Abstract
Objective
To examine the association between exposure to endotoxins and lung cancer risk by conducting a systematic review and meta-analysis of epidemiologic studies of workers in the cotton textile and agricultural industries; industries known for high exposure levels of endotoxins.
Methods
Risk estimates were extracted from studies published before 2009 that met predefined quality criteria, including 8 cohort, 1 case–cohort, and 2 case–control studies of cotton textile industry workers, and 15 cohort and 2 case–control studies of agricultural workers. Summary risk estimates were calculated using random effects meta-analyses. Potential sources of heterogeneity were explored through subgroup analyses.
Results
The summary risk of lung cancer was 0.72 (95% CI, 0.57–0.90) for textile workers and 0.62 (0.52–0.75) for agricultural workers. The relative risk of lung cancer was below 1.0 for most subgroups defined according to sex, study design, outcome, smoking adjustment, and geographic area. Two studies provided quantitative estimates of endotoxin exposure and both studies tended to support a dose–dependent protective effect of endotoxins on lung cancer risk.
Conclusion
Despite several limitations, this meta-analysis based on high-quality studies adds weight to the hypothesis that occupational exposure to endotoxin in cotton textile production and agriculture is protective against lung cancer.
Keywords: Endotoxins, Textile industry, Farmers, Lung cancer, Meta-analysis
Introduction
Reduced rates of lung cancer have been observed in several occupational groups who are exposed to high levels of organic dusts [1–3]. This reduced risk was initially attributed to inadequate adjustment for risk factors such as tobacco smoking [4, 5]. However, in 1973, Henderson and Enterline [1] proposed that endotoxins contaminating the dust inhaled by cotton textile workers might be responsible for the observed protective effect. Mastrangelo et al. [2] extended this proposition to dairy farmers as an explanation of their low mortality from lung cancer.
Endotoxin is a component of the outer membrane of Gram-negative bacteria and is released during replication and cell lysis. It is ubiquitous in indoor and outdoor environments. The highest endotoxin exposures have been measured in agricultural environments and certain occupational settings such as cotton textile mills [6]. Exposure primarily occurs through inhalation of airborne endotoxins present in organic dusts (also termed bioaerosols). Purified endotoxin is referred to as lipopolysaccharide (LPS), and the lipid-A portion, which is highly conserved across endotoxins originating from diverse bacterial species, is the biologically active component of endotoxins. Experimental studies in animals and a few trials in humans have shown that endotoxins can inhibit tumor initiation and growth and that endotoxins (or LPS) stimulate the production of endogenous antineoplastic mediators [7–9]. It has been suggested that immunomodulation is the primary anti-cancer mechanism, although evidence for mechanistic pathways is limited [10–12].
Previous meta-analyses found reduced lung cancer risks in studies of workers in the textile industry published before 1991 [13] and 2000 [3]. Several broad-based reviews have reported on cancer risks in farmers in the 1990s [14–16]. Unfortunately, few studies which investigated the association between lung cancer and employment in these industries included endotoxin or dust exposure measurements. This lack of exposure estimates hinders the ability to conclusively state that endotoxin is related to a decreased risk of lung cancer. We endeavored to examine more thoroughly the evidence on the relationship between occupational exposure to endotoxins and lung cancer risk, and to identify possible sources of heterogeneity in the relationship. We conducted a systematic review and selected high quality articles based on a priori set criteria for the meta-analysis. We focused on two industries which have been shown to involve high levels of endotoxin exposure—cotton textile manufacture and agriculture—for which several large scale studies had been published since the previously published meta-analyses.
Methods
Search strategy
We limited our review to studies of textile industry and agricultural workers due to the relatively low exposure levels in other occupational groups potentially exposed to endotoxins like waste-collection and treatment workers [17], veterinarians, and workers in paper factories [18], and due to the limited number of publications investigating risks of lung cancer in other occupational groups. We further narrowed our selection to cotton textile mill workers as there are few studies on lung cancer among workers in mills of other natural textile fibers (production of synthetic textiles entails negligible endotoxin exposures).
We searched the Medline and PubMed databases for all relevant articles published up to the end of 2008 using various combinations of the keywords endotoxin, farmers, agriculture, textile, cotton, lung and cancer. In addition, we scrutinized the reference lists of identified papers for additional relevant publications. If multiple articles were published on the same cohort, we included the most recent publication (n = 5 superseded publications [19–24]). We limited the retrieval to articles from English language peer-reviewed journals.
Criteria for inclusion and exclusion
Two investigators (I.B., V.L.) extracted information on the type of exposure assessment or classification used in the analysis; the subjects’ inclusion criteria; the comparison/control group; the statistical methods used; and the confounders considered in the analysis. We initially considered the quality components of the Newcastle–Ottawa Scale [25], a scale designed to assess non-randomized studies for meta-analyses. The scale evaluates selection of the cohort, exposure assessment, comparability of exposed and non-exposed cohorts, assessment of outcome, and adequacy of follow-up. We supplemented the quality assessment criteria based on the framework presented by Vlaanderen et al. [26], which specifically focuses on the quality of exposure assessment applied in human observational studies.
Proportionate mortality studies (PMR) were excluded (n = 12 [27–38]) as their results depend on the proportions of death from different causes [39]. Case–control studies that used cancer or respiratory disease patients as comparison groups were also excluded (n = 10 [40–49]) as such comparison is potentially biased [50]. Finally, studies that insufficiently described subject selection and statistical procedures were excluded (n = 1 [51]).
Data extraction
The risk estimates derived from the model including the largest number of covariates and their associated 95% confidence intervals (CI) were extracted. Among the studies included in the meta-analysis, risk estimates were expressed as standardized mortality ratios (SMR), standardized incidence ratios (SIR), relative risks (RR), and hazard ratios (HR) in the cohort and case–cohort studies, and as odds ratios (OR) in the case–control studies. If a study reported multiple estimates for independent subpopulations, for instance according to job classification, duration of exposure categories, or gender, these were reported in “Appendix 1”. If a cohort study did not report an overall risk estimate, estimates were combined into one risk estimate using the inverse of the variance as weight (Table 1). If subject groups overlapped within a study, the most inclusive estimate was used. Sex-specific estimates were also extracted. A 95% CI was calculated using Fisher’s exact method for those studies that did not report a CI [52]. Standard errors (SE) were derived from 95% CIs by taking the natural logs of the risk ratio and limits of the CI, calculating the CI legs, and applying the z-distribution standard deviate to the averaged CI legs.
Table 1.
Description of studies included in the meta-analysis: cotton textile workers
| Reference | Study design | Location | No. of cases | Study population | Follow-up | Source of controls | Variables included in adjustment | Exposure assessment | Effect estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Henderson [1] | C | Georgia, USA |
C1: 36.5 C2: 26.4 |
C1: 5822 C2: 6242 |
C1: 1938–1963 C2: 1948–1963 |
General white male population of Georgia | A, S, race | Industry, duration |
C1: M: SMR, 0.548 (0.385–0.757) C2: M: SMR, 0.303 (0.199–0.443) O: 0.43 (0.33–0.56) |
| Merchant [60] | C | North Carolina, USA | 18 |
M: 1,113 F: 393 |
1940–1975 | US general population | A, S, race | Job categories, duration |
M: SMR, 0.74 (0.44–1.17) F: (not reported) |
| Koskela [61] | C | Finland | 3 | F: 1,065 | 1950–1985 | General female population of Finland | A, S | Industry | F: SMR, 1.58 (0.32–4.47) |
| Hodgson [66] | C | UK | 42 | M/F: 3,458 | 1968–1984 | General population of England and Wales | A, S | Industry, duration |
M: SMR, 0.75 (0.51–1.06) F: SMR, 0.79 (0.39–1.41) O: 0.76 (0.55–1.04) |
| Szeszenia-Dabrowska [62] | C | Lodz, Poland | 140 |
M: 2,852 F: 4,693 |
1964–1993 | General population of Poland | A, S | Industry, department, duration |
M: SMR, 0.89 (0.71–1.10) F: SMR, 0.55 (0.28–0.96) O: 0.84 (0.69–1.04) |
| Fritschi [63] | C | Australia | F: 2 | M/F: 7,679 | 1982–1997 | General population of Australia | A, S | Industry | F: SIR, 1.06 (0.12–3.81) |
| Kuzmickiene [64] | C | Lithuania |
M: 70 F: 15 |
M: 5,495 F: 9,155 |
1978–2002 | General population of Lithuania | A, S |
Industry, department, duration, estimated dust concentrations |
M: SIR, 0.94 (0.73–1.19) F: SIR, 1.36 (0.76–2.25) O: 1.00 (0.80–1.25) |
| Mastrangelo [65] | C | Italy | 36 | M/F: 3,961 | 1970–1994 | Regional population, Veneto | A, S | Industry, duration | M/F: SMR, 1.03 (0.72–1.43) |
| Astrakianakis [67] | CCh | Shanghai, China | 628 | F: 3,188 | 1989–1998 | Randomly selected from the cohort population (267,400) | A, S, Sm | Duration, quintiles, and quartiles of exposure based on cumulative exposure derived from modelled cotton dust exposure estimates that were converted into endotoxin levels using previously measured job-specific endotoxin estimates | F: HR, 0.70 (0.52–0.95) |
| Levin [68] | CC | China | 169 | M: 128 | – | All males aged 35–64 from Shanghai, China | A, S, Sm | Industry, duration, tasks |
M: OR, 0.7 (0.5–0.7) F: OR, 0.8 (0.6–1.0) M/F: OR, 0.7 (0.6–0.9) |
| Wu-Williams [69] | CC | China | 31 | F: 44 | – | All females aged 29–70 from Shenyang and Harbin, China |
A, S, Sm, study area, education |
Industry, occupation category, duration | F: OR, 0.4 (0.7–1.1) |
Bold font denotes that the number of cases, the 95% CI, or the combined (overall) estimate was calculated from published data
C cohort study, CCh case–cohort study, CC case–control study, M males, F females, RR relative risk, SMR standardized mortality ratio, SIR standardized incidence ratio, HR hazard ratio, OR odds ratio, O overall estimate, A age, S sex, Sm smoking
Statistical analysis
Statistical analyses were performed with the “metan” and “metabias” commands in STATA, version 10.1 (STATA Corporation, College Station, TX). The data entered into this statistical package were the natural log-transformed risk ratios and associated SEs. The coefficient of inconsistency (I 2) was applied to assess heterogeneity between studies [53]. I 2 is an estimate of the percentage of total variation in study estimates due to heterogeneity rather than chance, and is considered substantial if it exceeds 50%. We deemed the random effects assumptions to be most appropriate for this set of heterogeneous studies, and therefore present only these results. Random effects models [54] were applied to calculate the (reverse transformed) pooled risk ratios and associated 95% CIs, and z scores of all studies in each occupational group; textile and agricultural.
We performed subgroup analyses to assess whether any observed between-study heterogeneity was due to study characteristics or differences in quality. Heterogeneity between subgroups was tested using the PROC MIXED procedure with maximum likelihood estimation, SAS 9.1 (SAS Institute Inc., Cary, NC), and was considered significant if the p value of the type 3 F-test was below 0.1. We refrained from assigning and weighting studies based on quality scores, as this can produce biased pooled estimates [55]. The subgroups defined a priori were, newer versus older studies, study design, mortality versus morbidity outcome, adjustment for smoking, geographic area, and sex. Too few studies stratified results by job title within the cotton industry or by farm type to perform subgroup analyses for these variables.
Publication bias was assessed by constructing funnel plots of the log risk ratio versus the SE of the log risk ratio [56], although we acknowledge the limitations of this method [57]. To supplement the funnel plot approach, the adjusted rank correlation method suggested by Begg and Mazumdar [58] and the regression asymmetry test proposed by Egger et al. [59] were applied.
Results
Characteristics of studies analyzed
Eight cohort [1, 60–66], 1 case–cohort [67], and 2 case–control [68, 69] studies in cotton textile industry workers, along with 15 cohort [2, 70–83] and 2 case–control [84, 85] studies in agricultural populations fulfilled the quality assessment criteria and were included in the meta-analysis. Tables 1 and 2 summarize characteristics of the studies included in the present meta-analysis; characteristics of all studies considered for inclusion, and application of the inclusion criteria are further elaborated in the “Appendices 1 and 2”, respectively.
Table 2.
Description of studies included in the meta-analysis: agriculture industry workers
| Reference | Study design | Location | No. of cases | Study population | Follow-up | Source of controls | Variables included in adjustment | Exposure assessment | Effect estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Burmeister [70] | C | Iowa, USA | 1,466 | M: 21,101 | 1971–1978 | White Iowa male population of Iowa | A, S, race | Occupation | M: SMR, 0.84 (0.80–0.88) |
| Wiklund [73] | C | Sweden | 1,155 | M: 254,417 | 1961–1979 | The 1,725,845 men working in other than farming occupations | A, S | Occupation | M: RR, 0.36 (0.34–0.38) |
| Stark 79] | C | NY, USA | 103 | M: 18,811 | 1973–1983 | 747,128 men from the rural areas of the NY state aged >25 | A, S | Occupation | M: SIR, 0.524 (0.428–0.636) |
| Gunnarsdottir [81] | C | Iceland | 20 | M: 5,922 | 1977–1987 | General male population of Iceland | A, S | Occupation | M: SIR, 0.41 (0.27–0.59) |
| Alberghini [74] | C | Italy | 65 | M: 4,580 | 1974–1987 | The regional general male population | A, S | Occupation | M: SMR, 0.68 (0.52–0.87) |
| Ronco [71] | C | Denmark | 810 | – | 1970–1980 | All persons economically active in 1970 | A, S | Occupation |
M: SMR, 0.45 (0.42–0.48)* F: SMR, 0.45 (0.34–0.57)* O: 0.45 (0.42–0.48) |
| Faustini [83] | C | Aprilia, Italy | 42 |
M: 1,701 F: 426 |
1970–1980 | General population of Italy | A, S | Occupation |
M: SMR, 1.02 (0.73–1.38) F: (only one case) |
| Wiklund [72] | C | Sweden | 94 | F: 50,682 | 1971–1987 | General female population of Sweden | A, S | Occupation | F: SIR, 0.46 (0.37–0.57) |
| Mastrangelo [2] | C | Padova, Italy | 39 | M: 2,283 | 1970–1992 |
General male population of region |
A, S (stratified by Sm) |
Occupation; diary vs. crop/orchard farming, duration, farm size |
Dairy: M: SMR, 0.49 (0.31–0.74) Crop/Orchard: M: SMR, 0.81 (0.46–1.31) O: 0.60 (0.43–0.84) |
| Sperati [75] | C | Viterbo, Italy | 46 |
M: 2,978 F: 2,586 |
1971–1996 | General population of the region | A, S | Occupation |
M: SMR, 0.54 (0.39–0.74) F: SMR, 0.67 (0.22–1.57) O: 0.55 (0.41–0.75) |
| Wang [76] | C | NY, USA | 21 | F: 6,310 | 1980–1993 | Women of same age living in rural areas of New York state | A, S | Occupation | F: SIR, 0.33 (0.20–0.51) |
| Alavanja [80] | C | Iowa and North Carolina, USA | 266 |
M: 51,211† Spouses (99% F): 31,350 |
1994/1997–2002 | General population in each of the two states | A, S, state, race | Occupation (private pesticide applicator); farm size, grew corn (yes, no), had animals (yes, no) |
M: SIR, 0.47 (0.41–0.53) F: SIR, 0.41 (0.32–0.52) O: 0.46 (0.41–0.51) |
| Mastrangelo [82] | C | Vicenza, Italy | 75 | M: 2,916 | 1970–1998 | General male population of Veneto region | A, S | Occupation, area of farm fields, diary cattle number, time since quitting diary farm work | M: SMR, 0.64 (0.51–0.81) |
| Lee [77] | C | USA | 34 | 3,540 | 1986–2002 | All other occupational categories | A, S, Sm | General occupation: farm workers and other agricultural workers; farm operators and managers |
HR, Farm operators and managers: M: 0.92 (0.59–1.44) M/F: 0.83 (0.51–1.35) Farm and other agriculture workers: M: 1.20 (0.63–2.29) F: 1.14 (0.28–4.71) M/F: 1.19 (0.79–1.89) M: 1.00 (0.70–1.45) O: 1.01 (0.73–1.40) |
| Laakkonen [78] | C | Finland |
Still farming in 1990s: 352 Quit: 1,443 |
M: 87,534 F: 75,552 |
1978–2005 | General population of Finland | A, S | General occupation, farm type (crop, beef, dairy, pig, poultry, other) |
Still farming in 1990 or 1994: SIR, 0.60 (0.54–0.66) Quit farming by 1990 or 1994: SIR, 0.73 (0.69–0.76) O: 0.70 (0.67–0.73) |
| Levin [85] | CC | Shanghai, China | 57 | M: 39 | – | All men aged 35–64 from Shanghai, China | A, S, Sm | Occupation, duration | M: OR, 1.6 (1.0–2.6) |
| Jahn [84] | CC | Germany | 128 | F: 125 | – | All German women | A, Sm, region | Occupation | F: OR, 1.20 (0.88–1.72) |
Bold font denotes that the 95% CI or the combined (overall) estimate was calculated from published data
C cohort study, CC case–control study, M males, F females, RR relative risk, SMR standardized mortality ratio, SIR standardized incidence ratio, HR hazard ratio, OR odds ratio, O overall estimate, A age, S sex, Sm smoking
* Combined self-employed, employees, and family worker categories
† Pesticide applicators, 97% male
Both an SMR and SIR analysis were reported for the same cohort of Icelandic farmers [81, 86]; only the latter estimate, published more recently, was included in the meta-analysis. Two studies reported on the Agricultural Health Study in the same year [80, 87]. The SIR from Alavanja et al. [80], rather than the SMR from Blair et al. [87], was included as the study had a longer follow-up and is based on cancer incidence rather than cancer mortality. This study was designed to investigate the risks associated with pesticide exposure, but was nevertheless included as close to 90% of subjects were farmers. We further restricted our analysis to the SIR reported for private applicators, who were almost exclusively farmers.
The numbers of lung cancer cases captured by these studies were 1,217 and 6,216 within the 11 textile industry studies and 17 agricultural industry studies, respectively.
Quantitative data synthesis and heterogeneity
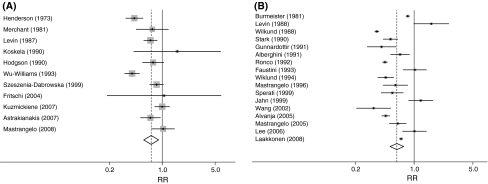
Tables 3 and 4 present the summary and subgroup risk estimates for cotton textile and agriculture workers, respectively. The summary risk (95% CI) of lung cancer was 0.72 (0.57–0.90) in the cotton textile industry and 0.62 (0.52–0.75) in the agricultural industry. Both occupational groups had reduced risks of lung cancer, as is presented graphically in Fig. 1.
Table 3.
Summary risks presented for meta-analyses of all studies and subgroups within the cotton textile industry
| No. of studies | Summary RR (95% CI) | Z (p value)* | Heterogeneity I 2 (p value)† | Test of heterogeneity between subgroups§ | References | |
|---|---|---|---|---|---|---|
| All studies | 11 | 0.72 (0.57–0.90) | 2.91 (0.004) | 82.5% (<0.001) | [1, 60–69] | |
| Published | ||||||
| Until 1990 | 5 | 0.66 (0.49–0.87) | 2.88 (0.004) | 69.4% (0.011) | 0.516 | [1, 60, 61, 66, 68 |
| Post-1990 | 6 | 0.76 (0.54–1.08) | 1.55 (0.120) | 87.8% (<0.001) | [62–65, 69] | |
| By study design | ||||||
| Cohort‡ | 9 | 0.78 (0.62–0.98) | 2.15 (0.031) | 73.4% (<0.001) | 0.152 | [1, 60–67 |
| Case–control | 2 | 0.53 (0.31–0.92) | 2.27 (0.023) | 92.4% (<0.001) | [68] | |
| By outcome | ||||||
| Mortality | 6 | 0.75 (0.55–1.03) | 1.80 (0.072) | 78.5% (<0.001) | 0.587 | [1, 60–62, 65, 66 |
| Morbidity | 5 | 0.68 (0.47–0.98) | 2.05 (0.041) | 87.9% (<0.001) | [63, 64, 67–69] | |
| By smoking adjustment | ||||||
| No | 8 | 0.79 (0.61–1.03) | 1.71 (0.087) | 76.4% (<0.001) | 0.179 | [1, 60–66] |
| Yes | 3 | 0.58 (0.40–0.85) | 2.81 (0.005) | 86.8% (0.391) | [67–69] | |
| By geographic area | ||||||
| Australia | 1 | 1.06 (0.19–5.97) | 0.07 (0.948) | – | 0.059 | [63] |
| China | 3 | 0.58 (0.40–0.85) | 2.81 (0.005) | 86.8% (<0.001) | [67–69 | |
| Europe | 5 | 0.91 (0.80–1.03) | 1.53 (0.126) | 0.0% (0.460) | [61, 62, 64–66] | |
| USA | 2 | 0.54 (0.32–0.92) | 2.28 (0.023) | 73.3% (0.053) | [1, 60] | |
Sex
| ||||||
| Male | 6 | 0.72 (0.57–0.91) | 2.79 (0.005) | 78.1% (<0.001) | 0.523 | [1, 60, 62, 64, 66, 68 |
| Female | 8 | 0.73 (0.52–1.03) | 1.81 (0.071) | 76.7% (<0.001) | [61, 62, 64, 66–69] | |
* Significance test of pooled effect estimate = 1
† Heterogeneity evaluated by I 2 and the p value of chi-squared test for heterogeneity
‡ Including one case–cohort study [67]
§ p value, considered significant if <0.1
 Sex-specific estimates, if provided, were combined in this analysis
Sex-specific estimates, if provided, were combined in this analysis
Table 4.
Summary risks presented for meta-analyses of all studies and subgroups within the agricultural industry
| No. of studies | Summary RR (95% CI) | Z (p value)* | Heterogeneity I 2 (p value)† | Test of heterogeneity between subgroups§ | References | |
|---|---|---|---|---|---|---|
| All studies | 17 | 0.62 (0.52–0.75) | 4.97 (<0.001) | 97.9% (<0.001) | [2, 70–85] | |
| Published | ||||||
| Until 1995 | 9 | 0.61 (0.45–0.84) | 3.09 (0.002) | 98.7% (<0.001) | 0.473 | [70–74, 79, 81, 83, 85] |
| Post-1995 | 8 | 0.64 (0.52–0.80) | 4.00 (<0.001) | 97.1% (<0.001) | [2, 75–78, 80, 82, 84] | |
| By study design | ||||||
| Cohort | 15 | 0.57 (0.47–0.69) | 5.62 (<0.001) | 98.0% (<0.001) | 0.005 | [2, 73–78, 80–83] |
| Case–control | 2 | 1.32 (1.00–1.74) | 1.98 (0.048) | 0.0% (0.334) | [84, 85] | |
| By outcome | ||||||
| Mortality | 8 | 0.69 (0.52–0.92) | 2.53 (0.011) | 97.2% (<0.001) | 0.108 | [2, 20, 74, 75, 77, 80–83] |
| Morbidity | 9 | 0.57 (0.43–0.76) | 3.90 (<0.001) | 98.0% (<0.001) | [72, 73, 76, 78, 81, 84, 85] | |
| By smoking adjustment | ||||||
| No | 14 | 0.55 (0.45–0.67) | 5.82 (<0.001) | 98.2% (<0.001) | 0.002 | [2, 70–76, 78–83] |
| Yes | 3 | 1.19 (0.94–1.50) | 1.47 (0.142) | 17.0% (<0.001) | [77, 84, 85] | |
| By geographic area | ||||||
| China | 1 | 1.60 (0.99–2.58) | 1.93 (0.054) | – | 0.069 | [85] |
| Europe | 11 | 0.60 (0.48–0.75) | 4.48 (<0.001) | 97.6% (<0.001) | [2, 20, 73–75, 78, 81–84] | |
| USA | 5 | 0.59 (0.41–0.86) | 2.79 (0.005) | 96.8% (<0.001) | [76, 77, 80] | |
Sex
| ||||||
| Male | 13 | 0.63 (0.49–0.81) | 3.66 (<0.001) | 98.1% (<0.001) | 0.366 | [2, 70, 71, 73–75, 77, 79, 81–83, 85] |
| Female | 7 | 0.54 (0.39–0.75) | 3.61 (<0.001) | 82.7% (<0.001) | [71, 72, 75–78, 80, 84] | |
* Significance test of pooled effect estimate = 1
† Heterogeneity evaluated by I 2 and the p value of chi-squared test for heterogeneity
§ p value, considered significant if <0.1
 Sex-specific estimates, if provided, were combined in this analysis
Sex-specific estimates, if provided, were combined in this analysis
Fig. 1.
Forest plots for study-specific and summary risk ratios with 95% CIs for lung cancer risk associated with working in a the cotton textile industry and b agriculture. Studies were pooled with the random effects method and ordered by publication year. Squares represent study-specific risk estimates (size of the square reflects the study-specific statistical weight); horizontal lines, the 95% CIs; diamond, the summary risk estimate and its corresponding 95% CI
The textile industry studies displayed considerable heterogeneity; the I 2 was greater than 69% in most subgroups (Table 3). The magnitude of the reduction in risk was greater for the studies from China and the USA than from Europe and greater than the one estimate from Australia (RR (95% CI) of 0.58 (0.40–0.85), 0.54 (0.32–0.92), 0.91 (0.80–1.03), and 1.06 (0.19–5.97), respectively). Textile industry studies that adjusted for smoking yielded a lower summary risk, 0.58 (0.40–0.85) (n = 3), than those that did not adjust for smoking, 0.79 (0.61–1.03) (n = 8), a difference that was of borderline statistical significance. The summary RR for cohort studies was 0.78 (0.62–0.98), and for case–control studies, 0.53 (0.31–0.92).
The risk estimates from agricultural studies exhibited even more heterogeneity than the textile industry studies, with an I 2 of greater than 82% for all subgroups with more than three studies (Table 4). Studies that adjusted for smoking (n = 3) had a summary RR of 1.19 (0.94–1.50) versus 0.55 (0.45–0.67) for the studies that did not. The two case–control studies yielded 1.32 (1.00–1.74), and the cohort studies had a lower summary RR (n = 15) of 0.57 (0.47–0.69). The meta-estimates of risk from the studies in Europe and the USA were similar, and significantly lower than the one estimate from China (0.60 (0.48–0.75), 0.59 (0.41–0.86), 1.60 (0.99–2.58), respectively).
In both textile industry and agricultural studies, meta-risk estimates were lower in earlier published studies, and in studies reporting morbidity versus mortality outcomes, although these differences were not statistically significant. The sex-specific meta-risk estimates did not differ in the textile industry cohorts, but a non-significant lower meta-risk estimate was found for female when compared with male agricultural workers.
Publication bias
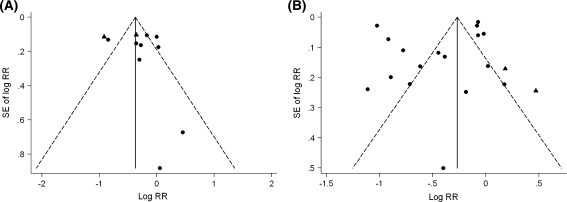
The graphical funnel plots appeared symmetrical for studies from both occupational groups (Fig. 2). There was little evidence of publication bias among either textile industry or agricultural studies, according to Begg and Mazumdar’s test and Egger’s test (p > 0.20).
Fig. 2.
Funnel plots of lung cancer risk ratios associated with working in a the cotton textile industry and b agriculture. Circles represent cohort studies; triangles represent case–control studies
Discussion
This meta-analysis substantiates the available evidence of a reduced risk of developing lung cancer among people occupationally exposed to organic dusts commonly associated with high levels of endotoxin. The apparent protective effect of endotoxin exposure on lung cancer risk has previously been postulated to result from healthy worker selection effect, inadequate adjustment for important confounders such as exposure to tobacco smoke, and/or inadequate comparison groups [4, 5]. Exploring the influence of several variables via subgroup analysis revealed that results were overall robust, and consistently indicated a reduced risk in both cotton textile mill workers and farmers, occupations known to involve high endotoxin exposure [88, 89]. In addition, the current meta-analysis illuminates sources of heterogeneity in this relationship which would need to be clarified in future studies on this topic.
Mastrangelo et al. [3] pooled cancer risks associated with working in a cotton textile factory from eight studies published up to 1999 [1, 42, 60, 62, 66, 68], and calculated a reduced risk for lung cancer of 0.87, 95% CI 0.81–0.93. The CI is narrower than the one presented in this study, probably because Mastrangelo et al. employed a fixed effect model. Given the large heterogeneity in study results observed, a random effects model seemed more appropriate at least for our analyses. For comparison purposes, applying a fixed effects model resulted in the meta-risk estimate of 0.69, 95% CI 0.63–0.75 for textile industry studies. Su et al. [13] pooled 5 SMRs from four cohorts also included in the current meta-analysis [1, 60, 61, 66] and reported a meta-SMR (95% CI) from presumably a fixed effect meta-analysis of 0.60 (0.39–0.73) for male textile workers and 0.89 (0.40–1.38) for female textile workers. In contrast with Su et al., we did not find an appreciable difference between female and male meta-risk estimates. This is possibly explained by our inclusion of the results from the large cohort of Shanghai female textile workers and several other recent studies.
A meta-analysis of cancers in farmers by Acquavella et al. [15] reported a summary lung cancer RR (95% CI) of 0.65 (0.58–0.73) for 29 studies (including 11 PMR studies excluded from the current analysis) pooled in a random effects model. This is similar to the result of the current meta-analysis. Davis et al. [16] added observed and expected cases of lung cancer from 24 studies published prior to 1991 and reported a combined relative risk (95% CI) of 0.66 (0.64–0.67) for farmers. We excluded some of the studies as they were PMRs, studies that employed other cancer patients as controls, or reports not published in peer-reviewed journals. As such, our meta-analysis is based solely on studies that met predefined quality control criteria. The evidence from currently available studies is insufficient to draw a definite conclusion about a causal association between endotoxin exposure and lung cancer risk. Few of the studies assigned quantitative estimates of exposure to the subjects or grouped subjects based on estimated categories of exposure, hindering meaningful dose–response analyses in this meta-analysis. Notably, however, there was evidence of a dose–response relationship in the two studies that did derive estimates of exposure. The study on Shanghai textile workers modelled and assigned endotoxin exposure to cases and controls and found an inverse dose–response relationship between endotoxin levels and lung cancer [67]. In a cohort of Lithuanian textile workers, lung cancer risk decreased for both males and females with increasing quartiles of cotton textile dust cumulative exposure [64].
In the cotton textile industry, airborne concentrations of endotoxin are higher in the early stages of cotton processing—the opening and carding operations—compared with later processes such as spinning and weaving [90, 91]. Of the studies that met the inclusion criteria, only three stratified by job title [62, 65, 68]. In a cohort of Italian cotton mill workers, Mastrangelo et al. [65] reported the greatest reduction in lung cancer SMRs for those workers in carding jobs, which presumably had the highest endotoxin exposure levels, and for those with the longest duration of employment. In the studies that stratified by job task, those workers who did other tasks (such as administration, packaging or maintenance) had higher lung cancer risks than those with job tasks likely to involve higher endotoxin exposures, such as preparers, spinners, and weavers [60, 62, 68]. A study on cotton textile workers in Lithuania also found that longer duration of employment (>10 years) and increasing exposure levels were associated with greater reductions in lung cancer risk in men, but these patterns were not observed in the female workers [64]. Most studies that investigated lung cancer patterns by duration of employment among both cotton textile and agricultural workers found deficits associated with increased years of employment [1, 64, 66, 69, 82], although several studies did not find this [60, 68]. Even though no quantitative information is available in most evaluated studies, results based on proxies of exposure intensity and duration seem in general to corroborate a dose–dependent effect.
Heterogeneity of the observed protective effect among textile studies may also be explained by factors related to exposure intensity such as the origin of the cotton, which is known to influence bale moisture and thus endotoxin levels [92]. This might explain the observed differences in the protective effect between China and the US and Europe. Endotoxin concentrations, expressed as Endotoxin Units per cubic meter of air (EU/m3) or per milligram of dust (EU/mg), have been measured with the Limulus amebocyte lysate (LAL) assay, albeit with inter-laboratory variation of up to one order of magnitude. Endotoxin levels generally range from 10 to 10,000 EU/m3 in the textile industry. As a precursory step in the exposure modeling for the Shanghai cohort, Astrakianakis et al. [88] combined data from five surveys, and showed that GM endotoxin levels ranged from approximately 60 EU/m3 near the spinning process to 3,600 EU/m3 near the carding process. Endotoxin levels generally decrease during the processing steps of opening, carding, drawing, combing, spinning, and weaving, as impurities are removed to produce a clean product. Median endotoxin concentrations in three Shanghai textile mills were 1,281 EU/m3 and 2,227 EU/m3 in samples collected from 14 areas and 41 personal samplers, respectively [93]. Other studies have reported geometric mean endotoxin values of 131 to 1,637 EU/m3 in four mills in Quebec, Canada [94]; a median level of 450 EU/m3 in a German cotton mill [95]; and a mean that ranged from 111.1 to 156.7 ng/m3 over 3 years in a cotton textile plant in Taiwan [96]. A study with 572 personal dust samples from workers in three Turkish cotton mills observed median levels of 2,135 EU/m3 for the open and card attendants, 5,857 EU/m3 for the waste room operatives, and much lower levels of 26–407 EU/m3 for the other occupational groups [97]. Comparisons of exposure levels between these studies should be made with caution as high inter-laboratory variation is known to exist. However, generally they are supportive of higher endotoxin exposure levels in the early stages of cotton processing.
An argument supporting a role for endotoxin in decreasing lung cancer risk is that lung cancer deficits have not been consistently observed in other (non-cotton) types of mills, in which exposure to endotoxin is lower. In the large cohort of Shanghai textile workers, lung cancer mortality was lowest in workers from the cotton textile spinning, weaving and knitting sector, versus the wool, silk, synthetic, mixed fiber textile sectors [21]. One study investigating lung cancer in a synthetic fiber mill found that lung cancer mortality was increased in workers exposed to increasing levels of dust [98]. Endotoxin levels are minimal in synthetic textile dusts, although levels slightly elevated compared to background levels (<5 EU/m3) have been documented in some mills due to contamination of humidification and lubricant mist systems [99]. Considering that covariate risk factors are unlikely to differ substantially between synthetic and cotton mills, the finding that lung cancer rates are lower in cotton versus synthetic mill workers is compelling evidence for a protective effect of endotoxin; however, these findings should be confirmed with further research.
It is possible that earlier cohorts exhibited greater deficits in lung cancer than more recent cohorts due to higher past exposures. While endotoxin and dust levels are only moderately correlated in cotton mills (r = 0.49, p < 0.01) [90], measures to control workers’ exposure to dust, such as improved ventilation and automation of processing, are likely to have resulted in lower cumulative dust and endotoxin exposures in more recent cohorts.
Determinants of endotoxin levels, such as process, sources, ventilation, and moisture, likely vary more between different agricultural settings than between cotton mills. Using a standardized protocol, Spaan et al. [89] reported a geometric mean (range) of 2,700 (96–41,200) EU/m3 for grain and legume primary production (n = 15), and 1,190 (73–19,500) EU/m3 at primary production animal farms (n = 377). High endotoxin levels have been measured during machine harvesting of certain crops. However, cumulative annual endotoxin levels are likely to be higher with livestock than crop farming due to the seasonal nature of the latter. Two agriculture studies from Italy and Finland included in the present meta-analysis, that stratified by production type, found that dairy farmers had lower risks of lung cancer than crop farmers, as did the Finnish male farmers with poultry and pigs [2, 78]. Furthermore, farmers who switched from dairy to crop farming increased their lung cancer risk [78]. Although there are indications among both the textile workers and farmers of a possible dose–response relation between endotoxin exposure and reduced lung cancer risk, this evidence is still rather weak.
Smoking is a major risk factor for lung cancer. Pooling risk estimates based on whether they were adjusted for smoking yielded different results for the textile industry and agricultural studies; the adjusted meta-RR was lower for textile industry studies, whereas the opposite pattern was observed in the agricultural studies, although this difference was non-significant in the textile industry and for each industry, there were only three studies which adjusted for smoking. Two of the three studies that adjusted for smoking in both industries were case–control studies, and the differences in smoking adjusted and unadjusted meta-risk estimates paralleled the differences between cohort and case–control studies. It is thus not possible to state whether factors related to the study design or the adjustment for smoking most influenced the overall risks. It is known that in US populations, farmers have lower rates of smoking than the general population [80]. Assuming this pattern holds in populations outside the US, unadjusted risk estimates with general population controls would over-estimate any protective effect of endotoxin exposure. However, there is some evidence from studies that present both unadjusted and adjusted risk estimates that upon adjustment for smoking, the protective effect associated with working as a farmer did not disappear [82, 84, 85].
A limitation of this meta-analysis is the fact that most of the cohort studies are based on administrative data, such as registries, and thus often lack information on risk factors and estimates of endotoxin exposure, and as such working in the cotton textile or agricultural industries was used as proxies of exposure. Studies may suffer from residual confounding from factors such as lifestyle (e.g., diet, physical activity) and/or other occupational exposures; however, some studies have shown that adjustment for risk factors such as smoking does not substantially change effect estimates [100]. Conversely, non-differential exposure classification may have attenuated the effect estimates. The studies are highly heterogeneous, and populations differ in baseline risk, lifestyle factors and contrast in exposure. The validity of combining and comparing risk estimates from observational studies to obtain a more precise meta-risk estimate is disputed [101], although it should be noted that synthesizing evidence from observational studies is also an issue in non-meta-analytic reviews. A counter argument is that heterogeneity can be used to attempt to discern which factors influence risk, and that consistency from findings from heterogeneous studies can justify the generalizability of meta-analysis results [102, 103]. We did not identify any obvious sources of heterogeneity, or find convincing evidence that one study characteristic variable contributed more to heterogeneity of the meta-risk estimates than the others as there was clustering of the variables we examined. The subgroup meta-analyses demonstrated that the protective relation of working in industries with high endotoxin levels on lung cancer is robust upon consideration of important study characteristics.
Endotoxin exposure is possibly beneficial with respect to reducing lung cancer risk. However, it should be noted that studies indicate that acute exposure to cotton dust can cause chest tightness, organic dust toxic syndrome and byssinosis, and long-term exposure is associated with accelerated decline in lung function and chronic respiratory disease [104–106]. The potential protective effect of endotoxin is relevant in understanding the etiology of lung cancer, and perhaps with respect to developing anti-carcinogenic therapeutics [107]. The lipid-A portion of endotoxin has been found to suppress tumor growth in animal models [10]. Explanations and evidence for plausible mechanistic pathways is limited. It seems that removing exposure—when farmers quit farming, or switch to a farming type with purportedly lower endotoxin exposures—causes deficits in lung cancer risk to disappear over time [78, 82]. It is unclear whether current exposure is protective, which intensity of exposure is relevant, and whether the protective effect diminishes with time elapsed since last exposure to endotoxins.
This meta-analysis of studies of two dissimilar occupational groups, agricultural and textile workers, despite several limitations, adds weight to previous evidence that exposure to endotoxin-contaminated organic dusts may lead to a reduced risk of developing lung cancer. Future research should investigate the dose–response relationship between endotoxin exposure and risk of lung cancer and focus on possible sources of heterogeneity in this relation. Quantifying exposures to organic dusts, endotoxin, and concurrent exposure to other biologically active agents may help solve the mechanistic pathways of the observed protective effect.
Acknowledgments
Acknowledgment of financial support
Not applicable.
Competing interests
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix 1
See Table 5.
Table 5.
Overview of all identified studies in cotton textile industry and agricultural workers that reported lung cancer mortality or morbidity risks
| Study | Design | Population | No. of cases | Lung cancer definition | Controls vs. group | Exposure | Confounders | Main results | Authors’ conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Textile industry | |||||||||
| Enterline [19] | Cohort |
6,242 male cotton textile workers in Georgia, US during 1948–1951 Follow-up: 1948–1963 |
7 | Identified from the national registry using ICD-7 | US general white male population | Industry | Age, sex, race | Overall: SMR = 27.3 | Authors attributed the low rate to a HWE |
| Henderson and Enterline [1] | Cohort |
Cohort 1: 5,822 men working during 1938–1941 Follow-up: 1938–1963 Cohort 2: 6,242 men working during 1948–1951 Follow-up: 1948–1963 All cotton textile workers in Georgia, US |
Cohort 1: 36.5 Cohort 2: 26.4 |
Identified from the national registry using ICD-7 | General white male population of Georgia, US | Industry, duration (1,772 workers who work in both 1938–1951 and 1948–1951 periods with >10 years of exposure and the remaining 4,936 from cohort 1 and 5,356 from cohort 2) | Age, sex, race |
SMRs: Overall: Cohort 1: 54.8 Cohort 2: 30.3 Duration: Cohort 1: 65.3 Cohort 2: 20.5 Worked in both periods: 42.4 |
Authors considered external data that showed no differences in smoking habits between textile workers and the general population and suggested that “something” in the working environment protects cotton textile workers from cancer |
| Buiatti et al. [51] | Cohort |
116,060 male and 51,415 female textile workers. All aged 14–60 from the Prato area, Italy. Comparison: The general population of Prato (79,697 males and 162,545 females) Follow-up: 1970–1974 |
45 | Identified from death certificates and confirmation through family interviews | The mean of the census data of the years 1961–1971 | Industry, tasks (four categories: selection of raw material, dyeing, spinning, weaving) | Age, sex |
Textile workers: Men: MR = 365 Women: MR = 5.8 Control population: Men: MR = 18.8, Women: MR = 1.2 Tasks: MR = 191.5, 118.9, 28.3, and 21.9 for selection of raw material, dyeing, spinning, and weaving, respectively |
Authors suggested that there is a link between some occupational tasks and lung cancer |
| Merchant and Ortmeyer [60] | Cohort |
2,119 men and 725 women in North Carolina, US. Analysis restricted to 1,113 men and 393 women with job histories Follow-up: 1940–1975 |
18 | Identified from death certificates using ICD-8 | US general population | Four job categories by exposure to cotton dust (preparation, yarn processing, slashing/weaving, other), duration of employment | Age, sex, race | Overall: SMR = 74 among white males. Preparation (SMR = 52), yarn processing (SMR = 30), slashing/weaving (SMR = 79), other (SMR = 174), p < 0.05 for all | Authors concluded that methodological issues like a HWE and different smoking habits were responsible for underestimating the cohort’s SMRs |
| Hodgson and Jones [66] | Cohort |
3,458 British cotton industry workers Follow-up: 1968–1984 |
42 | Identified from the national registry using ICD-8 and ICD-9 (depending on the period of death) | England and Wales general population | Industry, duration (<15, 15–29, >29 years) | Age, sex, smoking | SMRs: Overall: males: 0.75 (0.51–1.06), females: 0.79 (0.39–1.41). Duration: men: 127, 78, 63. Women: 0, 127, 65 for <15, 15–29 and >29 years, respectively (p > 0.05 for all). Nonsmokers: male: 12, female: 13 (p < 0.05 for both). Smokers: male = 97 female = 160 (p > 0.05) | Authors concluded that the deficit in lung cancer could not be explained from differences in smoking habits and suggested that their results support the proposed protective effect of endotoxin on the development of lung cancer |
| Koskela et al. [61] | Cohort | 1,065 women hired between 1950 and 1971 by 5 Finnish cotton mills. Follow-up: 1950–1985 | 3 | Identified from death certificates using ICD-8 | The general Finnish female population in 1977 | Industry | Age, sex | SMR = 158 (p > 0.05) | Authors reported inconsistency with previous studies |
| Szeszenia-Dabrowska et al. [62] | Cohort | 2,949 men and 4,943 women with at least 10 years of work in the Lodz (Poland) cotton plants. Follow-up: 1964–1993 | 140 | Identified from the related registry using ICD-9 | General Polish population | Industry, department (4 categories: chemical processing, weaving, spinning, other), duration | Age, sex | SMRs: Overall: men: 0.89 (71–110), women: 0.55 (0.28–0.96). Department: men: 0.83 (0.44–1.42), 0.79 (0.50–1.20), 0.85 (0.49–1.38), 1.02 (0.71–1.43) and women: 0 (0–0), 0.82 (0.37–1.56), 0.13 (–), 0.84 (0.10–3.03) for the 1st, 2nd, 3rd, and 4th departments, respectively | Authors focused on causal factors that showed elevated risks and attributed the decreased results in females to a HWE. However, they also reported consistency with previous regarding the results for lung cancer |
| Wernli et al. [21] | Cohort |
267,400 women born between 1925 and 1958 employed in 526 factories in the Shanghai textile industry Follow-up: 1989–1998 |
236 | Identified from the Shanghai cancer registry using ICD-9 | Shanghai general female population | Industry and 9 major textile sectors according to the material processed and the working task | Age, sex | SIR = 0.8 (0.74–0.86) for the hole, SIR = 0.72 (0.63–0.82) for cotton spinners, weavers and knitters | No conclusion was made since the study aimed to guide future research |
| Fritschi et al. [63] | Cohort |
7,679 (4,039 men and 3,640 women) textile workers joined the textile union of Australia before 1996 Follow-up: 1982–1997 |
2 | Identified from the National cancer statistics clearing house using ICD-9 | General population of Australia | Industry | Age, sex | Men: no deaths from lung cancer. Women: SIR = 106 (12–381) | Authors reported a very small number of lung cancer deaths and thus consistency with the results of a meta-analysis study |
| Kuzmickiene et al. [22] | Cohort | 5,495 men and 9,155 women employed for at least 1 year during 1969–1997 to the Alytus (Lithuania) factory. Follow-up: 1978–1997 | 53 | Identified from the National registry using ICD-9 | General Lithuanian population | Industry, department (1 category including spinning and weaving), duration (<10, >10 years) | Age, sex | Overall: SIR = 1.35 (0.99–1.81) for men and SIR = 1.11 (0.48–2.19) for women. Spinners/weavers: SIR = 1.12 (0.65–1.79) for men and SIR = 3.26 (0.50–3.58) for women. Deficits in SIR with increased years of work | Authors attributed the inconsistency with previous studies regarding lung cancer to unmeasured factors, low number of cases and to the multifactorial nature of carcinogenesis |
| Laakkonen et al. [24] | Cohort | All economically active Finns born between 1906 and 1945 who participated in the census of 1970 (667,121 men, 513,110 women). Follow-up: 1971–1995 | 270 | Identified from the National registry using ICD-10 | All the economically active population of Finland | Dust estimates obtained from the Nordic classification of Occupations (FINJEM). Exposure to textile dust was categorized as follows: none, low (<5mg/m3-year), medium (5–20mg/m3-year), and high (>20mg/m3-year) | Age, sex, social class, smoking | Textile dust: Men: SIR = 1.00 (0.99–1.01), 1.08 (0.92–1.26), 0.87 (0.69–1.08), 0.66 (0.43–0.97) and women: SIR = 1.01 (0.98–1.05), 0.99 (0.80–1.21), 0.79 (0.66–0.95), 0.60 (0.36–0.94) for the none, low, medium, and high groups of exposure, respectively | Authors concluded that their results supported the hypothesis that exposure to textile dust decreases the risk of lung cancer and that the stronger protective effect found in the highest exposure categories add to the hypothesis that endotoxin is responsible |
| Kuzmickiene and Stukonis [64] | Cohort | 5,495 men and 9,155 women employed for at least 1 year during 1969–1997 to the Alytus (Lithuania) factory. Follow-up: 1978–2002 | 85 | Identified from the National registry using ICD-9 | General Lithuanian population | Industry, department (cotton textile production unit; cotton textile finishing unit; maintenance unit), duration (<10, >10 years) | Age, sex |
Men: SIR = 0.94 (0.73–1.19), Women: SIR = 1.36 (0.76–2.25) SIRs for the low, medium, high and very high level of cumulative exposure were 1.91 (0.92–3.51), 1.30 (0.52–2.69), 0.77 (0.21–1.96), and 0.24 (CI 0.03–0.86), respectively An average level of exposure to textile dust was assigned to the four quartiles: low exposure (>0 to <8.0 mg/m3-year), medium exposure (from 8.1 to 19.7 mg/m3-year), high exposure (from 19.8 to 64.7 mg/m3-year), very high exposure (from 64.8 to 200.6) and no exposure Employed ≥10 years: SIR = 0.89 (0.57–1.31) for men; 1.27 (0.34–3.24) for women |
Authors conclude that their results confirm the lower risk of lung cancer in the cotton textile production workers compared with that in the general population. They acknowledge limitations of their study, including lack of detailed exposure records and a lack of cases |
| Mastrangelo et al. [65] | Cohort |
Italy M/F: 3,961 Follow-up: 1970–1994 |
36 | Identified from death certificates and coded according to ICD-9 | Regional population of Veneto, Italy | Duration (tertiles), department (working with carding (with high exposure), with spinning or weaving (with lower exposure to endotoxin)) | Age, sex |
Overall SMR = 1.03 (0.72–1.43). SMR was 0.93 (CI: 0.45 to 1.72; n = 10) and 1.07 (0.70 to 1.57; n = 26) for carders and non-carders, respectively. Statistically significant trend (p < 0.05) for linear decrease in risk with increasing duration of employment |
Authors conclude that the study supports earlier findings that cotton workers exposed to high levels of endotoxin-containing dust for prolonged periods of time have a lower risk of lung cancer |
| Astrakianakis et al. [67] | Case–cohort |
641 cases and 3,188 controls. Cohort population: 267,400 women born between 1925 and 58 from 526 textile factories in Shanghai. Follow-up: 1989–1998 |
– | Identified from the Shanghai cancer registry using ICD-9 | Randomly selected from the cohort population | Duration, quintiles, and quartiles of exposure based on cumulative exposure derived from modeled cotton dust exposure estimates that were converted into endotoxin using previously measured job-specific endotoxin estimates. Reference: the unexposed | Age, sex, smoking | Inverse dose–response trends for the risk of lung cancer with both increased cumulative exposure (unlagged) to endotoxin and time of exposure were observed e.g., HR = 1.06 (0.79–1.41), 0.98 (0.73–1.30), 0.79 (0.58–1.06), 0.88 (0.66–1.16), 0.70 (0.52–0.95) for the 1st, 2nd, 3rd, 4th, and 5th quintile, respectively for the whole follow-up period | Authors concluded that long-term and high-level exposure to endotoxin, compared with no exposure, is associated with a reduced risk of lung cancer and suggested that their results which are supported from other studies, show that endotoxin exposure has a protective effect on the development of lung cancer |
| Siemiatycki et al. [41] | Case–control | 499 (25 exposed to cotton) lung cancer cases and 920 controls. All incidence male cases aged 35–70 selected from 19 hospitals of Montreal, Canada | – | Histologically confirmed identified from the hospitals’ pathology department records | Patients with other types of cancer selected from the same database | Industry (exposure to specific types of dust in two levels: non-exposed and substantially exposed) | Age, social, and economical status, race, smoking, accuracy of job history | Overall for cotton dust: OR = 0.8 (0.4–1.3) | Authors were looking for potential carcinogens and thus did not comment the non-significant inverse associations between cotton dust and lung cancer |
| Levin et al. [68] | Case–control | 1,495 (169 textile workers) cases and 1,495 (241 textile workers) randomly controls aged 35 to 69 from Shanghai, China selected during 1984–1985 | – | Identified from the local registry using ICD-9 and reviewed by physicians | Randomly selected from the local area population | Industry, duration, 5 major task categories and self-determined exposure to textile dust. Comparison with non-textile workers (ref.) | Age, sex, smoking | Overall: OR = 0.7 (0.6–0.9). Tasks: OR = 0.8 (0.5–1.3), 0.8 (0.5–1.2), 1.0 (0.4–2.4), 0.3 (0.1–1.3), 1.1 (0.3–3.7) for preparers, weavers, dyers, mechanics and others. Decreased risk by increased years of exposure | Authors suggested that their results were not confounded by smoking and reported that the reduced risk of lung cancer in remains unclear due to the absence of info on dose–response relationships |
| Levin et al. [85] | Case–control | 733 (89 textile workers) lung cancer cases and 760 (128 textile workers) controls. All men aged 35–64 from Shanghai, China | – | Identified from the local registry using ICD-9 | Randomly selected from the local population | Industry, duration (<10, 10–19, 20–29, >30 years), occupation (1 category: textile, knitting, printing and dyeing workers) | Age, sex, smoking |
Overall: OR = 0.7 (0.5–1.0) Duration: OR = 0.9, 0.5, 0.9, 0.6 for <10, 10–19, 20–29, >30 years of employment, respectively. Textile, knitting, printing and dyeing workers: OR = 0.7 (0.5–1.2) |
Authors reported consistency with previous studies and concluded that the low rates of lung cancer could not be due to differences in smoking habits |
| Wu-Williams et al. [69] | Case–control | 1,049 (31 textile workers) cases and 960 (44 textile workers) controls. All women aged 29–70 from Shenyang and Harbin, China | – | Identified from the local registries, reviewed and confirmed by physicians | Randomly selected women from the general populations of the same cities | Industry, occupation (1 category including knitters, printers, and dyers), duration (<11, >11 years). All other workers used as reference | Age, sex, smoking, study area, education | Industry: OR = 0.7 (0.4–1.1). Duration: OR = 1.0 (0.4–2.5) and OR = 0.5 (0.3–1.0) for <11 and >11 years. Occupation: OR = 0.6 (0.3–1.0). Duration: 0.7 (0.3–1.8) and OR = 0.5 (0.2–1.0) for <11 and >11 years, respectively | Authors reported that the results are consistent with previous studies but also mentioned that the reasons for the decreased risks are unknown |
| Wang et al. [42] | Case–control | 4,806 male and 3,595 female cases and 14,685 male and 13,010 female controls from Tianjin, China | – | Identified from the local cancer registry using ICD-9 | Patients with other types of cancers selected with the same method | Industry. Reference: all other industries | Age, sex | OR = 0.86 (0.76–0.97) for men textile workers (339 cases, 1,171 controls). OR = 1.01 (0.86–1.18) for women textile workers (226 cases, 879 controls) | The Authors reported that the decreased risk was inconsistent in literature and suggested this to be a result of differences in the raw material and composition of the cotton dust |
| Jahn et al. [84] | Case–control | 686 (93 textile workers) cases and 712 (113 textile workers) controls, all German women | – | Histologically and/or cytologically confirmed | Randomly selected from the local population | Occupation, industry | Age, region, smoking, time since smoking cessation | Textile workers: OR = 0.7 (0.50–0.99). Leather and textile industry: OR = 0.8 (0.57–1.10) | Due to the large number of occupations included, authors focused on causal factors that showed elevated risks |
| Baccarelli et al. [47] | Case–control | 474 (8 exp. to cotton) men and 66 (6 exp. to cotton) women lung cancer cases and 453 (4 exp. to cotton) men and 129 (9 exp. to cotton) women controls. All from 88 hospitals of Leningrad, Russia | – | Pathologically diagnosed lung cancer cases identified from the central pathology laboratory autopsy records | Deceased subjects with autopsy based diagnosis of non-cancer and non-smoking-related diseases | Industry (type of dust), duration (<10, >10 years), Average intensity (below or above 75% of the related MAC), cumulative exposure score (2 levels: <5 and >5 calculated as the product of average intensity score per total duration) | Age, sex, smoking, region of residence | Cotton dust: Men: Overall: OR = 2.43 (0.67–8.82), duration: OR = 10.10 (1.02–100.2) and 0.65 (0.10–4.10) for <10 and >10 years, respectively. Average intensity: OR = 4.04 (0.67–24.4) and 1.32 (0.21–8.10) for <75% and >75% MAC, respectively. Cumulative exposure: OR = 0.23 (0.03–2.17) for >5. Women: Overall: OR = 1.34 (0.42–4.22) | Authors related their results to the increased risk observed among subjects exposed to cotton dust to the high lung cancer rates among textile workers reported in other previous investigations |
| Delzell and Grufferman et al. [27] | Proportionate mortality study | 42,355 deaths (4,462 textile workers) of white women in North Carolina (NC), US. Surveillance period 1976–1978 | 106 | Identified from the North Carolina, US DHR using ICD-8 | The proportion of deaths due to the same cause among all other NC white women decedents | Industry | Age, sex, race | Overall: 0.9 (0.7–1.0) | Authors made no comments on lung cancer mortality |
| Roman et al. [28] | Proportionate mortality study | 354,845 (73,394 with occupational history) deaths of women from England and Wales, aged 15–74. Surveillance period 1970–1972 | 74 | Identified from the National registry using ICD-8 | The proportion of deaths due to the same cause among all women in 1970–1972 | Occupation: five categories: (1) Fiber preparers, (2) spinners, processors, (3) laborers, (4) Winders, reelers, etc., and (5) Weavers | Age, sex | PMR = 59, 107, 34, 55, 59, 71 for Fiber preparers, spinners, processors, laborers, winders, and weavers, respectively. p < 0.01 only for weavers | Authors did not comment the low PMR for lung cancer among textile workers |
| Dubrow and Gute [29] | Proportionate mortality study | 45,482 deaths (6,113 textile workers) of males from the Rhode island. Surveillance period: 1968–1978 | 307 | Identified from the local registry using ICD-8 | Deaths due to the same cause in all non-textile occupations | Industry | Age, sex | PMR = 80 (72–88) | Authors reported consistency and attributed the low rates to smoking differences |
| O’Brien and Decoufle [30] | Proportionate mortality study | 311 white male carpet and textile workers in 5 northwest Georgia, US counties Surveillance period: 1970–1984 | 138 | Identified from mortality records of the Georgia Department of Human Resources | 38,062 deaths of the general state population during the same period | Industry | Age, sex, race | PCMR = 1.0 (0.9–1.1) | Authors reported inconsistency with previous studies |
| Agriculture industry | |||||||||
| Burmeister [70] | Cohort + Proportionate mortality study |
21,101 deaths of white males (6,402 farmers) in Iowa, US Follow-up: 1971–1978 |
1,466 | Identified from death certificates using ICD-? |
SMR: White Iowa male population PMR: Deaths of males in all non-farming occupations |
Occupation | Age, sex, race |
SMR: 0.84 (p < 0.01) PMR = 0.78 (p < 0.01) |
Authors attributed the lower rates of lung cancer among farmers to differences in smoking habits among farmers and non-farmers and to a HWE |
| Wiklund [20] | Cohort | 317,517 male and 36,711 female Swedish farmers identified from the 1960 census. Follow-up: 1961–1973 | 934 | Identified from the National cancer registry using ICD-7 | General population of Sweden | Occupation | Age, sex | SMR = 0.39 (0.36–0.43) for the total population. SMR = 0.38 for men | Authors attribute the low rates to light lifestyle and lower smoking |
| Wiklund and Steineck [73] | Cohort | 254,417 male farmers and 1,725,845 men working in other than farming occupations, all Swedish born between 1891 and 1940. Follow-up: 1961–1979 | 1155 | Identified from the National cancer registry using ICD-7 (excl. cancer in the pleura) | The 1,725,845 men working in other than farming occupations | Occupation | Age, sex | RR = 0.36 (0.34–0.38) | Authors suggested that smoking differences cannot explain the lower risks of lung cancer experienced by framers and that probably other carcinogens are responsible for them |
| Rafnsson and Gunnarsdottir [86] | Cohort |
5,923 male Icelandic farmers registered in the Farmers pension fund during 1977–1984. Follow-up: 1977–1985 |
15 | Identified from the National statistical registry using ICD-7 | General Icelandic male population | Occupation | Age, sex | SMR = 0.53 (0.30–0.87) | Authors attributed the lower mortality rate to the lower smoking among farmers and to the higher mortality of cancer experienced in large cities |
| Stark et al. [79] | Cohort |
18,811 NY farm Bureau members (>18 year old), registered for at least 1 year in the registry during 1973–1979 Follow-up: 1973–1983 |
103 | Identified from the local registry using ICD-9 | 747,128 men from the rural areas of the NY state aged >25 | Occupation | Age, sex | Overall: SIR = 52.4 p < 0.01 | Authors attributed the low rates of lung cancer to a HWE and to the lower smoking and alcohol consumption of farmers compared to the gen. population |
| Gunnarsdottir and Rafnsson [81] | Cohort |
5,922 male Icelandic farmers registered in the Farmers pension found during 1977–1983 Follow-up: 1977–1987 |
20 | Identified from the national cancer registry using ICD-7 | General Icelandic male population | Occupation | Age, sex | SIR = 0.41 (0.27–0.59) | Authors reported consistency with previous studies and attributed the lower mortality rate to the lower smoking and lifestyle differences between farmers and the gen population |
| Alberghini et al. [74] | Cohort |
4,580 male farmers from 13 municipalities in the provinces of Bologna, Modena, and Ferrara, Italy Follow-up: 1974–1987 |
65 | Identified from the municipality records using ICD-9 | (1) The national and (2) the regional general male population | Occupation | Age, sex | (1) With regional population as reference: SMR = 61 (47–77), (2) with national population as reference: SMR = 68 (52–87) | Authors reported consistency and mentioned that their results might be relatively light tobacco consumption, type of pesticides used |
| Ronco et al. [71] | Cohort + Mortality odds ratio analysis | Cohort: Farmers of both sexes, 15–74 years old, identified from the Danish Occupational Cancer registry. Follow-up: 1970–1980. Mortality odd ratio analysis: Farmers of both sexes aged 18–74 years, identified from the 1981 Italian census |
Cohort 1: 810 MOR analysis: 188 |
(1) Identified from the Danish Cancer Register using ICD-7 and (2) Record linkage between death certificates and the 1981 census classification using ICD-9 | (1) All persons economically active in 1970 (2) Subjects dying from all other causes of death were used as referents | Occupation, type of employment [two main categories: self-employed (SE) and employees (E), for Danish women 1 extra category: family worker (FM)] | Age, sex | (1) Cohort: Men: SMR = 0.40 and SMR = 0.72 for SE and E, respectively. (p < 0.05 for both). Women: SMR = 0.24, 0.45, 0.68 for SE, FW and E, respectively. (p < 0.05 for all). 2) MOR analysis: Men: MOR = 0.64 (p < 0.05) and MOR-0.84 for SE and E, respectively. Women: MOR = 0.91 and MOR = 2.12 for SE and E, respectively (p > 0.05 for both) | Authors attributed the lower rates among farmers to the lower levels of alcohol consumption and smoking among farmers |
| Faustini et al. [83] | Cohort |
1,701 male and 426 female farmers from Aprilia, Italy Follow-up: 1972–1988 |
42 | Identified from the regional registry using the ICD-8 | General population of Italy | Occupation | Age, sex |
Males: SMR = 1.02 (0.73–1.38). Females not calculated due to only one death |
Authors reported inconsistency with previous studies and concluded that confounding by smoking could not explain the results |
| Wiklund and Dich [72] | Cohort |
50,682 Swedish women reporting in the 1970 census who worked >20 h per week in agriculture Follow-up: 1971–1987 |
94 | Identified from the National registry using ICD-7 | General Swedish female population | Occupation | Age, sex | SIR = 0.46 (0.37–0.57) | Authors concluded that smoking could not be responsible for the low risks and suggested that physical activity might have played a role in the origin of lung cancer |
| Mastrangelo et al. [2] | Cohort |
2,283 male cattle and crop/orchard farmers from two areas in the province of Padova Follow-up: 1970–1992 |
39 | Identified from the local registry using ICD-9 | General male population of the region | Occupation, 2 types of farming (diary, crop), duration (12–74, 75–146, 147–248, and 249–587 months), size of farm (2–6, 7–11, 12–20, and 21–150 fields) | Age, sex, smoking | Diary farmers: overall SMR = 0.49 (0.31–0.74). Duration: SMR = 0.96 (0.41–1.89), 0.48 (0.19–0.99), 0.40 (0.13–0.93), and 0.25 (0.05–0.73) for the 1st, 2nd, 3rd, 4th quartiles. Farm size: SMR = 0.89 (0.46–1.56), 0.37 (0.12–0.86), 0.41 (0.11–1.05), 0.19 (0.02–0.69) for the 1st, 2nd, 3rd, 4th quartiles. Crop farmers: No significant associations | Authors concluded that the demonstrated decreased associations could not be attributed to a HWE and/or to confounding by smoking and suggested that their results were a result of endotoxin exposure since the protective effect was only found among diary farmers |
| Pukkala and Notkola [23] | Cohort | 119,681 male and 85,151 women farmers registered on 31 December 1978 in the Finish Farm register. Follow-up: 1979–1993 | 2,601 | Identified from the national registry using ICD-? | General Finish population | Occupation, 6 types of farming: Crop, small diary, diary, pig, poultry, and other farmers | Age, sex | Men: overall: SIR = 0.68 (0.66-0.71), Crop: 0.7, small diary: 0.7, Diary: 0.5, Pig: 0.5, Poultry: 0.5, Other: 0.8, all significant. Women: overall: SIR = 0.53 (0.45–0.62), Crop: 0.7, small diary: 0.5, Diary: 0.5, Pig: 0.1, Poultry: 0.9, Other: 0.4, all significant except poultry | Authors attributed the low rates of lung cancer to the lower consumption of smoke and to the different lifestyle of farmers |
| Sperati et al. [75] | Cohort | 2,978 male farmers licensed to buy pesticides during 1971–1973 and their 2,586 wives from Viterbo, Italy. Follow-up: 1971–1996 | 46 | Identified from the regional registry using ICD-9 | General population of the region | Occupation | Age, sex | Male farmers: SMR = 0.54 (0.39–0.74), Wives: SMR = 0.67 (0.22–1.57) | Authors mentioned consistency with previous studies |
| Wang et al. [76] | Cohort |
6,310 female farm residents aged 30 to 64 year, registered in the NY Farm Bureau, or members’ spouses or relatives Follow-up: 1980–1993 |
21 | Identified from the local registry using ICD-9 | Women of same age living in rural areas of NY | Occupation | Age, sex | SIR = 0.33 (0.20–0.51) | Authors attributed the low rates of lung cancer to the low smoking among farmers |
| Alavanja et al. [80] | Cohort | 52,395 private pesticide applicators (97% men), 32,347 spouses (99% women) of them and 4,916 commercial applicators from Iowa and North Carolina, US. Recruitment: 1994–1997, Follow-up until 2002 | 346 | Identified from the national death index and local registries using ICD-9 | General population in each of the two states | Occupation | Age, sex | Private applicators: SMR = 0.47 (0.41–0.53). Commercial applicators: SMR = 0.59 (0.3–1.03). Spouses: SMR = 0.41(0.32–0.52) | Authors attributed the findings to lower smoking consumption and differences in lifestyle factors |
| Blair et al. [87] | Cohort | 52,392 private pesticide applicators (97% men) and 32,345 spouses (99% women) of them from Iowa and North Carolina, US. Recruitment: 1994–1997. Follow-up until 2000 | 158 | Identified from the national and local registries using ICD-9 | General population in each of the two states | Occupation, farm size (<200, >200 acres), Grew corn (yes, no), Had animals (yes, no) | Age, sex, state, race, smoking | Overall: SMR = 0.4 (0.3–0.4). Private applicators: SMR = 0.4 (0.3–0.4), Spouses: SMR = 0.3 (0.2–0.5). Stratified analysis SMR: Corn: 0.5 for no and 0.3 for yes, Animals: 0.5 for no and 0.3 for yes, Farm size: 0.3 for no and 0.3 for yes, all statistically significant | Authors attributed the findings to a HWE, to lower smoking and alcohol consumption, and to higher physical activity experienced by farmers compared to the general population |
| Mastrangelo et al. [82] | Cohort + nested case–control |
Cohort: 2,916 male farmers from the province of Vicenza, Veneto region, Italy. Follow-up: 1970–1998 Case–control: 75 cases and 333 controls, all diary farmers |
75 | Diagnosed from the death certificates using ICD-9 | Cohort: The gen. male population of the region. Case–control: controls were systematically sampled | Occupation, tertiles of farm fields (<11, 11–21, >21) and diary cattle number (1–4, 5–13, >13). Time of quitting diary farm work (<15 year, >15 year) | Age, sex, smoking (case–control) | Overall: SMR = 0.64 (0.51–0.81). Cattle no: SMR = 0.76 (0.46–1.19), 0.37 (0.15–0.77), 0.26 (0.03–0.93) for the 1st, 2nd and 3rd tertiles. Case–control: Cattle no. OR = 0.60 (0.31–1.15) and OR = 0.18 (0.07–0.42) for the 2nd and 3rd tertile. Similar results for farm fields. Protective effect was removed for those that ceased diary farm work in more than 15 years ago | Authors concluded that increased levels of endotoxin (or other associated environmental factors) might be protective against lung cancer since protection diminishes over time after that exposure is removed |
| Laakkonen et al. [24] | Cohort | All economically active Finns born between 1906 and 1945 who participated in the census of 1970 (667,121 men, 513,110 women). Follow-up: 1971–1995 | 270 | Identified from the national registry using ICD-9 | All the economically active population of Finland | Dust estimates obtained from the Nordic classification of Occupations (FINJEM). Exposure categorization: none, low, medium, and high for both plant and animal dust | Age, sex, social class, smoking | Reduced SIRs among those exposed to plant and animal dust (predominantly farmers), e.g., Animal dust: men: SIR = 1.03 (1.01–1.04), 0.89 (0.87–0.92), 0.92 (0.87–0.97), 1.22 (0.93–1.57), women: SIR = 1.01 (0.97–1.05), 0.95 (0.79–1.12), 0.93 (0.83–1.05), 0.46 (0.06–1.65) for the 1st, 2nd, 3rd and 4th group, respectively | Authors concluded that their results supported the hypothesis that exposure to animal and plant dust decreases the risk of lung cancer and that the stronger protective effect found in the highest exposure categories add to the hypothesis that endotoxin is responsible |
| Lee et al. [77] | Cohort | 143,863 (1,412 male and 416 female agriculture workers, 1,377 male, and 335 female farm operators and managers) workers aged >18 years who participated in the1987, 1988, and 1990–1994 US National Health Interview Surveys. Follow-up: 1986–2002 | 16 and 18 for farm workers and operators respect | Identified from the National Death Index using ICD-10 | All other occupational categories | General occupation (2 categories: (1) Farm workers and other agricultural workers and (2) Farm operators and managers), Specific occupation (Farm workers) | Age, sex, smoking |
(1) Farm workers and other agricultural workers: Overall: OHR = 1.19 (0.74–1.89) Women: OHR = 1.14 (0.28–4.71) Men: OHR = 1.20 (0.63–02.29). Only farm workers: OHR = 0.91 (0.46–1.78) (2) Farm operators and managers: Overall: OHR = 0.83 (0.51–1.35), Men: OHR = 0.92 (0.59–1.44) |
Due to the large number of occupations and the non-significant results, authors paid little attention to the agriculture populations |
| Laakkonen and Pukkala [78] | Cohort |
All Finnish farmers (87,534 men and 75,552 women) on Dec. 1978 still living on Jan. 1995 Follow-up: 1995–2005 |
Still farming in 1990 or 1994: 352 Quit: 1443 | Identified from the Finnish Cancer Registry | General population of Finland | General occupation, farm type (crop, beef, dairy, pig, poultry, other) | Age, sex | Still farming in 1990 or 1994: SIR = 0.60 (0.54–0.66). Quit farming by 1990 or 1994: 0.73 (0.69–0.76) | Authors noted that farmers who changed production type from dairy to crop increased their overall cancer risk and tied this to the possibly protective effect of endotoxin exposure |
| Siemiatycki et al. [41] | Case–control | 499 (23 exposed to grain) lung cancer cases and 920 controls. All incidence male cases aged 35–70 selected from 19 hospitals of Montreal, Canada | – | Histologically confirmed identified from the hospitals’ pathology department records | Patients with other types of cancer selected from the same database | Industry (exposure to specific types of dust in two levels: non-exposed and substantially exposed) | Age, social, and economical status, race, smoking, accuracy of job history | Overall for grain (organic) dust: OR = 0.6 (0.4–1.1) | Authors were looking for potential carcinogens and thus did not comment the non-significant inverse associations between grain dust and lung cancer |
| Levin et al. [85] | Case–control | 733 (57 agriculture workers) lung cancer cases and 760 (39 agriculture workers) controls. All men aged 35–64 from Shanghai, China | – | Identified from the local registry using ICD-9 | Randomly selected from the local population | Occupation, duration (<10, 10–19, 20–29, >30 years) | Age, sex, smoking |
Overall: OR = 1.6 (1.0–2.6) Duration: OR = 1.6, 1.6, 1.3 for <10, 10–19, 20–29 years of employment, respectively |
Authors reported inconsistency with previous studies and mentioned that the use of pesticides might have altered the results |
| Brownson et al. [43] | Case–control | 4,115 (346 farmers) cases and 10, 885 (1,374 farmers) controls. All men from Missouri, US during 1984–1988 | – | Identified from the Local registry using ICD-Oncology | Patients with other types of cancers selected with the same method | Occupation | Age, sex, smoking | Adjusted only for age: OR = 0.67 (0.60–0.76), adjusted for age and smoking: OR = 0.76 (0.66–0.86) | Authors mentioned consistency with previous studies but they concluded that differences in smoking habits do not explain the low lung cancer rates |
| Reif et al. [40] | Case–control | 4,224 (517 farmers) cases and 15,680 (2529 farmers) controls. All males aged >20 years from New Zealand | – | Identified from the national registry using ICD-9 | Patients with other types of cancers selected with the same method | Occupation (reference: non-farmers) and 4 types of farming: Livestock, diary, crop and general (poultry farm workers, etc.) | Age, sex | Overall: OR = 0.70 (0.63–0.77). Analyses by type of farming: Livestock: OR = 0.81 (0.57–1.13), Diary: OR = 0.66 (0.48–0.92), Crop: OR = 0.87 (0.64–1.18), General farmers: OR = 0.67 (0.60–0.75) | Authors attributed the demonstrated lower risk of lung cancer among farmers to the lower proportion of current and ex-smokers among farmers than in the general population |
| Fincham et al. [44] | Case–control | 536 (107 farmers) lung cancer cases and 4,217 (1,023 farmers) controls. All men between 25 and 75 years from Alberta, Canada | – | Identified from the local cancer registry | Patients with other types of cancer from the same registry | Occupation | Age, sex, smoking, alcohol consumption |
Crude: OR = 0.78 (0.62–0.97) Adjusted for age and smoking: OR = 0.81 (0.65–1.02) |
Authors reported consistency and attributed the lower risk to the lower proportion of smokers among the farming population |
| Forastiere et al. [45] | Case–control | 508 (263 farmers) cases and 462 (274 farmers) controls, all male aged 35–80 years from Viterbo, Italy during 1980–1986 | – | Identified from the regional registry | Randomly sampled from the death registry | Occupation, duration (<10, >10 years), crop cultivation (wheat, crapes, olives, hazelnuts, fruits, potatoes, corn, strawberries) | Age, sex duration (only for type specific analysis) | OR: Overall: 0.76 (0.61–0.96). Duration: <10 years: 0.70 (0.52–0.93), >10 years: 0.81 (0.62–1.05). Cultivation: wheat, 0.79; crapes, 0.90; olives, 0.72; hazelnuts, 1.17; fruits, 0.88; potatoes, 1.18; corn, 0.92; strawberries, 0.89. All non-significant | The authors reported consistency with previous studies and suggested further investigation |
| Jahn et al. [84] | Case–control | 686 (128 farmers and agriculture workers) cases and 712 (125 farmers and agriculture workers) controls, all German women | – | Histologically and/or cytologically confirmed | Randomly selected from the local population | Occupation | Age, region, smoking, time since smoking cessation | Farmers, agricultural workers: OR = 1.2 (0.88–1.72). Fishing, forestry, farming, and horticulture: OR = 1.3 (0.92–1.75) | Due to the large number of occupations included, authors focused on causal factors that showed significant elevated risks |
| Pezzotto and Poletto [48] | Case–control | 367 (54 agriculture workers) lung cancer cases and 586 (66 agriculture workers) controls. All males from 3 medical institutions of Rosario, Argentina | – | Identified from the hospital records. All histologically confirmed and reviewed with ICD-O | Patients with non-smoking related diseases from the same hospitals | Occupation, duration (<33, >33 years) | Age, sex, smoking, and lifelong cigarette consumption |
Overall: OR = 1.8 (1.1–3.1) Duration: OR = 1.1 (0.4–3.1) and OR = 1.9 (1.1–3.6) for <33 and >33 years of employment, respectively (p fro trend < 0.05) |
Authors reported consistency with previous studies |
| Settimi et al. [46] | Case–control | 24 cases (8 employed in farming) and 897 (229 employed in farming) controls. All females hospitalized in 3 regions of Italy during 1990–1992 | – | Identified from the hospital records; histologically and cytologically confirmed | Other cancer patients excluding those with Bladder cancer | Occupation, duration (1–9, 10–19, >20 years), 6 types of crops: wheat, vine, vegetables and fruit trees. Reference group: administrative staff workers | Age, sex, smoking, family history of lung cancer | Overall: 1.7 (0.7–4.4), Type: OR = 0.3 (0.1–2.0), 4.1 (0.7–24.0), 1.3 (0.1–1.6), and 2.5 (0.7–9.5) for wheat, vine, vegetable and fruit tree growers, respectively. Duration: OR = 1.0 (0.1–8.1), 1.0 (0.1–8.7), and 2.3 (0.8–6.6) for 1–9, 10–19 and >20 years, respectively | Authors mentioned inconsistency with previous studies that showed low risk of lung cancer and consistency with other studies in agricultural related populations. The low risk among wheat farmers was attributed to lower chemical use |
| Matos et al. [49] | Case–control | 216 (36 agriculture workers) lung cancer cases and 397 (66 agriculture workers) control subjects. All males from 4 hospitals of Buenos Aires, Argentina | – | Identified from the hospital medical records | Patients with non-smoking related diseases and neoplasms | Occupation, Job title (1 major title: agriculture and animal husbandry workers and 2 subtitles: Agriculture and Field crop workers) | Age, sex, hospital, pack-years, industries |
Occupation: OR = 1.7 (1.0–2.8) Job title: agriculture and animal husbandry: OR = 1.6 (0.9–2.7), Agriculture: OR = 2.4 (0.9–6.1), Field crop workers: OR = 3.5 (1.1–10.5) |
Authors reported consistency with previous studies |
| Baccarelli et al. [47] | Case–control | 474 (50 exp. to grain, 32 to non-grain agriculture dust) men and 66 (5 exp. to non-grain dust) women lung cancer cases and 453 (48 exp. to grain, 22 to non-grain dust) men and 129 (15 exp. to non-grain dust) women controls. All from 88 hospitals of Leningrad, Russia | – | Pathologically diagnosed lung cancer cases identified from the hospital autopsy records | Deceased subjects with autopsy based diagnosis of non-cancer and non-smoking-related diseases | Industry (type of dust), duration (<10,>10 years), Average intensity (below or above 75% of the related MAC), cumulative exposure score (2 levels: <5 and >5 calculated as the product of average intensity score per total duration) | Age, sex, smoking, region of residence |
Grain dust: Men: Overall: OR = 0.94 (0.60-1.46) Women: Non-grain agriculture dust: Men: Overall: OR = 1.24 (0.68-2.26). Women: Overall: OR = 2.43 (0.67-8.82) |
As the analysis took place only to the overall level and due to the non-significant results, the authors paid little attention to the grain and to the non-grain agriculture dust |
| Gallagher et al. [31] | Proportionate mortality study |
254,901 deaths of males (28,032 farmers) in British Columbia, Canada Surveillance period: 1950–1978 |
742 | Identified from the provincial death registry using ICD-7 | Deaths due to the same cause in all non-farming occupations | Occupation | Age, sex | PMR = 0.66 (0.67–0.71) | Authors attributed the lower rates among farmers to the lower levels of alcohol consumption and smoking among farmers |
| Saftlas et al. [32] | Proportionate mortality study | 35,972 deaths of white male farmers aged >18 from Wisconsin, US. Surveillance period: 1968–1976 | 760 | Identified from the local registry office using ICD-8 | Deaths of males among all non-farmers in Wisconsin | Occupation | Age, sex, race | PMR = 0.52 (p < 0.05) | Authors attributed the lower rates among farmers to the lower levels of alcohol consumption and smoking among farmers |
| Une et al. [33] | Proportionate mortality study | 2,820 deaths of male farmers aged 35 to 84 (1,415 whites and 1,405 non-whites). From South Carolina, US. Surveillance period: 1983–1984 | 198 | Identified from the state registry office using ICD-9 | 23,129 deaths of non-farmers during the same period and from the same area | Occupation | Age, sex, race |
White: PMR = 0.80 (p < 0.05) Non-white: PMR = 0.87 (p>0.05) |
Authors reported consistency with previous studies and attributed the lower rates among farmers to the lower levels of alcohol consumption and smoking among farmers |
| Blair et al. [34] | Proportionate mortality study | 119,648 deaths of white men, 2,400 of white women,11,446 of non-white men and 2,066 of non-white women. All farmers from 23 US states. Surveillance period: 1984–1988 | 7,218 | Identified from the regional registry | The proportion of deaths due to the same cause among non-farmers in the specific 23 US states | Occupation | Age, sex, race | PMR: 0.87 (0.85–0.89), 0.82 (0.62–1.06), 0.98 (0.90–1.05) and 0.60 (0.38–0.89) for white male, white female, non-white male and non-white female farmers, respectively | Authors attributed the lower rates among farmers to the lower levels of alcohol consumption and smoking among farmers |
| Keller and Howe [35] | Proportionate mortality study + Case–control | All 21,186 cases of cancer among males reported to the ISCR during 1986–1988. (Complete information available only for 9,514). | 290 | Identified from the Illinois State Cancer Registry (ISCR) | Lung cancer among other occupations for both PMR and case–control | Occupation | Age, sex, race, smoking |
PMR = 0.80 (p < 0.01) Case–control: OR = 0.71 (0.62–0.83) |
Authors reported consistency with previous studies and concluded that other factors than smoking might also contribute to the low rate of lung cancer in farmers |
| Cerhan et al. [36] | Proportionate mortality study. |
88,090 deaths of white males aged >20 years from Iowa, US. Surveillance period: 1987–1993. |
1,412 | Identified from the Iowa State registry using ICD-9. | The proportion of deaths due to the same cause among non-farmers decedents. | Occupation | Age, sex | Overall: PMR = 0.70 (0.66–0.73) | Authors reported consistency and attributed the low rates to lower smoking and alcohol consumption by farmers |
| Colt et al. [37] | Proportionate mortality study. | 26,148 deaths of farm workers of 24 US states. Surveillance period: 1984–1993 | 1,546 | Identified from each State’s registry using ICD-9 | The proportion of deaths due to the same cause among all decedents in the specific 24 US states | Occupation | Age, sex, race | Overall: PCMR = 102 (97–107), White male: PCMR = 101 (94–107), White female: PCMR = 106 (97–116), Non-White male: PCMR = 124 (96–1570), Non-white female: PCMR = 79 (60–102) | Due to the many causes of death investigated and the non-significant results for lung cancer, authors paid little attention to these results |
| Lange et al. [38] | Proportionate mortality study | 229,549 and 44,930 deaths of crop and livestock US farmers, respectively. Surveillance period: 1984–1993 |
Crop: 12,091 Livestock: 2,201 |
Identified from the National occupational mortality surveillance using ICD-9 | The proportion of deaths due to the same cause among all database decedents | 2 types of farming: crop and livestock | Age, sex, race, smoking | Crop farmers: overall: RMR = 0.80 (0.78–0.81). Livestock farmers: PRM = 0.70 (0.67–0.73) | Authors considering also results from exposure assessment studies suggested that increasing exposure to endotoxin decreases the risk of lung cancer |
SMR standardized mortality ratio, SIR standardized incidence ratio, RR relative risk, OR odds ratio, HR hazard ratio, PMR proportionate mortality ratio, MR mortality ratio, HWE healthy worker effect
Appendix 2
See Table 6.
Table 6.
Quality assessment of the reviewed epidemiological studies on the effect of endotoxin to the development of lung cancer
| Reference | Design | Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure assessment | Relevant reference group? | Sufficient follow-up time? | Sufficient description of the inclusion criteria? | Adjustment for confounders | Sufficient description of the used statistical methods? | Excluded from the meta-analysis | |||||
| Industry | Tasks | Exposure Estimates | Smoking | Other | |||||||
| Textile industry | |||||||||||
| Enterline [19] | Cohort | Yes* | No | No | Yes | Yes | Yes | No | No | Yes | No§ |
| Henderson and Enterline [1] | Cohort | Yes* | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Buiatti et al. [51] | Cohort | Yes* | Yes | No | Yes | Yes | No | No | No | No | Yes |
| Merchant and Ortmeyer [60] | Cohort | Yes* | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Hodgson and Jones [66] | Cohort | Yes* | No | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Koskela et al. [61] | Cohort | Yes* | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Szeszenia-Dabrowska et al. [62] | Cohort | Yes* | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Wernli et al. [21] | Cohort | Yes* | Yes | No | Yes | Yes | Yes | No | No | Yes | No§ |
| Fritschi et al. [63] | Cohort | Yes* | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Kuzmickiene et al. [22] | Cohort | Yes* | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Laakkonen et al. [24] | Cohort | No | No | Yes | Yes | Yes | Yes | Yes | Social class | Yes | Yes|| |
| Kuzmickiene and Stukonis [64] | Cohort | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No§ |
| Mastrangelo et al. [65] | Cohort | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Astrakianakis et al. [67] | Case–cohort | No | No | Yes | Yes | – | Yes | Yes | No | Yes | No |
| Siemiatycki et al. [41] | Case–control | Yes* | No | No | No‡ | – | Yes | Yes | Social and economical status, accuracy of job history | Yes | Yes |
| Levin et al. [68] | Case–control | Yes* | Yes | No | Yes | – | Yes | Yes | No | Yes | No |
| Levin et al. [85] | Case–control | Yes* | No | No | Yes | – | Yes | Yes | No | Yes | No |
| Wu-Williams et al. [69] | Case–control | Yes | Yes | No | Yes | – | Yes | Yes | Study area, education level | Yes | No |
| Wang et al. [42] | Case–control | Yes | No | No | No‡ | – | Yes | No | No | Yes | Yes |
| Jahn et al. [84] | Case–control | Yes | No | No | Yes | – | Yes | Yes | Region, time since smoking cessation | Yes | Yes|| |
| Baccarelli et al. [47] | Case–control | Yes* | No | Yes | No | – | Yes | Yes | Region of residence | Yes | Yes |
| Delzell and Grufferman [27] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Roman et al. [28] | Proportionate mortality study | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Dubrow and Gute [29] | Proportionate mortality study | Yes* | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| O’Brien and Decoufle [30] | Proportionate mortality study | Yes | No | No | Yes | Yes | No | No | No | Yes | Yes |
| Agriculture industry | |||||||||||
| Burmeister [70] | Cohort+proportionate mortality study | Yes | No | No | Yes | Yes | No | No | No | Yes | No |
| Wiklund [20] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No§ |
| Wiklund and Steineck [73] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Rafnsson and Gunnarsdottir [86] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Stark et al. [79] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Gunnarsdottir and Rafnsson 1991 | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Alberghini et al. [74] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Ronco et al. [71] | Cohort + MOR analysis | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Faustini et al. [83] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Wiklund and Dich [72] | Cohort | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Mastrangelo et al. [2] | Cohort | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Pukkala and Notkola [23] | Cohort | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No§ |
| Sperati et al. [75] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Wang et al. [76] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Alavanja et al. [80] | Cohort | Yes | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Blair et al. [87] | Cohort | Yes | Yes | No | Yes | Yes | Yes | Yes | State | Yes | No |
| Mastrangelo et al. [82] | Cohort + case–control | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Laakkonen et al. [24] | Cohort | No | No | Yes | Yes | Yes | Yes | Yes | Social class | Yes | No§ |
| Lee et al. [77] | Cohort | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | No |
| Laakkonen and Pukkala [78] | Cohort | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | No |
| Siemiatycki et al. [41] | Case–control | Yes | No | No | No‡ | – | Yes | Yes | Social and economical status, accuracy of job history | Yes | Yes |
| Levin et al. [85] | Case–control | Yes | No | No | Yes | – | Yes | Yes | No | Yes | No |
| Brownson et al. [43] | Case–control | Yes | No | No | No‡ | – | Yes | No | No | Yes | Yes |
| Reif et al. [40] | Case–control | Yes | Yes | No | No‡ | – | Yes | No | No | Yes | Yes |
| Fincham et al. [44] | Case–control | Yes | No | No | No‡ | – | No | Yes | Alcohol use | Yes | Yes |
| Forastiere et al. [45] | Case–control | Yes | Yes | No | No‡ | – | No† | No | No | Yes | Yes |
| Jahn et al. [84] | Case–control | Yes | No | No | Yes | – | Yes | Yes | Region, time since smoking cessation | Yes | No |
| Pezzotto and Poletto [48] | Case–control | Yes | No | No | No | – | Yes | Yes | No | Yes | Yes |
| Settimi et al. [46] | Case–control | Yes | Yes | No | No‡ | – | Yes | Yes | Family history in lung cancer | Yes | Yes |
| Matos et al. [49] | Case–control | Yes | Yes | No | No | – | No | Yes | Hospital, industries | Yes | Yes |
| Baccarelli et al. [47] | Case–control | Yes* | No | Yes | No | – | Yes | Yes | Region of residence | Yes | Yes |
| Gallagher et al. [31] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Saftlas et al. [32] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Une et al. [33] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Blair et al. [34] | Proportionate mortality study | Yes | No | No | Yes | Yes | No† | No | No | Yes | Yes |
| Keller and Howe [35] | Proportionate mortality study +Case–control | Yes | No | No | No‡ | – | No† | Yes | No | Yes | Yes |
| Cerhan et al. [36] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Colt et al. [37] | Proportionate mortality study | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes |
| Lange et al. [38] | Proportionate mortality study | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
* Clearly stated that cotton industry workers were included
† Definition of lung cancer cases and/or selection is missing
‡ Reference group consisted from patients with other types of cancer
§ Studies that fulfilled the quality criteria but were superseded by more recent publications were excluded from the meta-analysis
|| Study subjects not clearly restricted to cotton textile workers
References
- 1.Henderson V, Enterline PE. An unusual mortality experience in cotton textile workers. J Occup Med. 1973;15:717–719. [PubMed] [Google Scholar]
- 2.Mastrangelo G, Marzia V, Marcer G. Reduced lung cancer mortality in dairy farmers: is endotoxin exposure the key factor? Am J Ind Med. 1996;30:601–609. doi: 10.1002/(SICI)1097-0274(199611)30:5<601::AID-AJIM8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Mastrangelo G, Fedeli U, Fadda E, Milan G, Lange JH. Epidemiologic evidence of cancer risk in textile industry workers: a review and update. Toxicol Ind Health. 2002;18:171–181. doi: 10.1191/0748233702th139rr. [DOI] [PubMed] [Google Scholar]
- 4.Lange JH. Reduced cancer rates in agricultural workers: a benefit of environmental and occupational endotoxin exposure. Med Hypotheses. 2000;55:383–385. doi: 10.1054/mehy.2000.1072. [DOI] [PubMed] [Google Scholar]
- 5.Enterline PE, Sykora JL, Keleti G, Lange JH. Endotoxin, cotton dust, and cancer. Lancet. 1985;2:934–935. doi: 10.1016/s0140-6736(85)90861-x. [DOI] [PubMed] [Google Scholar]
- 6.Liebers V, Brüning T, Raulf-Heimsoth M. Occupational endotoxin-exposure and possible health effects on humans. Am J Ind Med. 2006;49:474–491. doi: 10.1002/ajim.20310. [DOI] [PubMed] [Google Scholar]
- 7.Reisser D, Pance A, Jeannin J. Mechanisms of the antitumoral effect of lipid A. BioEssays. 2002;24:284–289. doi: 10.1002/bies.10053. [DOI] [PubMed] [Google Scholar]
- 8.Otto F, Schmid P, Mackensen A, et al. Phase II trial of intravenous endotoxin in patients with colorectal and non-small cell lung cancer. Eur J Cancer. 1996;32A:1712–1718. doi: 10.1016/0959-8049(96)00186-4. [DOI] [PubMed] [Google Scholar]
- 9.Chicoine MR, Won EK, Zahner MC. Intratumoral injection of lipopolysaccharide causes regression of subcutaneously implanted mouse glioblastoma multiforme. Neurosurgery. 2001;48:607–614. doi: 10.1097/00006123-200103000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Pance A, Reisser D, Jeannin JF. Antitumoral effects of lipid A: preclinical and clinical studies. J Investig Med. 2002;50:173–178. doi: 10.2310/6650.2002.33430. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 12.Liebers V, Raulf-Heimsoth M, Brüning T. Health effects due to endotoxin inhalation (review) Arch Toxicol. 2008;82:203–210. doi: 10.1007/s00204-008-0290-1. [DOI] [PubMed] [Google Scholar]
- 13.Su WL, Chen YH, Liou SH, Wu CP. Meta-analysis of standard mortality ratio in cotton textile workers. Eur J Epidemiol. 2004;19:989–997. doi: 10.1007/s10654-004-0917-3. [DOI] [PubMed] [Google Scholar]
- 14.Blair A, Zahm SH. Cancer among farmers. Occup Med. 1991;6:335–354. [PubMed] [Google Scholar]
- 15.Acquavella J, Olsen G, Cole P, et al. Cancer among farmers: a meta-analysis. Ann Epidemiol. 1998;8:64–74. doi: 10.1016/s1047-2797(97)00120-8. [DOI] [PubMed] [Google Scholar]
- 16.Davis DL, Blair A, Hoel DG. Agricultural exposures and cancer trends in developed countries. Environ Health Perspect. 1993;100:39–44. doi: 10.1289/ehp.9310039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wouters IM, Spaan S, Douwes J, Doekes G, Heederik D. Overview of personal occupational exposure levels to inhalable dust, endotoxin, β(1 → 3)-glucan and fungal extracellular polysaccharides in the waste management chain. Ann Occup Hyg. 2006;50:39–53. doi: 10.1093/annhyg/mei047. [DOI] [PubMed] [Google Scholar]
- 18.Prazmo Z, Dutkiewicz J, Skórska C, Sitkowska J, Cholewa G. Exposure to airborne Gram-negative bacteria, dust and endotoxin in paper factories. Ann Agric Environ Med. 2003;10:93–100. [PubMed] [Google Scholar]
- 19.Enterline PE. Mortality among asbestos products workers in the United States. Ann N Y Acad Sci. 1965;132:156–165. doi: 10.1111/j.1749-6632.1965.tb41098.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiklund K. Swedish agricultural workers. A group with a decreased risk of cancer. Cancer. 1983;51:566–568. doi: 10.1002/1097-0142(19830201)51:3<566::aid-cncr2820510334>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Wernli KJ, Ray RM, Gao DL, Thomas DB, Checkoway H. Cancer among women textile workers in Shanghai, China: overall incidence patterns, 1989–1998. Am J Ind Med. 2003;44:595–599. doi: 10.1002/ajim.10265. [DOI] [PubMed] [Google Scholar]
- 22.Kuzmickiene I, Didziapetris R, Stukonis M. Cancer incidence in the workers cohort of textile manufacturing factory in Alytus, Lithuania. J Occup Environ Med. 2004;46:147–153. doi: 10.1097/01.jom.0000111601.85534.12. [DOI] [PubMed] [Google Scholar]
- 23.Pukkala E, Notkola V. Cancer incidence among Finnish farmers, 1979–93. Cancer Causes Control. 1997;8:25–33. doi: 10.1023/a:1018474919807. [DOI] [PubMed] [Google Scholar]
- 24.Laakkonen A, Kyyronen P, Kauppinen T, Pukkala E. Occupational exposure to eight organic dusts and respiratory cancer among Finns. Occup Environ Med. 2006;63:726. doi: 10.1136/oem.2005.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O’Connell D, et al. (2006) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed June 18 2008
- 26.Vlaanderen J, Vermeulen R, Heederik D, Kromhout H. Guidelines to evaluate human observation studies for quantitative risk assessment. Environ Health Perspect. 2008;116:1700–1705. doi: 10.1289/ehp.11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delzell E, Grufferman S. Cancer and other causes of death among female textile workers, 1976–78. J Natl Cancer Inst. 1983;71:735–740. [PubMed] [Google Scholar]
- 28.Roman E, Beral V, Inskip H. Occupational mortality among women in England and Wales. Br Med J (Clin Res Ed) 1985;291:194–196. doi: 10.1136/bmj.291.6489.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubrow R, Gute DM. Cause-specific mortality among male textile workers in Rhode Island. Am J Ind Med. 1988;13:439–454. doi: 10.1002/ajim.4700130404. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien TR, Decouflé P. Cancer mortality among northern Georgia carpet and textile workers. Am J Ind Med. 1988;14:15–24. doi: 10.1002/ajim.4700140104. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher RP, Threlfall WJ, Jeffries E, Band PR, Spinelli J, Coldman AJ. Cancer and aplastic anemia in British Columbia farmers. J Natl Cancer Inst. 1984;72:1311–1315. [PubMed] [Google Scholar]
- 32.Saftlas AF, Blair A, Cantor KP, Hanrahan L, Anderson HA. Cancer and other causes of death among Wisconsin farmers. Am J Ind Med. 1987;11:119–129. doi: 10.1002/ajim.4700110202. [DOI] [PubMed] [Google Scholar]
- 33.Une H, Schuman SH, Caldwell ST, Whitlock NH. Agricultural life-style: a mortality study among male farmers in South Carolina, 1983–1984. South Med J. 1987;80:1137–1140. [PubMed] [Google Scholar]
- 34.Blair A, Dosemeci M, Heineman EF. Cancer and other causes of death among male and female farmers from twenty-three states. Am J Ind Med. 1993;23:729–742. doi: 10.1002/ajim.4700230507. [DOI] [PubMed] [Google Scholar]
- 35.Keller JE, Howe HL. Case–control studies of cancer in Illinois farmers using data from the Illinois State Cancer Registry and the U.S. census of agriculture. Eur J Cancer. 1994;30A:469–473. doi: 10.1016/0959-8049(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 36.Cerhan JR, Cantor KP, Williamson K, Lynch CF, Torner JC, Burmeister LF. Cancer mortality among Iowa farmers: recent results, time trends, and lifestyle factors (United States) Cancer Causes Control. 1998;9:311–319. doi: 10.1023/a:1008877204830. [DOI] [PubMed] [Google Scholar]
- 37.Colt JS, Stallones L, Cameron LL, Dosemeci M, Zahm SH. Proportionate mortality among US migrant and seasonal farmworkers in twenty-four states. Am J Ind Med. 2001;40:604–611. doi: 10.1002/ajim.1126. [DOI] [PubMed] [Google Scholar]
- 38.Lange J, Mastrangelo G, Fedeli U, Fadda E, Rylander R, Lee E. Endotoxin exposure and lung cancer mortality by type of farming: is there a hidden dose–response relationship? Ann Agric Environ Med. 2003;10:229–232. [PubMed] [Google Scholar]
- 39.Decouflé P, Thomas TL, Pickle LW. Comparison of the proportionate mortality ratio and standardized mortality ratio risk measures. Am J Epidemiol. 1980;111:263–269. doi: 10.1093/oxfordjournals.aje.a112895. [DOI] [PubMed] [Google Scholar]
- 40.Reif J, Pearce N, Fraser J. Cancer risks in New Zealand farmers. Int J Epidemiol. 1989;18:768–774. doi: 10.1093/ije/18.4.768. [DOI] [PubMed] [Google Scholar]
- 41.Siemiatycki J, Richardson L, Gérin M, et al. Associations between several sites of cancer and nine organic dusts: results from an hypothesis-generating case–control study in Montreal, 1979–1983. Am J Epidemiol. 1986;123:235–249. doi: 10.1093/oxfordjournals.aje.a114232. [DOI] [PubMed] [Google Scholar]
- 42.Wang QS, Boffetta P, Parkin DM, Kogevinas M. Occupational risk factors for lung cancer in Tianjin, China. Am J Ind Med. 1995;28:353–362. doi: 10.1002/ajim.4700280305. [DOI] [PubMed] [Google Scholar]
- 43.Brownson RC, Reif JS, Chang JC, Davis JR. Cancer risks among Missouri farmers. Cancer. 1989;64:2381–2386. doi: 10.1002/1097-0142(19891201)64:11<2381::aid-cncr2820641131>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Fincham SM, Hanson J, Berkel J. Patterns and risks of cancer in farmers in Alberta. Cancer. 1992;69:1276–1285. doi: 10.1002/cncr.2820690534. [DOI] [PubMed] [Google Scholar]
- 45.Forastiere F, Quercia A, Miceli M, et al. Cancer among farmers in central Italy. Scand J Work Environ Health. 1993;19:382–389. doi: 10.5271/sjweh.1458. [DOI] [PubMed] [Google Scholar]
- 46.Settimi L, Comba P, Carrieri P, et al. Cancer risk among female agricultural workers: a multi-center case–control study. Am J Ind Med. 1999;36:135–141. doi: 10.1002/(sici)1097-0274(199907)36:1<135::aid-ajim19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 47.Baccarelli A, Khmelnitskii O, Tretiakova M, et al. Risk of lung cancer from exposure to dusts and fibers in Leningrad Province, Russia. Am J Ind Med. 2006;49:460–467. doi: 10.1002/ajim.20316. [DOI] [PubMed] [Google Scholar]
- 48.Pezzotto SM, Poletto L. Occupation and histopathology of lung cancer: a case–control study in Rosario, Argentina. Am J Ind Med. 1999;36:437–443. doi: 10.1002/(sici)1097-0274(199910)36:4<437::aid-ajim4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 49.Matos EL, Vilensky M, Mirabelli D, Boffetta P. Occupational exposures and lung cancer in Buenos Aires, Argentina. J Occup Environ Med. 2000;42:653–659. doi: 10.1097/00043764-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Steenland K. Studies in occupational epidemiology. New York: Oxford University Press, Inc.; 1993. [Google Scholar]
- 51.Buiatti E, Baccetti S, Cecchi F, Tomassini A, Dolara P. Evidence of increased lung cancer rate among textile workers. Med Lav. 1979;70:21–23. [PubMed] [Google Scholar]
- 52.Breslow NE, Day NE. Statistical methods in cancer research. Volume II-The design and analysis of cohort studies. IARC Sci Publ. 1987;82:103–105. [PubMed] [Google Scholar]
- 53.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 55.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 56.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 57.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 59.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merchant JA, Ortmeyer C. Mortality of employees of two cotton mills in North Carolina. Chest. 1981;79:6S–11S. doi: 10.1378/chest.79.4_supplement.6s. [DOI] [PubMed] [Google Scholar]
- 61.Koskela RS, Klockars M, Järvinen E. Mortality and disability among cotton mill workers. Br J Ind Med. 1990;47:384–391. doi: 10.1136/oem.47.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szeszenia-Dabrowska N, Wilczynska U, Strzelecka A, Sobala W. Mortality in the cotton industry workers: results of a cohort study. Int J Occup Med Environ Health. 1999;12:143–158. [PubMed] [Google Scholar]
- 63.Fritschi L, Lakhani R, Nadon L. Cancer incidence in textile manufacturing workers in Australia. J Occup Health. 2004;46:493–496. doi: 10.1539/joh.46.493. [DOI] [PubMed] [Google Scholar]
- 64.Kuzmickiene I, Stukonis M (2007) Lung cancer risk among textile workers in Lithuania. J Occup Med Toxicol 2: doi:10.1186/1745-6673-2-14 (online 16 Nov 2007) [DOI] [PMC free article] [PubMed]
- 65.Mastrangelo G, Fadda E, Rylander R, et al. Lung and other cancer site mortality in a cohort of Italian cotton mill workers. Occup Environ Med. 2008;65:697–700. doi: 10.1136/oem.2007.036327. [DOI] [PubMed] [Google Scholar]
- 66.Hodgson JT, Jones RD. Mortality of workers in the British cotton industry in 1968–1984. Scand J Work Environ Health. 1990;16:113–120. doi: 10.5271/sjweh.1809. [DOI] [PubMed] [Google Scholar]
- 67.Astrakianakis G, Seixas NS, Ray R, et al. Lung cancer risk among female textile workers exposed to endotoxin. J Natl Cancer Inst. 2007;99:357–364. doi: 10.1093/jnci/djk063. [DOI] [PubMed] [Google Scholar]
- 68.Levin L, Gao Y, Blot W, Zheng W, Fraumeni JJ. Decreased risk of lung cancer in the cotton textile industry of Shanghai. Cancer Res. 1987;47:5777–5781. [PubMed] [Google Scholar]
- 69.Wu-Williams AH, Xu ZY, Blot WJ, et al. Occupation and lung cancer risk among women in northern China. Am J Ind Med. 1993;24:67–79. doi: 10.1002/ajim.4700240107. [DOI] [PubMed] [Google Scholar]
- 70.Burmeister L. Cancer mortality in Iowa farmers, 1971–78. J Natl Cancer Inst. 1981;66:461–464. [PubMed] [Google Scholar]
- 71.Ronco G, Costa G, Lynge E. Cancer risk among Danish and Italian farmers. Br J Ind Med. 1992;49:220–225. doi: 10.1136/oem.49.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiklund K, Dich J. Cancer risks among female farmers in Sweden. Cancer Causes Control. 1994;5:449–457. doi: 10.1007/BF01694759. [DOI] [PubMed] [Google Scholar]
- 73.Wiklund K, Steineck G. Cancer in the respiratory organs of Swedish farmers. Cancer. 1988;61:1055–1058. doi: 10.1002/1097-0142(19880301)61:5<1055::aid-cncr2820610534>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 74.Alberghini V, Luberto F, Gobba F, Morelli C, Gori E, Tomesani N. Mortality among male farmers licensed to use pesticides. Med Lav. 1991;82:18–24. [PubMed] [Google Scholar]
- 75.Sperati A, Rapiti E, Settimi L, Quercia A, Terenzoni B, Forastiere F. Mortality among male licensed pesticide users and their wives. Am J Ind Med. 1999;36:142–146. doi: 10.1002/(sici)1097-0274(199907)36:1<142::aid-ajim20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Lewis-Michl EL, Hwang SA, Fitzgerald EF, Stark AD. Cancer incidence among a cohort of female farm residents in New York State. Arch Environ Health. 2002;57:561–567. doi: 10.1080/00039890209602089. [DOI] [PubMed] [Google Scholar]
- 77.Lee DJ, Fleming LE, LeBlanc WG, et al. Occupation and lung cancer mortality in a nationally representative US cohort: The National Health Interview Survey (NHIS) J Occup Environ Med. 2006;48:823–832. doi: 10.1097/01.jom.0000225137.19863.4e. [DOI] [PubMed] [Google Scholar]
- 78.Laakkonen A, Pukkala E. Cancer incidence among Finnish farmers, 1995–2005. Scand J Work Environ Health. 2008;34:73–79. doi: 10.5271/sjweh.1167. [DOI] [PubMed] [Google Scholar]
- 79.Stark AD, Chang HG, Fitzgerald EF, Riccardi K, Stone RR. A retrospective cohort study of cancer incidence among New York State Farm Bureau members. Arch Environ Health. 1990;45:155–162. doi: 10.1080/00039896.1990.9936709. [DOI] [PubMed] [Google Scholar]
- 80.Alavanja MC, Sandler DP, Lynch CF, et al. Cancer incidence in the agricultural health study. Scand J Work Environ Health. 2005;31(Suppl 1):39–45. [PubMed] [Google Scholar]
- 81.Gunnarsdóttir H, Rafnsson V. Cancer incidence among Icelandic farmers 1977–1987. Scand J Soc Med. 1991;19:170–173. doi: 10.1177/140349489101900305. [DOI] [PubMed] [Google Scholar]
- 82.Mastrangelo G, Grange JM, Fadda E, Fedeli U, Buja A, Lange JH. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161:1037–1046. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 83.Faustini A, Forastiere F, Di Betta L, Magliola EM, Perucci CA. Cohort study of mortality among farmers and agricultural workers. Med Lav. 1993;84:31–41. [PubMed] [Google Scholar]
- 84.Jahn I, Ahrens W, Brüske-Hohlfeld I, et al. Occupational risk factors for lung cancer in women: results of a case–control study in Germany. Am J Ind Med. 1999;36:90–100. doi: 10.1002/(sici)1097-0274(199907)36:1<90::aid-ajim13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 85.Levin LI, Zheng W, Blot WJ, Gao YT, Fraumeni JF., Jr Occupation and lung cancer in Shanghai: a case–control study. Br J Ind Med. 1988;45:450–458. doi: 10.1136/oem.45.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rafnsson V, Gunnarsdottir H. Mortality among farmers in Iceland. Int J Epidemiol. 1989;18:146–151. doi: 10.1093/ije/18.1.146. [DOI] [PubMed] [Google Scholar]
- 87.Blair A, Sandler DP, Tarone R, et al. Mortality among participants in the Agricultural Health Study. Ann Epidemiol. 2005;15:279–285. doi: 10.1016/j.annepidem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Astrakianakis G, Seixas NS, Camp JE, et al. Modeling, estimation and validation of cotton dust and endotoxin exposures in Chinese textile operations. Ann Occup Hyg. 2006;50:573–582. doi: 10.1093/annhyg/mel018. [DOI] [PubMed] [Google Scholar]
- 89.Spaan S, Schinkel J, Wouters IM, et al. Variability in endotoxin exposure levels and consequences for exposure assessment. Ann Occup Hyg. 2008;52:303–316. doi: 10.1093/annhyg/men024. [DOI] [PubMed] [Google Scholar]
- 90.Simpson JCG, Niven RML, Pickering CAC, Oldham LA, Fletcher AM, Francis HC. Comparative personal exposures to organic dusts and endotoxin. Ann Occup Hyg. 1999;43:107–115. [PubMed] [Google Scholar]
- 91.Lane SR, Nicholls PJ, Sewell RDE. The measurement and health impact of endotoxin contamination in organic dusts from multiple sources: focus on the cotton industry. Inhal Toxicol. 2004;16:217–229. doi: 10.1080/08958370490277164. [DOI] [PubMed] [Google Scholar]
- 92.Olenchock SA, Christiani DC, Mull JC, Ye TT, Lu PL. Endotoxins in baled cottons and airborne dusts in textile mills in the People’s Republic of China. Appl Environ Microbiol. 1983;46:817–820. doi: 10.1128/aem.46.4.817-820.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mehta AJ, Wang XR, Eisen EA, et al. Work area measurements as predictors of personal exposure to endotoxin and cotton dust in the cotton textile industry. Ann Occup Hyg. 2008;52:45–54. doi: 10.1093/annhyg/mem061. [DOI] [PubMed] [Google Scholar]
- 94.Marchand G, Lalonde M, Beaudet Y, Boivin G, Villeneuve S, Pépin C. Documentation of the endotoxins present in the ambient air of cotton fiber textile mills in Québec. J Environ Monit. 2007;9:869–876. doi: 10.1039/b704087c. [DOI] [PubMed] [Google Scholar]
- 95.Oldenburg M, Latza U, Baur X. Exposure–response relationship between endotoxin exposure and lung function impairment in cotton textile workers. Int Arch Occup Environ Health. 2007;80:388–395. doi: 10.1007/s00420-006-0145-0. [DOI] [PubMed] [Google Scholar]
- 96.Su HJJ, Chen HL, Huang CF, Lin CY, Li FC, Milton DK. Airborne fungi and endotoxin concentrations in different areas within textile plants in Taiwan: a 3-year study. Environ Res. 2002;89:58–65. doi: 10.1006/enrs.2002.4345. [DOI] [PubMed] [Google Scholar]
- 97.Bakirci N, Kalaca S, Francis H, et al. Natural history and risk factors of early respiratory responses to exposure to cotton dust in newly exposed workers. J Occup Environ Med. 2007;49:853–861. doi: 10.1097/JOM.0b013e3180dca598. [DOI] [PubMed] [Google Scholar]
- 98.Hours M, Févotte J, Lafont S, Bergeret A. Cancer mortality in a synthetic spinning plant in Besançon, France. Occup Environ Med. 2007;64:575–581. doi: 10.1136/oem.2006.028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kateman E, Heederik D, Pal TM, Smeets M, Smid T, Spitteler M. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health. 1990;16:428–433. doi: 10.5271/sjweh.1764. [DOI] [PubMed] [Google Scholar]
- 100.Blair A, Stewart P, Lubin JH, Forastiere F. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50:199–207. doi: 10.1002/ajim.20281. [DOI] [PubMed] [Google Scholar]
- 101.Myers J, Thompson M. Meta-analysis and occupational epidemiology. Occup Med. 1998;48:99–101. doi: 10.1093/occmed/48.2.99. [DOI] [PubMed] [Google Scholar]
- 102.Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med. 1997;16:753–768. doi: 10.1002/(sici)1097-0258(19970415)16:7<753::aid-sim494>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 103.Berman NG, Parker RA. Meta-analysis: neither quick nor easy. BMC Med Res Methodol. 2002;2:10. doi: 10.1186/1471-2288-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schilling RS, Hughes JP, Dingwall-Fordyce I, Gilson JC. An epidemiological study of byssinosis among Lancashire cotton workers. Br J Ind Med. 1955;12:217–227. doi: 10.1136/oem.12.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christiani DC, Wegman DH, Eisen EA, Ye TT, Lu PL, Olenchock SA. Cotton dust and gram-negative bacterial endotoxin correlations in two cotton textile mills. Am J Ind Med. 1993;23:333–342. doi: 10.1002/ajim.4700230210. [DOI] [PubMed] [Google Scholar]
- 106.Wang XR, Zhang HX, Sun BX, et al. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J. 2005;26:881–886. doi: 10.1183/09031936.05.00125604. [DOI] [PubMed] [Google Scholar]
- 107.Rylander R. Endotoxin in the air: good or bad for you? Clin Pulm Med. 2007;14:140–147. [Google Scholar]