Abstract
Recombinant vaccinia virus (rVV) encoding tumor-associated antigens (TAAs) and adhesion or costimulatory molecules may represent important immunogenic reagents for cancer immunotherapy. Recently, intranodal (IN) antigen administration was suggested to be more immunogenic than intradermal (ID) vaccination. However, IN rVV administration has not been attempted so far. We used a rVV encoding gp100280–288, Melan-A/MART-127–35 and tyrosinase1–9 HLA-A0201 restricted epitopes and CD80 and CD86 costimulatory molecules in stage III and IV melanoma patients in a phase 1/2 trial. Of 15 patients initiating treatment, including two cycles of IN immunization, each comprising one rVV administration and three recall injections of the corresponding peptides, accompanied by subcutaneous granulocyte macrophage–colony stimulating factor supplementation, five withdrew due to progressing disease. Of 10 remaining patients seven showed evidence of induction of cytotoxic T lymphocytes (CTLs) directed against at least one epitope under investigation, as detectable by limiting dilution analysis (LDA) of specific precursors and multimer staining. Adverse reactions were mild (National Cancer Institute (NCI) grade 1–2) and mainly represented by fever, skin rashes, and pruritus. These data indicate that IN administration of rVV encoding melanoma-associated epitopes and costimulatory molecules is safe and immunogenic.
Introduction
Active specific tumor immunotherapy aims at the generation of immune responses directed against antigens uniquely or predominantly expressed by cancer cells. Indeed, a large number of tumor-associated antigens (TAAs) has been identified in the past.1 However, a majority of them are expressed to different extents in nontransformed cells and central or peripheral tolerance mechanisms2,3 might limit the generation of specific immunity to T cells characterized by the expression of antigen receptors of modest functional avidity.4 The current challenge is represented by the induction of TAA-specific immune responses able to elicit powerful effector activities of potential clinical significance.
To achieve this goal, different types of adjuvants have been used to boost the immunogenicity of antigenic peptides.5,6,7 Alternatively, genes encoding full TAA or their specific epitopes have been inserted into viral vectors, to generate effective immunogenic reagents.8,9,10,11 In a previous study, we have constructed a recombinant vaccinia virus (rVV) characterized by peculiar features12. First, it encodes HLA-A0201 restricted epitopes from three different melanoma differentiation antigens, MART-1/Melan-A, gp100 and tyrosinase. Second, antigenic epitopes are encompassed within a polypeptide including an adenovirus derived leader sequence driving the resulting gene products into the endoplasmic reticulum, thereby bypassing antigen processing steps. Third, genes encoding CD80 and CD86 costimulatory molecules have been added to this vector. Importantly, the transcription of each insert is driven by an individual promoter, thus granting a comparable expression of all the transgenes. This rVV was successfully tested in a phase 1/2 clinical trial, based on intradermal (ID) administration, accompanied by specific peptide boosts.13
The superiority of intranodal (IN) as compared to ID administration of immunogens in the induction of TAA-specific immune responses has recently been suggested.14 Most studies taking advantage of this injection route are based on the use of cultured dendritic cells loaded with antigenic peptides15,16,17,18,19 or autologous or allogeneic tumor lysates19,20 as immunogens. In contrast, rVV have yet not been administered IN to patients with cancers in active, antigen-specific immunotherapy trials.
In this study, we report for the first time a phase 1/2 clinical immunotherapy trial based on IN injection of a rVV and specific peptide boosts. The immunization protocol presented here appears to be well tolerated and able to induce immune responses in metastatic melanoma patients.
Results
Clinical trial design
This trial aimed at the assessment of safety and immunogenicity of IN immunization with a rVV inducing in infected cells the independent expression of HLA-A2 restricted epitopes MelanA/MART-127–35,21 GP100280–288 and tyrosinase1–9, targeted to the endoplasmic reticulum by an adenovirus 19K derived leader sequence (MRYMILGLLALAAVCSA), together with human costimulatory molecules CD80 and CD86 required for T cells activation (Figure 1a).22,23,24 To improve the safety of this reagent, viral DNA replication was suppressed by a limited treatment with psoralen and long-wave UV.25
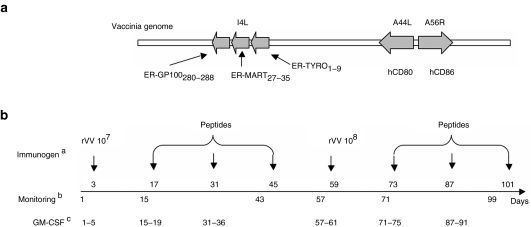
Figure 1.
Recombinant vaccinia virus and design of the study. (a) The recombinant vaccinia virus (rVV) used in this study encodes gp100280–288, Melan-A/MART-127–35, and tyrosinase1–9 tumor-associated antigen epitopes, in the form of fusion peptides targeted to the endoplasmic reticulum (ER), in the nonessential viral locus I4L and human CD80 and CD86 in the nonessential viral loci A44L and A56R, respectively. (b) aImmunogens were administered intranodally at the indicated days of the trial. bBlood samples for monitoring purposes were obtained at the indicated days. cGranulocyte macrophage–colony stimulating factor (GM–CSF) was administered subcutaneously for five consecutive days each week, as indicated.
Immunization protocol included IN rVV administration followed by IN boosts with antigenic peptides in the context of subcutaneous administration of granulocyte macrophage–colony stimulating factor, as detailed in Figure 1b, and in the “Materials and Methods” section.
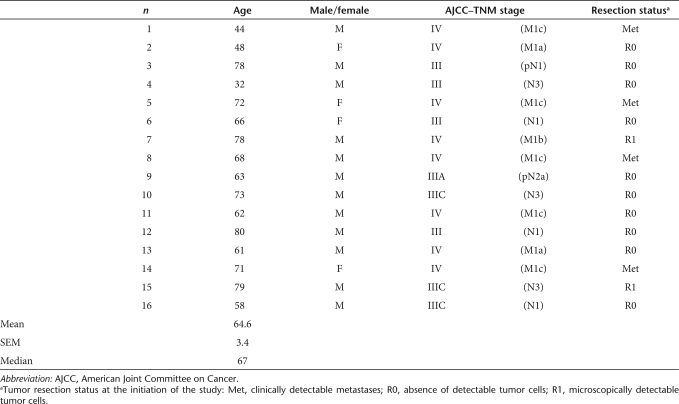
Demographics
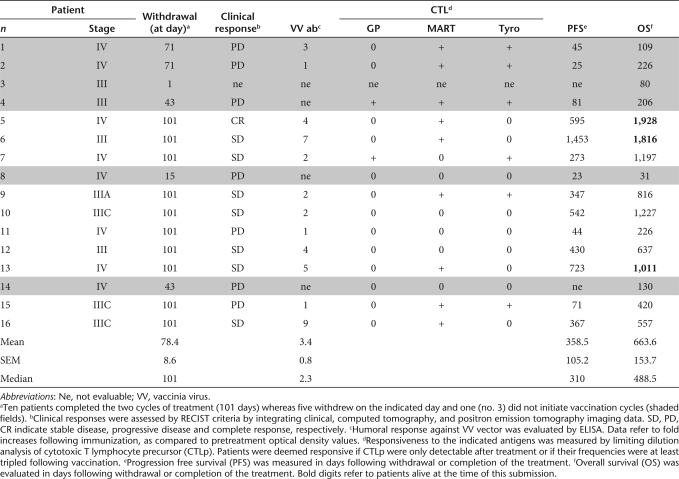
Sixteen patients (average age ± SEM: 64.6 ± 3.4 years, median = 67, range: 32–80) were enrolled in the study on an “intention-to-treat” basis (Table 1). One 78-year-old patient (no. 3) with a stage III tumor was unable to initiate the treatment because of a cerebrovascular accident. The other 15 patients underwent immunization.
Table 1.
Patient demographics
At the beginning of the trial, four of them showed clinical evidence of metastatic disease (patients no. 1, 5, 8, and 14). In two additional patients (no. 7 and 15), despite surgical resection, microscopical (R1) evidence of melanoma was still detectable. All other patients were considered clinically tumor free following surgery (R0).
Due to progressing disease, one patient, bearing a stage IV cancer (no. 8), had to interrupt the treatment before completion of the first vaccination cycle. Furthermore, four additional patients, bearing stage IV (no. 1, 2, and 14) and stage III (no. 4) melanoma could not complete the second cycle of immunization because of a rapid progression of their tumors. Thus, the full treatment consisting of two vaccination cycles and eventual extra cycles (see later text) was administered to 10 patients.
Immunocompetence in melanoma patients
To obtain an insight into the overall ability of patients admitted to the study to generate antigen-specific cytotoxic T lymphocytes (CTLs), we stimulated peripheral blood CD8+ cells from a group of them with influenza matrix (IM)58–66, HLA-A0201 restricted peptide, a classical “recall” epitope, frequently inducing cytotoxic T cell responses, detectable by limiting dilution analysis (LDA) and multimer staining, in healthy donors.26 In four of five patients, bearing stage IV (no. 1, 2, and 5) or stage III (no. 6) tumors, IM58–66 specific CTL could indeed be expanded following “in vitro” stimulation (IVS) with average frequencies of 75, 3, 7, and 12/106 CD8+ cells, respectively. Specific multimer staining was also observed (18.8, 1.7, 8.5, and 15.5%, respectively) in expanded CD8+ cells. In one patient (no. 4) with a stage III tumor, however, no response was observed. Taken together, these values did not significantly differ from those observed in a simultaneously tested group of five healthy donors, including four responders (data not shown). Notably, unresponsiveness to IM58–66 in melanoma patients was not predictive of unresponsiveness to rVV treatment (see later text Figures 3 and 4 and Supplementary Tables S1 and S2). Interestingly, however, two of the IM58–66 responsive patients (no. 5 and 6) ranked among those experiencing long term survival in our study (see later text).
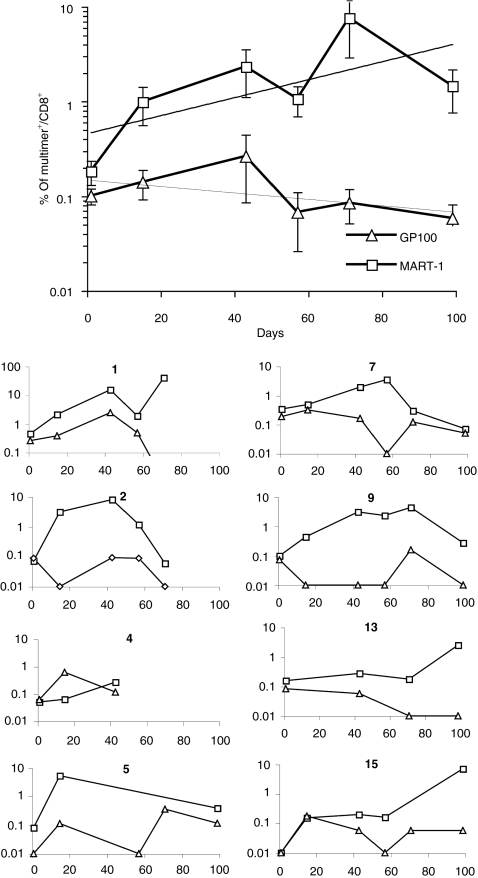
Figure 3.
Phenotypic monitoring of CD8+ T cell response by multimer staining. CD8+ T cells from the indicated patients participating to the study were cultured in the presence of gp100280–288 (triangles) or Melan-A/MART-127–35 (squares) peptides as detailed in “Materials and Methods”. Cells were then stained with the corresponding HLA-A0201 multimers and anti-CD8. Specific binding was evaluated by flow cytometry. Data are reported as percentages of CD8+ T cells. The top panel refers to the average ± SEM including trend lines, of all observed values from the 15 patients treated. The 10 small panels, labeled with patients' numbers detail data from each individual responsive patient.
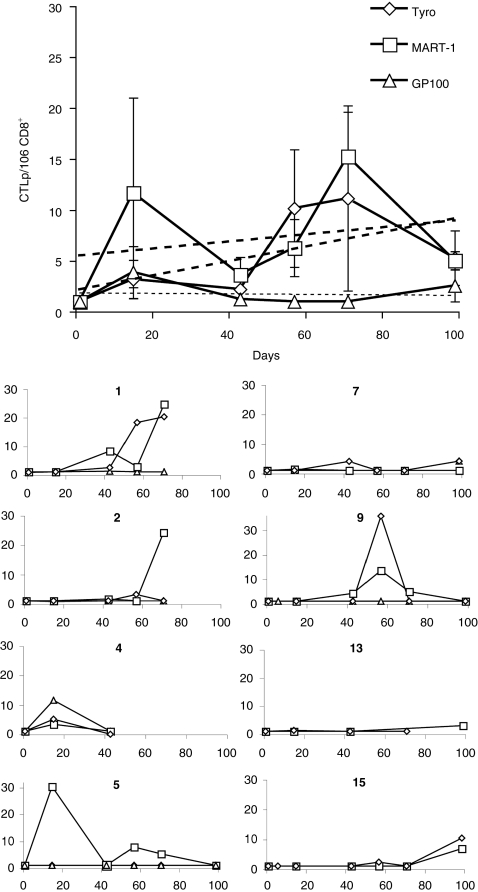
Figure 4.
Monitoring of tumor-associated antigen–specific CTLp frequency by limiting dilution analysis during treatment. CD8+ T cells from the indicated patients participating to the study were cultured in the presence of gp100280–288 (triangles), Melan-A/MART-127–35 (squares), or tyrosinase1–9 (diamonds) peptides in limiting dilution conditions, as detailed in “Materials and Methods”. Cytotoxic activity of split wells was then evaluated by 51Cr release assays, using, as targets T2 cells pulsed with specific or control peptides. Data are reported as specific cytotoxic T lymphocyte precursor (CTLp) per 106 CD8+ T cells. The top panel refers to the average ± SEM including trend lines of all values observed in the 15 patients. The 10 small panels labeled with patients' numbers detail the results of each individual responsive patient.
IN delivery of rVV is feasible and safe
Lymph nodes from the groin area were utilized for vaccination purposes. Their visualization and IN administration of immunogenic reagents including rVV and peptides could be successfully performed under ultrasound assisted guidance with the help of experienced radiologists (R.W.H. and A.L.J.). Injections usually resulted in the observation of node swelling, thus confirming that the procedure had been correctly carried out.16 After a total of 114 IN injections, we did not observe any complications related to the application of this technique.
“In vitro” stimulated immune response to TAA: multimer staining
IVS is used to promote the expansion of TAA-specific T cells, thereby increasing the sensitivity of detection of multimer positive lymphocytes and allowing the performance of cytotoxicity assays.14,27,28,29 Figure 2 shows representative stainings of cells obtained from one patient (no. 9) during the clinical trial. Figure 3 reports gp100280–288 and MART-126–35 multimer staining data, following IVS of CD8+ T cells, from all patients included in the study (average ± SEM top large panel), at the different time points under investigation. The 10 individual graphical representations correspond to the 10 patients (no. 1, 2, 4, 5, 6, 7, 9, 13, 15, and 16) who showed significant variations in multimer staining or induction of CTL precursor (CTLp) (see later text) during the treatment. Values from each individual patient are also reported in the Supplementary Table S1.
Figure 2.
Evolution of multimer staining during treatment. CD8+ T cells from patient no.9 were sampled at the indicated days during the clinical trial and stimulated “in vitro” with Melan-A/MART-127–35 peptide, as detailed in the “Materials and Methods” section. Cells were then stained with phycoerythrin-labeled HLA-A0201-Melan-A/MART-126–35 multimers and fluorescein isothiocyanate labeled anti-CD8 monoclonal antibodies. Digits refer to percentages of CD8+ T cells stained with specific multimers.
CD8+ T cell staining by HLA-A0201-gp100280–288 multimers was usually modest (below 0.1% positivity) with only 1/74 measurements exceeding 1% of CD8+ cells. With the exception of patient no.1, only minor variations in the percentages of positive cells were observed during the treatment (Figure 3).
Regarding HLA-A0201-Melan-A/MART-126–35 multimer staining, in at least eight cases (patients/panel no. 1, 2, 5, 6, 7, 9, 13, and 15) percentages of positive cells displayed marked (tenfold or more) increases as compared to pretreatment values. In the remaining patients percentages of multimer positive cells were low (<1%) throughout the monitoring and did not display major immunization related variations. It is of note that percentages of multimer positive cells in responsive patients frequently (five of eight responders) tended to decline at the end of the trial (Figure 3, patients no. 2, 5, 6, 7, and 9), although, on average, an increasing frequency of Melan-A/MART-126–35-specific cells could be consistently observed throughout the immunization protocol.
“In vitro” stimulated immune response to TAA: CTLp frequency
TAA-specific CTLp frequency was evaluated by LDA in the 15 patients undergoing immunization (Figure 4). Pretreatment responsiveness to the three target epitopes was always undetectable or low (CTLp ≤1/106 CD8+ T cells). Vaccination induced “de novo” CTLp ≥3/106 CD8+ T cells or increased at least threefold baseline CTLp frequencies in two patients for gp100280–288 (Figure 4; patients no. 4 and 7), nine for Melan-A/MART-127–35 (Figure 4; patients no. 1, 2, 4, 5, 6, 9, 13, 15, and 16) and six for tyrosinase1–9 (Figure 4; patients no. 1, 2, 4, 7, 9, and 15). Notably, peak frequencies of CTLp specific for one or more of the epitopes under investigation were frequently, albeit not exclusively, observed following rVV (day 3 and day 59), rather than peptide administration. Values from individual patients are also reported in the Supplementary Table S2).
Of note, regarding Melan-A/MART-127–35, a highly significant correlation (r = 0.35; P = 0.00007) was detectable between specific multimer staining and CTLp frequency.
“Ex vivo” immune responses to TAA
Multimer staining “ex vivo” is less sensitive than that performed following IVS. However, it has been suggested that it might better reflect ongoing “in vivo” immune responsiveness. In order to complement our monitoring data, Melan-A/MART-127–35 multimer staining of freshly isolated CD8+ T cells was also attempted. At least 50.000 purified CD8+ T cells were analyzed. HLA-A0201-Melan-A/MART-126–35 multimers stained 0.05, 0.1, and 0.14% of the CD8+ T cells from patients no. 9, 11, and 12, respectively, on day 1. In all three cases IN rVV administration induced an increase in percentages of positive cells (0.09, 0.64, and 0.25%, respectively) within 2 weeks. Notably, however, IVS of cells from the same specimens resulted in the expansion of Melan-A/MART-127–35-specific T cells from patient 9, but not from patients 11 and 12, suggesting that multimer positive cells detectable “ex vivo” could be, at least in part, characterized by poor proliferation and cytotoxic potential.30
Clinical correlations
Five patients experienced progressive disease during the treatment (see earlier text and Table 2) forcing them to withdraw from the trial before the completion of the two immunization cycles. Of the 10 remaining evaluable patients, seven had developed a CTL response against at least one of the TAA epitopes included in the rVV during the treatment (see “Materials and Methods” section). In contrast, no responsiveness was detectable in the three remaining patients. Among immunologically responsive patients, only one of seven experienced rapidly progressing disease. Among the three unresponsive patients one progressive and two stable diseases were observed. During their follow-up, responsive patients where CTL responsiveness had been induced were characterized by a similar progression free survival as compared to unresponsive patients (mean ± SEM = 547 ± 173 versus 338 ± 153 days, P = 0.57). Accordingly, overall survival in immunologically responsive and nonresponsive patients did not significantly differ (mean ± SEM = 1,106 ± 224 versus 696 ± 295 days, P = 0.42). At the time of this submission three immunologically responsive (patients no. 5, 6, and 13) but none of the unresponsive patients are still alive (Table 2).
Table 2.
Summary of clinical and immunological data
Anti-vector immune responses
Humoral responses against vaccinia virus (VV) were measured in patients completing the two immunization cycles by enzyme-linked immunosorbent assay. On average, optical density values were markedly increased (mean ± SEM = 3.4 ± 0.8-fold, median: 2.3-fold) over pretreatment values (Table 2). However in two patients (no. 11 and 15) no increase of humoral responsiveness was detected. Notably, no significant differences in the extent of humoral response against the vector were observed between patients responsive or unresponsive to the recombinant TAA epitopes (Table 2).
Viral shedding
Replicating wild-type VV is a potentially pathogenic microorganism. Although our attenuated recombinant vector was always used in replication inactivated form, upon request from local regulatory authorities, we monitored potential viral shedding in plasma and urines of the immunized patients. Indeed, despite the use of a highly sensitive quantitative PCR technique (see “Materials and Methods”), no viral DNA was detectable in probes from five of five patients tested.
Toxicity
The immunization protocol used in this trial proved to be endowed with low toxicity upon ID administration in a previous study.13 IN injection also resulted in modest National Cancer Institute (NCI) grade 1–2 adverse reactions, most frequently represented by fever (10 of 15 patients: 9 NCI grade 1 and 1 NCI grade 2), skin rashes likely related to granulocyte macrophage–colony stimulating factor coadministration (13 of 15 patients: 11 NCI grade 1 and 2 NCI grade 2)31 and skin pruritus (12 of 15 patients: 11 NCI grade 1 and 1 NCI grade 2). Notably, in two cases a transient (2–4 days) NCI grade 2 leukopenia was observed. It is of note that one major adverse event unrelated to the study, namely an atrioventricular block, was also observed.
Minor adverse events including anemia (7 of 15 patients: all NCI grade 1) were deemed unrelated to the vaccine but, rather, to the underlying cancer. None of the adverse events led to withdrawal from the study. In a stage IV patient (no. 7) showing evidence of successful induction of CTL responses against all three TAA under investigation, NCI grade 1 vitiligo was observed. As expected,32 this patient was characterized by a relatively long survival despite the advanced stage of his disease (Table 2).
Preclinical models suggest that ocular autoimmunity might represent a hallmark of effective immunization against melanoma differentiation antigens.33 However, in keeping with other reports,34 we did not observe any adverse effect of immunization on eyes or vision in our patients, irrespective of immunological or clinical response.
Discussion
VV-based vectors are characterized by high immunogenicity and capability of expressing a variety of transgenes. Safety, also due to their lack of integration into the host genome, qualifies them as reagents of choice for immuno/gene therapy of a multiplicity of cancers.35 Different types of reagents have been proposed, encoding TAA, adhesion or costimulatory molecules or their combinations.36
We have constructed a rVV encoding CD80 and CD86 costimulatory receptors together with HLA-A0201 restricted epitopes from three differentiation TAA.12,13 At difference with other reagents encoding TAA as full gene products or strings of HLA class I restricted epitopes37 the rVV used in this study presents specific features. Epitopes are encoded in the form of independent minigenes with own promoters and are produced as fusion peptides together with an adenovirus derived leader sequence driving them into the endoplasmic reticulum.38 Furthermore, this reagent encodes “natural” epitopes and not their analogue counterparts. Indeed, “natural” epitopes have been suggested to promote the expansion of CTL endowed with higher functional avidity and tumoricidal capacity as compared with analogues.39 A previous study by our group13 indicates that ID immunization with rVV accompanied by boost injections with antigenic peptides may represent a well tolerated protocol, capable of inducing TAA-specific immune responses.
The expansion of antigen-specific T cells usually takes place in lymph nodes. Thus, antigen-presenting cells need to reach secondary lymphoid organs along chemokine gradients in order to effectively prime specific responses. In line with this concept, it has been suggested14,16 that IN immunization might be more effective than ID vaccination. Most of these works were performed by using pulsed dendritic cells as immunogens. Their IN injection is meant to maximize the likelihood of productive encounters with antigen-specific T cells.
In contrast, direct IN injection of rVV has not been explored as yet, in cancer immunotherapy. We reasoned that direct expression of TAA derived epitopes in lymph nodes might result in the production of high amounts of antigen easily accessible to responding T cell. Furthermore, overexpression of costimulatory molecules within the lymph nodes could also favor effective antigen presentation by resident antigen-presenting cells, and prevent the induction of specific anergy.
Indeed CTL responses against at least one of the antigenic epitopes under investigation were detectable in 10 of 15 patients initiating the IN immunization treatment. Notably, in six of them two or three antigens could be successfully targeted.
In the rVV under investigation, the expression of each epitope is driven by an independent promoter (see earlier text). Therefore their expression level is likely to be reasonably similar.13 However, CTL responsiveness to gp100280–288 epitope was less frequently detectable, than responses to tyrosinase1–9 and, most of all, to Melan-AMART-127–35. This low immunogenicity could be, at least in part, related to a low stability of this epitope.40
Kinetics of TAA-specific immune responses, as detectable in peripheral blood T cells, appeared to reflect that observed in experimental models following vaccination against major histocompatibility complex class I restricted antigens41 with prominent expansion and retraction phases.
Increased humoral responsiveness to vector VV was also detected, but, consistent with a majority of pox-vector-based vaccination trials, it did not appear to impair the generation of transgene-specific responses.
Adverse reactions were usually mild and limited to fever, skin rashes and pruritus of NCI grade 1–2 observed in a majority of patients. Furthermore, two episodes of transient grade 2 leukopenia were also observed. Remarkably, the rVV under investigation could be administered up to four times in the apparent absence of major side effects. Thanks to its lack of severe toxicity, the treatment was well accepted by patients, as witnessed by the willingness of three patients (no. 5, 6, and 13) to undergo extra (1–3) immunization cycles on a compassionate basis.
It has recently been suggested that evaluation of clinical responsiveness to tumor immunotherapy should rely more on “soft” criteria rather than on those commonly applying to chemo or radiotherapy, focusing on clinical course, e.g., “patient” rather than “tumor” response.42 It is of note, in this context, that, in at least three of seven patients immunologically responsive to treatment, relapses emerged in the form of single metastases that could be relatively easily surgically excised.
Generation of TAA-specific immune responses is a relatively slow process. In our study, the highest frequencies of CTL specific for the TAA epitopes under investigation were usually detectable after three or more vaccinations, including one or more administrations of rVV, and additional extra cycles. One-third (5 of 15) of patients beginning our immunization protocol was unable to complete the two vaccination cycles due to rapidly progressing disease. These data suggest that early vaccination would likely display the highest impact on the clinical course of the disease.
Taken together our data indicate that IN rVV administration coupled with peptide boosts in patients with metastatic melanoma is safe and immunogenic, although they do not support the notion of an obvious superiority to ID administration. Irrespective of the injection route, however, they further urge the clinical evaluation of the rVV used in this study in randomized studies aimed at assessing its clinical effectiveness.
Materials and Methods
Patients. Sixteen HLA-A0201+ melanoma patients with AJCC (American Joint Committee on Cancer) stage III (n = 8) and stage IV (n = 8)43 tumors (Table 1) provided informed consent according to the Declaration of Helsinki to the participation to a clinical trial approved by the Swiss Agency for Therapeutic Products (Swissmedic, no.:GT1999017) and by the ethical committee of the University Hospital of Basel (no.:175/02) (NIH registration: ClinicalTrials.gov Identifier NCT00116597). Eligible patients had no chemotherapy, radiotherapy, or immunotherapy for at least 4 weeks prior to the participation to the trial, had a Karnofsky performance status >70%, a life expectancy of at least 2 months and no other malignancy. Treatment was performed on an outpatient basis and complete clinical examination was done at the beginning and the end of the protocol.
Immunogens. The rVV used in this trial (Figure 1a) was produced and tested according to GMP (Good Manufacturing Practice) standards (BioReliance, Stirling, UK). The capability of this replication-incompetent rVV to induce the expression of surface markers, the presentation of each recombinant epitope, and to stimulate specific CD8+ CTL responses have been verified “in vitro” and “in vivo”, as previously reported.21,24,38 GMP grade synthetic peptides, corresponding to the three transgenic epitopes under investigation, resuspended in phosphate buffered saline (PBS)–20% dimethyl sulfoxide, were commercially obtained (Orpegen Therapeutica, Heidelberg, Germany).
Vaccination protocol. The vaccination protocol included two cycles of immunization of 7 weeks each, alternating a week of treatment with a week of rest (Figure 1b). The weeks of treatment included five, daily, subcutaneous injections of granulocyte macrophage–colony stimulating factor (5 µg/kg body weight; Laboratorio Pablo Cassarà, Buenos Aires, Argentina) except for the last week of each cycle (week 7 and 15). On day 3 of each treatment week, the antigenic formulation was injected under ultrasound guidance into a superficial inguinal lymph node. The inguinal lymph nodes were chosen because of their relatively long axes and they were not adjacent to any major blood vessel. The antigens were delivered through the administration of the nonreplicating rVV on day 3 and 59 at 107 pfu and 108 pfu doses (in a volume of 0.2 ml Tris 10 mmol/l), respectively, or in the form of soluble peptides on days 17, 31, 45, 73, 87, and 101 (100 µg of each peptide for a total volume of 0.6 ml). Due to their responsiveness to the treatment and/or performance status, some patients (no. 5, 6, and 13) received supplementary immunizations, on a compassionate basis. These extra-vaccinations were performed either as a full, third, “extra-cycle” (similar to cycle 2—week 9 to 15) or, more simply, as a single injections of 108 pfu of the rVV under investigation.
Monitoring of TAA-specific immune response. Multimer staining (see later text) and LDA of CTLp frequency were used to monitor antigen-specific immune responsiveness.
Briefly, ficoll-purified peripheral blood mononuclear cells were obtained from all patients on the first day of each week of treatment (days 1, 15, 43, 57, 71, and 99). CD8+ cells were isolated from peripheral blood mononuclear cells by using magnetic beads (MACS; Miltenyi Biotech, Bergish Gladbach, Germany). They were set in culture in individual 96 micro-well plates for each epitope under investigation, at three different numbers of cells per well in 28 replica wells and in bulk cultures. T lymphocytes from each plate were stimulated on day 1 of culture with autologous irradiated CD8− peripheral blood mononuclear cells, as antigen-presenting cells, following pulsing with each of the three peptides (20 µg/ml) under investigation. Soluble peptides (1 µg/ml final concentration) were again added to the cultures on day 7. IL-2 (Hoffmann-La Roche, Basel, Switzerland) was added on day 3, 7, and 10 (10, 10, and 100 U/ml, respectively). After 2 week cultures, the frequency of specific CD8+ CTLp was assessed by multimer staining on cells from bulk cultures and by cytotoxicity assays for each of the three epitopes on the LDA cultures.
For multimer staining, cells were resuspended in 100 µl culture medium and 3 µl of specific pentamers (0.05 mg/ml, ProImmune, Oxford, UK) were added for 10 minutes incubation at room temperature. For MelanA/MART-1, we used multimers bearing the 26–35 analogue 27L peptide, that stain T cell specific for either natural or analogue epitopes,39 whereas for gp100, reagents bearing the natural 280–288 peptide were utilized. In contrast, due to its low solubility, tyrosinase1–9 peptide cannot be efficiently used to generate specific multimer preparations. Therefore, our study was limited to MelanA/MART-1 and gp100 specific multimer staining. Anti-CD8 monoclonal antibodies (Pharmingen, SanDiego, CA, 5 µl) were added to the samples for additional 30 minutes incubation. Cells were then washed and analyzed by flow cytometry (FACScalibur, Becton Dickinson, SanDiego, CA).
Antigen-specific cytotoxic activity was assessed by 51Cr release assays. Each microculture plate was split in two fresh plates to serve as effectors for specific and control targets. As target cells, 103 51Cr labeled T2 cells were added to microwells from each plate following a 2 hours pulsing with the peptide used for IVS or a control peptide, in the presence of 105 unlabeled K562 cells per well. After 4 hours at 37 °C, supernatants were transferred onto LumaPlates-96 (PerkinElmer, Meriden, CT) and dried. Luminescence was counted in a scintillation counter (TopCount, Packard Instruments, Meriden, CT). A microculture was considered positive if lysis observed upon coculture with specific peptide-pulsed target cells was at least three standard deviations above the average of spontaneous release (P ≤ 0.05) and at least 12% above values detected upon coculture with target cells pulsed with control peptide.12,13,44 Frequencies of CTLp were calculated by multiwell distribution analysis.44
Inter- and intra-assay variabilities of CTLp detection could not be evaluated by using patients' cells due to obvious limitations in the amount of blood available for monitoring. However, they were measured for the responses to HLA-A0201 restricted IM58–66 epitope (see earlier text) in healthy donors. Four measurements of frequencies of CTLp specific for this epitope were performed in CD8+ T cells sampled over a two months time from three healthy donors. Variations of CTLp frequencies observed in the four different assays of each donor never exceeded threefold of initial levels. Based on these data, patients were considered immunologically responsive if frequencies of CTLp specific for the epitopes under investigation observed during the trial were at least more than threefold higher than pretreatment values.
Clinical evaluation. The clinical course of the patients admitted to the study was evaluated according to the RECIST (Response Evaluation Criteria In Solid Tumors) criteria45,46,47 by integrating clinical data, computed tomography and positron emission tomography imaging.
Monitoring of humoral response against the VV vector. Plasma from each blood sample was collected to evaluate the antivaccinia vector antibody response. Enzyme-linked immunosorbent assays were performed on plates (MaxiSorb, Nalge Nunc, Rochester, NY) coated with 100 µl of a solution containing 107pfu/ml of cushion purified inactivated wild-type virus diluted in PBS. Wells were saturated with 200 µl of PBS–3% BSA for 2 hours and washed with PBS–0.05% Tween 20 (PBS-T). Diluted plasma samples (100 µl) were then added for 1 hour incubation. After three washes with PBS-T, 100 µl of a 1/1,000 antihuman-Ig-biotinylated antibody preparation (Pharmingen, SanDiego, CA) diluted in 3% BSA were added for a 45̀ incubation. Unbound antibody was removed by multiple PBS-T washes. Specific binding was revealed by the addition of 100 µl of 1/10,000 diluted Avidin-HRP reagent (SigmaFast; Sigma-Aldrich, St. Louis, MO). Optical densities of duplicate samples were measured and analyzed, by using, as references, pretreatment values from each patient.
rVV assessment in biological fluids. In order to verify the possibility of viral shedding, blood, and urines from immunized patients were collected from patients before and 1 and 24 hours after the administration of rVV. DNA was extracted from 200 µl plasma and urine according to the Blood and Body Fluid Spin Protocol of the QIAmp DNA Blood Mini Kit (Qiagen, Hombrechtikon, Switzerland). VV DNA detection was attempted by utilizing the ABI prism 7700 sequence detection system, the TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) and the following primers and probe specific for the I3L viral gene:
Fwd: 5′CGGCTAGTCCTATGTTGTATCAACTTC3′
Rev: 5′TGCAAAGAATTTGGAATGCG3′
Probe: FAM-CTGGCCCAGGCAGTCAGATCATCTT-TAMRA.
To comply with the settings of the study, standard curves and positive controls were constructed by spiking plasma and urine samples with inactivated VV. Detection limit of quantitative PCR ranged between 100 and 300 copies/ml.
SUPPLEMENTARY MATERIALTable S1. Monitoring of CD8+ T cell response by multimer staining.Table S2. Limiting dilution analysis of CTL precursor frequencies.
Supplementary Material
Monitoring of CD8+ T cell response by multimer staining.
Limiting dilution analysis of CTL precursor frequencies.
Acknowledgments
We thank the patients who participated to the trial, their families and to the nurses of the Tageschirurgie of the Kantonsspital Basel Stadt. This study was partially supported by grants from the Swiss Nationalfonds for Scientific Research, the Cancer Leagues of Basel Stadt and Basel Land (Basel, Switzerland) and the Freie Akademische Gesellschaft (FAG, Basel, Switzerland). W.P.W. was partially supported by the Swiss Bridge Foundation. The authors have no conflict of interest to declare.
REFERENCES
- Novellino L, Castelli C., and , Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter J, Brors B, Hergenhahn M., and , Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. [PubMed] [Google Scholar]
- Schaed SG, Klimek VM, Panageas KS, Musselli CM, Butterworth L, Hwu WJ, et al. T-cell responses against tyrosinase 368-376(370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund's adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8:967–972. [PubMed] [Google Scholar]
- Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato RJ, Drury N, Naylor S, Jac J, Saxena S, Cao A, et al. Vaccination of prostate cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother. 2008;31:577–585. doi: 10.1097/CJI.0b013e31817deafd. [DOI] [PubMed] [Google Scholar]
- Hofbauer GF, Baur T, Bonnet MC, Tartour E, Burg G, Berinstein NL, et al. Clinical phase I intratumoral administration of two recombinant ALVAC canarypox viruses expressing human granulocyte-macrophage colony-stimulating factor or interleukin-2: the transgene determines the composition of the inflammatory infiltrate. Melanoma Res. 2008;18:104–111. doi: 10.1097/CMR.0b013e3282f702cf. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Deraffele G, Mitcham J, Moroziewicz D, Cohen SM, Hurst-Wicker KS, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115:1903–1912. doi: 10.1172/JCI24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- Oertli D, Marti WR, Zajac P, Noppen C, Kocher T, Padovan E, et al. Rapid induction of specific cytotoxic T lymphocytes against melanoma-associated antigens by a recombinant vaccinia virus vector expressing multiple immunodominant epitopes and costimulatory molecules in vivo. Hum Gene Ther. 2002;13:569–575. doi: 10.1089/10430340252809856. [DOI] [PubMed] [Google Scholar]
- Zajac P, Oertli D, Marti W, Adamina M, Bolli M, Guller U, et al. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum Gene Ther. 2003;14:1497–1510. doi: 10.1089/104303403322495016. [DOI] [PubMed] [Google Scholar]
- Bedrosian I, Mick R, Xu S, Nisenbaum H, Faries M, Zhang P, et al. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- Figdor CG, de Vries IJ, Lesterhuis WJ., and , Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarrière N, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- Hersey P, Halliday GM, Farrelly ML, DeSilva C, Lett M., and , Menzies SW. Phase I/II study of treatment with matured dendritic cells with or without low dose IL-2 in patients with disseminated melanoma. Cancer Immunol Immunother. 2008;57:1039–1051. doi: 10.1007/s00262-007-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother. 2006;55:819–829. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac P, Oertli D, Spagnoli GC, Noppen C, Schaefer C, Heberer M, et al. Generation of tumoricidal cytotoxic T lymphocytes from healthy donors after in vitro stimulation with a replication-incompetent vaccinia virus encoding MART-1/Melan-A 27-35 epitope. Int J Cancer. 1997;71:491–496. doi: 10.1002/(sici)1097-0215(19970502)71:3<491::aid-ijc30>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Protti MP, Rugarli C., and , Bellone M. B7.1 expression on tumor cells circumvents the need of professional antigen presentation for in vitro propagation of cytotoxic T cell lines. Cancer Res. 1996;56:11–15. [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL., and , Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Marti WR, Zajac P, Spagnoli G, Heberer M., and , Oertli D. Nonreplicating recombinant vaccinia virus encoding human B-7 molecules elicits effective costimulation of naive and memory CD4+ T lymphocytes in vitro. Cell Immunol. 1997;179:146–152. doi: 10.1006/cimm.1997.1158. [DOI] [PubMed] [Google Scholar]
- Tsung K, Yim JH, Marti W, Buller RM., and , Norton JA. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol. 1996;70:165–171. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Thurner B, Dieckmann D, Keikavoussi P, Bender A, Maczek C, Jonuleit H, et al. Mage-3 and influenza-matrix peptide-specific cytotoxic T cells are inducible in terminal stage HLA-A2.1+ melanoma patients by mature monocyte-derived dendritic cells. J Immunol. 2000;165:3492–3496. doi: 10.4049/jimmunol.165.6.3492. [DOI] [PubMed] [Google Scholar]
- Chianese-Bullock KA, Irvin WP, Jr, Petroni GR, Murphy C, Smolkin M, Olson WC, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J Immunother. 2008;31:420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- Meijer SL, Dols A, Jensen SM, Hu HM, Miller W, Walker E, et al. Induction of circulating tumor-reactive CD8+ T cells after vaccination of melanoma patients with the gp100 209-2M peptide. J Immunother. 2007;30:533–543. doi: 10.1097/CJI.0b013e3180335b5e. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–2050. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Liénard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- Holmes JP, Gates JD, Benavides LC, Hueman MT, Carmichael MG, Patil R, et al. Optimal dose and schedule of an HER-2/neu (E75) peptide vaccine to prevent breast cancer recurrence: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2008;113:1666–1675. doi: 10.1002/cncr.23772. [DOI] [PubMed] [Google Scholar]
- Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straatsma BR, Nusinowitz S, Young TA, Gordon LK, Chun MW, Rosen C, et al. Surveillance of the eye and vision in clinical trials of CP-675,206 for metastatic melanoma. Am J Ophthalmol. 2007;143:958–969. doi: 10.1016/j.ajo.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Shen Y., and , Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005;11:180–195. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Arlen PM, Gulley JL, Madan RA, Hodge JW., and , Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol. 2007;27:451–462. doi: 10.1615/critrevimmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
- Smith CL, Mirza F, Pasquetto V, Tscharke DC, Palmowski MJ, Dunbar PR, et al. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J Immunol. 2005;175:8431–8437. doi: 10.4049/jimmunol.175.12.8431. [DOI] [PubMed] [Google Scholar]
- Zajac P, Schütz A, Oertli D, Noppen C, Schaefer C, Heberer M, et al. Enhanced generation of cytotoxic T lymphocytes using recombinant vaccinia virus expressing human tumor-associated antigens and B7 costimulatory molecules. Cancer Res. 1998;58:4567–4571. [PubMed] [Google Scholar]
- Speiser DE, Baumgaertner P, Voelter V, Devevre E, Barbey C, Rufer N, et al. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci USA. 2008;105:3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazza A, Adamina M, Ausiello CM, Giardina B, Marini M, Palazzo R, et al. Hydrolysis of the tumor-associated antigen epitope gp100(280-288) by membrane-associated and soluble enzymes expressed by immature and mature dendritic cells. Clin Immunol. 2004;111:252–261. doi: 10.1016/j.clim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Schluns KS., and , Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Schlom J, Arlen PM., and , Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- Chaux P, Vantomme V, Coulie P, Boon T., and , van der Bruggen P. Estimation of the frequencies of anti-MAGE-3 cytolytic T-lymphocyte precursors in blood from individuals without cancer. Int J Cancer. 1998;77:538–542. doi: 10.1002/(sici)1097-0215(19980812)77:4<538::aid-ijc11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. National Cancer Institute (2006Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials J Nucl Med 471059–1066. [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monitoring of CD8+ T cell response by multimer staining.
Limiting dilution analysis of CTL precursor frequencies.