Abstract
Cardiomyocyte contraction is regulated by phosphorylation of sarcomeric proteins. Throughout the heart regional and transmural differences may exist in protein phosphorylation. In addition, phosphorylation of sarcomeric proteins is altered in cardiac disease. Heterogeneity in protein phosphorylation may be larger in hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) as it may be caused by multiple mutations in genes encoding different sarcomeric proteins. Moreover, HCM is characterized by asymmetric remodelling of the heart. In the present study we assessed if local differences in sarcomeric protein phosphorylation are more evident in primary HCM or DCM than in non-failing donors. Thereto, phosphorylation of the two main target proteins of the beta-adrenergic receptor pathway, troponin I (cTnI) and myosin binding protein C (cMyBP-C) was analysed in different parts in the free left ventricular wall of end–stage failing HCM and DCM patients and donors obtained during transplant surgery. Intra-patient variability in protein phosphorylation within tissue samples of approximately 2 g wet weight was comparable between donor, HCM and DCM samples and could partly be attributed to the precision of the technique. Thus, our data indicate that within the precision of the measurements small, biopsy-sized cardiac tissue samples are representative for the region of the free left ventricular wall from which they were obtained.
Keywords: Cardiomyopathy, Phosphorylation, Physiology
Introduction
Primary hypertrophic cardiomyopathy (HCM) is characterized by asymmetrical hypertrophy of the left ventricle and septum, without underlying cardiac or vascular abnormalities (Richardson et al. 1996) The prevalence of primary HCM is 0.2% in the general population and in about 60% of the cases a mutation is identified in one of the 13 genes encoding the sarcomeric proteins (Ramaraj 2008; Richard et al. 2003, 2009). The clinical presentation and development of HCM is very diverse, ranging from no symptoms at all to end–stage heart failure. Primary dilated cardiomyopathy (DCM) is characterized by eccentric remodelling of the heart (Richardson et al. 1996). Although 20–48% of DCM cases are familial, only a small number of mutations are known to date (Chang and Potter 2005). The asymmetric remodelling observed in HCM and the variable geno- and phenotypes in familial HCM and DCM raise the question how representative small cardiac tissue sample are for the region of the left ventricle (LV) they are taken from.
A characteristic feature of end-stage heart failure is down regulation and desensitization of the β-adrenergic receptor pathway, as a consequence of chronic stimulation of the sympathetic system (Bristow et al. 1993; Hamdani et al. 2008). Consequently, the downstream sarcomeric target proteins cardiac troponin I (cTnI) and myosin binding protein C (cMyBP-C) may become less phosphorylated by protein kinase A (PKA) (Bodor et al. 1997; El Armouche et al. 2007; van der Velden et al. 2003; van Dijk et al. 2008). Therefore, we compared the variability in phosphorylation of the β-adrenergic target proteins cTnI and cMyBP-C within the left ventricle from HCM and DCM tissue samples to cardiac tissue from donors.
Methods
Cardiac tissue
Tissue from the free LV wall from end-stage heart failure patients clinically characterized with familial hypertrophic (n = 5) or dilated (n = 5) cardiomyopathy was obtained during cardiac transplantation surgery. Control cardiac LV tissue was obtained from donor hearts (n = 5) for which no suitable transplant recipient was found. The donors had no history of cardiac disease, a normal ECG and normal ventricular function on echocardiography performed within 24 h prior to heart explantation.
The tissue was collected in cold cardioplegic solution, divided into pieces of approximately 2 g wet weight and immediately frozen in liquid nitrogen. Samples were obtained after informed consent and with approval of the local ethical committee (St. Vincent’s Hospital Human research Ethics Committee: File number H03/118; Title: Molecular Analysis of Human heart Failure).
Protein phosphorylation
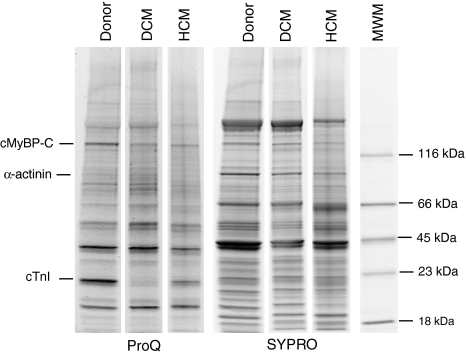
From each patient, four or five small pieces of about 0.5–1 mg wet weight were taken randomly from one LV tissue sample. All samples were treated with trichloro acetic acid prior to protein analysis to preserve the endogenous phosphorylation status of the sarcomeric proteins. Proteins in cardiac tissue homogenates were separated on 4–15% Tris-HCl gels (Biorad) (Fig. 1). Each sample was run in duplo on the gels. To detect protein phosphorylation, the gels were stained with ProQ Diamond. Subsequently the gels were stained with SYPRO Ruby to determine protein content. Staining of the gels was visualized using the Las-3000 Image Reader (Fuji; 460/605 nm) and analyzed with AIDA, as described before (Zaremba et al. 2007). cMyBP-C phosphorylation level was expressed relative to cMyBP-C content and cTnI phosphorylation to α-actinin content to correct for loading differences.
Fig. 1.
A representative gradient gel stained with ProQ Diamond and SYPRO Ruby, demonstrating the phosphorylation status of cMyBP-C and cTnI. MWM indicates molecular weight marker
Data analysis
Data are presented as mean ± standard error of the mean (SEM). The variance within each sample was expressed as the coefficient of variation. A comparison was made between the mean coefficient of variation in HCM, DCM and donor with one-way ANOVA. P < 0.05 was considered significant.
Results
Inter- and intra-patient variability can partly be attributable to imprecision of the technique used to determine protein phosphorylation. The precision of the technique was estimated by analyzing the difference between duplicate measurements. The coefficient of variation of the duplicate measurements of cMyBP-C phosphorylation was higher in HCM and DCM samples compared to donor (38 ± 10 and 39 ± 9 vs. 11 ± 2%, respectively). Lower variability was seen in cTnI phosphorylation, though again the coefficient of variation was higher in HCM and DCM than in donor (17 ± 3 and 24 ± 6 vs 10 ± 2%, respectively). The variabilities between duplicate measurements are inversely related with the absolute phosphorylation levels (i.e. higher variability on low phosphorylation levels), indicating that differences in variability are largely accountable to noise in the measurement.
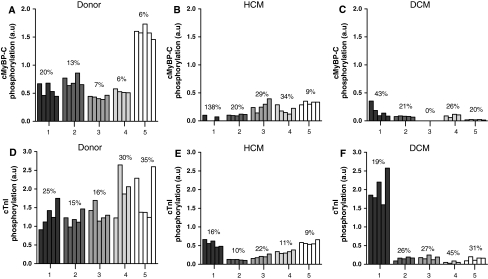
The variability within each LV sample was assessed by determining how much each of the 4–5 randomly selected tissue samples differed from the average of that heart and expressed as the coefficient of variation. The phosphorylation of cMyBP-C and cTnI in the different tissue samples within the three groups is shown in Fig. 2, with the coefficient of variation depicted above the bars for each individual. The coefficient of variation of cMyBP-C phosphorylation ranged from 6 to 20% in donor, from 9 to 138% in HCM and from 0 to 43% in DCM samples (Fig. 2a, b, c). Similarly, the variability in cTnI phosphorylation ranged from 15 to 35% among donor, from 9 to 22% between HCM and from 19 to 45% in DCM samples (Fig. 2d, e, f).
Fig. 2.
Intra-heart variability in protein phosphorylation. a–c cMyBP-C phosphorylation. d–f cTnI phosphorylation. The coefficient of variation is indicated above the bars for each heart
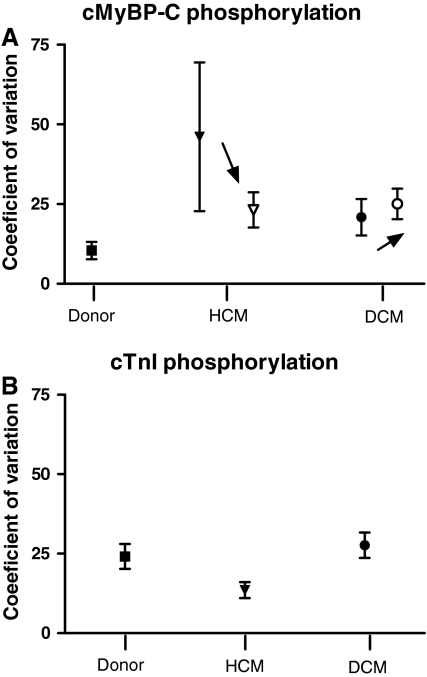
The variability within LV samples from each group was compared using one-way ANOVA (Fig. 3). The average coefficient of variation of cMyBP-C phosphorylation was slightly higher in HCM (46 ± 23%) and DCM (21 ± 6%) compared to donor (11 ± 3%), but the difference was not significant (Fig. 3a, closed symbols). The relatively wide spread in variability of cMyBP-C phosphorylation in HCM and DCM can be explained by the zero phosphorylation level in some of the samples from HCM 1 and DCM 3. The cMyBP-C phosphorylation in these samples was presumably below the detection level of the ProQ Diamond stain. Exclusion of HCM 1 and DCM 3 lowered the average coefficient of variation of cMyBP-C phosphorylation in HCM to 23 ± 6%, while the mean coefficient of variation became slightly higher in DCM (25 ± 5%). Furthermore, the large SEM of the coefficient of variation in HCM was lowered. Even after exclusion of HCM 1 and DCM 3 the difference in average variance between HCM and DCM and donor remained not significant (Fig. 3a, open symbols). Accordingly, it can be concluded that the average coefficient of variation for cTnI phosphorylation (Fig. 3b; closed symbols) did not differ significantly among groups (14 ± 3% in HCM; 28 ± 4% in DCM and 24 ± 4% in donor).
Fig. 3.
The coefficient of variation, a measure of how much the individual values of each heart differed from the mean, was on average not significantly different between donor, HCM and DCM for the phosphorylation status of cMyBP-C (a) or cTnI (b) (closed symbols). Within the analysis of cMyBP-C phosphorylation, exclusion of outliers (HCM 1 and DCM 3) lowered the mean coefficient of variation and the SEM in HCM, while the average coefficient of variation of the DCM group was slightly increased (as indicated by the arrow, open symbols). After exclusion of the outliers there was still no significant difference in coefficient of variation among the groups. Groups were compared by one-way ANOVA, P < 0.05 was considered significant
The average phosphorylation of cMyBP-C was lower in HCM and DCM than in donor (HCM 0.2 ± 0.05, DCM 0.1 ± 0.03 and donor 0.4 ± 0.01). Post hoc analysis indicated that cMyBP-C phosphorylation was significantly lower in DCM compared to donor. The average cTnI phosphorylation was significantly lower in both HCM and DCM compared to donor (0.4 ± 0.01 and 0.6 ± 0.4 vs. 1.5 ± 0.1, respectively).
Discussion
In this study the variability in phosphorylation of β-adrenergic target proteins within patients with HCM and DCM was determined. A comparison was made between protein phosphorylation status of cMyBP-C and cTnI in HCM and DCM patients and in donor.
Although some variation was found between different pieces from one heart sample, this variation was not significantly different in HCM and DCM samples compared to donor samples. Furthermore, the coefficient of variation of the cMyBP-C and cTnI phosphorylation in hearts was in the same range as the variability of the duplicate measurements on the ProQ stained gels, indicating that part of the variability within hearts can be ascribed to imprecision of the technique. Since the samples weighted approximately 2 g, our results do not rule out the possibility of transmural differences in protein phosphorylation (Cazorla et al. 2005; Davis et al. 2001).
Figure 2 demonstrates inter-patient variability in protein phosphorylation. Furthermore, it can be appreciated that in general the phosphorylation of cMyBP-C and cTnI is lower in HCM and DCM compared to donor, in accordance with previous data obtained in ischemic heart disease (van der Velden et al. 2003).
In conclusion, we demonstrate comparable variability of cMyBP-C and cTnI phosphorylation within hearts from HCM and DCM patients and donors, indicating that, within the precision of our measurements, small left ventricular tissue samples of end stage-failing hearts from primary cardiomyopathy patients are representative for the region they were taken from.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Minobe WA, Raynolds MV, Port JD, Rasmussen R, Ray PE, Feldman AM. Reduced beta 1 receptor messenger RNA abundance in the failing human heart. J Clin Invest. 1993;92:2737–2745. doi: 10.1172/JCI116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J. 2005;19:88–90. doi: 10.1096/fj.04-2066fje. [DOI] [PubMed] [Google Scholar]
- Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/S0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- El Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- Ramaraj R. Hypertrophic cardiomyopathy: etiology, diagnosis, and treatment. Cardiol Rev. 2008;16:172–180. doi: 10.1097/CRD.0b013e318178e525. [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Richard P, Villard E, Charron P, Isnard R. The genetic bases of cardiomyopathies. J Am Coll Cardiol. 2009;48:A79–A89. doi: 10.1016/j.jacc.2006.09.014. [DOI] [Google Scholar]
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end–stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res . 2003;57:37–47. doi: 10.1016/S0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- van Dijk SJ, Hamdani N, Stienen GJ, van der Velden J. Myocardial adaptations in the failing heart: cause or consequence? J Muscle Res Cell Motil. 2008;29:159–162. doi: 10.1007/s10974-009-9169-x. [DOI] [PubMed] [Google Scholar]
- Zaremba R, Merkus D, Hamdani N, Lamers JMJ, Paulus WJ, dos Remedios C, Duncker DJ, Stienen GJM, van der Velden J. Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomic Clin Appl. 2007;1:1285–1290. doi: 10.1002/prca.200600891. [DOI] [PubMed] [Google Scholar]