHCRC (Fred Hutchinson Cancer Research Center). All dogs were immunized for leptospirosis, distemper, hepatitis, papillomavirus, and parvovirus, dewormed and observed for disease for at least 2 months before study. Dogs weighed from 10.2 to 15.3 (median, 12.25) kg and were between 1.5 and 3 years old.
Vector production. A packaging/helper plasmid carrying the capsid sequence for AAV1-E351K was generated by site-directed mutagenesis of an AAV1 plasmid (A. Arnett, J.S. Chamberlain et al., unpublished results). Recombinant AAV vectors were produced as previously described.13,31 Briefly, the recombinant AAV-CMV-canine factor IX plasmid was co-transfected into HEK293 cells with the packaging/helper plasmid pDGM carrying the appropriate capsid sequence5 by means of CaPO4 transfection. The cellular pellets and supernatants were collected and processed through a Microfluidizer (Microfluidics, Newton, MA). Empty capsids were removed by double CsCl gradient centrifugation followed by dialysis into Hank's buffered salt solution (Invitrogen, Carlsbad, CA). The vector genomes were determined relative to plasmid standards using Southern blot hybridization.
Intramuscular injection and muscle biopsy. In preparation for intramuscular injection of rAAV vectors, dogs were anesthetized with isofluorane and placed in a lateral decubitus position. Skin of a hindlimb was opened to expose semimembranosus, semitendinosus, and c femoris muscles. Nonabsorbable sutures were placed in the muscles followed by injection of 250 µl of Ringer's solution containing the appropriate amount of vectors into the muscle belly 4 mm beneath the sutures and the aponeurosis using 31-gauge syringes (Becton Dickinson, Franklin Lakes, NJ). The skin was closed with 4-0 Maxon, and all dogs were monitored daily for recovery. Muscle biopsy samples were obtained 2, 4, and 12 weeks after vector injection, embedded in O.C.T. medium (Tissue-Tek, Hatfield, PA), frozen in liquid nitrogen–cooled isopentane, and stored at −80 °C until use.
Histological analysis and immunofluorescence staining. Six-micrometer cryostat sections were cut for analysis. For basic histology evaluation, sections were fixed in methanol, stained in hematoxylin and eosin-phloxine, and mounted in VectaMount (Vector Laboratories, Burlingame, CA). For immunofluorescence, sections were blocked in 4% bovine serum albumin in 1× Dulbecco's phosphate-buffered saline (PBS; Gibco-Invitrogen, Carlsbad, CA) and incubated for 60 minutes with the following primary antibodies: mouse anti-canine CD4 and CD8 monoclonal antibody (IE4 0.97 mg/ml and JD3 1.2 mg/ml, respectively, gift from Peter Moore, UC Davis, Davis, CA, produced by FHCRC Biologics), and rabbit anti-canine factor IX (Affinity Biologicals, Ancaster, Ontario, Canada). Sections were then rinsed three times in PBS and incubated for 30 minutes with goat anti-mouse FITC-conjugated or goat anti-rabbit rhodamine red–conjugated secondary antibody (Jackson Laboratories, West Grove, PA). Sections were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma, St Louis, MO), then rinsed in PBS, and mounted in VectaShield (Vector Laboratories). Normal mouse and rabbit isotype (Invitrogen) antibodies were used as negative controls. Muscle biopsy samples taken from uninjected sites were used as negative controls. Staining was examined and photographed using a Nikon Eclipse 800 fluorescent microscope (Nikon Instruments, Melville, NY).
Peptide synthesis and peptide pool generation. An overlapping peptide library encompassing the entire AAV capsid sequence was synthesized by NMI Peptides, Reutlingen, Germany. Peptides were >98% pure as indicated by analytical high-performance liquid chromatography and were dissolved in 100% DMSO at a concentration of 100 mg/ml. Peptide pools each containing 13 or 14 peptides in equal amounts were prepared and stored at −80 °C until use.
Cell isolation and expansion. Heparinized blood samples were diluted with the same amount of PBS (Gibco-Invitrogen), overlaid on Ficoll-Hypaque (1.074; FHCRC) using a ratio of 2:1, and PBMCs were isolated by density gradient centrifugation. Cells were washed twice in Waymouth medium (Gibco-Invitrogen) containing 2% heat-inactivated dog serum, and frozen down in heat-inactivated dog serum containing 10% DMSO. Cells were then thawed and used directly in ELISpot assays (see below), or after stimulation with individual peptide pools containing 0.2 µg/ml of each peptide in 50% Waymouth 50% Iscove medium (Gibco-Invitrogen) for 10 days. Half of the medium supplemented with 25U/ml IL-2 (R&D Systems, Minneapolis, MN) was changed on days 3, 5, and 8.
ELISpot assay. 96-well PVDF microplates (Millipore, Bedford, MA) were coated with mouse anti-canine IFN-γ antibody according to manufacturer's recommendation (R&D Systems) at 4 °C overnight. The plates were washed with 0.05% Tween 20 in PBS (Gibco-Invitrogen) the next day, and blocked with 1% bovine serum albumin and 5% sucrose in PBS. PBMCs collected at different time points before and after viral injection were thawed and adjusted to a concentration of 2 × 106/ml in 50% Waymouth 50% Iscove medium (Gibco-Invitrogen) supplemented with 1% nonessential amino acids (Mediatech, Herndon, VA), 1% sodium pyruvate (Mediatech), 1% penicillin and streptomycin (Gibco-Invitrogen), 5% L-glutamine, and 10% heat-inactivated dog serum. The cells were then added at 2 × 105/well to the microtiter wells together with 4 µg/ml of either a peptide pool or an individual peptide. Cells were incubated at 37 °C for 3 days, and the plates were then washed with 0.05% Tween 20 in PBS, and incubated with biotinylated goat anti-canine IFN-γ antibody (R&D Systems) overnight at 4 °C. The plates were washed again the next day with 0.05% Tween 20 in PBS, incubated with streptavidin-conjugated alkaline phosphatase (R&D Systems) for 2 hours, washed again, and incubated with BCIP/NBT (R&D Systems) for color development. Positive spots were counted with an automatic reader (CTL, Cleveland, OH).
MRI. Conventional 1H imaging protocols were used to characterize limb skeletal muscle structure and water relaxation properties (transverse relaxation time, T2).29,32 Dogs were sedated with acepromazine 0.025 mg/kg; glycopyrrolate 0.011 mg/kg, and butorphanol (0.1–0.2 mg/kg IV), placed laterally on an MRI table, begun on propofol at 0.05 mg/kg/minute, and monitored for vital signs. Both hindlimbs were taped on top of each other and inserted up to the knee with a cylindrical T2 calibration standard into a two flexible element SENSE surface coil with a wider diameter of 17 cm and a less wide diameter of 13 cm (Philips Sense Flex M coil; Philips Healthcare, Best, the Netherlands) using a Philips 3T Achieva (v 2.5 software; Philips Healthcare, Best, the Netherlands). The protocol consisted of T1-weighted water excitation images for fast anatomical information and a set of T2-weighted images with the echo time ranging from 20 to 170 ms to highlight damaged muscle. The latter included turbo spin echo sequences to enable quantitative analysis of T2 relaxation times in the regions of injection. T2 maps were postprocessed by multiecho images with 16 echo time values ranging from 20 to 170 ms. The same or the contralateral hindlimb injected with buffer or without injection served as a control. T2 values and T2-weighted images of muscles from untreated and AAV-injected limbs were compared.
SUPPLEMENTARY MATERIALFigure S1. ELISpot assay with individual peptides from positive pools after cell expansion.
Supplementary Material
ELISpot assay with individual peptides from positive pools after cell expansion.
Acknowledgments
We thank Jianhong Cao for his assistance on ELISpot assays, and Barry Storer for statistical analysis. We thank E. Zellmer, Diana Jensen, Jenee O'Brian, C. Doremis, and E. Finn for technical assistance; A. Joslyn, B. Steinmetz and their team, and M. Spector, DVM, for their care of the dogs. We thank Kathy High for providing the c-FIX plasmid. We further thank S. Carbonneau, H. Crawford, B. Larson, K. Carbonneau, J. Fleenor, and D. Gayle for administrative assistance and manuscript preparation. This work was supported by NIH U54-HD47175; NIH P30-CA15704; NIH R01-AR056949; NIH R01-AR041928; NIH R21-EB00816; a grant from the Muscular Dystrophy Association, and by a Career Development Grant (to Z.W.) from the Muscular Dystrophy Association (MDA 114979).
REFERENCES
- Athanasopoulos T, Graham IR, Foster H., and , Dickson G. Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD) Gene Ther. 2004;11 Suppl 1:S109–S121. doi: 10.1038/sj.gt.3302379. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chamberlain JS, Tapscott SJ., and , Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009;50:187–198. doi: 10.1093/ilar.50.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KH., Jr, and , Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- High K.2002AAV-mediated gene transfer for hemophilia Genet Med 46 suppl.): 56S–61S. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC, et al. Identification of mouse AAV capsid-specific CD8+ T cell epitopes. Mol Ther. 2005;12:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu Q, Yang P, Hsu HC., and , Mountz JD. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Rutledge EA, Halbert CL., and , Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M., and , Samulski RJ. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittel A, Keilholz U., and , Scheibenbogen C. Evaluation of the interferon-gamma ELISPOT-assay for quantification of peptide specific T lymphocytes from peripheral blood. J Immunol Methods. 1997;210:167–174. doi: 10.1016/s0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- Daly TM. Overview of adeno-associated viral vectors. Methods Mol Biol. 2004;246:157–165. doi: 10.1385/1-59259-650-9:157. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC., and , Salvetti A. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr Gene Ther. 2005;5:265–271. doi: 10.2174/1566523054064977. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello J, Carrero-González B, Avilés P, Santisteban C, Méndez RJ, Ferreirós J, et al. Magnetic resonance imaging in the evaluation of inflammatory lesions in muscular and soft tissues: an experimental infection model induced by Candida albicans. Magn Reson Imaging. 1999;17:1327–1334. doi: 10.1016/s0730-725x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW., and , Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KA. Adeno-associated virus-mediated gene transfer for hemophilia B. Int J Hematol. 2002;76:310–318. doi: 10.1007/BF02982689. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Schultz BR, Allen JM, Halldorson JB, Blankinship MJ, Meznarich NA, et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers CD, Schedel H, Fleckenstein JL, Nägele M, Witt TN, Pongratz DE, et al. Magnetic resonance imaging of skeletal muscles in idiopathic inflammatory myopathies of adults. J Neurol. 1994;241:306–314. doi: 10.1007/BF00868438. [DOI] [PubMed] [Google Scholar]
- Wishnia A, Alameddine H, Tardif de Géry S., and , Leroy-Willig A. Use of magnetic resonance imaging for noninvasive characterization and follow-up of an experimental injury to normal mouse muscles. Neuromuscul Disord. 2001;11:50–55. doi: 10.1016/s0960-8966(00)00164-4. [DOI] [PubMed] [Google Scholar]
- Cahill KS, Gaidosh G, Huard J, Silver X, Byrne BJ., and , Walter GA. Noninvasive monitoring and tracking of muscle stem cell transplants. Transplantation. 2004;78:1626–1633. doi: 10.1097/01.tp.0000145528.51525.8b. [DOI] [PubMed] [Google Scholar]
- Walter G, Cordier L, Bloy D., and , Sweeney HL. Noninvasive monitoring of gene correction in dystrophic muscle. Magn Reson Med. 2005;54:1369–1376. doi: 10.1002/mrm.20721. [DOI] [PubMed] [Google Scholar]
- Walter GA, Cahill KS, Huard J, Feng H, Douglas T, Sweeney HL, et al. Noninvasive monitoring of stem cell transfer for muscle disorders. Magn Reson Med. 2004;51:273–277. doi: 10.1002/mrm.10684. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and , Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LM, Baker RE., and , Anderson JE. Magnetic resonance imaging of regenerating and dystrophic mouse muscle. Biochem Cell Biol. 1998;76:532–541. doi: 10.1139/bcb-76-2-3-532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ELISpot assay with individual peptides from positive pools after cell expansion.