Introduction
Ricin toxin is a 64 kDa protein produced by castor beans (Ricinus communis) [reviewed in 1,2]. The holotoxin consists of two polypeptide chains (A and B) joined by a disulfide bond. The A chain (RTA) is a ribosome inactivating protein (RIP) that inhibits protein synthesis in mammalian cells. The B chain (RTB) is a lectin that binds to galactose residues on the surface of cells. Once internalized by a cell, RTA translocates into the cytosol where it enzymatically inactivates 60S ribosomes. A single molecule of RTA in the cytoplasm of a cell completely inhibits protein synthesis. The reported estimated lethal dose of ricin in humans is 1–25 μg/kg when inhaled, injected, or ingested [2–4]. Because of its wide availability and extraordinary toxicity, ricin represents a potential agent for use in bioterrorism [3] and is therefore classified by the Centers for Disease Control, Atlanta GA (CDC) as a level B biothreat. Ricin intoxication can be prevented in experimental animals by vaccination with toxoid [5] or deglycosylated ricin A chain (dgRTA) (Leonard Smith, USAMRIID, personal communication), or by passive immunization with anti-ricin antibodies [5,6]. However the toxoid is considered to be too toxic for routine use in humans and dgRTA is difficult and expensive to produce, and also retains both active sites and could induce toxic side effects in humans [7]. Passive immunization with anti-ricin antibodies is only effective if the ricin dose is relatively low and the antibody is administered within a few hours after exposure [5, 8–9].
In order to avoid these limitations, we developed a recombinant RTA vaccine, named RiVax, into which we have incorporated two point mutations, Y80A and V76M, to greatly reduce or eliminate both of its known toxicities, i.e. ribotoxicity and vascular leak-inducing ability [10,11]. In the absence of adjuvant, RiVax is non-toxic and immunogenic in mice, rabbits and humans [10–12]. Immune sera from all three species contained ricin-neutralizing antibodies, which could passively protect non-vaccinated mice from a lethal i.p. challenge with ricin. Mice immunized three times at four week intervals with at least 1.0 μg of vaccine were consistently protected against ricin challenges of 10 LD50s administered by one of three routes, intraperitoneal (i.p.) injection, oral gavage or inhaled aerosol [4].
It is anticipated that the target group for this vaccine will be personnel in the military as well as emergency first-responders. Since the conditions in some countries are less than ideal, with it being logistically difficult to maintain continuous cold chains, especially −20°C or −80°C, for transport and distribution of drugs, we sought to develop a formulation that was stable at 4°C or 25°C for extended periods of time. In the present study, we describe the development of a lyophilized formulation of the vaccine that shows no loss of function for at least one year when stored at either temperature, as determined by protection of mice given a lethal challenge of ricin by all three challenge routes.
Materials and Methods
Vaccine, ricin and antibodies
The construction, production and purification of RiVax have been described [10, 11]. Briefly, the gene for the enzymatically active wild type RTA, kindly provided by Dr. J. Michael Lord, Department of Biology Sciences, University of Warwick, Coventry, U.K. [13, 14], was genetically altered to eliminate both its cytotoxic activity (Y80A) and its ability to induce vascular leak (V76M). This construct was then inserted into pET28a (Novagen, Madison, WI), which relies on kanamycin rather than ampicillin for selection. Overnight cultures of E. coli strain BL21 (DE3) (Novagen) freshly transformed with this plasmid in Terrific Broth [15] containing 30 μg/mL kanamycin grown with agitation at 37°C were used to inoculate the same media in two 5 L fermentors (New Brunswick Scientific, Edmon, N.J.). Cultures were grown at 37°C with aeration to an OD600 of 0.6 to 0.8, then at 30°C to an OD600 of 1.0. Cultures were induced with 1.0 mM isopropyl-B-D-thiogalactopyranoside (IPTG) and grown overnight. Cells were harvested, resuspended in 2–3 mL phosphate-buffered saline (PBS) (50 mM phosphate buffered saline, pH 7.0) per gram of cell paste, and lysed by sonication. Cell debris was removed by centrifugation at 25,000 g for 30 min and supernatants were filtered (0.45 μm). This lysate was dialyzed overnight at 4°C against 10 mM phosphate buffer pH 6.5 and chromatographed on CM-Sepharose FF (Pharmacia, Piscataway, NJ) equilibrated with the same buffer. After washing, a 100 to 175 mM NaCl gradient was used for elution. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to identify the RiVax peak. The fractions containing this peak were pooled, dialyzed against PBS pH 7.5 buffer, and the preparation chromatographed over an Acticlean column (Sterogene Bioseparations, Carlsbad, CA) to remove endotoxin (this and all subsequent steps were carried out using water and reagents that were endotoxin-low). The collected fractions containing purified RiVax (flow through) were pooled and the amount of endotoxin was measured using a Limulus Amebocyte Lysate (LAL) assay (Associates of Cape Cod, East Falmouth, MA) as per the manufacturer's instructions. The protein concentration of the final product was usually between 0.5 and 1.0 mg/mL and the endotoxin levels were 0.5 to 10 EU/mg. This preparation was formulated in 10 mM Histidine-HCl, 144 mM NaCl, pH 6.0, 50% v/v glycerol, stored at −20°C, which has been determined to be an ideal formulation for stability [16]. This material was dialyzed overnight into 10 mM Histidine-HCl, 144 mM NaCl, pH 6.0 before use as control vaccine in the protection experiments or to prepare the 26 lyophilized formulations that we tested, as described in the results. The various formulations of vaccine (described in Results) were sterilized by filtration through a 0.22 μm filter, aliquotted into sterile vials, frozen at −80°C, lyophilized at ≤100 mBar at room temperature and sealed under vacuum. The vials were removed from the lyophilizer, their aluminum seals were crimped, and they were stored at either 4°C or room temperature for periodic stability testing. Random lyophilized vials were tested for water content by net weight determinations before and after 2 h at 150°C and found to be 1–2%.
Antibodies specific for the vaccine were purified from pooled immune serum using ricinconjugated CL-B4 Sepharose affinity chromatography in the presence of 0.1 M galactose [17]. Radioimmunoassay (RIA) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were used to verify the purity and integrity of the antibody.
Stability testing
At intervals, vials of the various formulations of lyophilized vaccine were reconstituted with water to their original concentration and each was inspected for insoluble particles immediately upon reconstitution and again one hour later. Samples that remained clear after one hour were concentrated to 1 mg/mL and evaluated by SDS-PAGE to detect breakdown products. Formulations that retained integrity for extended periods were also analyzed by HPLC (BioLogic, BioRad, Hercules CA) with a 7.8mm ID × 30 cm, 5μm.TSK G2000sw column (TOSHO BioScience) at 1 mL/min Histidine NaCl buffer, to determine the extent of aggregation of the preparations.
Radioimmunoassay (RIA) of anti-RiVax antibody
96 well plates (Costar, Corning, NY) were coated overnight at 4°C with 100 μL wild type (wt) RTA, or RiVax at 20 μg/mL in PBS. After washing and blocking the plates with 5% fetal calf serum (FCS), 100 μL of mouse anti-RiVax antibody at concentrations ranging from 1 to 1000 ng/mL (standard curve) or 100 μL of appropriate dilutions of the sera from vaccinated or control mice were added in triplicate. After overnight incubation at 4°C the plates were washed and [125I]-labeled affinity purified rabbit anti-mouse Ig (105 cpm/100 μL) was added. After a 2 hour incubation at room temperature, the plates were washed again and the radioactivity in the wells was counted in a gamma-counter (Pharmacia).
Vaccinations
Lyophilized RiVax was reconstituted with water to its original volume on the day of each vaccination. As a positive control, RiVax stored in 50% glycerol at −20°C was dialyzed overnight in Histidine NaCl buffer and its concentration adjusted to have the dose per 50 μL for each dose group. When using aluminum hydroxide, the vaccine was adsorbed onto alum on the day of each vaccination (final concentration 1 mg/mL). Vaccine doses that were used are described in the Results section. Female Swiss Webster mice, 6–8 weeks of age, (Taconic, Germantown, NY) were vaccinated with an intramuscular (i.m.) injection of 50 μL three times at 4 week intervals.
Ricin challenges
Mice were challenged 14 days after the final vaccination. The day prior to challenge, 100–150 μL of blood was collected from each mouse to determine levels of anti-RiVax antibodies. Ricin challenges in mice were performed as described [4] using ten times the LD50 as previously determined for each of the three challenge routes [4,10]. Briefly, for all i.p challenges with ricin, mice were injected with ricin in a volume of 100 μL PBS. For all gavage challenges, mice were moved to a clean cage without food 20 h prior to the intragastric gavage challenge. Mice were single-hand restrained and a 1.25 mm diameter gavage needle was gently inserted directly into their stomachs. The mice were dosed with a volume corresponding to 1% of their body mass (10 μL/g). Food was withheld for an additional 4 hours following the challenge. For the ricin aerosol exposures, mice were exposed in a nose-only exposure chamber (InTox, Moriarty, NM) with a total system airflow rate of 10 L min−1, precisely measured using a DryCal air flow meter (Bios, Butler, NJ). A small-particle aerosol with a median particle diameter of 2 μm was generated using a Lovelace nebulizer (InTox) in conjunction with a particle dryer. Respiratory minute volumes (total volume of air inhaled per minute) were estimated by using the formula, Log10 (V) (mL min−1) = −0.899 + 1.725 (Log10 body weight) (g), which is based on animal weight [18]. The estimated inhaled and retained dose of ricin was then determined from the minute volume, the calculated ricin concentration per L air, published particle retention rates, and exposure time. The exposed mice were observed daily for 14 days. Lung function in the mice was measured using a Buxco 12 chamber whole body plethysmograph (Wilmington, NC). This technique has been used to assess airway obstruction [19 and references therein]. For all challenge routes, mice were monitored and weighed daily for 14 days, a period of time sufficient for almost all surviving vaccinated animals to regain their initial weight. Mice were euthanized by CO2 asphyxiation followed by spinal dislocation when moribund or following 25% weight loss.
Results
Formulation and Testing
We evaluated 26 different formulations of our vaccine as lyophilized preparations. Batches of 0.2 mg/mL vaccine were prepared in two different concentrations of various sugars, all in 10 mM histidine, 144 mM NaCl pH 6.0,: 1. Sucrose (10% and 20%); 2. Trehalose (10% and 20%); 3. Dextran (5% and 10%); 4. Mannose (5% and 10%); 5. Sorbitol (10% and 20%); 6. Arginine (2% and 5%); 7. no additions (histidine/NaCl only). All were prepared with or without 0.04% Tween 80. These preparations were filtered-sterilized, aliquotted into sterile vials, frozen at −80°C, lyophilized at ≤100 mBar at room temperature and sealed under vacuum. The vials were removed from the lyophilizer, the aluminum seals were crimped and stored at either 4°C or room temperature for periodic stability testing.
Initial testing over a 16 week period involved reconstituting one vial of each formulation/concentration/temperature, and inspecting each for insoluble particles immediately upon reconstitution and again one hour later. Samples that remained clear after one hour were concentrated and run on SDS-PAGE to detect breakdown products. The dextran, mannose, and no sugar formulations were often cloudy. Though initially stable, the arginine and sorbitol formulations were occasionally cloudy or had insoluble aggregates (likely dimers and trimers etc.) and both began to fail after 2–4 weeks. All of these formulations were eventually eliminated from consideration, leaving only trehalose and sucrose formulations as candidates.
During this period we conducted a `pilot' vaccination study to determine if we retained immunogenicity following lyophilization. We chose to use the 20% sucrose with Tween stored at 4°C for this study. Groups of mice were vaccinated four times at weekly intervals and challenged one week later with 10 LD50s of ricin i.p.; groups of two male and female mice were vaccinated with formulation only for a negative control. All of the vaccinated mice survived the challenge and none lost more than 11% of their pre-challenge body weight, while none of the naïve mice survived the challenge (data not shown). From this we concluded that lyophilization does not decrease immunogenicity.
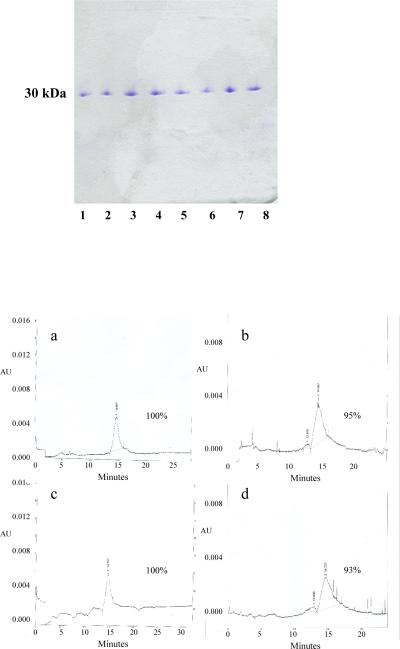
Select sucrose and trehalose formulations remained stable (clear and particle-free and >90 of the major band on SDS-PAGE) through 12 months. In general, formulations stored at 4°C were more stable than those at room temperature, and formulations with Tween were stable for longer than without Tween. In particular, all four sucrose preparations, with and without Tween, and both trehalose preparations without Tween, all stored at RT, failed the visual tests within 16 weeks. HPLC testing of the remaining contenders at one year showed that the sucrose preparations often had multiple peaks, containing what appeared to be both breakdown products and aggregates, often with <80% remaining in the main peak, so we eliminated sucrose. There remained under consideration four trehalose formulations of which we ultimately chose 20% trehalose with 0.04% Tween since, without Tween, both 10 and 20% trehalose were unstable at room temperature and we eliminated 10% with Tween because, on a single gel at 16 weeks of a vial stored at room temperature, it had multiple bands (though it passed all subsequent tests, through one year). SDS-PAGE and HPLC analysis of vials of this preparation, stored at either temperature for up to one year, are shown in Figure 1. Electrophoresis showed no detectable breakdown products and, as determined by HPLC, 93–100% of the material was in a single peak.
Figure 1. Stability of the lyophilized vaccine over the course of one year.
Lyophilized vaccine formulated with 20% Trehalose and 0.04% Tween was reconstituted with water to the original concentration of 0.2 mg/mL. A. SDS-PAGE Analysis. The vaccine was concentrated to 1 mg/mL and electrophoresed under non-reducing conditions on an 8% – 25 % gradient gel: Lyophilized vaccine was stored at room temperature for 12 months (lanes 1 and 2) or 6 months (lanes 3 and 4), or stored at 4°C for 12 months (lanes 5 and 6) or 6 months (lanes 7 and 8); the even numbered samples were filtered following reconstitution and concentration, the odd numbered samples were not. B. HPLC Analysis. 0.2 mg/mL samples were dialyzed against 10 mM histidine/144 mM NaCl, concentrated to 0.3 – 0.6 mg/mL, and analyzed on a TOSOH G2000SWxl 7.8 mm × 30 cm column (MW standards were also run but not shown). Panels A and B: storage at 4°C for 12 months; panels C and D: storage at room temperature for 12 months.
Vaccination
Having chosen our lyophilization formulation, we prepared >300 lyophilized vials of 20% trehalose with 0.04% Tween (T20T) and stored half at each temperature for long term stability testing, and to use for future studies with adjuvants and different routes of administration. Using this batch we conducted a series of mouse experiments to test immunogenicity over time as well as the effects of the adjuvant alum, which was added to the formulation after reconstitution.
Our first potency experiment was to compare T20T stored at both temperatures with vaccine freshly prepared from the glycerol stock. For the potency experiment, groups of mice were immunized three times every four weeks with 50 μL i.m. injections of 10 μg, 1 μg, or 0.1 μg doses of lyophilized vaccine stored at either 4°C or room temperature or with freshly dialyzed and identically formulated vaccine, or with formulation only. Two weeks after the final vaccination, serum samples were collected and the mice were challenged i.p. with a 10X LD50 dose of ricin . This experiment was conducted three times over the course of a year and the results are summarized in Table 1.
Table 1.
Summary of Antibody Titers and Survival Rates in Mice Following Ricin
| formulation1 | storage temp. | dose (μg × 3) | serum titer averages (μg/mL ± S.D.)2 | % survival3 (total) | ||||
|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | 3 mo | 6 mo | 12 mo | |||
| T20T | 4°C | 10 | 342 ± 58 | 204 ± 30 | 232 ± 4.3 | 100 (4) | 100 (7) | 100 (5) |
| 1 | 92 ± 38 | 107 ± 21 | 92 ± 2.4 | 100 (3) | 100 (8) | 100 (5) | ||
| 0.1 | 11 ± 7 | 75 ± 10 | 34 ± 4.7 | 50 (4) | 88 (8) | 20 (5) | ||
| T20T | RT | 10 | 150 ± 10 | 286 ± 28 | 237 ± 26 | 100 (4) | 100 (8) | 100 (5) |
| 1 | 113 ± 7 | 161 ± 28 | 82 ± 14 | 100 (4) | 100 (8) | 80 (5) | ||
| 0.1 | 24 ± 14 | 50 ± 13 | 7.5 ± 1.9 | 50 (4) | 75 (8) | 0 (5) | ||
| fresh | −20°C | 10 | 224 ± 53 | 399 ± 52 | 241 ± 19 | 100 (3) | 100 (7) | 100 (5) |
| 1 | 59 ± 9 | 127 ± 18 | 99 ± 15 | 100 (4) | 100 (8) | 100 (5) | ||
| 0.1 | 17 ± 8 | 68 ± 12 | 13 ± 4.5 | 50 (4) | 86 (7) | 0 (5) | ||
| control | 0 | 1 ± 0 | 1 ± 0 | 0.6 ± 0.1 | 0 (4) | 0 (8) | 0 (3) | |
Vaccine preparations used: T20T = trehalose, 20%, with 0.04% Tween 80; stored at 4°C or room temperature (RT); `fresh' vaccine = dialyzed from frozen 50% glycerol stock overnight as described in methods; `control' = formulation buffer only.
Serum titer averages: geometric means of the total antibody titers, as measured by RIA, with the standard deviation; number of mice for each value shown as the denominator in the survival section of this table; no significant differences between titers of the same dose levels except for: 0.1 μg T20T RT vs. fresh at 3 months (P < 0.05), 0.1 μg T20T 4°C vs RT at 12 months (P < 0.01); 0.1 μg T20T RT vs fresh at 12 months (P < 0.05), 0.01 μg T20T 4°C at 6 months vs 0.01 μg T20T RT at 3 months (P < 0.05), and 0.01 μg T20T 4°C at 6 months vs 0.01 μg T20T RT at 12 months (P < 0.05).
% Survival: the percentage of animals surviving the challenge at each timepoint. Experiments done once (3 and 12 month time points) or twice (6 month time point) with 3 to 5 mice per experiment, with the total number of mice as indicated in parentheses.
For each time point, the pre-challenge titers of the mice given the same dose levels were the same (no statistical differences by Student's T test) regardless of the storage conditions, except that 0.1 μg T20T RT was better than the freshly prepared vaccine at the 3 month timepoint (P < 0.005), and 0.1 μg T20T 4°C was better than both the 0.1 μg T20T RT and freshly prepared vaccine at the 12 month timepoint (P < 0.01 and P < 0.05 respectively), indicating that the 4°C preparation is somewhat more stable than the vaccine stored at room temperature. Comparisons between time points showed that there were no significant differences between the titers except for 0.01 μg T20T 4°C was significantly better at 6 months than either at 3 months or at 12 months (P < 0.05 for both); since there was no statistical difference between the 3 and 12 month time points, we do not consider this to indicate that the preparation was deteriorating.
At each time point we observed no significant difference between survival of the mice receiving the same dose levels of the differently stored material. However we did observe less protection in some mice vaccinated with the lowest dose level at the third time point. Additionally, a mouse in the 1 μg T20T RT dose group died at the 12 month time point. Since we see a moderate reduction in potency in all three test groups, including the group vaccinated with the fresh vaccine, we consider this to be an anomaly of the experiment and not a significant reduction in potency. This was substantiated in experiments that followed using our other two challenge routes.
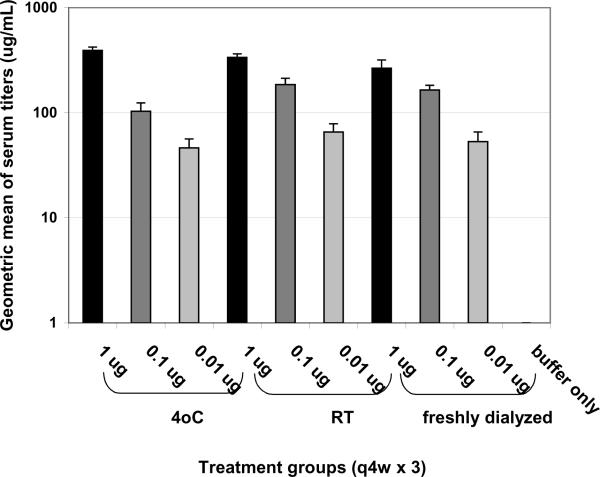
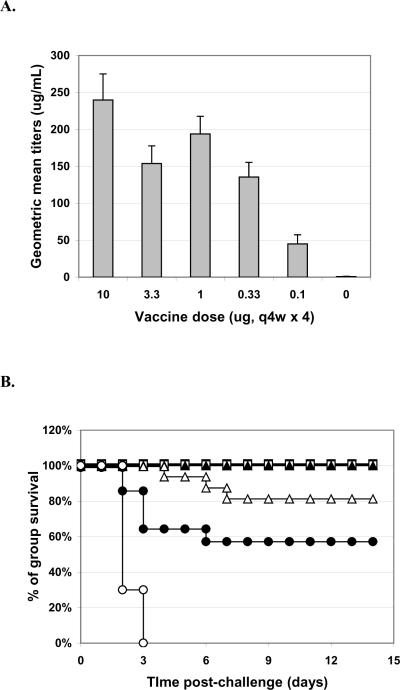
We also tested the potency of this batch of lyophilized vaccine adsorbed to alum at the six month time point. This experiment was performed exactly as above except: 1) that we used 10-fold lower doses of vaccine in order to be within the correct range to determine the minimum dose required for protection (based on past experience); and 2) for the addition of 1 mg/mL aluminum hydroxide (Alhydrogel or `alum') following reconstitution of the lyophilized vaccine or freshly dialyzed vaccine. As shown in Figure 2, the vaccine on alum induced titers comparable to vaccine without alum (Table 1) but it required 10-fold less vaccine. Similarly, the vaccine on alum protects the mice in a dose dependent manner (Figure 3) and was as protective as 10-fold more vaccine without alum (Table 1).
Figure 2. Pre-challenge serum titers.
Mice were vaccinated q4w × 3 with RiVax lyophilized and stored at 4°C or room temperature, or with freshly dialyzed vaccine, and all adsorbed to alum, as indicated. There were no statistical differences in titers between the same dose levels of different storage conditions. N = 8 mice/group, combined data from two experiments with four mice per group. Assays are run twice with triplicate wells per sample.
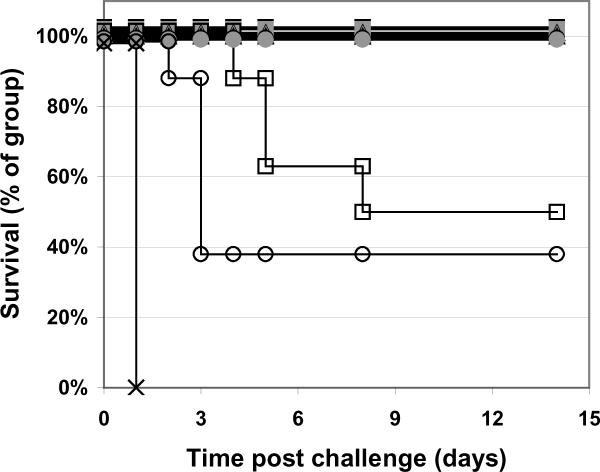
Figure 3. Survival curves of mice vaccinated with RiVax on alum; i.p. challenge.
Mice were immunized q4w × 3 with: 1 μg (■), 0.1 μg (■), or 0.01 μg (□) vaccine lyophilized and stored at 4°C, 1 μg (▲), 0.1 μg (▲), or 0.01 μg (Δ) vaccine lyophilized and stored at room temperature, or 1 μg (●), 0.1 μg (●), or 0.01 μg (∘) freshly dialyzed vaccine, of buffer only (×) N = 8 mice/group, combined data from two experiments with four mice per group.
Aerosol and Gavage Challenge
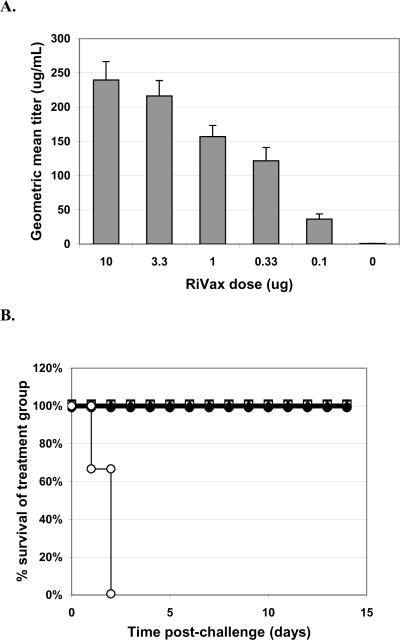
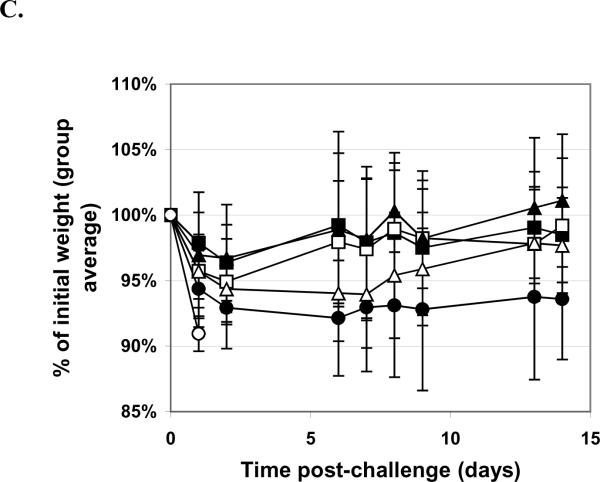
We also conducted experiments to test the lyophilized vaccine in our other two challenge models, oral gavage and aerosol. In these experiments, we tested five different dose levels of vaccine (10, 3.3, 1.0, 0.33, and 0.1 μg/dose q4w × 3) plus negative control; 14 days after the third dose mice were bled for serum titers and challenged with 10X LD50 ricin given either by oral gavage or by inhaled aerosol. These results were compared with historical data in mice receiving the same vaccine doses, regimen and challenge that were tested using freshly prepared vaccine. The results of the gavage experiments are shown in Figure 4. The lyophilized vaccine protected at even the very lowest dose level (0.1 μg/dose) while historically we had 100% survival in only the two highest dose groups and only 20% survival in the lowest dose group (historical data not shown; [4]). As shown in the weight data plot, the lowest two dose groups did have more weight loss than the highest three dose groups but with the standard error these differences are probably not significant
Figure 4. Gavage Challenge With Ricin.
Mice were vaccinated q4w × 3, with doses as indicated, with RiVax lyophilized and stored at 4°C. A. Pre-challenge serum titers. Geometric mean titers (each sample assayed twice) are presented with SD. B. Survival curves. RiVax dosing: 10 μg (■), 3.3 μg (□), 1 μg (▲), 0.33 μg, (Δ) or 0.1 μg (●) vaccine lyophilized and stored at 4°C, or buffer only (∘). C. Weight plots. Symbols as for B. N = 16 mice/group, combined data from four experiments with four mice per group.
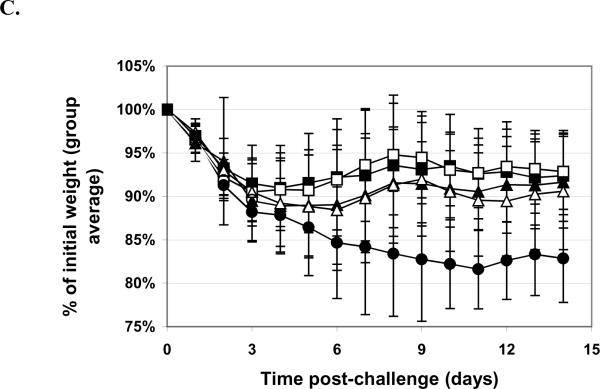
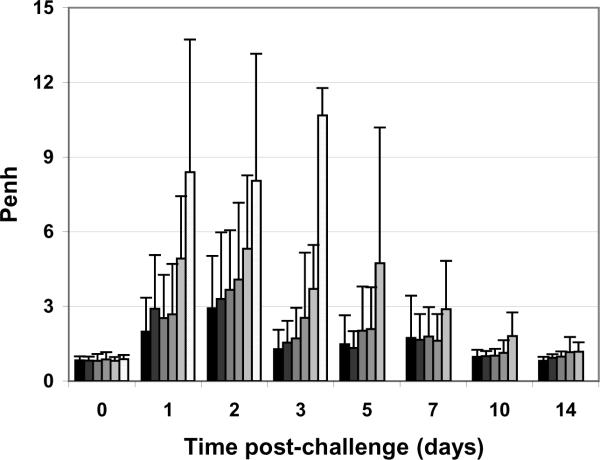
The data for the inhaled aerosol ricin challenges are shown in Figures 5. The results are indistinguishable from those obtained previously using freshly dialyzed RiVax. The lung function of the mice was monitored by whole body plethymosgraphy and, as shown in Figure 6, the vaccine protects the airway of the mice in a dose dependent manner.
Figure 5. Aerosol Challenge with Ricin.
Mice were vaccinated q4w × 3, with doses as indicated, with RiVax lyophilized and stored at 4°C. A. Pre-challenge serum titers. Geometric mean titers (each sample assayed twice) are presented with SD. B. Survival curves. RiVax dosing: 10 μg (■), 3.3 μg (□), 1 μg (▲), 0.33 μg , (Δ) or 0.1 μg (●) vaccine lyophilized and stored at 4°C, or buffer only (∘). C. Weight plots. Symbols as for B. N = 16 mice/group, combined data from four experiments with four mice per group.
Figure 6. Lung function analysis of mice vaccinated with lyophilized RiVax and challenged by aerosol.
Mice were immunized q4w × 3 with: 10 μg, 3.3 μg, 1.0 μg, 0.33 μg or 0.1 μg vaccine, or buffer only (greyscale dark to light). N = 16 mice/group, combined data from four experiments with four mice per group.
Discussion
Most vaccines are supplied as liquid formulations and must be stored at 4°C to ensure stability and potency. The removal of bulk water from vaccine could reduce the physical and chemical degradation of vaccine ingredients, and permit extended shelf lives and possibly the avoidance of the cold chain. Lyophilized vaccines that are currently approved for human use or being tested experimentally consist primarily of dead or attenuated organisms. Examples include live attenuated hepatitis A [20], influenza [21], Herpes Simplex 2 [22], and yellow fever [23]. Most of these are lyophilized in sugars such as sucrose [22,24–5], trehalose [22,24], glucose [24], mannitol [21], Pluronic F68P [21], dextran [21,22], lactose [23] and sorbitol glutamate [23] or mixtures of several of these [21–23]. Some include Tween [21] or histidine [23]. In addition, some are lyophilized with alum [24,26]. Most are as stable or more stable than liquid formulations and most are stored at 4°C. In contrast, there are only a few examples of lyophilized protein vaccines. These include group B Strep [25], CHO-derived Hepatitis (B) [24], diphtheria toxoid [26] and tetanus toxoid [26].
The goal of this study was to prepare a number of lyophilized RiVax formulations and evaluate them for stability and efficacy over time. The major findings to emerge were: 1) The vaccine was most stable as a lyophilized preparation when formulated in 10 mM histidine-HCl, pH 6.0 with 144 mM NaCl, 20% trehalose, 0.04% Tween 80 and stored at 4°C; 2) This formulation shows no deterioration of potency, in terms of total anti-vaccine titers or survival following an i.p. ricin challenge in mice, after one year; 3) When adsorbed to aluminum hydroxide following reconstitution, it is equipotent to vaccine/alum prepared fresh; 4) The lyophilized formulation protects as well in our two mucosal ricin challenge models as freshly prepared vaccine, which are the likely routes of ricin exposure if used as a terrorist weapon.
In total, 26 preparations, each stored at two different temperatures, 4°C and room temperature, were assessed for stability. Both sucrose- and trehalose-containing formulations (with and without the addition of Tween 80) performed well over one year but only the 20% trehalose with 0.04% Tween 80 passed every single stability test conducted at intervals throughout the entire year, and so it was chosen as our test formulation.
We conducted a series of experiments to compare the lyophilized vaccine formulation with vaccine freshly prepared from our reference standard, vaccine in Histidine-HCl buffer with 50 % glycerol stored at −20°C. We compared the vaccines directly at various dose levels using our `standard' efficacy model in mice, using a challenge dose of 10 times the LD50 delivered i.p. over the course of one year and found no significant loss if potency of the lyophilized preparation stored at 4°C and a slight loss in efficacy of the preparation stored at room temperature.
Based on previous studies with our vaccine, comparing the degree and durability of the immune response with or without adjuvant, it is clear that the vaccine is much more effective with the addition of an adjuvant, as expected. Thus, we also tested the lyophilized formulation of RiVax as an alum formulation; we adsorbed the vaccine to alum following reconstitution and compared it to freshly prepared alum vaccine preparations. The results from these studies indicate that there was no difference in potency. We made no attempt to develop a lyophilized alum formulation, since freezing aluminum salts commonly results in agglomeration, often with loss of potency [26–28]. There are reports that describe alum-formulated lyophilized vaccines prepared by spray freeze-drying techniques [26,29] or that utilize the addition of stabilizers [30] but these were not the subject of this study. We may consider evaluating these approaches in future since a lyophilized alum formulation would be optimal..
We also tested the potency of our lyophilized vaccine in our two mucosal challenge models, as these are the exposure routes that would be expected if ricin were used as a weapon of mass destruction either domestically or in battle. The lyophilized preparation was tested in both the gavage and aerosol models and we found that there was no loss of potency as compared to historical data.
Thus far we have testing the lyophilized formulation through one year and have found no measurable loss of stability or potency during this time. We will continue to assess this formulation over time but this formulation should be suitable for development into a licensed product for field use where the availability of a cold-chain is undependable. These results contribute to the ultimate goal of the development of the vaccine for use in those identified as being at risk for ricin intoxication, primarily military personnel and first responders. While the data presented here pertain to material stored for up tp one year, we are encouraged that we have devised a formulation that stabilizes our vaccine through lyophilization, storage, and reconstitution, which will enable use of the vaccine without requiring a cold chain. We will continue to test our vaccine over time to determine it's actual shelf life.
Acknowledgements
We thank Phyllis Barron for help with the mice, Steve Ruback, Kelly Mapes, JueYang, and Drew Ivey, for the production of vaccine, antibodies and running the assays, and Linda Berry for administrative assistance. We are also indebted to Drs. Rob Brey and John Schindler for helpful discussions.
Supported by NIH grant AI-056372
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doan LG. Ricin: mechanism of toxicity, clinical manifestations, and vaccine development. A review. Journal of Toxicology - Clinical Toxicology. 2004;42(2):201–8. doi: 10.1081/clt-120030945. [DOI] [PubMed] [Google Scholar]
- 2.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294(18):2342–51. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 3.Franz DR, Jaax NK. Ricin Toxin. In: Zajtchuk R, Bellamy RF, editors. Textbook of Military Medicine. Office of the Surgeon General at TMM Publications, Borden Inst., Walter Reed Army Medical Center; Washington, DC: 1997. pp. 631–642. [Google Scholar]
- 4.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25(42):7459–69. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewetson JF, Rivera VR, Creasia DA, Lemley PV, Rippy MK, Poli MA. Protection of mice from inhaled ricin by vaccination with ricin or by passive treatment with heterologous antibody. Vaccine. 1993;11(7):743–6. doi: 10.1016/0264-410x(93)90259-z. [DOI] [PubMed] [Google Scholar]
- 6.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–21. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths GD, Bailey SC, Hambrook JL, Keyte MP. Local and systemic responses against ricin toxin promoted by toxoid or peptide vaccines alone or in liposomal formulations. Vaccine. 1998;16(5):530–5. doi: 10.1016/s0264-410x(97)80007-2. [DOI] [PubMed] [Google Scholar]
- 8.Poli MA, Rivera VR, Pitt ML, Vogel P. Aerosolized specific antibody protects mice from lung injury associated with aerosolized ricin exposure. Toxicon. 1996;34(9):1037–44. doi: 10.1016/0041-0101(96)00047-5. [DOI] [PubMed] [Google Scholar]
- 9.Foxwell BM, Detre SI, Donovan TA, Thorpe PE. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology. 1985;34(1):79–88. doi: 10.1016/0300-483x(85)90080-0. [DOI] [PubMed] [Google Scholar]
- 10.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20(27–28):3422–7. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 11.Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005;23(39):4775–84. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci USA. 2006;103(7):2268–73. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Hare M, Brown AN, Hussain K, Gebhardt A, Watson G, Roberts LM, et al. Cytotoxicity of a recombinant ricin-A-chain fusion protein containing a proteolytically-cleavable spacer sequence. FEBS Lett. 1990;273(1–2):200–204. doi: 10.1016/0014-5793(90)81084-2. [DOI] [PubMed] [Google Scholar]
- 14.Simpson JC, Lord JM, Roberts LM. Point mutations in the hydrophobic C-terminal region of ricin A chain indicate that Pro250 plays a key role in membrane translocation. Eur. J. Biochem. 1995;232:458–463. doi: 10.1111/j.1432-1033.1995.tb20831.x. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd edition Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 16.Peek LJ, Brey RN, Middaugh CR. A rapid, three-step process for the preformulation of a recombinant ricin toxin A-chain vaccine. Journal of Pharm Sci. 2007;96(1):44–60. doi: 10.1002/jps.20675. [DOI] [PubMed] [Google Scholar]
- 17.Simmons BM, Russell JH. A Single Affinity Column Step Method for the Purification of Ricin Toxin from Castor Beans (Ricinus communis) Analytical Biochemistry. 1985;146(1):206–10. doi: 10.1016/0003-2697(85)90417-8. [DOI] [PubMed] [Google Scholar]
- 18.Depledge MH. Respiration and lung function in the mouse, Mus musculus (with a note on mass exponents and respiratory variables) Respiration Physiology. 1985;60(1):83–94. doi: 10.1016/0034-5687(85)90041-6. [DOI] [PubMed] [Google Scholar]
- 19.Rios AM, Mejias A, Chavez-Bueno S, Fonseca-Aten M, Katz K, Hatfield J, et al. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrobial Agents & Chemotherapy. 2004;48(8):2897–904. doi: 10.1128/AAC.48.8.2897-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Tan S, Shao C, Jin W, Ji Q, He J, et al. Safety and immunogenecity of lyophilized live attenuated hepatitis A vaccine in non-human primate model. Chinese Medical Journal. 2000;113(4):332–335. [PubMed] [Google Scholar]
- 21.Maa YF, Ameri M, Shu C, Payne LG, Chen D. Influenza vaccine powder formulation development: Spray freeze-drying and stability evaluation. J Pharm Sci. 2004;93(7):1912–23. doi: 10.1002/jps.20104. [DOI] [PubMed] [Google Scholar]
- 22.Zhai S, Hansen RK, Taylor R, Skepper JN, Sanches R, Slater NK. Effect of freezing rates and excipients on the infectivity of a live viral vaccine during lyophilization. Biotechnol. 2004;20(4):1113–20. doi: 10.1021/bp034362x. [DOI] [PubMed] [Google Scholar]
- 23.Monath TP. Stability of yellow fever vaccine. Dev Biol Stand. 1996;87(1):219–25. [PubMed] [Google Scholar]
- 24.Diminksy D, Moav N, Gorecki M, Barenholz Y. Physical, chemical and immunological stability of CHO-derived hepatitis B surface antigen (HBsAg) particles. Vaccine. 1999;18(1–2):3–17. doi: 10.1016/s0264-410x(99)00149-8. [DOI] [PubMed] [Google Scholar]
- 25.Paoletti LC. Potency of clinical group B streptococcal conjugate vaccines. Vaccine. 2001;19(15–16):2118–26. doi: 10.1016/s0264-410x(00)00437-0. [DOI] [PubMed] [Google Scholar]
- 26.Maa YF, Zhao L, Payne LG, Chen D. Stabilization of alum-adjuvanted vaccine dry powder formulations: mechanism and application. J Pharm Sci. 2003;92(2):319–32. doi: 10.1002/jps.10294. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, Tyagi A, Carpenter J, Perkins S, Sylvester D, Guy M, et al. Characterization of the freeze sensitivity of a hepatitis B vaccine. Hum Vaccines. 2009;5(1):26–32. doi: 10.4161/hv.5.1.6494. [DOI] [PubMed] [Google Scholar]
- 28.Clausi A, Cummiskey J, Merkley S, Carpenter JF, Braun LJ, Randolph TW. Influence of particle size and antigen binding on effectiveness of aluminum salt adjuvants in a model lysozyme vaccine. J Pharm Sci. 2008;97(12):5252–62. doi: 10.1002/jps.21390. [DOI] [PubMed] [Google Scholar]
- 29.Amorij JP, Huckriede A, Wilschut J, Frijlink HW, Hinrichs WL. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25(6):1256–73. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun LJ, Tyagi A, Perkins S, Carpenter J, Sylvester D, Guy M, et al. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine. 2009;27(1):72–9. doi: 10.1016/j.vaccine.2008.10.027. [DOI] [PubMed] [Google Scholar]