Abstract
The role of interferon-γ in autoimmune diabetes was assessed by breeding a null mutation of the interferon-γ receptor α chain into the nonobese diabetic mouse strain, as well as into a simplified T cell receptor transgenic model of diabetes. In contrast to a previous report on abrogation of the interferon-γ gene, mutation of the gene encoding its receptor led to drastic effects on disease in both mouse lines. Nonobese diabetic mice showed a marked inhibition of insulitis—both the kinetics and penetrance—and no signs of diabetes; the transgenic model exhibited near-normal insulitis, but this never evolved into diabetes, either spontaneously or after experimental provocation. This failure could not be explained by perturbations in the ratio of T helper cell phenotypes; rather, it reflected a defect in antigen-presenting cells or in the islet β cell targets.
Keywords: nonobese diabetic mice, knock-out mice, autoimmune disease, cytokine
Insulin-dependent diabetes mellitus (IDDM) is an autoimmune disease characterized by specific destruction of the insulin-producing β cells of the islets of Langerhans of the pancreas (for reviews, see refs. 1 and 2). Many of the key features of this disease are mimicked in the nonobese diabetic (NOD) strain of mice, rendering it a precious small-animal model (1, 2).
Diabetes in both human patients and NOD mice is a frustratingly complex disease. The genetics entail a constantly climbing number of loci, scattered on many chromosomes throughout the genome, although dominated by the major histocompatibility complex (for review, see ref. 3). Pathogenesis involves multiple stages: the initial provocation of islet-reactive lymphocytes, invasion of the islets by leukocytes (known as insulitis), and the final destruction of β cells (with ensuing diabetes). There is clear evidence that the different stages are discrete entities and that progression from one to the next is subject to regulation (see discussion in ref. 4). Not surprisingly, then, there appears to be a rich cast of cellular players. T lymphocytes, both CD4+ and CD8+, are considered major disease mediators (1, 2), but a critical role for B lymphocytes (5) and macrophages (6) also has been evidenced.
To circumvent this labyrinth complexity, several groups have generated transgenic variants of the NOD strain, highlighting a particular element of the immune system. One such strain is the BDC2.5 T cell receptor (TCR) transgenic (tg) line, which allows one to focus on the contribution of CD4+ T cells (4, 7). The BDC2.5 line carries the rearranged TCR genes from a diabetogenic CD4+ T cell clone isolated from the lymphoid organs of a diseased NOD mouse. The transgene-encoded specificity makes an exaggerated contribution to the peripheral T cell repertoire, avoiding thymic and peripheral deletion or anergy induction. The transgenic mice show no abnormalities at 2 weeks of age, a rampant insulitis beginning at 3 weeks, and eventually diabetes, although not before several months in NOD-background mice (7) (A.G., J.D.K., M. G. Mattei, H. Kikutami, C.B. and D.M., unpublished data). The relative simplicity of the BDC2.5 TCR tg model has permitted important insights into the genetics (Gonzalez et al., unpublished data) and pathogenesis (4, 7–9) of diabetes.
One experiment made possible by the BDC2.5 tg line was a direct test of the role of the T helper (Th)-1 subset of CD4+ cells in diabetes progression. Transfer of Th-1 but not Th-2 cells, both displaying the BDC2.5 specificity, into newborn NOD mice rapidly provoked diabetes. This result substantiated the notion that diabetes is primarily a Th-1 cell-mediated disease, corroborating a body of more indirect data (reviewed in refs. 10–12). The preeminence of Th-1 cells prompts one to question which of the characteristic properties of this subset make the most important contributions to disease and precisely how they act. An obvious candidate is secretion of interferon (IFN)-γ, the prototypical Th-1 cytokine. Treatment of NOD mice with an anti-IFN-γ monoclonal antibody (mAb) blocked the diabetes normally induced by cyclophosphamide injection or splenocyte transfer (13, 14), and localized overproduction of IFN-γ in the islets provoked disease (15). It was surprising, then, that introduction of a null mutation of the IFN-γ gene had such mild effects: normal generation of diabetogenic splenocytes, no effect on insulitis, and only a delay in diabetes (16).
Here, we present data on NOD and BDC2.5 TCR tg mice carrying an incapacitating mutation at the IFN-γ receptor (R) α locus, Ifngr. These findings corroborate the importance of IFN-γ in diabetes pathogenesis and provide information on the multiple means by which it exerts its influence.
MATERIALS AND METHODS
Mice.
The BDC2.5 transgenic line has been described (7); in the crosses described here, transgenic animals used as breeders were at the 17th backcross to NOD/Lt. Mice carrying the Ifngr-null mutation (17) originally were produced on a 129 genetic background. They were backcrossed with NOD mice for up to eight generations, with intercrosses to produce experimental animals (mutant −/− as well as +/− and +/+ control littermates) at the fourth and eighth generations (see figure legends). The mutation was followed in heterozygotes by Southern blots (BamHI digest of genomic DNA, probed with a 2-kb HindIII fragment from plasmid pSPHF1.9, revealing a wild-type band at 7.3 kb and a mutant band at 4.7 kb; ref.17). Control animals with the 129 allele of the wild-type Ifngr locus were generated by similar backcrosses, starting from 129/SV mice, after the segregation of the normal loci by Southern blot (HindIII digest of genomic DNA probed as above, yielding 2.5- and 1.9-kb bands for 129 and NOD alleles, respectively). After several backcrosses, the control line was intercrossed to generate animals with nod/nod, nod/129, or 129/129 genotypes at Ifngr.
Insulitis and Diabetes.
Insulitis was evaluated on hematoxylin/eosin-stained paraffin sections of pancreas, taken at several levels throughout the organ (>50 islets per mouse), as described (18). For insulitis on the straight NOD background, pancreata were dissected at 12 weeks of age; for insulitis in the context of the BDC2.5 transgene, at 5 weeks. Diabetes was monitored by the presence of glucose in urine (Uristix, Bayer, Tarrytown, NY), with confirmation by blood glucose measurements (Glucofilm strips read in a Glucometer 3, Bayer). Animals were considered diabetic when they gave positive readings in urine and when they had two consecutive blood glucose measurements above 250 mg/dl. Cyclophosphamide-induced diabetes in BDC2.5-positive transgenics was elicited by a single intraperitoneal injection of 200 mg/kg Cy (Sigma).
Cytokine and Cytokine mRNA Production.
Graded numbers of splenocytes were activated with Concanavalin-A (5 μg/ml) or in anti-CD3-coated plates (coated with 5 μg/ml purified KT3 monoclonal antibody (19) in 96-well plates. Supernatants were collected [at 24 h for interleukin 2 (IL-2), at 48 h for other cytokines] and tested for release of cytokines by bioassay (proliferation of the IL-2-dependent CTLL-2 line, see ref. 7) or by ELISA (IFN-γ, IL-10, IL-6) with monoclonal antibodies obtained from PharMingen, following the manufacturer’s protocol.
The evaluation of cytokine mRNA in islets will be described in detail elsewhere (I.A., unpublished data). Briefly, islets were picked after collagenase digestion of the pancreas, and total RNA was extracted with guanidinium thiocyanate and used for cDNA synthesis. mRNAs for various cytokines were amplified by PCR from graded amounts of this cDNA, and the products were detected on Southern blots and quantified by radio-imaging. The values in the linear portion of the dose-response curve were converted into arbitrary units and standardized by amplification of thymidine kinase and Thy-1 mRNAs in the same cDNA samples.
Transfer Experiments.
To transfer diabetes with BDC2.5 transgenic T cells, we followed largely the protocol of ref. 8. Ten-week-old BDC2.5-positive mice were used as donors (insulitic but nondiabetic). Splenocytes (4 × 108) were cultured in 200 ml RPMI 1640 medium, 10% FCS, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1 mM glutamine, and 5 μg/ml concanavalin-A in 175-cm2 flasks for 40 h at 37°C. Viable cells (107) were injected intraperitoneally into neonatal recipients (between 1 and 3 days of age).
RESULTS
Effect on Insulitis in NOD Mice.
To explore the role of IFN-γ in the development of diabetes, we first bred the Ifngr-null mutation onto the NOD background. This mutation abrogates expression of the IFN-γRα chain; consequently, the high-affinity receptor is not displayed at the cell surface and cells are unresponsive to IFN-γ (17). NOD/Ifngr−/− mice appeared outwardly ordinary and developed ostensibly normal immune systems, consistent with the original description of mixed background (B6 × 129) animals lacking IFN-γRα (17).
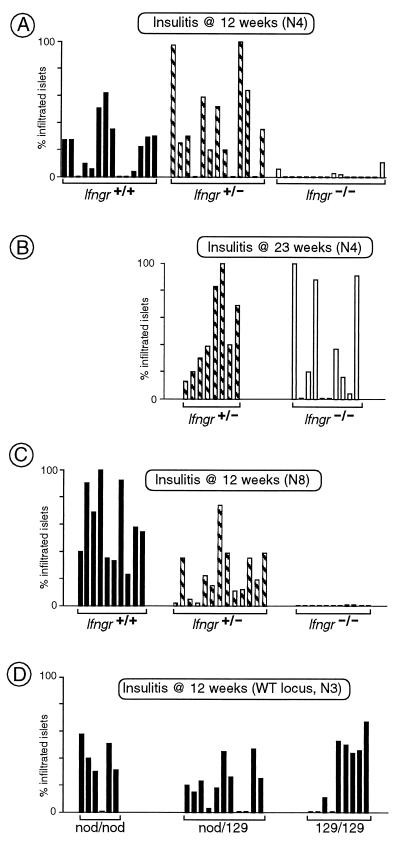
As illustrated in Fig. 1A, homozygous mutant mice backcrossed four generations (N4) onto the NOD background did not develop insulitis at 12 weeks of age, in sharp contrast to heterozygous mutant and wild-type littermates. Insulitis was delayed in the Ifngr−/− animals, rather than prevented, because some of a 23-week-old N4 cohort had clearly infiltrated islets (Fig. 1B). The dampening effect of the mutation was still evident, however, because some of the Ifngr−/− animals had no islet infiltration at this advanced age, which is never seen in Ifngr+ mice.
Figure 1.
IFN-γRα is necessary for efficient autoimmune insulitis. (A) Insulitis in NOD mice (at the fourth generation of backcross), homozygous for the Ifngr-null mutation, and heterozygous or wild-type controls. Pancreata were examined histologically at 12 weeks of age, and insulitis was scored on several sections for each mouse. Each bar represents an individual mouse and shows the percentage of islets displaying infiltration. (B) As in A, except that the mice were 23 weeks of age. (C) As in A, except that the intercross was set up after eight generations of backcross. (D) After three generations’ backcross to NOD of the Ifngr locus from the 129 inbred strain, a large cohort of littermates was produced by intercrossing, and mice of the three resulting genotypes were tested for insulitis as in A.
Although the difference in insulitis between mice expressing or not expressing IFN-γRα seemed clear, it was important to rule out potential artifacts resulting from the complex genetics of the disease in NOD mice. First, we made additional crosses onto the NOD background to minimize the non-NOD genetic material. Homozygous mutants at the eighth-generation backcross (N8) were also devoid of insulitis at 12 weeks (Fig. 1C). Second, we generated a control line by introducing the region surrounding the Ifngr locus from wild-type 129 mice (the source of the embryonic stem cells used for the targeting). This type of control line permits an assessment of the influence of allelic variants at loci surrounding the engineered mutation. Strain 129-derived genes in the vicinity of the Ifngr locus did not influence insulitis at 12 weeks whether they were in the heterozygous or homozygous state (Fig. 1D).
Effect on Insulitis in BDC2.5 TCR tg Mice.
The Ifngr-null mutation was also introduced into the BDC2.5 TCR tg line, in hopes that this simpler, exaggerated model would facilitate the dissection of IFN-γ’s mechanism of action. Interesting in this light are previous indications (18, 20) that BDC2.5 transgenic mice are immune to some of the factors critical at early stages of disease at the point of insulitis initiation.
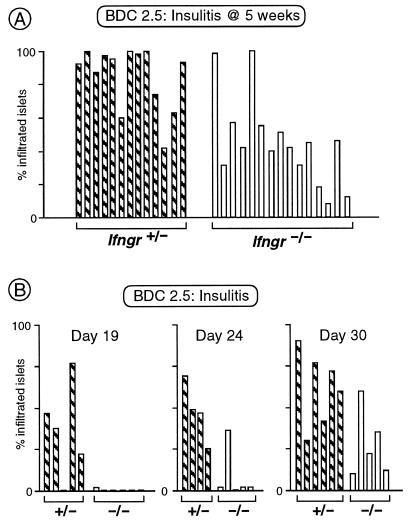
As documented in Fig. 2A, homozygous mutant BDC2.5 transgenics developed the usual impressive degree of insulitis at 5 weeks of age. It was also of typical appearance histologically (not shown). Insulitis was a bit delayed in the mutant animals, however. Ifngr−/− mice showed the first signs between 24 and 30 days of age, whereas Ifngr+ littermates already demonstrated significant infiltration at day 19 (Fig. 2B), the latter reflective of the kinetics of infiltration previously determined for wild-type animals (onset between day 15 and 18) (Gonzalez et al. unpublished data; F. Lühder, P. Höglund, J. P. Allison, C.B. and D.M., unpublished data).
Figure 2.
Absence of IFN-γRα delays insulitis in the context of the BDC2.5 transgene. (A) Insulitis scored as in Fig. 1 in littermates carrying the BDC2.5 TCR transgene, and heterozygous or homozygous for the Ifngr-null mutation (in 5-week-old mice, because insulitis progresses faster in the transgenic context). (B) The onset of insulitis was tested in littermates carrying the BDC2.5 TCR transgene, and heterozygous or homozygous for the Ifngr-null mutation, at three different ages.
Effect on Diabetes.
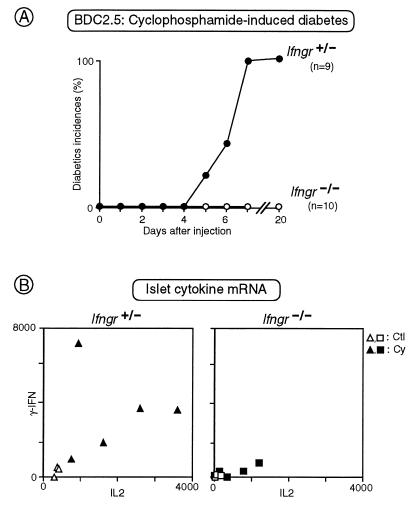
Spontaneous diabetes did not occur in Ifngr−/− mice. We did not perform a systematic study of disease but, among the intercross animals at various stages of backcross, 0 out of 14 homozygous mutants became diabetic, compared with 4 out of 18 heterozygous controls (in animals aged 30 weeks or more). For a more sensitive test, independent of the incidence of insulitis, we turned to cyclophosphamide-induced disease in BDC2.5 transgenics. It is possible to induce diabetes in both NOD and BDC2.5 tg animals by injection of cyclophosphamide but only in animals that have infiltrated islets (refs. 4 and 21; I.A., J.D.K., C.B. and D.M., unpublished data). Because BDC2.5 transgenic mice lacking IFN-γRα did show insulitis, we could hope to induce diabetes with cyclophosphamide. However, as illustrated in Fig. 3A, the Ifngr−/− animals were completely resistant to such provocation, as opposed to Ifngr+ littermates, 100% of which had succumbed by 7 days after treatment.
Figure 3.
IFN-γRα is required for cyclophosphamide-induced diabetes in BDC2.5 transgenics. (A) Littermates carrying the BDC2.5 TCR transgene, and heterozygous or homozygous for the Ifngr-null mutation, were treated with a single injection of cyclophosphamide, and diabetes was monitored in the following days. The cumulative incidence of diabetes is plotted here. (B) The amount of IL-2 and IFN-γ mRNAs present in islets were evaluated by semiquantitative PCR either before (open symbols) or after (solid symbols) treatment with cyclophosphamide, in littermates carrying the BDC2.5 TCR transgene, and heterozygous (Left) or homozygous (Right) for the Ifngr-null mutation. Each point represents an individual mouse; units are on an arbitrary scale.
Although cyclophosphamide has been a useful diabetes-inducing reagent for years, the precise mechanism of its action remains unknown. Because hyperglycemia develops during a narrow time window in cyclophosphamide-treated BDC2.5 transgenics (days 4 to 7 after injection), we have monitored the evolution of diverse parameters incipient to diabetes (ref. 4; I.A. et al., unpublished data). Two of the more notable changes are a striking increase in the aggressivity of insulitis (increased heterogeneity and invasiveness) and a pronounced local augmentation of mRNA transcripts of the genes encoding several of the inflammatory cytokines (IL-2, IFN-γ, IL-6, IL-1β). BDC2.5 TCR tg animals lacking IFN-γRα showed the typical evolution of insulitis after cyclophosphamide treatment (not shown). Their islets did show some increase in the levels of inflammatory cytokine gene transcripts, but this response was very muted compared with islets from control mice. This latter point is illustrated by the IFN-γ/IL-2 transcript plots in Fig. 3B; similar results were seen for IL-6 and IL-1β gene transcripts (not shown). Parenthetically, IL-4 transcript levels were marginally detectable both before and after cyclophosphamide induction in both Ifngr−/− and Ifngr+ mice.
A Defect in β cells or Antigen-Presenting Cells Rather Than T Cells.
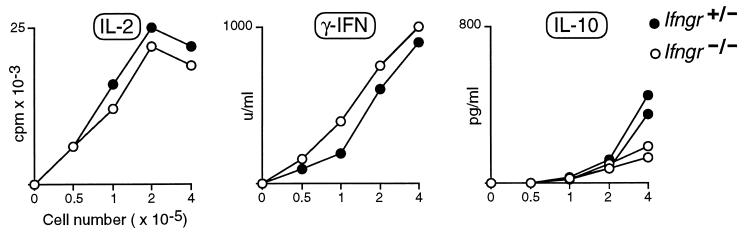
A popular notion, encouraged by some recent results but contradicted by others, is that the progression of insulitis to diabetes in NOD mice is regulated by the phenotype of invading T helper lymphocytes, Th-1 cells promoting a destructive insulitis that culminates in the obliteration of β cells, and Th-2 cells promoting a protective infiltration that inhibits β cell death (10–12). Following this idea, it might be supposed that the insulitis in Ifngr-null BDC2.5 TCR transgenics does not evolve into diabetes because the mutation inhibits the differentiation of Th-1 cells, enhances the differentiation of Th-2 cells, or both. This would not be supported by most previous studies on the Ifngr−/− mice, which demonstrated efficient development of Th-1 cells and no overproduction of Th-2 cells in a variety of contexts (16, 22–25). Nevertheless, we reexamined this point in the BDC2.5 TCR tg system. As illustrated by the representative experiment depicted in Fig. 4, splenocytes from mutant and wild-type animals secreted very similar levels of Th-1-derived cytokines after in vitro activation by anti-CD3. If anything, slightly more IFN-γ was produced in the receptor-null mice, a consistent finding in several experiments. IL-4 was produced at indistinguishable, barely detectable levels in the two cases (not shown); IL-10 was made at only slightly reduced levels by the mutant mice (Fig. 4). Similar results were obtained after Con A stimulation and in the presence or absence of the BDC2.5 transgenes. Thus, it would appear that the quiescence of the infiltrate in the Ifngr−/− TCR transgenics does not reflect a global imbalance in the Th-1/Th-2 cell ratio.
Figure 4.
Comparable cytokine production by splenocytes from IFN-γRα-deficient and control animals. Splenocytes from littermates heterozygous or homozygous for the Ifngr-null mutation were stimulated in vitro with anti-CD3, and the cytokines released into the medium evaluated by ELISA (IFN-γ, IL-10) or bioassay (IL-2). These profiles are representative of four different experiments, with or without the BDC2.5 transgene.
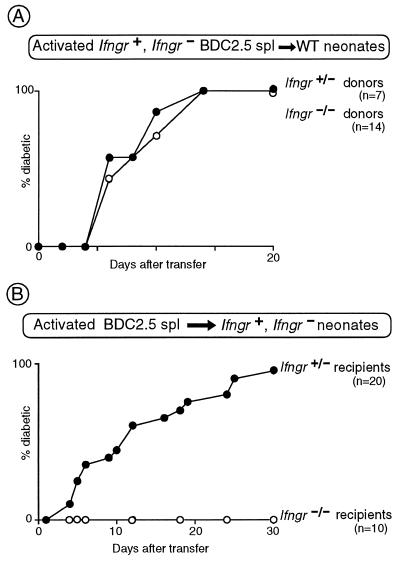
This result prompted us to question whether the defect even resided in the T cells, which could be directly tested in transfer experiments. Initially, Con A-activated splenocytes from 10-week-old (prediabetic) Ifngr+/− and Ifngr−/− BDC2.5 transgenics were injected into wild-type NOD neonates. The two donor populations were equally diabetogenic (Fig. 5A). In a reciprocal experiment, activated splenocytes from standard prediabetic BDC2.5 transgenics were introduced into either wild-type or mutant NOD neonates. Diabetes could not be induced in the mutant hosts, even though BDC2.5 T cells are fully capable of homing to the islets in the mutant strain (Fig. 2A). Clearly, then, the defect does not reside in the T cells; rather, IFN-γRα has to be expressed on β islet cells and/or antigen-presenting cells like macrophages and dendritic cells for the full evolution to diabetes to occur.
Figure 5.
IFN-γRα is required on stromal cells, not on T cells. (A) Con A-activated splenocytes were prepared from littermates carrying the BDC2.5 TCR transgene, and heterozygous or homozygous for the Ifngr-null mutation, and transferred to NOD neonates. The recipients were monitored for diabetes, and the cumulative incidence of disease is plotted. (B) Splenocytes from 10-week-old BDC2.5 transgenic mice (wild type at the Ifngr locus) were activated with Con A in vitro and transferred to neonatal mice, heterozygous or homozygous for the Ifngr-null mutation, as indicated. The recipients were monitored for diabetes, and the cumulative incidence of disease is plotted.
DISCUSSION
The potent effects of the Ifngr-null mutation in both the NOD and BDC2.5 TCR tg systems are in sharp contrast to the reportedly mild influence of the IFN-γ “knock-out” mutation in NOD mice. The latter caused no change in insulitis and only a relatively minor delay in diabetes. The stronger effect in our experiments cannot be attributed to the influence of 129-derived genes introduced during the backcross onto the NOD background (Fig. 1 C and D). Differences between the strains lacking IFN-γ and IFN-γRα have been described before (see, for example, discussion in ref. 23) and have been hypothesized to reflect an additional receptor for IFN-γ or ligand for IFN-γR. Interestingly, one of the major differences between the two strains is in their propensity to produce Th-1 vs. Th-2 cells, in mice lacking IFN-γ, but not those devoid of IFN-γRα, showing a markedly reduced Th-1/Th-2 ratio.
Our results are more in line with the reported influence of the IFN-γ knock-out mutation on disease in a diabetes model based on lymphocytic choriomeningitis virus infection of transgenic strains artificially expressing viral proteins specifically on β cells. In this case, the mutation did not affect the generation of islet-reactive T cells but blocked insulitis and diabetes (26). The inhibition of diabetes in this model may not be surprising because CD8+ T cells are the primary effectors and INF-γ is known to be the major mediator secreted by this subset.
According to our data, IFN-γ impacts at multiple points during the progression of autoimmune diabetes. First, NOD mice showed a striking inhibition of insulitis—a very retarded onset and a reduced penetrance. This type of effect has several possible explanations, reflecting the pleiotropic nature of this cytokine (27). It could be that the initial triggering of islet-reactive T cells does not occur because IFN-γ controls the expression of major histocompatibility complex class I and II molecules as well as several other proteins involved in antigen processing and presentation. It might also be that homing of the islet-reactive cells to the pancreas is ineffective, given IFN-γ’s potent influence on leukocyte–endothelial cell interactions via control of expression of adhesion molecules, chemokines, and their receptors. Additionally, the inhibition of insulitis could reflect the fact that IFN-γ is one of the major effector molecules secreted by CD8+ T lymphocytes. Several recent studies have uncovered a crucial role for CD8+ cells in initiating the insulitic lesion (18, 20, 28–32). Suggestively, the requirement for both CD8+ cells and IFN-γ is bypassed in the BDC2.5 TCR tg model.
That Ifngr-null BDC2.5 transgenics develop insulitis with only a slight delay permitted us to test for an additional role of IFN-γ in later events. In the mutant transgenics, the insulitis remained quite innocuous, never progressing to the destructive form that culminates in diabetes. However, it was not completely inert because some response to cyclophosphamide did occur (histological changes, limited cytokine production). It would have been tempting to attribute the impotence to an enrichment for cells of the Th-2 rather than Th-1 phenotype, but our data provided no evidence in support of this notion. This is perhaps best illustrated by comparing the phenotypes of mice lacking IFN-γ vs. IFN-γR: the former demonstrated an impoverished Th-1/2 ratio but only mild inhibition of disease; the latter showed quite normal T helper phenotypes but a block in diabetes development. Instead, it seemed that a function associated with the β cells or antigen-presenting cell populations was defective. Again, it is possible that ineffective antigen presentation is the explanation, preventing diversification of the anti-islet response, e.g., by inhibiting epitope spreading. More likely perhaps is that the inability to respond to INF-γ does not allow efficient killing of β cells, because this cytokine is known to influence apoptosis, inducible nitric oxide synthetase production, and respiratory burst, and it has already been implicated as a major mediator in β cell death (for review, see ref. 33).
Coupling the Ifngr mutation with the BDC2.5 TCR tg line should prove valuable in more precisely elucidating the role of IFN-γ in diabetes development. Use of the receptor rather than the ligand mutation will allow one to focus on the individual target cells influenced by IFN-γ action rather than having to deal with pleiotropic systemic effects. Exploiting the simpler, easily manipulated BDC2.5 TCR tg model should facilitate unraveling of the molecular mechanism of IFN-γ’s operation, an otherwise daunting task given that this cytokine is known to regulate the expression of more than 200 genes (27).
Acknowledgments
We thank W. Magnant for diabetes testing, J. Hergueux for typing the mice, and F. Fischer and V. Louerat for maintaining the mice. This work was supported by grants to D.M. and C.B. from the Juvenile Diabetes Foundation International (196078) and the Human Frontier Science Program, and by institutional funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Centre Hospitalier Universitaire Régional, and Bristol–Myers Squibb. B.W. was supported by a fellowship from the Université Louis Pasteur, I.A. by a fellowship from the Association pour la Recherche contre le Cancer, and A.G. by fellowships from the European Union and the Ministerio de Educacion y Ciencia de Espana.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IDDM, insulin-dependent diabetes mellitus; NOD, nonobese diabetic; TCR, T cell receptor; tg, transgenic; Th, T helper; IFN, interferon; R, receptor.
References
- 1.Tisch R, McDevitt H. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 2.Bach J F. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 3.Wicker L S, Todd J A, Peterson L B. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 4.André I, Gonzalez A, Katz J, Wang B, Benoist C, Mathis D. Proc Natl Acad Sci USA. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serreze D V, Chapman H D, Varnum D S, Hanson M S, Reifsnyder P C, Richard S D, Fleming S A, Leiter E H, Shultz L D. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchings P, Rosen H, O’Reilly L, Simpson E, Gordon S, Cooke A. Nature (London) 1990;348:639–641. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- 7.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 8.Katz J D, Benoist C, Mathis D. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 9.Kurrer M O, Pakala S V, Hanson H L, Katz J D. Proc Natl Acad Sci USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovitch A. Diabetes. 1994;43:613–621. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- 11.Liblau R S, Singer S M, McDevitt H O. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 12.Charlton B, Lafferty K J. Curr Opin Immunol. 1995;7:793–798. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 13.Debray-Sachs M, Carnaud C, Boitard C, Cohen H, Gresser I, Bedossa P, Bach J-F. J Autoimmunity. 1991;4:237–248. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 14.Campbell I L, Kay T W H, Oxbrow L, Harrison L C. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarvetnick N, Liggitt D, Pitts S L, Hansen S E, Stewart T A. Cell. 1988;52:773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 17.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Gonzalez A, Benoist C, Mathis D. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 19.Tomonari K. Immunogenetics. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 20.Katz J, Benoist C, Mathis D. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 21.Harada M, Makino S. Diabetologia. 1984;27:604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- 22.Matthys P, Froyen G, Verdot L, Huang S, Sobis H, Van Damme J, Vray B, Aguet M, Billiau A. J Immunol. 1995;155:3823–3829. [PubMed] [Google Scholar]
- 23.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Guidice G, Aguet M, Louis J A. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schijns V E, Haagmans B L, Rijke E O, Huang S, Aguet M, Horzinek M C. J Immunol. 1994;153:2029–2037. [PubMed] [Google Scholar]
- 25.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier M-C, Fournier C. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 26.Von Herrath M G, Oldstone M B A. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehm U, Klamp T, Grott M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 28.Serreze D V, Leiter E H, Christianson G J, Greiner D, Roopenian D C. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 29.Wicker L S, Leiter E H, Todd J A, Renjilian R J, Peterson E, Fischer P A, Podolin P L, Zijlstra M, Jaenisch R, Peterson L B. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 30.Sumida T, Furukawa M, Sakamoto A, Namekawa T, Maeda T, Zijlstra M, Iwamoto I, Koike T, Yoshida S, Tomioka H, Taniguchi M. Int Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 31.Kay T W H, Parker J, Stephens L A, Thomas H E, Allison J. J Immunol. 1996;157:3688–3693. [PubMed] [Google Scholar]
- 32.Serreze D V, Chapman H D, Varnum D S, Gerling I, Leiter E H, Shultz L D. J Immunol. 1997;158:3978–3986. [PubMed] [Google Scholar]
- 33.Benoist C, Mathis D. Cell. 1997;89:1–3. doi: 10.1016/s0092-8674(00)80174-9. [DOI] [PubMed] [Google Scholar]