Abstract
Corneal alkali burns are a serious clinical problem that often leads to permanent visual impairment. In this process, transforming growth factor (Tgf)-β1 is upregulated and involved in the response to corneal injury and the process of corneal stromal scarring. To develop an efficient compound to inhibit Tgf-β1 in the cornea, we designed GB1201, a pyrrole–imidazole (PI) polyamide targeting rat Tgf-β1 gene promoter to the activator protein-1 (AP-1) binding site. GB1201 showed a high binding affinity to the target DNA sequence in the gel mobility shift and Biacore assays. GB1201 significantly inhibited the rat Tgf-β1 gene promoter activity in HEK (human embryonic kidney) 293 cells in a concentration-dependent manner. Topically administrated GB1201 was distributed immediately to the nuclei of all cell layers of the cornea and remained for 24 hours. A corneal alkali burn model in rats was used to evaluate the therapeutic efficacy of GB1201. GB1201 suppressed the upregulation of Tgf-β1 in the burned cornea, both in the mRNA and protein levels. Moreover, daily treatment with GB1201 for a week significantly improved the corneal tissue wound healing, reduced corneal stromal scarring, and prevented corneal haze formation. Our data suggest that PI polyamide may open new opportunities for therapeutic intervention in the treatment of chemically burned corneas.
Introduction
More than 65,000 work-related eye injuries, causing significant morbidity and disability, are reported in the United States annually. Ocular chemical burns make up a significant percentage of work-related eye injuries, especially injuries from alkali.1,2 The cornea is a highly organized transparent tissue that must remain transparent to refract light properly. Exposure to alkali may produce extensive damage to corneal tissues, and severe burns may cause permanent visual impairment or even blindness.3 Alkali-burned corneas seldom heal properly, and they show reduced corneal transparency (hazy cornea) and opacity, except for a grade 1 injury.3 Transforming growth factor (Tgf)-β1 is a pivotal molecule in tissue repair. In the case of a corneal alkali injury, Tgf-β1 has been shown to promote migration of corneal epithelial cells and keratocytes, and to induce transdifferentiation of the keratocytes to myofibroblasts, consequently contributing to the repair of the corneal epithelium and stromal layer. Many studies have shown that Tgf-β1 is upregulated in corneal tissues following alkali exposure.3,4,5,6,7,8 However, Tgf-β1 has also been reported as a potent chemotactic factor for monocytes, neutrophils, and macrophages. Tgf-β1 also has the ability to induce expression of other cytokines, such as matrix metalloproteinase 9 (Mmp-9), vascular endothelial growth factor, and monocyte/macrophage chemotactic protein-1, which is believed to be involved in matrix degradation, local neovascularization and inflammation, respectively.4,5 Overexpression of Tgf-β1 has been noted to exacerbate damage to the injured cornea.4,5 Besides, although Tgf-β1 can induce keratocyte transdifferentiation to myofibroblasts, overexpressed Tgf-β1 reveals myofibroblast accumulation in the stromal layer of the burned cornea, which was suggested to be the main mechanism of corneal haze formation after injury because myofibroblasts have an altered crystalline production and, consequently, are less transparent than keratocytes.6,7
All of the above-mentioned information allows us to hypothesize that inhibition of Tgf-β1 gene induction might prevent corneal tissue destruction resulting from alkali burns and result in a positive impact on corneal wound healing and a negative impact on corneal haze formation. The regulation of gene expression may occur at multiple levels. Pyrrole–imidazole (PI) polyamides are an example of a tool for the pretranscriptional regulation of target gene induction.9 We have reported previously that PI polyamide targeted to the activator protein-1 (AP-1) binding site of a Tgf-β1 gene promoter could be a potential and feasible agent for the treatment of progressive renal diseases and in-stent restenosis.10,11 In this study, we designed and synthesized GB1201, a novel PI polyamide targeted to the Tgf-β1 promoter. It was designed to span the 3′ boundary of the AP-1 binding site, which was slightly different with our previously used one and provided us a similar binding affinity to the AP-1 site as well as distribution in eye tissues. The biological effects of GB1201 were investigated by using a corneal alkali burn model in rats. Here, we show, for the first time, the feasibility and therapeutic efficacy of GB1201 to alkali-burned corneas.
Results
Binding specificity and affinity of GB1201 to the target DNA
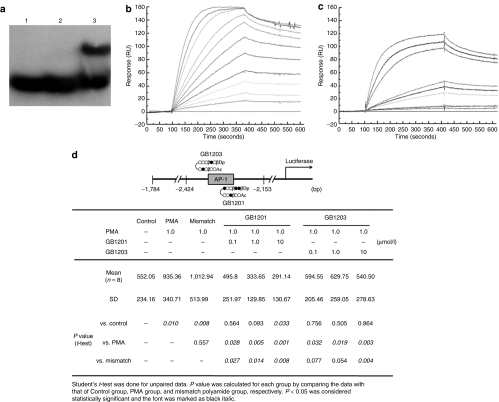
Gel mobility shift and Biacore assays allow for determination of the binding affinity and specificity of PI polyamides to target DNA. As shown in Figure 1a, a clear mobility band was observed when GB1201 was incubated with the appropriate target double-strand DNA, indicating the specific binding of GB1201 to the target double-strand DNA, whereas no mobility band was detected for mismatch polyamide, indicating mismatch polyamide did not bind to the appropriate double-strand DNA.10 The kinetics of interaction between GB1201 or mismatch polyamide with target double-strand DNA was measured by Biacore assay (Figure 1b,c). In surface plasmon resonance sensorgrams, the high binding affinity of GB1201 to the match-oligo was demonstrated and KD = (5.12 ± 3.43) × 10−9 was calculated. The kinetic constants calculated from fitting resulting sensorgrams are described in Table 1. GB1201 had 149 times higher affinity in respect to the mismatch polyamide, although the binding of GB1203 is 3.6 times better.10 These data indicated that GB1201 also had a specific and strong affinity to the target nucleotide sequence.
Figure 1.
Binding specificity and affinity of GB1201 to the target DNA. (a) Gel mobility shift assay. Lane 1, single-stranded DNA; lane 2, double-stranded DNA; lane 3, double-stranded DNA with GB1201. (b,c) Biacore assay. Typical surface plasmon resonance sensorgrams for the interaction of GB1201 with the DNA immobilized on the surface of a sensor plasmon SA. All experiments were done using the same DNA immobilization level sensor chips in the same reaction conditions. In the sensorgram, the Biacore Surface Plasmon Response expressed in resonance units (RU) was plotted versus time. Concentrations for GB1201 are 0, 1, 5, 10, 20, 30, 50, 75, 100, 150, 200, and 300 nmol/l, from lowest to top curves. Concentrations for mismatched polyamide are 0, 100, 200, 600, and 800 nmol/l, and 1, 5, 7.5, and 10 µmol/l, from lowest to top curves. (d) Effect of GB1201 and previously used polyamide GB1203 (ref. 10) on rat Tgf-β1 promoter activity. bp, base pair; PMA, phorbol 12-myristate acetate.
Table 1.
Kinetic constants for polyamide binding and mismatch binding
Inhibiting effect of GB1201 on Tgf-β1 promoter activity
As shown in Figure 1d, phorbol 12-myristate acetate (PMA) (1.0 µmol/l) markedly increased the luciferase activity in HEK (human embryonic kidney) 293 cells that were transfected with rat Tgf-β1 promoter plasmid. GB1201 (0.1, 1, and 10 µmol/l) significantly decreased the luciferase activity stimulated by PMA in a concentration-dependent manner. This inhibition was more efficient than that shown in previous report of GB1203. However, the mismatch polyamide (1.0 µmol/l) did not affect the Tgf-β1 promoter activity.
Distribution of FITC-labeled GB1201 in rats' eyes
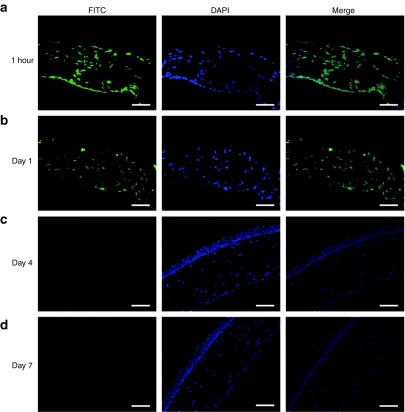
FITC-labeled PI polyamide permits us to trace the distribution and subcellular localization of the small molecule in vivo, as well as providing us with pharmacodynamic information on the use of GB1201 in the eyes. As shown in Figure 2, 1 hour after a single-dose topical administration (Figure 2a), strong fluorescent signals from FITC-labeled GB1201 and DAPI dye were colocalized exclusively to the nuclei of all cell layers of corneal tissues, including the remaining corneal epithelial cells, stromal keratocytes, and endothelial cells, suggesting that GB1201 quickly permeated from the injured corneal epithelial layer into the corneal stromal layer and endothelial layer, and distributed throughout the cell nucleus. A fluorescence signal was also detected in the anterior chamber of the eyes (data not shown). Fluorescence signals remained in the nuclei and stayed above a detectable level in corneal tissues for 24 hours (Figure 2b), but were not detectable on the fourth and seventh days (Figure 2c,d).
Figure 2.
Distribution of FITC-labeled GB1201 in rat cornea by one dose of drug application after alkali exposure. (a) Representative images that were taken for corneas harvested at 1 hour, (b) day 1, (c) day 4, and (d) day 7. FITC: viewed by FITC filter; DAPI: viewed by Hoechst filter. Merge: a merged image of fluorescence signals from both FITC and DAPI dye. Up: epithelial layer side; down: endothelial layer side. Bar = 50 µm.
Corneal re-epithelialization time
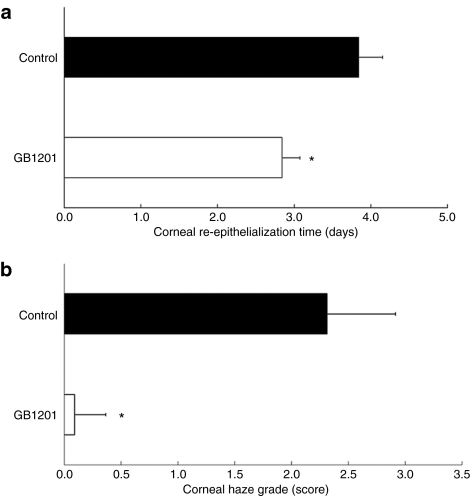
After an alkali burn, the corneal epithelium and basement membrane are disrupted, giving inflammatory mediators as well as microorganisms direct access to the stroma, where they initially contribute to keratocyte apoptosis and necrosis in the anterior stroma. Corneal epithelial defects must be rapidly resurfaced to maintain tissue homeostasis by avoiding microbial infection and further damage to the underlying stroma. In our study, the process of corneal re-epithelialization was significantly promoted after GB1201 treatment. As indicated in Figure 3a, for the GB1201-treated group, corneal re-epithelialization was completed in 6 out of 18 eyes (33%) after 60 hours (2.5 days) and in 15 out of 18 eyes (>83%) after 72 hours on day 3. On day 4, corneal epithelium defects were completely resurfaced in all of the eyes, and no fluorescence staining was detected. However, in the control group, re-epithelialization was delayed by 1–2 days. On day 3, only 1 of the 18 eyes (5%) was resurfaced; on day 4, 3 out of the 18 eyes (17%) still showed defects and, even on day 7, spot fluorescence staining was still detected in some of the eyes.
Figure 3.
Effects of GB1201 on corneal wound healing after alkali exposure. (a) Time required for cornea re-epithelialization in the GB1201-treated and the control groups. Complete re-epithelialization was defined as no fluorescein staining in the injured area. *P < 0.001. (b) Grade of corneal opacity. Corneal haze was graded according to the haze grading system reported by Fantes et al.31 *P < 0.001.
Corneal wound healing and opacity evaluation
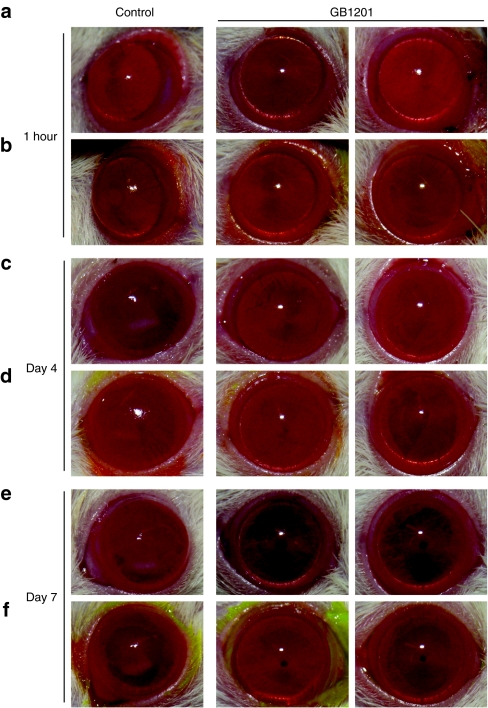
Representative photos in Figure 4 depict the corneal wound healing process in both groups. After an alkali burn, the corneal epithelial layer was largely destroyed and defective in all of the injured eyes. As shown in Figure 4a,b, a large area of corneal epithelium defects was present in all of the eyes after 1 hour of alkali exposure, as detected by fluorescence staining. Epithelial defects with opacity were observed and got worse on days 1 and 2 in both groups (data not shown). On day 3, defects seen in the corneas treated with GB1201 were typically smaller and less frequent than that of the corneas in the control group. On day 4 (Figure 4c,d), epithelial defects were completely resurfaced in the GB1201-treated group, whereas in the control group, the process was delayed, spot fluorescence staining was still observed on day 4, and mild-to-moderate corneal haze was visible in the anterior stromal layer. On day 7 (Figure 4e,f), in the GB1201-treated group, the wounds were completely healed and the corneas were clear again. The gross appearance of the eyes was similar to that of healthy eyes, whereas, in the control group, spot fluorescence staining was still detected and haze remained on the surface of the corneas. The column chart in Figure 3b indicates the grade of corneal opacity evaluated on day 7 in both groups. There were significant differences in mean opacity scores between the GB1201-treated group and the control group. After alkali exposure, the polyamide-treated group showed improved corneal resurfacing and reduced corneal scarring compared with that of the control group.
Figure 4.
Representative photos of cornea during the process of wound healing. Representative photos were taken by digital camera for each group at (a,b) 1 hour, (c,d) day 4, and (e,f) day 7. Fluorescence staining (b,d,f) was performed at the same time in order to give a clear visualization of the corneal epithelium defects. Bar = 50 µm.
Immunohistochemistry
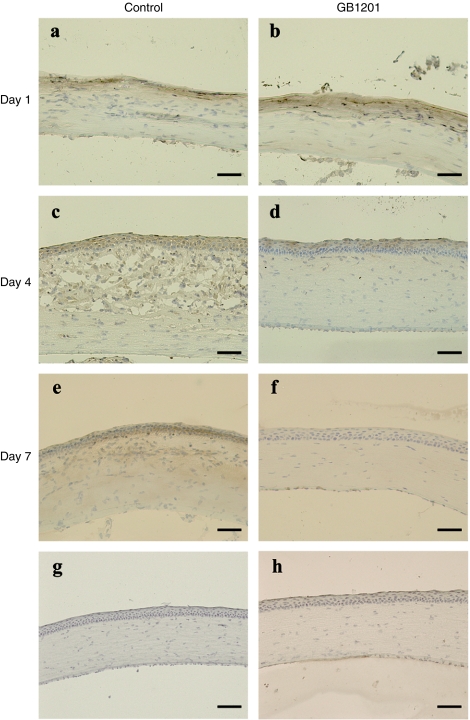
Our immunohistochemistry data showed that Tgf-β1 was faintly expressed in unburned cornea, and the expression was detected only in the epithelial layer (Figure 5g). After an alkali injury, the corneal epithelial layer was disrupted and totally lost. Keratocytes were activated and proliferated to repair the defects. As shown in Figure 5a,b, on day 1, Tgf-β1 was strongly expressed in the activated keratocytes in the anterior stroma. No histological differences were seen in the GB1201-treated group and the control group. On day 4 (Figure 5c,d), strong Tgf-β1 immunoreactivity was detected in both regenerated epithelial cells and proliferated keratocytes in the stromal layer in both groups. GB1201-treated corneas (Figure 5d) exhibit a well-stratified epithelium with less cellularity in the stroma, whereas in corneas in the control group (Figure 5c), the stroma is thicker and swollen with more spindle-shaped Tgf-β1+ keratocytes and infiltrated inflammation cells gathered in the anterior part. On day 7, GB1201-treated corneas (Figure 5f) showed a well-regenerated epithelium and stroma nearly identical to that of a normal cornea. Corneas in the control group (Figure 5e) still showed a significant hypercellularity (presumed to be proliferated keratocytes and transdifferentiated myofibroblasts) and strong Tgf-β1 immunoreactivity in the stromal layer.
Figure 5.
Immunohistochemical analysis of stromal healing and TGF-β1 expression in burned cornea. (a,b) Representative images that were taken for burned corneas harvested at day 1, (c,d) day 4, and (e,f) day 7. (g) Unburned corneas, (h) negative control.
Quantitative real-time RT-PCR assay
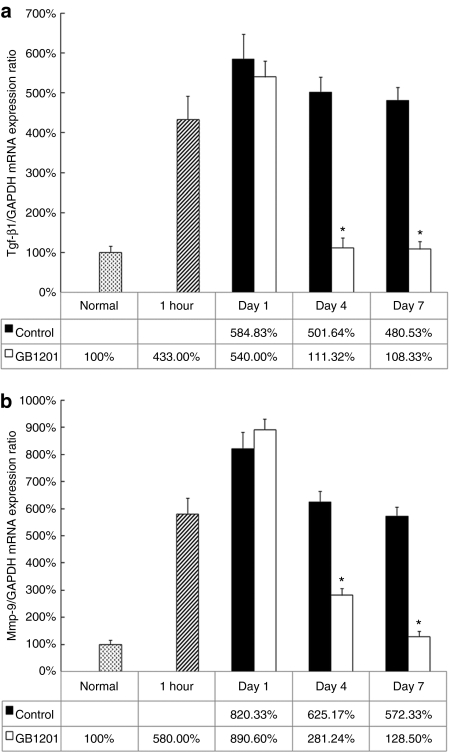
A quantitative real-time RT-PCR was done in order to evaluate the mRNA expression level of the Tgf-β1 gene in the burned cornea after GB1201 application. Our data (Figure 6a) indicated a marked upregulation of Tgf-β1 mRNA after alkali exposure. At 1 hour after injury (before we applied GB1201), the expression level of Tgf-β1 mRNA increased up to fourfold. In both groups, on the 1st day, the expression consistently went up to more than fivefold; no significant difference was found between them. Four days after drug treatment, this upregulation was dramatically suppressed by GB1201, and the expression of Tgf-β1 mRNA fell to almost the same level as that of a normal cornea, whereas it remains at a higher level (almost fivefold) in the control group even at day 7. Meanwhile, we examined the mRNA expression of another secretory protein, Mmp-9, one of the representative downstream regulation targets of the Tgf-β1 gene. As indicated in (Figure 6b), after alkali exposure, the Mmp-9 mRNA level increased up to sixfold at 1 hour and eightfold in the first day. The expression of Mmp-9 mRNA shows marked downregulation on day 4 and decreased to a normal level on day 7 in the GB1201-treated group, while remaining at a higher level in the control group.
Figure 6.
Effects of GB1201 on the expression of Tgf-β1 mRNA and Mmp-9 mRNA in rat corneas after alkali exposure. (a) Expression of Tgf-β1 mRNA. (b) Expression of Mmp-9 mRNA. The expression level of target genes was normalized by that of the GAPDH housekeeping gene. *P < 0.001.
Discussion
The recovery of an intact and phenotypically normal cornea is the most important determinant of a favorable outcome following alkali injury. Aggressive medical management to facilitate corneal re-epithelialization and reduce scar formation is especially important in grade 2 and grade 3 corneal alkali injuries where the major concern is delayed re-epithelialization, stromal haze formation, and secondary complications.3 Corneal haze is a mild, diffuse, homogeneous white opacity in the anterior stroma of cornea. Mild haze can lead to decreased visual acuity, glare, halos, and night vision problems. Severe haze can dramatically impair visual acuity once localized near the visual axis and may have to be modified by corneal transplantation.
Corneal stromal scarring and opacification are believed to be dependent, in part, on upregulation of Tgf-β1, causing stromal cells to express extracellular matrix components qualitatively and quantitatively different from those found in uninjured stroma.5,6,7 In the present study, we produced a grade 2 corneal alkali burn model in rats and evaluated corneal epithelial healing, stromal repair, expression of Tgf-β1 mRNA and protein, and expression of Mmp-9 mRNA. Our preliminary data suggest that GB1201 could effectively regulate Tgf-β1 gene expression, prevent tissue destruction, facilitate the rate of corneal re-epithelialization and improve the healing of injured corneal tissue, as well as reducing the frequency of corneal haze formation. Corneas in the control group exhibit delayed corneal re-epithelialization, stromal edema, hypercellularity, and a mild-to-moderate grade of haze formation. In contrast, burned cornea treated with GB1201 was resurfaced with a cornea-like well-stratified epithelium, healed with undetectable or minimal scarring, and restored to transparency by day 7. In our study, Tgf-β1-targetting PI polyamide also demonstrated regulation of other downstream cytokines targeted by Mmp-9, which is also overexpressed after corneal injury and is a target for corneal injury therapy,12,13,14,15 as indicated by the quantitative real-time RT-PCR assay. This, in part, contributed to the improved corneal re-epithelialization, which was delayed by persistent inflammation and degradation of the corneal epithelial basement membrane, the scaffold for regenerated epithelial cells.
Recent studies indicate a favorable outcome in mice corneal alkali burn models by altering the Smad7/Tgf-β signal pathway with adenovirus expression vectors.5 Adenoviral vectors are commonly used for gene therapy with certain advantages, such as high transduction efficiency and high viral titers, and infect both replicating and differentiated cells. However, researchers are facing the challenges associated with their inherent limitations, such as short-term expression, which requires repeated infection, vector-mediated immunogenicity, tissue-specific targeting, etc. PI polyamides are cell-permeable small molecules; the cellular permeability depends to a large extent on the size and linker used for polyamide design.16 In the current study, the in vivo distribution data demonstrated a high level of specific nuclear uptake of GB1201 in corneal tissues. FITC-labeled GB1201 was distributed immediately and sufficiently in the nuclei of corneal epithelial cells, keratocytes, and endothelial cells, and was localized in the nuclei for 24 hours without any delivery system, allowing one-drop once-daily treatment. Although fixation of cells could potentially compromise the integrity of the cellular membrane and allow increased polyamide traffic, it would not be representative of the permeability in live cells and of exclusive nucleus localization. Besides a cellular permeability, PI polyamides are completely resistant to nucleases; this is another marked advantage compared to other nucleic acid reagents, such as antisense DNA, ribozymes, siRNA, and decoys, which have a problem with stability. A drug-delivery system is also required to distribute the target sequence of living cells sufficiently for binding.17 Based on this, PI polyamides show more potential as a gene regulation agent for the treatment of corneal injury.
Based on the architecture of natural products such as netropsin, distamycin A, and duocarmycin A, 18,19 they are capable of distinguishing and binding to all Watson–Crick base pairs in the DNA minor groove with a high affinity and specificity comparable to that of naturally occurring DNA-binding proteins.9,18,20,21 PI polyamides have been the subject of intense study, along with many other classes of minor groove binders.9 Sequence-specific DNA recognition depends on side-by-side aromatic amino-acid pairings in the minor groove. The antiparallel pairing of imidazole (Im) opposite pyrrole (Py) recognizes a G·C base pair from C·G A·T and T·A, whereas a Py/Im combination distinguishes C·G from G·C, T·A and A·T. A Py/Py pair is degenerate and recognizes either an A·T or T·A base pair. These pairing rules have proven useful for programmed recognition of a broad repertoire of DNA sequences,9,18,21 especially for the binding sequence of transcription factors. The initiation of transcription requires the binding of transcription factors to the cognate DNA response elements in the gene promoter. Interfering with protein–DNA interfaces may block the binding of transcription factors, consequently inhibiting the initiation of gene transcription and modulating target gene induction. It has been revealed that PI polyamides targeting transcription factors were able to effectively interfere with protein–DNA interfaces and regulate target gene expression.9
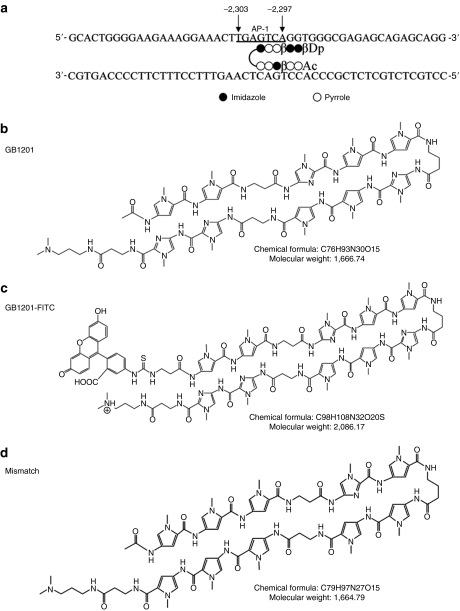
To design a polyamide that targets rat Tgf-β1, we analyzed the rat Tgf-β1 promoter structure and PMA-stimulated activity in promoter deletion mutants. Positive regulatory elements that were stimulated by PMA were found at bases −2,424 to −2,153, which contain an AP-1 binding site.10
AP-1 elements respond to AP-1 transcription factors such as Jun homodimers or Fos/Jun heterodimers, PMA, angiotensin II, and v-Src. Stimulation of Tgf-β1 promoter activity occurs by binding to the AP-1 element.10,22,23 The polyamide GB1201, targeting the Tgf-β1 promoter, showed specific binding to the target DNA in gel mobility shift and Biacore assays. GB1201 showed better Tgf-β1 promoter activity inhibition than that of GB1203 after PMA stimulation. GB1201 was designed not to cover AP-1 consensus sequences but spans the 3′ boundary of AP-1 binding site, allowing for a specific binding to the Tgf-β1 promoter, whereas GB1203 spans the 5′ boundary. Besides, GB1201 has a “PyPy-β-ImPyPy-γ-ImPyPy-β-ImIm-βDp” (3-β-2) structure. According to the ribbon model, β-alanine residue can relax the ligand curvature of the double-stranded DNA spiral structure, providing for optimal hydrogen bond formation between the floor of the minor groove, and both Im and Py residues within the Py-β-Im polyamide subunit.24 In our in vivo experiments, fluorescein-labeled GB1201 exhibited a good nuclear uptake in the corneal tissues without any delivery systems and localized in the nuclei of corneal cells for 24 hours, allowing for one-application-per-day drug treatment. Our data showed topical administration of GB1201 once a day was sufficient to improve corneal healing and reduce stromal scarring. The pharmacodynamic properties of PI polyamides show more dramatic advantages than other reported agents, such as GM600125,26 and TIMP-1 (ref. 27), and synthetic MMP inhibitors, which need to be applied by subconjunctival injection or topically applied every 2 hours, respectively, in order to maintain an effective concentration in the eyes.
Our data lead us to suggest that PI polyamide may open new opportunities for therapeutic intervention in the treatment of chemically burned corneas, as well as giving new hope for the treatment of other corneal diseases with aberrant TGF-β1 gene expression. Among them, one representative case in ophthalmology is the potential application in the prevention and treatment of corneal haze after refractive surgery: PRK (photorefractive keratectomy) and laser-assisted in situ keratomileusis. Clinically significant corneal haze after PRK has been noted in 2–4% of eyes, and much higher percentages have been reported after high myopic corrections have been done. The pathogenesis of haze after PRK and alkali burn is almost similar; the only difference is that in the case of PRK, the epithelium and basement membrane are surgically disrupted.
Materials and Methods
Synthesis of PI polyamides (GB1201) targeting rat Tgf-β1 gene promoter. GB1201 was designed to span the 3′ boundary (−2,300 to −2,295) of the AP-1 binding site (−2,303 to −2,297) of the Tgf-β1 gene promoter (Figure 7a,b). The number refers to the start of the open reading frame as +1 (ref. 28). Detailed information on polyamide design was given in our previous report.10 FITC-labeled GB1201 (Figure 7c) was synthesized to trace the in vivo distribution and subcellular localization of the small compound in rats' eyes. Mismatched polyamide (Figure 7d) was designed to have almost the same composition of pyrrole and imidazole rings, but targeted to a different DNA sequence and not able to bind to the AP-1 site. All of the PI polyamides were synthesized with a machine-assisted automatic synthesis system, the PSSM-8 Peptide Synthesizer (Shimadzu, Kyoto, Japan), according to previously established methods.24,29,30 PI polyamides were dissolved in a 0.01% acetic acid solution at a concentration of 1 mmol/l as a stock and diluted to 1:1,000 to a final concentration of 1 µmol/l as a working solution.
Figure 7.
Target sequence and chemical structure of pyrrole–imidazole polyamides targeting rat Tgf-β1 gene promoter. (a) GB1201 was designed to span the 3′ boundary (−2,300 to −2,295) of the AP-1 binding site (−2,303 to −2,297) of the Tgf-β1 gene promoter. Number refers to the start of the open reading frame as +1. (b) Structure of the match polyamide, GB1201. (c) Structure of the FITC-labeled GB1201. (d) Structure of the mismatch polyamide used in this study.
DNA-binding assays. Fluorescein-labeled match-oligos corresponding to the Tgf-β1 promoter, including the AP-1 binding site, were synthesized for gel mobility shift assays. The appropriate sense and antisense oligo-DNA were annealed for preparing double-stranded oligo. 1 µmol/l of DNA was incubated with 50 µmol/l polyamide for 1 hour at 37 °C, and then was separated by electrophoresis on 20% polyacrylamide gel and visualized by a luminescent image analyzer LAS-3000 (Fujifilm, Tokyo, Japan). A Biacore assay was performed to measure the real-time binding affinity of GB1201 to target DNA. Biotin-labeled oligos were annealed for a double strand and were immobilized to a streptavidin-functionalized sensor chip SA (Biacore, Uppsala, Sweden). The kinetics of interaction between polyamide, mismatched, and biotin-labeled double-stranded oligos were measured using a Biacore 2000 system (Biacore). Data processing was performed on a Biacore 2000 system according to recommended protocols.
Inhibition effect of GB1201 on Tgf-β1 promoter activity. HEK 293 cells were seeded onto 24-well plates and grown in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO) with 20% calf serum. Rat Tgf-β1 promoter was cloned into the KpnI site of the pGL3-basic vector. For evaluation of the effects of polyamide on promoter activity, rat Tgf-β1 promoter plasmid with luciferase was transfected into HEK 293 cells, then the cells were incubated with 0.1, 1.0, or 10.0 µmol/l GB1201, previously used polyamide GB1203 (ref. 10) or 1.0 µmol/l mismatched polyamide in the presence of 1.0 µmol/l PMA for use as a stimulator for Tgf-β1 promoter activity for 24 hours. Promoter activity was then measured with a dual-luciferase reporter assay system, as previously described.10,11
Animals and the corneal alkali burn model. This study was approved by the Nihon University School of Medicine Institutional Review Board and Institutional Animal Care and Use Committee. Eight-week-old adult male rats (Wistar strain) were used in this study. The rats were anesthetized with diethyl ether. An alkali burn was produced in both eyes by placing a drop of 5 µl 0.5 N NaOH solution in the center of the cornea. After a 10-second alkali exposure, the ocular surface was irrigated with 10 ml phosphate-buffered saline. One hour after the alkali burn, 5 µl of 1 µmol/l GB1201 solution was applied to the right eye, and 0.01% acetic acid mock solution was applied to the left eye as a negative control in the experiment. The drugs were administrated at 9 AM every day, and this was continued for 7 days. In order to reduce the risk of bacterial infection, an antibiotic ointment (0.3% Tarivid; Santen Pharmaceutical, Osaka, Japan) was applied every night. Eighteen eyes were used in each group. Six rats with unburned cornea were used as a normal control. The animals were examined every day until they were killed.
Distribution of FITC-labeled GB1201 in rats' eyes. In order to check the distribution and subcellular localization of GB1201 in alkali-burned eyes, we topically applied 5 µl 1 µmol/l FITC-labeled GB1201 1 hour after the alkali burn. Polyamide was applied only once, and then the rats were killed at 1 hour, day 1, day 4, and day 7 after drug application. The eyeballs were enucleated immediately with scissors and forceps, and put into a plastic mold, and then they were immediately embedded in OCT compound (Miles, Elkhart, IN) and frozen in liquid nitrogen. All samples were kept at −80 °C until analysis. Cyrosections, 5 µm in thickness, were cut and fixed in 4% formalin for 30 minutes. Cell nuclei were counterstained by incubating with DAPI/Hoechst solution, and the sections were examined with a fluorescence inverted microscope (Axiovert 200M inverted microscope; Carl Zeiss, Jena, Germany) with appropriate filters (FITC filter: excitation 460–500 nm, emission 510–560 nm; DAPI/Hoechst filter: excitation 325–375 nm, emission 435–485 nm).
Corneal re-epithelialization time. The eyes were examined by microscope every morning just before drug application. The time required to complete re-epithelialization was recorded for each group. Fluorescence staining allows an easy visualization of the corneal epithelium defects. Complete re-epithelialization was defined as no fluorescein staining in the injured area.
Corneal opacity evaluation. The extent of corneal haze was evaluated using a slit-lamp biomicroscope and the grading system of Fantes et al.31 Grade 0, a totally clear cornea such that no opacity could be seen by any method of slit-lamp microscopic examination; grade 0.5, a trace or a faint haze seen only by indirect broad tangential illumination; grade 1, haze of minimal density seen with difficulty with direct and diffuse illumination; grade 2, a mild haze easily visible with direct focal slit-illumination; grade 3, a moderately dense opacity that partially obscured the iris details; and grade 4, a severe dense opacity that completely obscured the details of intraocular structures. During the corneal tissue wound healing process, representative photos were taken by digital camera for each group at 1 hour, day 1, day 4, and day 7.
Immunohistochemical examination. At 1 hour and then at 1, 4, and 7 days after the corneal burn, the eyeballs were enucleated and embedded in OCT compound. Cryosections, 5 µm in thickness, were cut and processed for histological and immunohistochemical analysis. Rabbit–antihuman Tgf-β1 polyclonal antibody (cat. no. Y241; Yanaihara Institute, Fujinomiya, Shizuoka, Japan) was used as the primary antibody, with a dilution of 1:1,000. Four unburned corneas were used as the normal control for comparing the amount of healing between GB1201-treated eyes and the mock solution–treated eyes. A negative control was also included in order to determine the specificity of the primary antibody. All immunohistochemical analysis was done by technical specialists at Kyodo Byori, Kobe, Hyogo, Japan.
Real-time quantitative RT-PCR assay. One hour and 1, 4, and 7 days after the corneal alkali burn, the corneas, 5 mm in diameter in the PI polyamide-treated group and in the control group, were carefully excised under biomicroscope. Four unburned corneas were also excised as a normal control. The total cellular RNA was isolated using an RNeasy Mini Kit (Qiagen Science, Germantown, MD), and then 500 ng RNA was reverse-transcribed using the PrimeScript RT reagent Kit (Takara Bio, Ohtsu, Shiga, Japan), according to the manufacturer's instructions. Then gene expression was analyzed by real-time quantitative PCR assay (Thermal Cycler Dice Real Time System; Takara Bio), using SYBR Premix Ex Taq (Takara Bio). The expression level of the GAPDH gene was used as the internal control to normalize for differences in complementary DNA input. Primers for RT-PCR were designed to span two adjacent exons separated by an intron so as to easily detect the existence of possible contamination of the genomic DNA. All primers used in this study and corresponding primer sequences are listed in Supplementary Table S1.
Statistical analysis. All values are reported as mean ± SD. Statistical analysis was done with the Student's t-test for unpaired data. P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIALTable S1. Primer sets for real-time RT-PCR.
Supplementary Material
Primer sets for real-time RT-PCR.
Acknowledgments
We thank Motoaki Kataba and Yuki Yamada for technical support. This work is supported by the Academic Frontier Project for 2006 Project for Private Universities, a matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology (MEXT) to H.N., the National Institute of Environmental Health Services to H.N. (ES012249-01), the National Cancer Institute Center support grant CA16056 (to Roswell Park Cancer Institute) and Monbukagakusho:MEXT Scholarship to M.C., kindly provided by the Japanese government.
REFERENCES
- Peate WF. Work-related eye injuries and illnesses. Am Fam Physician. 2007;75:1017–1022. [PubMed] [Google Scholar]
- McGwin G, Jr, Xie A., and , Owsley C. Rate of eye injury in the United States. Arch Ophthalmol. 2005;123:970–976. doi: 10.1001/archopht.123.7.970. [DOI] [PubMed] [Google Scholar]
- Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- Saika S.2004TGF-beta signal transduction in corneal wound healing as a therapeutic target Cornea 238 suppl.): S25–S30. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, et al. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol. 2005;166:1405–1418. doi: 10.1016/S0002-9440(10)62358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AE, Guo X, Chung EH, Joyce NC. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001;42:1465–1471. [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, et al. 1999The cellular basis of corneal transparency: evidence for ‘corneal crystallins' J Cell Sci 112Pt 5): 613–622. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Igarashi T, Fujimoto C, Ozaki N., and , Ishizaki M. Immunohistochemical observation of amniotic membrane patching on a corneal alkali burn in vivo. Jpn J Ophthalmol. 2007;51:3–9. doi: 10.1007/s10384-006-0389-y. [DOI] [PubMed] [Google Scholar]
- Dervan PB, Doss RM., and , Marques MA. Programmable DNA binding oligomers for control of transcription. Curr Med Chem Anticancer Agents. 2005;5:373–387. doi: 10.2174/1568011054222346. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Fukuda N, Ueno T, Tahira Y, Ayame H, Zhang W, et al. Development of gene silencing pyrrole-imidazole polyamide targeting the TGF-beta1 promoter for treatment of progressive renal diseases. J Am Soc Nephrol. 2006;17:422–432. doi: 10.1681/ASN.2005060650. [DOI] [PubMed] [Google Scholar]
- Yao EH, Fukuda N, Ueno T, Matsuda H, Nagase H, Matsumoto Y, et al. A pyrrole-imidazole polyamide targeting transforming growth factor-beta1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc Res. 2009;81:797–804. doi: 10.1093/cvr/cvn355. [DOI] [PubMed] [Google Scholar]
- Sakimoto T, Shoji J., and , Sawa M. Active form of gelatinases in tear fluid in patients with corneal ulcer or ocular burn. Jpn J Ophthalmol. 2003;47:423–426. doi: 10.1016/s0021-5155(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT., and , Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–679. [PubMed] [Google Scholar]
- Fini ME, Cook JR., and , Mohan R.1998Proteolytic mechanisms in corneal ulceration and repair Arch Dermatol Res 290suppl.): S12–S23. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Girard MT, Kublin CL, Cintron C., and , Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991;147:425–439. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- Liu B., and , Kodadek T. Investigation of the relative cellular permeability of DNA-binding pyrrole-imidazole polyamides. J Med Chem. 2009;52:4604–4612. doi: 10.1021/jm9002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best TP, Edelson BS, Nickols NG., and , Dervan PB. Nuclear localization of pyrrole-imidazole polyamide-fluorescein conjugates in cell culture. Proc Natl Acad Sci USA. 2003;100:12063–12068. doi: 10.1073/pnas.2035074100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauger JW, Baird EE., and , Dervan PB. Recognition of DNA by designed ligands at subnanomolar concentrations. Nature. 1996;382:559–561. doi: 10.1038/382559a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Lian C, Isomura M, Saito I., and , Wang AH. Distamycin A modulates the sequence specificity of DNA alkylation by duocarmycin A. Proc Natl Acad Sci USA. 1996;93:14405–14410. doi: 10.1073/pnas.93.25.14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcamone F, Penco S, Orezzi P, Nicolella V., and , Pirelli A. Structure and synthesis of distamycin A. Nature. 1964;203:1064–1065. doi: 10.1038/2031064a0. [DOI] [PubMed] [Google Scholar]
- White S, Baird EE., and , Dervan PB. On the pairing rules for recognition in the minor groove of DNA by pyrrole-imidazole polyamides. Chem Biol. 1997;4:569–578. doi: 10.1016/s1074-5521(97)90243-x. [DOI] [PubMed] [Google Scholar]
- Pertovaara L, Sistonen L, Bos TJ, Vogt PK, Keski-Oja J., and , Alitalo K. Enhanced jun gene expression is an early genomic response to transforming growth factor beta stimulation. Mol Cell Biol. 1989;9:1255–1262. doi: 10.1128/mcb.9.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989;264:19373–19378. [PubMed] [Google Scholar]
- Turner JM, Swalley SE, Baird EE., and , Dervan PB. Aliphatic/aromatic amino acid pairings for polyamide recognition in the minor groove of DNA. J Am Chem Soc. 1998;120:6219–6226. [Google Scholar]
- Schultz GS, Strelow S, Stern GA, Chegini N, Grant MB, Galardy RE, et al. Treatment of alkali-injured rabbit corneas with a synthetic inhibitor of matrix metalloproteinases. Invest Ophthalmol Vis Sci. 1992;33:3325–3331. [PubMed] [Google Scholar]
- Kato T, Saika S., and , Ohnishi Y. Effects of the matrix metalloproteinase inhibitor GM6001 on the destruction and alteration of epithelial basement membrane during the healing of post-alkali burn in rabbit cornea. Jpn J Ophthalmol. 2006;50:90–95. doi: 10.1007/s10384-005-0287-8. [DOI] [PubMed] [Google Scholar]
- Paterson CA, Wells JG, Koklitis PA, Higgs GA., and , Docherty AJ. Recombinant tissue inhibitor of metalloproteinases type 1 suppresses alkali-burn-induced corneal ulceration in rabbits. Invest Ophthalmol Vis Sci. 1994;35:677–684. [PubMed] [Google Scholar]
- Yang Y, Mumy M, Romeo D., and , Wakefield LM. Identification of the start sites for the 1.9- and 1.4-kb rat transforming growth factor-beta1 transcripts and their effect on translational efficiency. Gene. 1998;219:81–89. doi: 10.1016/s0378-1119(98)00402-8. [DOI] [PubMed] [Google Scholar]
- Bando T, Narita A, Saito I., and , Sugiyama H. Molecular design of a pyrrole-imidazole hairpin polyamides for effective DNA alkylation. Chemistry. 2002;8:4781–4790. doi: 10.1002/1521-3765(20021018)8:20<4781::AID-CHEM4781>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Murty MS., and , Sugiyama H. Biology of N-methylpyrrole-N-methylimidazole hairpin polyamide. Biol Pharm Bull. 2004;27:468–474. doi: 10.1248/bpb.27.468. [DOI] [PubMed] [Google Scholar]
- Fantes FE, Hanna KD, Waring GO, 3rd, Pouliquen Y, Thompson KP., and , Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sets for real-time RT-PCR.