Summary
A hallmark of Yersinia type III machines is the presence of needles extending from the bacterial surface. Needles perform two functions, serving as the conduits for the transport of effectors into immune cells but also acting as a sensor. The polymerized needle protein, YscF, is thought to perceive threshold levels of environmental calcium ions to trigger secretion. yopR (yscH) is a gene downstream of yscEFG, encoding the chaperones and principal building blocks of the needle. Here we investigated the contribution of YopR towards type III secretion and pathogenesis. Yersinia pestis KIM D27 mutants lacking yopR were defective for virulence in a mouse model of septicemic plague. yopR variants of Yersinia enterocolitica W22703 displayed a reduced ability to inject effectors into macrophages and required lower calcium concentrations to activate type III secretion than wild-type yersiniae. Further, yopR mutants failed to assemble YscF into needle complexes and instead secreted YscF into the medium. These results imply that YopR may be involved in controlling the secretion of YscF, thereby impacting the assembly of type III machines. An alternative possibility, that YopR participates directly in the polymerization of YscF, seems less likely as YopR is not associated with purified needles.
Keywords: type III secretion, needle polymerization, YopR, Yersinia
Introduction
Three Yersinia species -- Yersinia enterocolitica, Yersinia pestis and Yersinia pseudotuberculosis-- are pathogenic to animals as well as humans (Butler, 1995) and employ a virulence plasmid-encoded type III secretion system to evade the host immune system (Cornelis et al., 1998). Type III secretion machines provide for the transport of Yop effectors from the bacterial cytoplasm into the cytosol of host target cells (Marketon et al., 2005), thereby disabling phagocytes and other innate immune responses to bacterial infections (Rosqvist et al., 1994, Galán & Wolf-Watz, 2006). Common features of type III machines are their assembly from many different proteins in the cytoplasm, inner and outer membranes as well as the extension of the machine via a hollow ‘needle’ filament (Kubori et al., 1998, Hoiczyk & Blobel, 2001, Cornelis, 2006).
Needles are formed via the polymerization of YscF and decorated with a cap-like tip comprised of LcrV (Hoiczyk & Blobel, 2001, Mueller et al., 2005). Two ring structures, one in the bacterial plasma membrane and one in the outer membrane, are joined via a central rod that is formed from YscI, which is also thought to connect with the needle protein at the level of the outer YscC ring (Marlovits et al., 2004, Marlovits et al., 2006). Needle polymerization is controlled by YscP, a secreted machine component (Journet et al., 2003, Payne & Straley, 1999). Without yscP, Yersinia polymerize extended needles and are unable to inject effectors into host cells (Agrain et al., 2005). LcrV, the tip protein, as well as YopB and YopD are essential for type III injection, but dispensable for polymerization of needles (Håkansson et al., 1993, Lee et al., 2000, Mueller et al., 2008). All three proteins are thought to gate type III needles by forming a translocation structure in the plasma membrane of host cells (Mueller et al., 2008). The structural attributes of such membrane translocation complexes (injectisomes) remain however unknown.
Pseudomonas aeruginosa PscF, a homolog of YscF, binds two putative chaperones, PscE and PscG, that are conserved in yersiniae (YscE and YscG) (Quinaud et al., 2005, Quinaud et al., 2007). yscH, located downstream of the genes for needle protein/chaperones (yscEFG), encodes a secreted product, named YopR (Allaoui et al., 1995). Y. enterocolitica W22703 yopR mutants are still able to catalyze type III injection of host cells (Lee & Schneewind, 1999). However, as investigated in a mouse model of intraperitoneal challenge, yopR mutants display a 34 fold reduction in virulence (Lee & Schneewind, 1999). YopR is secreted into the extracellular medium early during development, while needle complexes assemble, and the protein has been investigated as a model substrate of type III machines (Sorg et al., 2006, Blaylock et al., 2008). The X-ray structure of YopR revealed the unexpected similarity with YopN (Schubot et al., 2005), another secreted protein that blocks recognition of effector substrates by type III machines until host cell contact has been established (Yother & Goguen, 1985, Cheng et al., 2001, Torruellas et al., 2005). YscF has been proposed to bind calcium and may respond to changes in the abundance of this ion by altering the needle structure (Ferracci et al., 2005, Davis & Mecsas, 2007). Such a mechanism may trigger YopN injection into host cells and enable the subsequent transport of effectors along the conduit of type III needles (Ramamurthi & Schneewind, 2002). Regulation of Yersinia type III machines can be monitored on agar plates as the “low calcium response (LCR)”; chelation of calcium ions prevents growth of the organisms at 37°C due to the massive release of effectors (Goguen et al., 1984, Michiels et al., 1990) and mutations in type III machine genes, for example yscF, cause an LCR phenotype (Allaoui et al., 1995).
Here we examined the functional phenotypes of yersiniae associated with loss of YopR. Y. pestis KIM D27 lacking yopR showed reduced virulence in a mouse model of septicemic plague and displayed an LCR phenotype on agar plates. Y. enterocolitica W22703 yopR mutants secreted YscF into the extracellular medium but did not assemble needle complexes on the bacterial surface. In agreement with a specific perturbation of type III needles, yopR mutants injected reduced amounts of effectors into macrophages and required lower calcium concentrations than wild-type yersiniae to respond with type III secretion. Thus, yopR may be involved in controlling the secretion of YscF and thereby impact the assembly of type III machines. An alternative possibility, that YopR participates directly in the polymerization of YscF, seems less likely to us as YopR was not found to be associated with purified needles complexes.
Results
Loss of virulence by Y. pestis KIM D27 harboring a ΔyopR mutation
We sought to ascertain whether the expression of yscH (yopR) is required for the pathogenesis of bacterial infections other than Y. enterocolitica. yscH homologues are located downstream of the needle protein chaperone genes (homologues of yscEFG) in Aeromonas solmonicida, Photorhabdus species, Pseudomonas aeroginosa, Y. enterocolitica, Y. pseudotuberculosis and Y. pestis. Earlier studies developed mouse models for plague infection that permit accurate measurements of virulence contributions (Overheim et al., 2005, DeBord et al., 2006). The high pathogenicity island (HPI)/pigmentation locus (pgm) of the plague pathogen, Y. pestis, is absolutely required for the establishment of bubonic and pneumonic plague (Girard, 1955). Non-pigmented variants are generated frequently from virulent isolates via spontaneous loss of HPI/pgm through recombination at flanking IS100 sites (Buchrieser et al., 1999, Parkhill et al., 2001). The pgm strain KIM D27 (KIM 5) causes septicemic plague and death of BALB/c mice following intravenous inoculation with an LD50 dose of 102 CFU (Miller et al., 2009). Mutations that abrogate type III secretion of KIM 5 increase the LD50 dose by a factor >5×104 (Straley & Cibull, 1989). In our own experiments, cohorts of 10 BALB/c mice were infected by intravenous injection with Y. pestis KIM D27 or an isogenic yopR mutant; animal morbidity was monitored over the next fourteen days. As expected, mice infected with KIM D27 experienced 90% mortality with just 250 bacteria (Figure 1A). In contrast, yopR mutant-infected animals required an infectious dose more than one hundred times greater to cause just 30% mortality (Figure 1B). These findings establish that the yopR homologue makes a very important contribution to the pathogenesis of plague, which is even greater than the relative contribution of yopR during Y. enterocolitica infections (Lee & Schneewind, 1999).
Fig. 1.
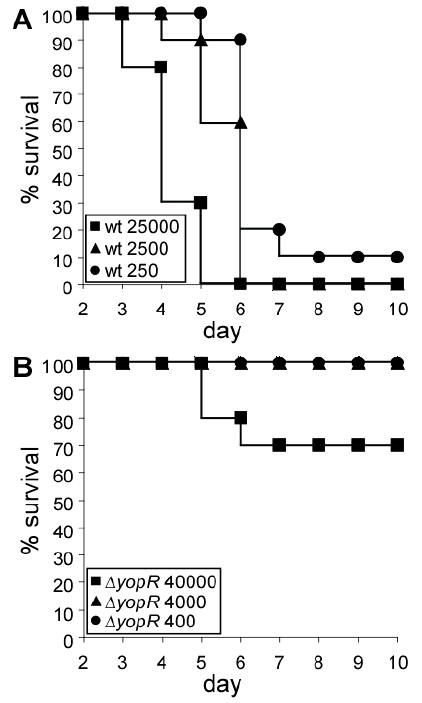
yopR is required for Y. pestis virulence. Cohorts of 10 BALB/c mice were infected intravenously with wild-type Y. pestis KIM D27 (A) or an isogenic yopR mutant (B). Percent mouse survival is plotted as a function of time in days. Inset designates the bacterial inoculum for each cohort of animals in colony-forming units.
YopR contribution to macrophage infection
The purpose of type III secretion by Yersinia species is to protect the bacteria from host cellular defenses. To assess the contribution of YopR to secretion in this context, a murine macrophage cell line was infected with wild-type or ΔyopR Y. enterocolitica. Two measures of effector injection were monitored over a 10 hour course of infection: cell shape and death (Rosqvist et al., 1990, Bashaw et al., 2007). Over the first two hours of infection, macrophage cell shape was obliterated by both bacterial strains (compare Figure 2A and 2B to 2D, a negative control for injection, and also (Blaylock et al., 2008)), signifying that effectors impacting actin polymerization were translocated to functionally indistinguishable levels. Given that Y. enterocolitica secretes no fewer than four effectors that ablate the actin cytoskeleton within the first 90 minutes of infection (Trosky et al., 2008), we sought a more sensitive measure of injection by enumerating cell death over time, a process dependent upon just one effector (YopP) and that takes about 8 hours (Figure 2E)(Bashaw et al., 2007, Mills et al., 1997). In this assay, we observed that macrophage killing by ΔyopR Yersinia lagged significantly that by wild-type after 4, 6 and 8 hours of infection; this phenotype could be complemented with a plasmid encoding yopR (Figure 2C and 2E). Eventually, ΔyopR Yersinia were able to trigger apoptosis of most tissue culture cells (see 10 hour time point, Figure 2E).
Fig. 2. Macrophage death is delayed by infection with ΔyopR Y. enterocolitica.

A-D. J774 macrophages were infected for five hours with the indicated Y. enterocolitica strains. Superimposed differential interference contrast (DIC) and fluorescence microscopy images of propidium iodide-stained macrophages interrogate the cell shape and viability of infected macrophages.
E. The number of propidium iodide positive (dead) cells is reported as a percentage of the total for wild-type (wt), ΔyopR, and complemented ΔyopR, pyopR Y. enterocolitica W22703. For enumeration, five images (as in A-D) were captured for each strain at two hour intervals over six hours starting with hour four of the infection. Error bars represent one standard deviation. Statistical significance was calculated with the two-tailed Student’s t test and p values are indicated.
In vitro type III secretion studies with yopR mutants
Having established a contribution of YopR to type III secretion in vivo, we next analyzed secretion in vitro. Protein transport by Yersinia type III machines in the mammalian host has two destinations, the export of substrates into extracellular fluids of the host (a high calcium environment) and type III injection directly into host cells (a low calcium environment) (Rosqvist et al., 1994, Lee et al., 1998). We assessed the contribution of YopR to each of these in turn. Tissue culture medium (containing 1.8 mM Ca2+) conditioned for two days by macrophages was used to culture wild-type and ΔyopR Y. enterocolitica at 37°C. Cultures were centrifuged to sediment bacteria into a pellet (P) that was separated from the extracellular medium in the supernatant (S) and proteins in both fractions were analyzed by immunoblotting (Figure 3A). The abundance of proteins secreted by type III machines under these conditions (YopD, LcrV and YscP) was indistinguishable between wild-type and ΔyopR mutants (Figure 3A).
Fig. 3. Secretion profile of yopR mutant in defined and complex media.

A. Wild-type and ΔyopR Y. enterocolitica were grown in tissue culture medium which had previously supported the growth of macrophages. Secreted proteins (S) and intact bacteria (P) were blotted and probed with antisera against secreted proteins and a fractionation control (RpoA).
B. Wild-type, ΔyopR and the complementing ΔyopR, pyopR Y. enterocolitica were grown in TSB (complex) or M9 (defined) media chelated for calcium. Secreted proteins were analyzed by Coomassie blue staining after separation by SDS-PAGE; the size of molecular weight standards is indicated. For immunoblots, secreted proteins (S) were run next to the corresponding whole cell (P) fractions. Samples were blotted and probed with the indicated antisera where RpoA is a fractionation control.
Wild type, ΔyopR and a complementing strain were also grown at 37°C in media chelated for calcium, conditions that promote the secretion of all Yops, including effectors reserved for injection of eukaryotic cells in vivo, for example YopE. Bacteria were sedimented by centrifugation and the secreted proteins in the supernatant were analyzed by Coomassie-stained SDS-PAGE and immunoblot (Figure 3B). Wild-type Y. enterocolitica secreted Yops vigorously into culture supernatants under these conditions (Figure 3B). Curiously, secretion by ΔyopR Y. enterocolitica was indistinguishable from wild-type in defined (M9) media but was impaired in complex media, for example tryptic soy broth (TSB) (Figure 3B). This conditional secretion defect was fully complemented by the restoration of yopR on a plasmid. Moreover, the nearly identical abundance of secretion substrates in the bacterial pellet (P) of ΔyopR in both types of media suggests a secretion defect rather than a role for YopR in regulating Yop synthesis. We took the variable secretion of ΔyopR Yersinia in calcium-chelated medium to beg the question whether this strain may have difficulty in sensing or responding to environmental signals that promote export of type III substrates.
The low-calcium response of ΔyopR mutant yersiniae is defective
To investigate further the sensory capabilities of yopR null Y. enterocolitica, this strain was grown in defined media containing increasing concentrations of calcium. As above, secretion was examined with Coomassie blue-stained SDS-PAGE and immunoblot for exported Yops. Wild-type Yersinia secreted Yops maximally until a threshold of 0.6 mM calcium before secretion levels dropped off (Figure 4A). In contrast, addition of the smallest increment of calcium (0.3 mM) to ΔyopR Y. enterocolitica drastically reduced the abundance of effectors (Figure 4A).
Fig. 4. Altered calcium sensitivity of the yopR mutant.
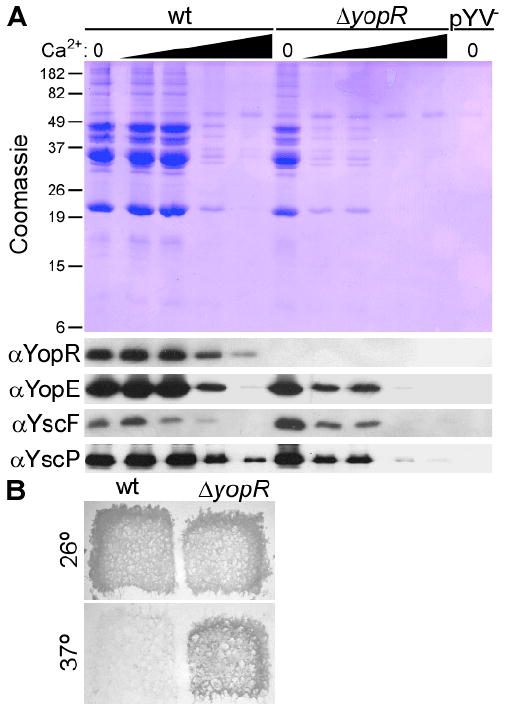
A. Secretion by wild-type, ΔyopR and pYV- (lacking the type III secretion-encoding plasmid) Y. enterocolitica W22703 strains was monitored in defined (M9) media. Where indicated by a wedge, an increasing concentration (0.3, 0.6, 1.2 or 2 mM) of CaCl2 was added to the media. Secretion was monitored by Coomassie-stained SDS-PAGE and immunoblot as in Figure 1.
B. Calcium-chelated agar plates were replica-plated with wild-type Y. pestis KIM D27 and an isogenic ΔyopR mutant and incubated at 26° or 37°C.
To test the universality of calcium sensitivity by yopR mutant Yersinia, we took advantage of the low calcium response of the plague pathogen (Kupferberg & Higuchi, 1958, Goguen et al., 1984). Y. pestis KIM D27 ceases to grow and form colonies under conditions that induce type III secretion, for example brain heart infusion agar chelated for calcium and incubated at 37°C (Figure 4B). We tested the ability of Y. pestis ΔyopR to grow under the same conditions, reasoning that if secretion is impaired, then growth may be observed. Our prediction was borne out (Figure 4B). Collectively, these data suggest that in Yersinia species lacking YopR, the sensory or responsive attributes of the type III secretion machine is confused by the environmental calcium signal.
Needle polymerization defect of ΔyopR yersiniae
Having documented the various defects of ΔyopR bacteria to secrete under a variety of conditions, the question remains: what is the underlying molecular mechanism of these observations? The hypersensitivity of ΔyopR bacteria to extracellular calcium reminded us of a report that linked YscF function to altered calcium sensitivity (Torruellas et al., 2005). YscF is the principal component of the needle filament (Hoiczyk & Blobel, 2001, Broz et al., 2007) where LcrV sits at the tip (Mueller et al., 2005). We therefore decided to investigate YscF polymerization into needles biochemically and microscopically.
Wild-type and yopR mutant strains were grown in defined media under conditions that maximally induce secretion; the profiles of exported proteins were indistinguishable (‘S’ lanes, Figure 5). Needles were sheared off the bacteria (‘P’ lanes, Figure 5) and floated on sucrose gradients to remove impurities. Wild-type bacteria yielded sucrose fractions containing YscF and LcrV, and by electron microscopy, we saw needles (Figure 5). Surprisingly, yopR mutant bacteria secreted needle components without polymerizing a structure that could be sheared and thereby visualized (Figure 5). To ensure that the absence of needles was indeed the result of the lesion in yopR and not due to a second-site mutation, bacteria complemented with a plasmid-borne copy of yopR were examined for needle polymerization; in this strain, needles were once again observed (Figure 5).
Fig. 5. Needle polymerization defect of ΔyopR Yersinia.
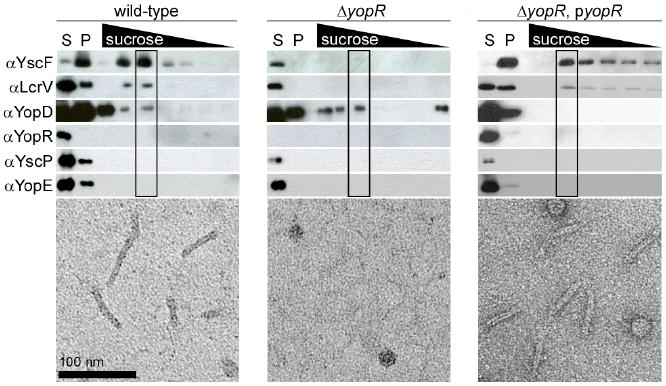
Wild-type, ΔyopR and the complementing ΔyopR, pyopR Y. enterocolitica were induced for type III secretion and secreted proteins (S) were collected and blotted with sera to components of the needle and to other secreted Yops. Needles on the bacterial surface were sheared and enriched by centrifugation (P). This complex mixture was loaded onto a sucrose gradient and electron micrographs of the boxed fractions are reproduced (bottom).
The absence of needles in the sheared fraction of yopR mutant Y. enterocolitica could have a number of explanations: needles might be stronger, shorter or nonexistent. To distinguish between these possibilities, the bacterial surface was examined directly by electron microscopy (Figure 6G & H) and needle components were stained and visualized by immuno-fluorescence microscopy (Figure 6A-F). Fluorescent staining of wild-type bacteria grown in calcium-chelated defined medium at 37°C with YscF- or LcrV- specific antibodies decorated the surface of wild-type Yersinia (Figure 6A & B). These same bacteria always displayed an abundance of type III needles (needles are highlighted with yellow shadows in Figure 6G). By comparison, yopR mutant bacteria showed reduced staining for YscF and almost none for LcrV (Figure 6C-F) by immuno-fluorescence and were devoid of needles by electron microscopy (Figure 6H).
Fig. 6. ΔyopR mutant Y. enterocolitica do not assemble needles.

A-F. Wild-type, ΔyopR and the complementing ΔyopR, pyopR Y. enterocolitica were grown under conditions that induce type III secretion. The presence of needle components YscF and LcrV was detected by immuno-fluorescence microscopy and cell shape captured by the corresponding DIC images. The white scale bar in A is 2μm. G and H. Needles on the surface of wild-type and ΔyopR strains were observed with electron microscopy; each needle has a pseudocoloured yellow ‘shadow’. Black scale bars represent 100 nm.
Discussion
yopR mutant bacteria present a curious paradox: How does a strain with no obvious needles (Figures 5 and 6) inject eukaryotic cells and cause disease (Figures 1 and 2)? We do not imagine that yopR mutant bacteria enable the translocation of Yops by means of some heterologous or non-canonical route. Indeed, we tested the possibility that a yopR mutant could compensate for loss of LcrV and found that it could not; a double mutant in lcrV and yopR cannot inject Yops into macrophages (data not shown).
The data can be accounted for one of two ways: perhaps the abundance of needles is so reduced (i.e. by >95%) that we do not detect them. There are a number of problems with this model: (i) at best, a bacterium constructs 50-100 needles (Journet et al., 2003), so a reduction in needles of the order contemplated would leave individual cells almost defenseless in vivo, (ii) during macrophage infection experiments (Figure 2), we examined the pools of injected Yops and found them reduced only by a factor of 2-3 (data not shown), and (iii) the abundance of secreted Yops was not in the least reduced when secretion of the yopR mutant was interrogated in minimal medium (Figure 3). We therefore discount the possibility that YopR regulates the number of needles per bacterium. Our alternative model instead envisions that needles constructed by yopR null strains may be too short to shear or visualize and that YopR promotes needle assembly.
How does YopR contribute to needle polymerization? The possibility of YopR acting as a scaffold is undermined by the absence of YopR from sheared needles (Figure 5). We therefore propose a model where the action of YopR occurs in the bacterial cytoplasm (Figure 7). Our model is rooted in the observations that YopR and YopN share structural similarity (Schubot et al., 2005) and a common interaction with the secretion system’s ATPase (Sorg et al., 2006, Botteaux et al., 2009). YopN is thought to act at the site of secretion to block the premature export of effectors destined for the eukarytotic cytosol (Cheng et al., 2001, Ferracci et al., 2005). We suggest that YopR acts in a similar capacity during needle assembly, turning away substrates that might prematurely cap the structure, preventing further YscF polymerization.
Fig. 7. Model of YopR function.

Selective access of early (YscF, YscI, YscP) and middle (YopB, YopD, and LcrV) substrates to the type III machines of yersiniae is controlled by YopR and YopN, respectively. YopR and YopN are structural homologs and secreted by the type III machine. These mobile regulatory components are thought to function as checkpoints, probing the completion of discrete intermediary stages in the assembly of the type III injection pathway. The location of secreted YopR (into the medium) and YopN (within host cells) differs, reflective of their corresponding checkpoints.
Needle length may thus be the result of a dynamic conversation between the ‘architect’—YscP—and the ‘traffic cop’—YopR—of type III secretion during needle polymerization at each construction site. While the architect requires more building blocks, the traffic cop blocks all other traffic to the construction site; when YscP is satisfied, YopR dissociates from the injectisome (and is maybe now secreted) and the structure is capped to prevent further elongation.
The length of the type III needle is tightly regulated because needles of the wrong length are problematic to bacteria: (i) longer needles cannot coordinate with adhesins (Mota et al., 2005) and other surface features (West et al., 2005), and (ii) environmental sensation may be misregulated (Figures 3 and 4, and (Torruellas et al., 2005)).
In conclusion, this study revealed the phenotypes associated with loss of yopR expression. These phenotypes can be explained as a series of causal relationships: the absence of YopR precludes polymerization of needles; the resultant secretion machine is compromised for its sensory capability. Further, misguided secretion impairs bacterial survival as Yersinia face host innate immune defenses.
Experimental procedures
Bacterial strains and growth conditions
Y. enterocolitica W22703 and ΔyopR strains are the same as those described previously (Cornelis et al., 1987, Blaylock et al., 2008), as was the complementing plasmid with a copy of yopR under the control of Ptac (Blaylock et al., 2008). The Y. pestis strain used for all experiments was KIM D27 (KIM 5 (pgm-1)(Brubaker, 1969). To generate a yopR mutant in this strain, a 2 kb DNA fragment centered on the gene was PCR-amplified from pCD1 with primers 5’-ntctagaatgatgaattgaatttcgagg-3’ and 5’-nctcgaggcgacatctgactcctcaacc-3’. This DNA fragment and pLC28 (Cheng et al., 1997) were digested with XbaI and XhoI and ligated. The resulting plasmid was mutated by PCR amplification with a primer 5’- atgacggttacccttaattaaagcttagaggttccattacatcg-3’ and its reverse complement to introduce a stop codon and frameshifted restriction site (underlined in primer sequence) after the sixth codon of yopR. The resulting plasmid was mated into KIM 5 and resolved as described previously (Cheng et al., 1997). For the propagation of Y. enterocolitica, TSB (Bacto) was used; Y. pestis was grown in Brain Heart Infusion (Bacto). For secretion assays, EGTA was added to TSA to a final concentration of 5 mM; alternatively, bacteria were grown in the defined media, M9 with EGTA or CaCl2 where indicated (Cheng et al., 1997). To generate conditioned tissue culture medium, J774 macrophages were grown to confluence in DMEM supplemented with 10% FBS and 1% glutamax; the growth medium was exchanged with DMEM. After 48 hours, this medium was decanted and filter-sterilized.
Needle purification and visualization
Needle purification was carried out as described earlier (Mota et al., 2005). Briefly, 50 ml of overnight culture (Y. enterocolitica W22703, ΔyopR or the mutant with complementing plasmid) was inoculated into 1 liter of M9 and incubated for 2 hours at 30°C and then grown for 3 hours more at 37°C. Bacteria were sedimented by centrifugation at 2,700 ×g for 10 minutes, then suspended in 40 ml 1 M Tris-HCl, pH 7.5. Needles retained on the bacterial surface were sheared off by vortexing for 3 minutes. The bacteria were again sedimented by centrifugation at 8,000 ×g for 10 minutes. The needle-containing supernatant was passed through a 0.45-μm cellulose acetate membrane filter (Whatman) to remove all bacteria and centrifuged at 40,000 ×g for 30 minutes using a Beckman Coulter Ti 80 rotor. The sediment, the crude needle preparation, was suspended in 500 μL of 20 mM Tris-HCl, pH 7.5, 5% sucrose and loaded onto a 6 ml step-gradient of 70%, 20% and 10% sucrose (2 ml each) and centrifuged at 40,000 ×g overnight in the Ti 80 rotor. One ml fractions were collected and examined by immunoblot and electron microscopy. For electron microscopy, all samples were placed on a carbon-coated copper grid, washed with H2O and stained with 2% uranyl acetate before viewing on a Tecnai F30 electron microscope at 300 kV.
Immuno-fluorescence
Bacteria were adhered to poly-L-lysine-coated microscope slides while fixing with 3% paraformaldehyde, 0.1% glutaraldehyde for 20 minutes at room temperature. The fixative and non-adherent bacteria were washed away with PBS, and samples blocked with 2% BSA in PBS for 20 minutes. Primary antibody to YscF or LcrV (polyclonal sera) was diluted 1:250 and added to samples overnight. The samples were washed ten times in PBS, and Alexa Fluor-conjugated secondary antibody (Invitrogen) was incubated for one hour with the bacteria. After ten washes, the samples were visualized by DI contrast and fluorescence and images collected on a Leica SP5 tandem scanner spectral 2-photon confocal with a 100× objective.
Macrophage infection
J774 macrophages were propagated and infected in Dulbecco’s modified Eagle medium supplemented with fetal bovine serum (10% final concentration) and glutamax (Invitrogen, 1% final concentration). Bacterial strains destined for infection were refreshed from overnight cultures for one hour at 26°C, and added to the macrophages at a multiplicity of infection of 5. Four hours later, propidium iodide was added to a final concentration of 5μg/ml. Differential image contrast and fluorescence images were collected on a TE-2000 inverted microscope (Nikon) at 200× magnification from that point on every two hours. Live and dead cells were enumerated from five fields of view, and these numbers were subjected to Student’s two-tailed t test to determine statistical significance.
Plague infection of mice
Plague strains destined for animal infection were refreshed from overnight cultures for two hours at 26°C in BHI, serially diluted to the desired optical density in PBS and 100 μl were injected intravenously into the periorbital plexus of anesthetized BALB/c mice. Mice were monitored for morbidity and mortality over 14 days. Mice were observed for morbidity, mortality, and recovery over 14 days following infection. All animal experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC) at The University of Chicago. Statistical analysis of animal mortality following plague challenge used the Fisher’s exact test.
Acknowledgments
This work was supported by United States Public Health Service award AI 042797 from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch. All authors acknowledge membership in and support from the Region V ‘Great Lakes’ Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH award 1-U54-AI-057153). We thank Y. Chen and V. Bindokas for help with electron and light microscopy respectively. We are grateful to Lauriane Quénée, Nancy Ciletti and Kristy Skurauskis of the Animal Research & Immunology Core of the GLRCE for help with plague infection experiments.
References
- Agrain C, Sorg I, Paroz C, Cornelis GR. Secretion of YscP from Yersinia enterocolitica is essential to control the length of the injectisome needle but not to change the type III secretion substrate specificity. Mol Microbiol. 2005;57:1415–1427. doi: 10.1111/j.1365-2958.2005.04758.x. [DOI] [PubMed] [Google Scholar]
- Allaoui A, Schulte R, Cornelis GR. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- Bashaw J, Norris S, Weeks S, Trevino S, Adamovicz JJ, Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol. 2007;14:605–616. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock B, Sorg JA, Schneewind O. Yersinia enterocolitica type III secretion of YopR requires a structure in its mRNA. Mol Microbiol. 2008;70:1210–1222. doi: 10.1111/j.1365-2958.2008.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteaux A, Sory MP, Biskri L, Parsot C, Allaoui A. MxiC is secreted and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol. 2009;71:449–460. doi: 10.1111/j.1365-2958.2008.06537.x. [DOI] [PubMed] [Google Scholar]
- Broz P, Mueller CA, Muller SA, Phillipsen A, Sorg I, Engel A, Cornelis GR. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol. 2007;65:1311–1320. doi: 10.1111/j.1365-2958.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Brubaker RR. Mutation rate to non-pigmentation in Pasteurella pestis. J Bacteriol. 1969;98:1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchrieser C, Rusniok C, Frangeul L, Couve E, Billault A, Kunst F, Carniel E, Glaser P. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosis strains. Infect Immun. 1999;67:4851–4861. doi: 10.1128/iai.67.9.4851-4861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T. Yersinia species. In: Mandell GL, Douglas RG, Bennett JE, editors. Infectious Diseases. New York: Churchill Livingstone; 1995. pp. 1748–1756. [Google Scholar]
- Cheng LW, Anderson DM, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- Cheng LW, Kay O, Schneewind O. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J Bacteriol. 2001;183:5293–5301. doi: 10.1128/JB.183.18.5293-5301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G, Laroche Y, Balligand G, Sory M-P, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Mecsas J. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J Bacteriol. 2007;189:83–97. doi: 10.1128/JB.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBord KL, Anderson DM, Marketon MM, Overheim KA, DePaolo RW, Ciletti NA, Jabri B, Schneewind O. Immunogenicity and protective immunity against bubonic and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect Immun. 2006;74:4910–4914. doi: 10.1128/IAI.01860-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracci F, Schubot FD, Waugh DS, Plano GV. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol Microbiol. 2005;57:970–987. doi: 10.1111/j.1365-2958.2005.04738.x. [DOI] [PubMed] [Google Scholar]
- Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Girard G. Plague. Annu Rev Microbiol. 1955;9:253–277. doi: 10.1146/annurev.mi.09.100155.001345. [DOI] [PubMed] [Google Scholar]
- Goguen JD, Yother J, Straley SC. Genetic analysis of the low calcium response in Yersinia pestis Mud1(Ap lac) insertion mutants. J Bacteriol. 1984;160:842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S, Bergman T, Vanooteghem J-C, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galán JE, Aizawa S-I. Supermolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- Kupferberg LL, Higuchi K. Role of calcium ions in the stimulation of growth of virulent strains of Pasteurella pestis. J Bacteriol. 1958;76:120–121. doi: 10.1128/jb.76.1.120-121.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VT, Anderson DM, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- Lee VT, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- Lee VT, Tam C, Schneewind O. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlovits TC, Kubori T, Lara-Tejero M, Thomas DR, Unger VM, Galán JE. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature. 2006;441:637–640. doi: 10.1038/nature04822. [DOI] [PubMed] [Google Scholar]
- Marlovits TC, Kubori T, Sukhan A, Thomas DR, Galán JE, Unger VM. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NC, Berube BJ, Schneewind O. On the contribution of a heterologous lcrV gene towards Y. pestis escape from plague protective immunity. Infect Immun. 2009 submitted. [Google Scholar]
- Mills SD, Boland A, Sory M-P, van der Smissen P, Kerbouch C, Finlay BB, Cornelis GR. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota LJ, Journet L, Sorg I, Agrain C, Cornelis GR. Bacterial injectisomes:needle length does matter. Science. 2005;307:1278. doi: 10.1126/science.1107679. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, Brubaker RR, Jabri B, Schneewind O. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005;73:5152–5159. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Thompson NR, Titball RW, Holden MT, Prentice MB, Sebaihia M, James KD, Churcher C, Mungall KL, Baker S, Dasham D, Bentley SD, Brokks K, Cerdeno-Tarraga AM, Chillingworth T, Cronin A, Davies RM, Davis P, Dougan G, Feltwell T, Hamlin N, Holroyd S, Jagels K, Karlyshev AV, Leather S, Moule S, Oyston PC, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- Payne PL, Straley SC. YscP of Yersinia pestis is a secreted component of the Yop secretion system. J Bacteriol. 1999;181:2852–2862. doi: 10.1128/jb.181.9.2852-2862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinaud M, Chabert J, Faudry E, Neumann E, Lemaire D, Pastor A, Elsen S, Dessen A, Attree I. The PscE-PscF-PscG complex controls type III secretion needle biogenesis in Pseudomonas aeruginosa. J Biol Chem. 2005;280:36293–36300. doi: 10.1074/jbc.M508089200. [DOI] [PubMed] [Google Scholar]
- Quinaud M, Plé S, Job V, Contreras-Martel C, Simorre J, Attree I, Dessen A. Structure of the heterotrimeric complex that regulates type III secretion needle formation. Proc Natl Acad Sci USA. 2007;104:7803–7808. doi: 10.1073/pnas.0610098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Schneewind O. Type III protein secretion in Yersinia species. Annu Rev Cell Dev Biol. 2002;18:107–133. doi: 10.1146/annurev.cellbio.18.012502.105912. [DOI] [PubMed] [Google Scholar]
- Rosqvist R, Forsberg Å, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of yersinia obstructs the primary host defense. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubot FD, Jackson MW, Penrose KJ, Cherry S, Tropea JE, Plano GV, Waugh DS. Three-dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. J Mol Biol. 2005;346:1147–1161. doi: 10.1016/j.jmb.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Sorg JA, Blaylock B, Schneewind O. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc Nat Acad Sci USA. 2006;103:16490–16495. doi: 10.1073/pnas.0605974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley SC, Cibull ML. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989;57:1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruellas J, Jackson MW, Pennock JW, Plano GV. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol. 2005;57:1719–1733. doi: 10.1111/j.1365-2958.2005.04790.x. [DOI] [PubMed] [Google Scholar]
- Trosky JE, Liverman AD, Orth K. Yersinia outer proteins: Yops. Cell Microbiol. 2008;10:557–565. doi: 10.1111/j.1462-5822.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- West NP, Sansonetti PJ, Mounier J, Exley RM, Parsot C, Guadagnini S, Prevost M-C, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. Optimization of Virulence Functions Through Glucosylation of Shigella LPS. Science. 2005;307:1313–1317. doi: 10.1126/science.1108472. [DOI] [PubMed] [Google Scholar]
- Yother J, Goguen JD. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


