Abstract
Respiratory diseases are a major cause of mortality and morbidity worldwide. Current treatments offer no prospect of cure or disease reversal. Transplantation of pulmonary progenitor cells derived from human embryonic stem cells (hESCs) may provide a novel approach to regenerate endogenous lung cells destroyed by injury and disease. Here, we examine the therapeutic potential of alveolar type II epithelial cells derived from hESCs (hES-ATIICs) in a mouse model of acute lung injury. When transplanted into lungs of mice subjected to bleomycin (BLM)-induced acute lung injury, hES-ATIICs behaved as normal primary ATIICs, differentiating into cells expressing phenotypic markers of alveolar type I epithelial cells. Without experiencing tumorigenic side effects, lung injury was abrogated in mice transplanted with hES-ATIICs, demonstrated by recovery of body weight and arterial blood oxygen saturation, decreased collagen deposition, and increased survival. Therefore, transplantation of hES-ATIICs shows promise as an effective therapeutic to treat acute lung injury.
Introduction
The most distal region of the lung is a complex organization of alveoli where O2/CO2 exchange occurs. The alveolar epithelium is composed of two types of epithelial cells, type I (ATICs) and type II (ATIICs). The ATICs are large yet highly flattened cells with multiple apical surfaces that extend into adjacent alveoli. These cells, together with the endothelium of the surrounding capillaries, form the very thin blood–air interface that is essential for O2/CO2 exchange. In contrast, ATIICs are small cuboidal cells that secrete surfactant, which reduces surface tension, preventing collapse of the alveolus. The lung is constantly exposed to environmental toxins and pathogens that can destroy alveolar epithelial cells, in particular the thin injury prone ATICs. The ability of the injured alveolar epithelium to quickly and efficiently self-repair is therefore very important for maintaining normal pulmonary function. Although repair of the lung alveolar epithelium may include respiratory stem or progenitor cells not yet identified,1,2,3 it is well established that ATIICs have an important role in repopulating the injured alveolus by not only proliferating into new ATIICs but also by differentiating into ATICs.4
Despite the endogenous repair capacity of the alveolar epithelium, it is often not sufficient. Inadequate, delayed, or impaired re-epithelialization of the injured alveolus is regarded as a key factor in the pathogenesis of several life-threatening pulmonary diseases, including acute lung injury, acute respiratory distress syndrome, and chronic obstructive pulmonary disease. Current treatments for lung alveolar epithelial injury at best provide symptomatic relief but offer no prospect for repair of the damaged epithelium or preventing lung fibrosis. Consequently, there is a pressing need for the development of novel therapies that facilitate the regeneration of alveolar epithelium destroyed by acute and chronic lung diseases.
Embryonic stem cells (ESCs) are self-renewing pluripotent cells, which can be induced to differentiate into a wide range of different cell types.5,6 The potential use of ESCs in the treatment of pulmonary diseases has evoked extensive interest in developing methods that promote ESC differentiation into lung progenitor cells. Because of their ability to proliferate and to differentiate into ATICs, ATIICs derived from ESCs may be promising as a transplantable source of cells that could be used therapeutically to treat distal lung injury. Recently, ESCs were shown to differentiate into ATIICs in culture,7,8,9 but these procedures yielded a mixture of ESC derivatives with only a small percentage of the cells being ATIICs. A mixed population of cells will not be suitable for transplantation, and remaining pluripotent cells in these mixed cultures carry a significant risk of producing teratomas after transplantation. Therefore, a prerequisite for using ATIICs therapeutically is to develop a method that will routinely yield a pure population of ATIICs. Our laboratory has recently achieved this goal by generating stable hESC lines that can be differentiated and enriched into a pure population of ATIICs.10 The purpose of this investigation is to determine in a mouse model of bleomycin (BLM)-induced acute lung injury if transplanted ATIICs derived from hESCs (hES-ATIICs) will differentiate and functionally repair the epithelium of the acutely injured alveolus.
Results
Stable transfected hESC lines
Aquaporin-5 (AQP5) is a mercury-sensitive water channel that is found in the apical membrane of ATICs with little to no expression in ATIICs.11,12 T1α is a cell surface mucin-like glycoprotein with no known function that is expressed by ATICs but not ATIICs.13,14,15,16 Both AQP5 and T1α are used frequently as phenotypic markers for ATICs. Therefore, to visualize the differentiation of hES-ATIICs into ATICs two new stable human embryonic stem cell (hESC) lines (AQP5P.65 and T1αP.53) were generated in which either AQP5 or T1α transcriptional promoters were cloned upstream of the LacZ gene (Figure 1). The construction of the vectors and the generation and characterization of the stable hESC lines are described in Materials and Methods.
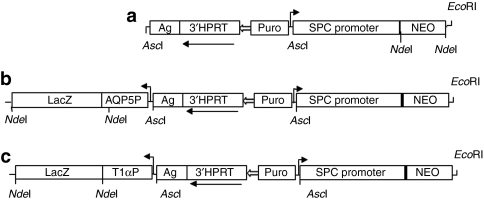
Figure 1.
Structure of Surfactant Protein C (SPC) promoter–NEOr transgene vectors. The structures of the three SPC promoter–Neor transgene vectors used to generate hES-ATIIC lines are schematically diagramed. Each diagram is drawn to depict relevant information, so they are not to exact scale. The details regarding construction of the transgene vectors are described in Materials and Methods. (a) The original vector (3′hprt.SPCP.NEOr) used to generate a pure population of the hES-ATII cells.10 A 3.8-kb human genomic DNA fragment containing the SPC promoter and 170 bp of noncoding sequence of exon 1 was cloned into the targeting vector backbone,45 containing the hypoxanthine phosphoribosyl transferase 3′-cassette (3′-HPRT), the puromycin-resistant gene (Puro), the K14Agouti transgene (Ag), and a LoxP site (open arrow). The Neor gene was added downstream of the SPC promoter. The other two transgene vectors were generated to allow the differentiation of hES-ATIICs to ATICs to be detected by LacZ staining. (b) This vector was constructed by adding 1.26-kb human AQP5 promoter and 3.1-kb LacZ cDNA cassette into 3′hprt.SPCP.NEOr vector. (c) This vector was generated by cloning 1.2-kb human T1α promoter and 3.1-kb LacZ complementary DNA cassette into 3′hprt.SPCP.NEOr vector. The EcoRI site located downstream of Neor gene was used to linearize the plasmid before transfection of the hES cells.
Proliferation and differentiation of hES-ATIICs in vitro
One of the important biological functions of ATIICs is to proliferate and to serve as progenitor cells for ATICs. To determine whether hES-ATIICs proliferated in vitro, hES-ATIICs generated from the SPC/NEO.74 hESC line10 were cultured with mouse embryonic fibroblast–conditioned Dulbecco's modified Eagle's medium (DMEM). Recombinant keratinocyte growth factor (rhKGF), which is an ATIIC growth factor,17 was added to some of the cultures to determine whether rhKGF could be used to enhance proliferation of hES-ATIICs. After 7 days of culture, the number and size of the hES-ATII colonies had increased significantly (Figure 2a,b). In the absence of rhKGF, each well contained on average 12 colonies with each colony comprising 7–12 cells. The addition of rhKGF for 7 days caused the number and size of the hES-ATII colonies to increase (average 35 colonies/well and 25–35 cells/colony) (Figure 2b). These results show that hES-ATIICs proliferate in vitro and that the addition of rhKGF significantly increases the proliferation of the hES-ATIICs in culture.
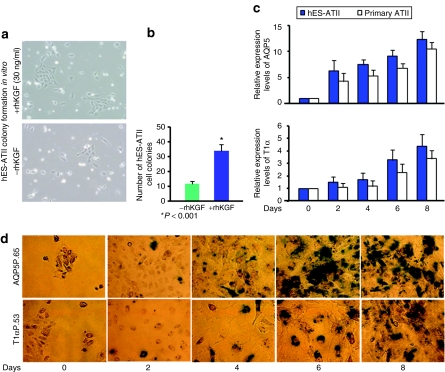
Figure 2.
Proliferation and differentiation of hES-ATIICs in vitro. (a) Shown are representative phase-contrast images of cultured hES-ATIICs. Small hES-ATIIC-derived colonies containing 7–10 cells were observed in cultures on matrigel-coated dishes with mouse embryonic fibroblast–conditioned Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (bottom image, original magnification = ×200). Large hES-ATIIC-derived colonies containing 25–35 cells were seen in the cultures treated with 30 ng/ml of rhKGF (upper image, original magnification = ×200). (b) There were significantly more total colonies in the rhKGF-treated cultures compared to the untreated (12 colonies/well) (P < 0.001; n = 7). (c) Expression of markers for ATICs during differentiation of hES-ATIICs. Aquaporin-5 (AQP5)- and T1α-specific quantitative reverse transcriptase-PCR was performed using total RNA isolated from differentiating cultures of hES-ATIICs. Bar graphs depict RNA expression levels of AQP5 and T1α in the cultures on day 0, 2, 4, 6, and 8 (n = 7). (d) Differentiation of ATII cells to ATI cells as visualized by LacZ staining. A few LacZ+ cells (blue-green) were seen in the differentiating culture of hES-ATII cells as early as on day 2. The number of LacZ+ cells significantly increased over time, with 100% of the cells showing at least some LacZ+ staining on day 8 (original magnification= ×200).
To assess the ability of hES-ATIICs to differentiate into ATICs in vitro, hES-ATIICs and control primary human ATIICs were cultured with DMEM and left to spontaneously differentiate. Differentiation was assessed by real-time quantitative reverse transcriptase-PCR (QRT-PCR) using specific primer pairs to detect gene expression of AQP5 and T1α. As shown in Figure 2c, cultured hES-ATIICs and primary human ATIICs exhibited increased expression of RNA specific for AQP5 and T1α, indicating that hES-ATIICs differentiate into ATICs in vitro at a similar rate as do primary ATIICs (Figure 2c). To confirm these findings, hES-ATIICs derived from the AQP5P.65 and T1αP.53 cell lines, were cultured as above and stained for LacZ expression. In support of the data shown in Figure 2c, fresh hES-ATII cultures did not express LacZ (day 0), but did exhibit increased LacZ expression over time with essentially all cells expressing some level of LacZ after 8 days of culture (Figure 2d). Collectively, the QRT-PCR and LacZ data indicate that hES-ATIICs spontaneously differentiate in culture in the absence of mouse embryonic fibroblast to cells expressing ATICs phenotypic markers.
Transplantation and in vivo differentiation of hES-ATIICs in a mouse model of acute lung injury
The antineoplastic drug BLM when administered intratracheally to mice primarily targets the pulmonary epithelium and reproduces the pattern and numerous features of acute lung injury in humans, including rapid onset of inflammation, alveolar injury that heals with fibrosis, and severe hypoxemia.18 As described in Materials and Methods, female SCID/C57BL/6 mice were subjected to BLM-induced acute lung injury. Following BLM treatment, hES-ATIICs were administered by intratracheal intubation into the mouse terminal airways, and end point lung sections were examined by immunohistochemistry. To examine whether transplantation efficiency would be affected by the time in which cell therapy was provided, hES-ATIICs were administered 1 or 2 days following BLM-induced acute lung injury. Transplanted hES-ATIICs were identified using an antihuman pro-SPC antibody and a mouse antihuman nuclei monoclonal antibody.19,20 As shown in Figure 3, control mice that received either saline or BLM, but not hES-ATIICs showed no antihuman nuclei staining. In contrast, lung tissue from mice that were subjected to acute lung injury and administered hES-ATIICs did show significant antihuman nuclei staining. In addition, most of the cells positive for human nuclei co-stained with antihuman pro-SPC (Figure 3 and Table 1), demonstrating that the hES-ATIICs were capable of being transplanted into the BLM-injured alveoli. The time that the hES-ATIICs were administered (1 or 2 days after lung injury) did not affect significantly the percentage of SPC+/human nuclei+ cells found in the mouse end point lung sections (Table 1). Lung tissue sections from saline-treated control mice that were administered hES-ATIICs were devoid of antihuman nuclei staining, indicating that lung injury is required for transplantation of hES-ATIICs to occur. In addition, BLM-injured mouse lungs that were provided human monocytes rather than hES-ATIICs also exhibited no antihuman nuclei staining, suggesting that transplantation and possible cell engraftment was specific for the hES-ATIICs.
Figure 3.
Immunofluorescent staining of hES-ATIICs transplanted in the lungs of bleomycin (BLM)-challenged mice. Shown are representative slides of lung sections from BLM-challenged mice with and without transplanted hES-ATIICs. The slides were immunostained with a mouse antihuman nuclei monoclonal antibody and rabbit antihuman pro-SPC antibody as described in Materials and Methods. Mouse and human nuclei are stained blue by 4',6-diamidino-2-phenylindole (DAPI); cells of human origin (hES-ATIICs) are stained red by the antihuman nuclei monoclonal antibody; both mouse and human cells expressing SPC (ATIICs) are stained green by the rabbit antihuman pro-SPC. Human cells expressing SPC (hES-ATIICs) are co-stained red and green giving a yellowish color. As expected the number of mouse ATIICs that expressed SPC (green) in the control lung section (control: original magnification = ×400) was greatly decreased in the BLM-challenged mouse lung (BLM: original magnification = ×400). Human specific (red) cells were identified only in the BLM-challenged lungs that had been transplanted with hES-ATIICs (BLM/ATII day 1 and BLM/ATII day 2: original magnification = ×400). Most of the human-specific cells co-stained for SPC, indicating that these cells are transplanted hES-ATIICs (indicated by white arrows in the far right panels BLM/ATII day 1 and BLM/ATII day 2). As depicted in the BLM/ATII day 2, there were some transplanted human cells that were not SPC+ (indicated by yellow arrows), suggesting that these cells were ATICs that had differentiated from the transplanted hES-ATIICs.
Table 1.
Relative human type II alveolar cell content in bleomycin-mouse lung tissue
Although the majority of the transplanted hES-ATIICs co-stained positive for both human nuclei and SPC, there were numerous examples of transplanted cells staining positive for human nuclei but not for SPC within the counted area of 400 SPC+ cells (Figure 3 and Table 1), suggesting that a number of the transplanted hES-ATIICs had differentiated into ATICs within 9 days after transplantation. Immunohistochemistry of lung sections stained for anti-T1α supported this observation (Figure 4a). Moreover, LacZ+ cells in similar numbers as the SPC‐/nuclei+ cells were observed in the lungs of BLM-treated mice that received hES-ATIICs derived from the T1αP.53 and AQP5P.65 cells lines, but not in the saline or BLM control lungs (Figure 4b). Collectively, these data demonstrate that the hES-ATIICs have the ability to transplant into the damaged alveoli of BLM acutely injured mouse lungs and to differentiate into cells expressing phenotypic markers of ATICs.
Figure 4.
Identification of ATICs differentiated from transplanted hES-ATIICs. (a) Representative slides are shown (original magnification = ×200) of lung sections from BLM-challenged mice with and without transplanted hES-ATIICs that were immunostained by mouse antihuman nuclei monoclonal antibody (red) and rabbit antihuman T1α antibody (green) as described in Materials and Methods. Slides were nuclear-counterstained by 4',6-diamidino-2-phenylindole (DAPI) (blue). Cells expressing T1α were observed in control lungs and BLM-challenged lungs, because the anti-T1α antibody recognized both human and mouse ATI cells (control and BLM panels). Cells expressing both T1α and human-specific nuclei proteins were only present in BLM-challenged lungs that had been transplanted with hES-ATIICs (BLM/ATII panel). Human cells expressing T1α are thought to be ATICs differentiated from the transplanted hES-ATIICs (indicated by arrows). (b) Shown are slides from control lung sections and BLM-challenged mice with and without transplanted hES-ATIICs generated from the AQP5P.65 and T1αP.53 cell lines. Slides were stained for LacZ expression as described in Materials and Methods. Cells expressing LacZ were found in the BLM-injured lungs when transplanted with hES-ATIICs from both hES-ATII cell lines, supporting the data shown in Figure 3 and a that some of the transplanted hES-ATIICs had or were in the process of differentiating to ATICs (note that the LacZ figures shown were selected so that several different LacZ+ cells could be visualized in a single panel). However, most panel photographs had only one to two LacZ+ cells—similar to the number of SPC‐/human nuclei+ cells (Figure 3) and the T1α+ cells in a.
Transplantation of hES-ATIICs abrogates BLM-induced acute lung injury in mice
Following intratracheal exposure to BLM, hES-ATIICs were transplanted into the injured alveoli of the mouse to determine whether the hES-ATIICs could prevent or reverse the acute lung damage caused by BLM. Direct initial damage to the alveolar epithelial cells occurred followed by acute inflammation within 24 hours. Ten days after BLM-challenge, ~50–75% of the lung alveolar epithelium was severely injured, noted by interstitial thickening, alveolar collapse, cystic air spaces, extensive interstitial infiltration of inflammatory cells, and collagen deposition (Figure 5a). Transplantation of hES-ATIICs, after BLM-challenge greatly reduced the extent of damage within the lung as evidenced by only a few isolated, small areas of injured tissue surrounded by much larger areas of normal alveolar structure (Figure 5a). In contrast, transplantation of human monocytes after BLM-challenge did not reduce BLM-induced lung injury. Hydroxyproline content was also determined to evaluate BLM-mediated collagen deposition in the lungs (Figure 5b). The hydroxyproline content was increased significantly after BLM exposure and remained elevated when treated with human monocytes. In contrast, the amount of hydroxyproline in the BLM-injured lungs that received hES-ATIICs was reduced and near the hydroxyproline content found in the saline control lungs.
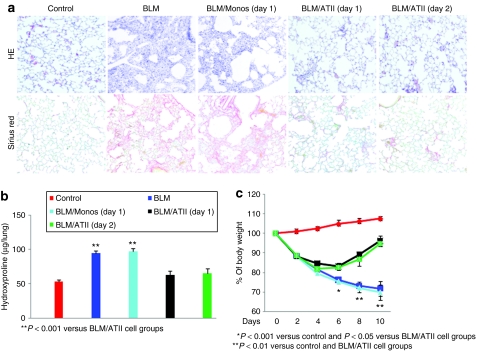
Figure 5.
Transplantation of hES-ATIICs reduced BLM-induced acute lung injury in mice. (a) Shown are hematoxylin–eosin (H&E) and Sirius Red stained slides of representative end point (day 10) lung sections from BLM-challenged mice with and without transplanted hES-ATIICs (original magnification = ×200). H&E and Sirius Red staining were performed as described in Materials and Methods. BLM-challenge caused extensive alveolar structural damage (50–75%) with extensive cellular infiltration, interstitial thickening (H&E/BLM, second panel) and collagen deposition (Sirius Red/BLM, second panel) compared with control lungs treated with saline (H&E/control and Sirius Red/control far left panels). Lungs that were transplanted with hES-ATIICs 1 (BLM/ATII day 1) or 2 days (BLM/ATII day 2) after BLM-induced acute lung injury showed significantly reduced damage with only a few (5%) isolated areas of the lung found to exhibit signs of cellular infiltration, interstitial thickening, and collagen deposition. Lungs that were transplanted with human monocytes 1 day after lung injury showed the same extensive alveolar structural damage (50–75%) as the BLM-treated lungs not transplanted with hES-ATIICs. (b) Collagen deposition was also evaluated by analysis of the hydroxyproline content in the experimental mouse lungs as described in Materials and Methods. The hydroxyproline content was significantly increased from 53.36 ± 2.48 to 94.58 ± 2.99 µg/lung 10 days after BLM exposure (P < 0.001, n = 10). As was similarly concluded visually by Sirius Red staining, transplantation of hES-ATIICs 1 (62.72 ± 5.89 µg/lung; P < 0.001, n = 10) or 2 days (65.1 ± 7.17 µg/lung; P < 0.001, n = 10) after BLM-challenge significantly reduced collagen deposition to levels near that of uninjured lungs. In support of the Sirius Red staining data, monocyte transplantation did not result in reduction of hydroxyproline content (96.49 ± 3.18 µg/lung) in the BLM-injured mouse lungs. (c) This graph illustrates the percentage of body weights of control saline-treated mice (red), to that of BLM-treated mice (dark blue), and BLM-injured mice provided human monocytes (light blue) or hES-ATIICs 1 (black) or 2 (green) days after BLM–acute lung injury. The body weight of BLM-challenged mice as well as BLM-challenged mice transplanted with human monocytes was decreased significantly over time and by day 10 was only 72% of the body weight of the control mice (day 6: P < 0.001, n = 8; day 8: P < 0.001, n = 8; day 10: P < 0.001, n = 8). Weight loss occurred initially as well in the mice that were transplanted with hES-ATIICs 1 or 2 days after BLM exposure. However, by 4 days after lung injury these mice started to show body weight recovery, and by day 6 the body weights of the mice transplanted with hES-ATIICs were significantly increased compared to the BLM-injured mice (day 6, hES-ATIICs day 1, P < 0.05, n = 8) (day 6, hES-ATIICs day 2, P < 0.05, n = 8). By day 10, the BLM-challenged mice transplanted with hES-ATIICs had recovered ~95% of their initial body weights.
BLM-induced lung injury is associated with significant loss of body weight. To determine whether the transplantation of hES-ATIICs could either arrest or reverse the loss of weight in the BLM-challenged animals, mice treated with or without hES-ATIICs were weighed for 10 days after BLM-challenge (Figure 5c). Intratracheal administration of BLM caused a significant weight loss by day 2. The loss of weight in the BLM-injured mice that did not receive hES-ATIICs or were provided with human monocytes continued over time and by day 10 had lost 28% of their initial body weight. The BLM-injured mice that were treated with hES-ATIICs also experienced an initial drop in weight, but by day 6 these mice experienced a significant increase in weight, and by day 10 they had recovered 95% of their weight before BLM-induced lung injury (Figure 5c).
Recovery of lung tidal volume and blood arterial oxygen saturation in BLM-treated mice transplanted with hES-ATIICs
As demonstrated by the experimental results shown in Figure 5a,b, exposure of the lungs of severe combined immunodeficient (SCID) mice to BLM caused extensive alveolar epithelial cell damage, resulting in airway structure distortion, interstitial tissue thickening, and collagen deposition. In contrast, most of the alveolar damage was arrested or repaired if hES-ATIICs were administered to these mice following BLM-induced acute lung injury. Therefore, it was hypothesized that normal lung function could be restored to the BLM-challenged mice after transplantation with hES-ATIICs. To test this hypothesis, lung tidal volumes and blood arterial oxygen saturation levels were measured in mice that were treated with and without hES-ATIICs following BLM-induced acute lung injury. As illustrated in Figure 6a, the lung tidal volume during spontaneous respiration was significantly decreased after BLM lung injury; in contrast, the lung tidal volume in the BLM-challenged mice that were treated with hES-ATIICs was completely normal. Similar to the lung tidal volume, the blood oxygen levels in the BLM-challenged mice declined significantly 4 days after BLM lung injury (Figure 6b) and by day 13 all the BLM-treated mice succumbed to respiratory failure (Figure 6c). Impressively, the BLM-injured mice that had been transplanted with hES-ATIICs exhibited normal blood arterial oxygen saturation levels at 4 days after BLM-challenge. To determine whether transplantation of the hES-ATIICs could provide a long-term benefit and to ensure that administration of these cells would not lead to teratoma formation, 28 female SCID mice were exposed to 3.5 units/kg of BLM as before; 6 of these mice received hES-ATIICs after BLM exposure. None of the mice subjected to BLM-induced acute lung injury and not provided treatment with hES-ATIICs lived >13 days after BLM exposure (Figure 6c). In stark contrast, all the BLM-challenged mice that were treated with hES-ATIICs remained alive and healthy and without teratoma formation for the entire duration of the study (300 days). Collectively, these data indicate that the BLM-injured alveolar epithelium can be functionally repaired long-term by transplantation of hES-ATIICs.
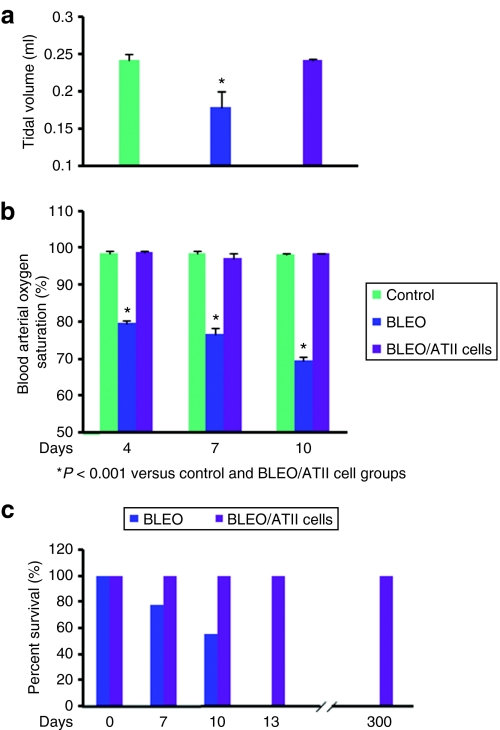
Figure 6.
Pulmonary function and survival rate of BLM-challenged mice with and without transplanted hES-ATII cells. (a) Shown graphically are the lung tidal volumes that were determined during spontaneously breathing using a rodent pulmonary plethysmograph (Buxco Electronics) as described in Materials and Methods. The tidal volumes measured 10 days after acute lung injury were significantly reduced in the BLM-challenged mice (0.179 ± 0.02 ml) compared to normal control mice (0.242 ± 0.007 ml) (P < 0.001, n = 8/group). In contrast, when the BLM-challenged mice were transplanted with hES-ATIICs 1 day after BLM-induced acute lung injury their lung tidal volumes had returned to normal (0.241 ± 0.002 ml) by day 10 after BLM-challenge (P = 0.605, n = 8/group). (b) Shown graphically are the blood arterial oxygen saturation levels recorded on days 4, 7, and 10 after BLM-challenge using a small rodent oximeter sensor mounted on the thigh of each mouse (MouseOX; STARR Life Sciences) as described in Materials and Methods. Compared to normal mice (98.0%), the blood arterial oxygen saturation levels in the BLM-challenged mice (79.55%) were significantly decreased by day 4 after BLM-challenge (P < 0.001, n = 12/group) and continued to decrease for the remainder of the 10 day study (75.66% on day 7 and 69.7% on day 10) (day 7, P < 0.001, n = 12/group) (day 10, P < 0.001, n = 12/group). In contrast, the BLM-challenged mice that were transplanted with hES-ATIICs showed complete recovery of normal arterial oxygen saturation levels by 4 days after BLM-induced acute lung injury (P = 0.673 normal versus BLM mice treated with hES-ATIICs, n = 12/group) (P < 0.001 BLM mice versus BLM mice treated with hES-ATIICs, n = 12/group). (c) Percent survival of BLM-challenged mice with and without transplantation of hES-ATIICs is graphically presented. During the first 7 days following BLM-challenge 5 of 22 mice had died. Between 7 and 10 days, another 5 mice from this group of 22 had died. All of the remaining BLM-treated mice (12) had died by 13 days after BLM-induced acute lung injury. In contrast, all of the BLM-challenged mice that were transplanted with hES-ATIICs survived (6/6). In addition, all 6 of these mice remained completely healthy to the end point of the study (300 days).
Discussion
Current treatments for lung injury do very little to induce cellular repair or to prevent the onset of lung fibrosis. Considerable interest has developed in the potential use of stem cells to repair lung epithelium damaged or destroyed by injury and disease. Initial investigations were performed using bone marrow–derived stem cells, which at first were thought to engraft and differentiate into pulmonary epithelial cells following lung injury.21,22,23,24,25,26 However, subsequent studies revealed that the original conclusions drawn from these rodent lung injury models were incorrect due to staining artifacts.27,28 It is now generally agreed that following lung injury little, if any, engraftment of lung cells originating from the bone marrow occur.
Because of their ability to differentiate into essentially any cell in the body, ESCs may provide an attractive alternative to bone marrow stem cells in regenerating lung tissue. However, much of the enthusiasm for using hESCs to regenerate damaged tissue has been tempered by the observation that direct application of hESCs in vivo may cause teratoma formation, resulting in a possible lethal outcome.29,30 Primary ATIICs have shown promise in their ability to reverse lung injury in rats subjected to BLM treatment,31 indicating that endogenous lung cells with progenitor cell properties may prove useful in repairing damaged or diseased lung tissue. However, it will be extremely difficult to obtain sufficient quantities of human primary ATIICs that could be used for treatment of lung injury and disease. Accordingly, our laboratory recently developed a reliable culture and genetic selection procedure to generate a pure population of transplantable ATIICs from hESCs.10 In the current study, hES-ATIICs were evaluated in a mouse model of BLM-induced acute lung injury for their ability to functionally repair damaged lung tissue without causing teratoma formation.
As with whole-organ transplantation, hES-ATII cells may experience immune rejection when transplanted into the lungs of allogeneic recipients. Immune rejection of transplanted allogeneic hES-ATII cells could be controlled by immunosuppresive drugs; or possibly in the near future by using ATIICs generated from induced pluripotent stem cells derived from the patient's own dermal fibroblasts. No matter how graft rejection issues with allogeneic cells are resolved, proof of principal transplantation studies in animal models such as those in this investigation must be performed before any therapeutic use of hES-ATII cells can be proposed. Xenogenic transplantation of human cells into recipient animals with a fully intact immune system may also cause rejection of the hES-ATIICs. Therefore, to evaluate the therapeutic potential of hES-ATIICs in acute lung injury, immune-compromised SCID mice were used to prevent rejection of the transplanted hES-ATIICs. Because of their severely impaired immune system, SCID mice also provided an excellent model32 in which to evaluate the hES-ATII-treated mice for teratoma formation should there be remaining hESCs in the ATIIC genetically selected cultures. A relatively high dose of BLM (3.5 units/kg) was given to SCID mice by intratracheal intubation. This dose of BLM was used so that at the time of hES-ATIIC transplantation the lung damage resembled that of acute lung injury, including extensive interstitial inflammatory cell infiltration, interstitial thickening, and collagen deposition. Moreover, 3.5 units/kg of BLM caused sufficient lung injury so that endogenous cell repair was negligible and ineffective, leading to 100 % mortality of all BLM-treated mice by 13 days after BLM-challenge, thereby allowing the impact of transplanted hES-ATIICs on survival to be evaluated without possible confounding results due to endogenous lung stem cell repair.
Failure to efficiently repopulate the alveolar epithelium after injury is a major factor in the development of pulmonary fibrosis.33 Alveolar cell damage results in denuded epithelial basement membranes and the release of chemoattractant molecules, which mediate the migration of fibroblasts into the alveolar space and the development of intra-alveolar fibrosis. Although it could not be definitively proven in our studies that the transplanted hES-ATIICs were physically engrafted into the mouse alveolar epithelial basement membranes denuded by BLM injury, there was considerable evidence to support this possibility, such as: (i) numerous hES-ATIICs remained in the mouse lung sections obtained 10 days following acute injury even after exhaustive lavage, (ii) many of the transplanted hES-ATIICs had differentiated into cells expressing phenotypic markers of ATICs, (iii) no hES-ATIICs were found when transplanted into mouse lungs not subjected to BLM-induced acute injury, i.e., lack of denuded basement membranes to facilitate engraftment, and (iv) in contrast to the hES-ATIICs, human monocytes did not remain in the BLM-injured mouse lungs following transplantation. Therefore, re-epithelialization of BLM-damaged alveolar epithelium by engrafted hES-ATIICs may have prevented the development of severe fibrosis observed in the BLM-injured mice that were not treated with hES-ATIICs.
Although our data strongly argue that engraftment and differentiation of hES-ATIICs play a major role in repairing or preventing tissue damage caused by BLM, it is unlikely that these two events alone accounted for all the noted therapeutic benefits provided by the hES-ATIICs. Instead, it is probable that engraftment is one part of a complex series of biological events initiated by the transplantation of hES-ATIICs. For example, transplantation of hES-ATIICs prevented or reversed BLM-induced lung damage in some areas of the alveolar epithelium that did not harbor engrafted hES-ATIICs. Bone marrow stem cells have been reported to cause the release or production of anti-inflammatory cytokines, thereby causing some reversal of lung injury despite their lack of engraftment.34,35,36 Perhaps transplanted hES-ATIICs secrete factors that are either anti-inflammatory or cause the release of anti-inflammatory molecules as well, thereby arresting the inflammation and fibrosis caused by BLM. In addition, even though endogenous progenitor or stem cells of the lung were not sufficient to repair or arrest tissue damage in our model of acute lung injury, it is possible that hES-ATIICs through the release of growth factors trigger the expansion of endogenous lung progenitor or stem cells that in concert with the hES-ATIICs re-epithelialize the damaged alveolar epithelium.
Whether or not lung injury prevention or repair was dependent on engraftment and differentiation of the transplanted hES-ATIICs, it is unequivocal that their presence in the damaged alveolus provided a major therapeutic benefit to mice subjected to BLM–acute lung injury. For example, the loss of body weight by BLM-treated mice was irreversible resulting in death if the mice were not provided hES-ATIICs. However, when given hES-ATIICs the BLM-injured mice experienced an almost complete reversal of weight loss, indicating that the transplanted hES-ATIICs were required for body weight recovery. In addition, treatment with hES-ATIICs not only prevented or reversed visual hallmarks of pulmonary injury, but also restored near normal lung function to mice subjected to BLM–acute lung injury. Moreover, this benefit was specific for hES-ATIICs, as treatment with purified human monocytes did not prevent or reverse BLM-induced acute lung injury. Importantly, the therapeutic benefits provided by hES-ATIICs were long-term and without teratoma formation, indicating that the cultures of hES-ATIICs used in these studies were highly purified and free of any remaining hESCs. Staining of the long-term lung sections indicated that ~5% of the hES-derived ATI or ATII cells remained in the lungs 300 days after BLM injury and transplantation (data not shown). It has been estimated that the turnover of the adult mammalian alveolar epithelium is 4–5 weeks (ref. 37). Therefore, the percentage of transplanted human lung epithelial cells remaining in the mouse lungs at 300 days appears reasonable, given that the lung alveolar epithelium was almost fully repaired by 10 days preventing long-term proliferation of the engrafted hES-ATII cells. Further investigations are needed to determine the proliferation capability of hES-ATII cells in vivo.
In summary, the data from this study have shown for the first time that lung progenitor ATIICs derived from hESCs can indeed be transplanted into acutely damaged alveoli of mice and that these hES-ATIICs arrested or reversed BLM-induced pathological changes of acute lung injury, including fibrosis. Moreover, the therapeutic benefits provided by the transplantation of hES-ATIICs were long- term and without the development of teratomas.
Materials and Methods
Construction of human SPC promoter–NEOr+T1α/AQP5 promoter–LacZ transgene vectors. The 3′hprt.SPCP.NEOr vector containing the human SPC promoter–NEOr transgene and puromycin-resistant gene (Figure 1a) was used to generate two new transgene vectors, 3′hprt.SPCP.NEOr.AQP5P.LacZ (Figure 1b) and 3′hprt.SPCP.NEOr.T1α.LacZ (Figure 1c). To clone the AQP5 or T1α promoter–LacZ transgene cassettes into the 3′hprt.SPCP.NEOr vector, the NEOr gene and the cloning NdeI site were removed and replaced with a NEOr gene containing an AseI restriction cloning site and HpaI, NdeI, and EcoRI restriction sites were downstream of the NEOr gene. Either a 1.2-kb human genomic DNA fragment containing the T1α promoter and 202 bp of noncoding sequence of exon 1 of the T1α gene (ref. 38) or a 1.26-kb genomic DNA fragment containing the human AQP5 promoter (‐1,260 to ‐1) (ref. 39) was cloned into the engineered HpaI and NdeI sites of the 3′hprt.SPCP.NEOr vector. A 3.1-kb LacZ cDNA was cloned at the NdeI restriction site downstream of either the AQP5 or T1α promoters. The 3′hprt.SPCP.NEOr.T1αP.LacZ and 3′hprt.SPCP.NEOrAQP5P.LacZ vectors were linearized by EcoRI before transfection.
Transfection and selection of hESC lines. The National Institutes of Health approved hESC line, H9.2 (passages 40–65) (WiCell, Madison, WI), was used throughout this study. Undifferentiated hES cells cultured on mitotically inactivated mouse embryonic fibroblasts in 6-well plates were transfected with either the 3′hprt.SPCP.NEOr.T1αP.LacZ or the 3′hprt.SPCP.NEO.AQP5P.LacZ vectors using the Nucleofector II (Amaxa, Gaithersburg, MD), and selected in the presence of 0.25 µg/ml puromycin (Sigma, St Louis, MO) for 14 days as described previously.10 Surviving hES clones were examined for the Neor and LacZ transgene by PCR analysis. The hES clones T1α.LacZ.53 and AQP5P.LacZ.65 containing only a single copy of the corresponding transgene were selected for further analysis.
In vitro differentiation, selection, culture, and characterization of hES-ATIICs. The hESC lines, SPCP.NEO.74, T1α.LacZ.53, and AQP5P.LacZ.65, were cultured on matrigel-coated 10-cm plates and allowed to spontaneously differentiate in differentiation medium composed of 80% knock-out DMEM (Gibco Invitrogen, Carlsbad, CA), 20% fetal bovine serum, 1% nonessential amino acids, 1 mmol/l L-glutamine, 100 µg/ml penicillin, and 100 µg/ml streptomycin. The hES-ATIICs in the cultures were selected with G418 (20 µg/ml; Gibco Invitrogen) as reported previously.10 For transplantation, G418-selected hES-ATII cells on day 14 were trypsinized and then seeded back onto fresh matrigel–coated 10-cm culture plates with differentiation medium. The hES-ATII cells were harvested the following day, washed once with normal saline, and resuspended in normal saline (107 cells/ml). For spontaneous differentiation studies, G418-selected hES-ATIICs as well as normal human ATIICs were seeded onto 6-well culture plates with DMEM (Gibco Invitrogen) containing 10% fetal bovine serum (day 0) with medium change every other day. The primary ATIICs were isolated and cultured as previously described.40 ATIICs derived from the new LacZ hESC lines were evaluated for normal characteristics of primary ATIICs as described previously for SPCP.NEO.74 (ref. 10). As was shown for SPCP.NEO.74, hES-ATIICs derived from T1α.LacZ.53 and AQP5P.LacZ.65 were morphologically normal containing lamellar bodies, which are characteristic hallmarks of primary ATIICs. In addition, hES-ATIICs from these two new cell lines synthesized surfactant proteins A, B, and C as well as the complement proteins C3 and C5. They also expressed RNA specific for cystic fibrosis transmembrane conductance regulator and α-1AT. To examine the ability of hES-ATIICs to proliferate in vitro, G418-selected hES-ATII cells were resuspended in mouse embryonic fibroblast–conditioned DMEM containing 10% fetal bovine serum with or without 30 ng/ml of rhKGF (R&D Systems, Minneapolis, MN) and plated on matrigel-coated 6-well plates (1.0 × 104/well). The medium was changed everyday for 8 days.
QRT-PCR analysis. Total RNA was isolated on days 0, 2, 4, 6, and 8 to analyze the expression of human ATIC phenotypic markers, T1α and AQP5, by QRT-PCR. Total RNA was isolated using RNA Bee (Tel-Test, Friendswood, TX). TaqMan One-Step RT-PCR Master Mix Kit (AB Applied Biosystems, Foster City, CA) was used for QRT-PCR analysis following manufacturer's instructions employing 100 ng of total RNA for AQP5 and T1α and 100 pg of total RNA for endogenous control 18S with the following primers and probes: (i) AQP5 forward (5′-CCA TGG TGG TGG AGC TGA TTC TG-3′), AQP5 reverse (5′-TG CGG CGG GAG TCA GT-3′) and AQP5 probe (5′-6-FAM-CTT CCA GCT GGC ACT CTG CAT CTT CGC C-TAMRA-3′); (ii) T1α forward (5′-GCT GCT TTG TTC TGG AAT ATG GAT ATC TC-3′), T1α reverse (5′-TTG AGC CTC TAG CAC CAT TAA GCA-3′) and T1α probe (5′-FAM-AGC AGC TTC CTC GGC ATC CAG G –TAMRA-3′); and (iii) 18S forward (5′-TAA CGA ACG AGA CTCTGG CAT-3′), 18S reverse (5′-CGG ACA TCT AAG GGC ATC ACA G-3′) and 18S probe (5′-FAM-TGG CTG AAC GCC ACT TGT CCC TCT AA-TAMRA-3′). QRT-PCR was carried out at 48 °C for 30 minutes and 95 °C for 10 minutes followed by 40 cycles at 95 °C for 15 seconds and 60 °C for 1 minute in a 7900HT Sequence Detection Systems (AB Applied Biosystems).
Transplantation of hES-ATIICs into BLM-injured mouse lungs. To impair possible graft rejection of the hES-ATIICs, the BLM-induced acute lung injury model was established using immunodeficient SCID mice. Pathogen-free, 8- to 10-week-old, female SCID mice with body weights of 16–18 g, on a C57BL/6 genetic background (Jackson Laboratories, Bar Harbor, ME) received 50 µl of either BLM (3.5 units/kg; Bristol-Myers Squibb, New York, NY) or sterile normal saline endotracheally via oropharynx intubation41 using a BioLITE Intubation Illumination System (Braintree Scientific, Braintree, MA). Oropharynx intubation required no surgery and thus no tracheal inflammation, thereby allowing subsequent noninvasive administration of hES-ATII cells on days 1 or 2 after BLM-challenge. The BLM-treated mice were transplanted with hES-ATIICs derived from the three different stable transfected hESC lines, SPCP.NEO.74, T1α.LacZ.53, and AQP5P.LacZ.65, at a dose of 0.5 × 106 cells in 50 µl of sterile normal saline via an endotracheal catheter as above. Control mice received 50 µl of sterile normal saline or 0.5 × 106 human monocytes. The body weights were determined every other day. BLM-exposed mice with or without transplantation of hES-ATII cells were killed on day 10 and lungs harvested for histological analysis. All mice were housed in a dedicated pathogen-free facility and cared for by a licensed veterinarian supervised staff. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Houston.
LacZ staining. The differentiating cultures of hES-ATIICs on days 0, 2, 4, 6, and 8 were washed three times with phosphate-buffered saline (PBS) before fixation in 0.5% glutaraldehyde in PBS for 10 minutes at room temperature. After washing with PBS containing 1 mmol/l MgCl2, the cells were incubated overnight at 37 °C with X-gal solution composed of 0.1% X-gal, 5 mmol/l potassium ferrocyanide, 5 mmol/l potassium ferricyanide, and 1 mmol/l MgCl2 in PBS. β-Galactosidase activity was visualized with X-gal precipitates after washing with PBS. For LacZ staining of lung tissue,23 lungs which had been lavaged three times with 500 µl of PBS containing 2 mmol/l MgCl2, were fixed for 10 minutes by instillation of 0.5% glutaraldehyde in PBS via a tracheal catheter. The fixative was removed by washing three times with PBS containing 2 mmol/l MgCl2 through the tracheal catheter. Each lung was instilled with 500 µl of X-gal solution before tracheal ligation and then immersed in X-gal solution overnight at 37 °C. Lungs were fixed again in 4% paraformaldehyde at 4 °C overnight, embedded in paraffin, and sectioned. The lung sections were counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA) before mounting with Cytoseal (Richard Allan Scientific, Kalamazoo, MI).
Histological analysis and injury scoring of mouse lung tissues. On day 10 after BLM-challenge, the mouse lungs were lavaged three times with 500 µl of PBS, inflated, and fixed with 500 µl of 4% paraformaldehyde, and then immersed in the fixative solution at 4 °C overnight. After embedding in paraffin, tissue sections from the frontal, middle, and posterior coronal planes were prepared on slides. Collagen deposition was evaluated by Sirius Red staining.42 Briefly, lung sections were incubated with 1% Sirius Red and 0.1% Fast Green FCF (Sigma, St Louis, MO) in saturated picric acid for 1 hour. The slides were then washed twice with 0.5% acetic acid in distilled H2O. The slides were then dehydrated and mounted with Cytoseal. Another set of slides from the same lung were stained with hematoxylin–eosin for routine histology. BLM-induced lung damage was graded according to the lung area (0, <10, 10–25, 25–50, 50–75, and >75%) involved with cellular infiltration, interstitial thickening, structure distortion as well as abnormal collagen deposition (n = 10 for experimental and control mice; at least two slides for each different plane/mouse).43
Hydroxyproline assay. Collagen deposition in the BLM-injured mouse lungs was also determined by analysis of hydroxyproline content by slight modification of a previously described method.44 Briefly, lungs were minced and homogenized in saline. An aliquot of homogenate was hydrolyzed in 2 ml of 6 N HCl at 110 °C overnight, neutralized with 2 ml of 6 N NaOH, and filtered through a 0.45-mm nylon membrane. The samples (0.1 ml) were then incubated for 20 minutes at room temperature with 1 ml of 1.4% chloramine T (Sigma) containing 10% n-propanol, and 0.5 mol/l sodium acetate, pH 6.0. Ehrlich's reagent (1 ml) was then added and incubated at 65 °C for 15 minutes. Hydroxyproline content was measured at 550 nm against a standard curve.
Immunofluorescent staining and cell quantization. Lung section slides prepared as described above were deparaffinized, hydrated, and incubated with 20 µg/ml proteinase K solution containing 50 mmol/l Tris and 1 mmol/l EDTA, pH 8.0 at 37 °C for 15 minutes. After rinsing twice in PBS-Tween (0.05% Tween-20 in PBS), slides were incubated in 1% Triton X-100 in PBS for 30 minutes before blocking for 1 hour with 5% of normal goat serum in PBS containing 0.2% Triton X-100. To block the endogenous mouse IgG, the sections were further incubated with 0.12 mg/ml of unconjugated affiniPure Fab fragment goat anti-mouse IgG (H+L) (cat. no. 115-007-003; Jackson Laboratories) for 1 hour. To identify hES-ATIICs, the sections were incubated with 1:10 diluted mouse antihuman nuclei monoclonal antibody and 1:100 diluted rabbit antihuman pro-SPC antibody (Chemicon, Temecula, CA) in PBS for 1 hour. The human nuclei and SPC+ cells were visualized with Alexa Fluor 546 F(ab′)2 fragment of goat anti-mouse IgG (H+L) and Alexa Fluor 488 F(ab′)2 fragment of goat anti rabbit IgG (H+L), respectively. The percentage of SPC+ cells that were also human nuclei+ were determined by visually counting 200–400 SPC+ cells in at least two slides per mouse lung (8 mice/group). The number and percentage of the counted SPC+ cells that were also human nuclei+ were then determined. The relative number of SPC‐/human nuclei+ cells was determined by counting the number of SPC‐/human nuclei+ cells were present in the exact area in which the SPC+ cells were counted. To determine whether any of the transplanted hES-ATIICs had differentiated into cells expressing the ATIC phenotypic marker T1α, immunofluorescent staining was performed with the mouse antihuman nuclei monoclonal antibody and a 1:50 diluted rabbit antihuman T1α antibody (Abgent, San Diego, CA), and secondary antibodies as above.
Measurements of lung tidal volume and blood arterial oxygen saturation. The spontaneous lung tidal volume of BLM-injured mice with or without transplanted hES-ATIICs was recorded during normal respiration without ventilation using a rodent pulmonary plethysmograph model PLY 3111 and a specifically designed software program designed for lung tidal volume analysis (Buxco Electronics, Sharon, CT). In addition, blood arterial oxygen saturation was recorded using a small rodent oximeter sensor mounted on the thigh of each tested mouse (MouseOX; STARR Life Sciences, Oakmont, PA). Data were collected for a minimum of 10 seconds without any error code for the two measured parameters, six measurements (6 × 10 seconds) per mouse during a 3-minute period.
Statistical analysis. The data shown in Figures 2b,5b,c, and 6a,b were analyzed statistically by the Student's t-test (two-tailed) using Microsoft Excel software. P values <0.05 (α level = 0.05) were considered significant.
Acknowledgments
This article is dedicated to the memory of Pierce Runnells. The generosity of Clive and Nancy Runnells and their immense love for their son made this work possible. R.A.W. is grateful for the support provided by William S. Kilroy, Sr., Chair in Pulmonary Disease. Support was provided by US Public Health Service National Institutes of Health Grants RO1 AI25011 (to R.A.W.), RO1 HL07433 (to R.A.W.), and a pilot award (to R.A.W.) from the Center for Clinical and Translational Sciences, which is funded by Clinical and Translational Award UL1RR024148 from the National Center For Research Resources. We thank Dr Gigli for critique of the manuscript, and Dr Simmons for the providing the purified human monocytes. D.W. and R.A.W. designed the research; D.W. and R.A.W. generated genetically modified hESC lines; D.W. and R.A.W. analyzed the data; D.W established the BLM injury model and conducted transplantation study; D.W and J.E.M conducted tissue staining; D.W and D.G.C. performed hESC cultures; J.L.A. prepared and cultured human primary alveolar type II cells; D.W. and R.A.W. wrote the paper. The authors have no conflict of interests of competing financial interests to declare.
REFERENCES
- Rawlins EL, Ostrowski LE, Randell SH., and , Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell. Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11 Suppl:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Wobus AM. Potential of embryonic stem cells. Mol Aspects Med. 2001;22:149–164. doi: 10.1016/s0098-2997(01)00006-1. [DOI] [PubMed] [Google Scholar]
- Odorico JS, Kaufman DS., and , Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM., and , Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng. 2005;11:1177–1187. doi: 10.1089/ten.2005.11.1177. [DOI] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E., and , Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC., and , Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jin N, Narasaraju T, Chen J, McFarland LR, Scott M, et al. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem Biophys Res Commun. 2004;319:774–780. doi: 10.1016/j.bbrc.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC., and , Williams MC. T1α, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Vanderbilt JN, Allen L, Gonzalez RF, Tigue Z, Edmondson J, Ansaldi D, et al. Directed expression of transgenes to alveolar type I cells in the mouse. Am J Respir Cell Mol Biol. 2008;39:253–262. doi: 10.1165/rcmb.2008-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen Z, Narasaraju T, Jin N., and , Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest. 2004;84:727–735. doi: 10.1038/labinvest.3700095. [DOI] [PubMed] [Google Scholar]
- Millien G, Spira A, Hinds A, Wang J, Williams MC., and , Ramirez MI. Alterations in gene expression in T1α null lung: a model of deficient alveolar sac development. BMC Dev Biol. 2006;6:35. doi: 10.1186/1471-213X-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich TR, Yi ES, Cardiff R, Yin S, Bikhazi N, Biltz R, et al. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994;144:862–868. [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW., and , Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, et al. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, et al. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ., and , Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Summer R, Sun X, Fitzsimmons K., and , Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S, Takaoka K, Hattori T, Miyazawa N, Iwanaga T, Takeda S, et al. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 2003;42:162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A., and , Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC., and , Dunn NR.2009Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies Stem Cell Resepub ahead of print). [DOI] [PubMed]
- Selman M, King TE., and , Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V., and , Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40:1700–1705. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantusch B, Kalt R, Krieger S, Puri C., and , Kerjaschki D. Sp1/Sp3 and DNA-methylation contribute to basal transcriptional activation of human podoplanin in MG63 versus Saos-2 osteoblastic cells. BMC Mol Biol. 2007;8:20. doi: 10.1186/1471-2199-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MD, Bhakta KY, Raina S, Yonescu R, Griffin CA, Copeland NG, et al. The human Aquaporin-5 gene. Molecular characterization and chromosomal localization. J Biol Chem. 1996;271:8599–8604. doi: 10.1074/jbc.271.15.8599. [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Smith ME, Smith JF, Margraf LR., and , Mendelson CR. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol. 1997;17:672–682. doi: 10.1165/ajrcmb.17.6.2858. [DOI] [PubMed] [Google Scholar]
- Brown RH, Walters DM, Greenberg RS., and , Mitzner W. A method of endotracheal intubation and pulmonary functional assessment for repeated studies in mice. J Appl Physiol. 1999;87:2362–2365. doi: 10.1152/jappl.1999.87.6.2362. [DOI] [PubMed] [Google Scholar]
- Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ., and , Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med. 1998;158:1936–1944. doi: 10.1164/ajrccm.158.6.9712073. [DOI] [PubMed] [Google Scholar]
- Chen ES, Greenlee BM, Wills-Karp M., and , Moller DR. Attenuation of lung inflammation and fibrosis in interferon-γ-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001;24:545–555. doi: 10.1165/ajrcmb.24.5.4064. [DOI] [PubMed] [Google Scholar]
- WOESSNER JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Zheng B, Mills AA., and , Bradley A. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res. 1999;27:2354–2360. doi: 10.1093/nar/27.11.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]